Abstract
Aging populations are more sensitive to noxious stimuli as a result of altered somatosensory systems. In these experiments, we examined pain-like behaviors in young, middle-aged, and old mice during peripheral inflammation to determine if the same sensitivity exists in pre-clinical animal models. Immediately following injury, middle-aged and old mice exhibited more spontaneous pain-like behaviors than young mice, matching pain prevalence in clinical populations. Middle-aged and old mice also developed persistent mechanical hypersensitivity in the injured paw. Furthermore, old mice developed mechanical hypersensitivity in the non-injured paw suggesting age-dependent changes in central nociceptive systems. To address this end, pain-related protein expression was examined in the central nucleus of the amygdala (CeA), a limbic brain region that modulates somatic pain. Following injury, increased phosphorylation of extracellular-signal regulated kinase 1 (ERK1), a protein with known nociceptive functions, was observed in the right CeA of old mice and not middle-aged or young animals. These findings suggest that age-dependent changes in supraspinal nociceptive systems may account for increased pain-like behaviors in aging populations.
Keywords: pain, amygdala, ERK1/2, mGluR5
Graphical Abstract
1. Introduction
As humans age, pain frequency and sensitivity increase, perhaps due to gradual changes in nociceptive sensory systems (Andersson et al., 1993; Bouhassira et al., 2008; Miaskowski, 2000; Patel et al., 2013). To date, very few basic science studies have investigated the changes that occur in nociceptive systems, specifically those in the brain, during natural aging; the focus of most published articles has been on peripheral nociceptive systems due to their direct, and possibly easier-to-treat link to pain. However, in the context of persistent pain, the central nervous system, and brain regions like the central nucleus of the amygdala (CeA) are critical for maintaining increased pain sensitivity and the affective abnormalities that often accompany these conditions (Veinante et al., 2013).
In the present study, we investigated the behavioral and molecular changes that occur in young, middle-aged, and old male C57Bl/6 mice following intraplantar formalin injection. Intraplantar formalin injection induces spontaneous pain-like behaviors in mice and rats that last for 60 minutes and mechanical hypersensitivity that lasts for several additional hours (Carrasquillo and Gereau, 2007; Fu et al., 2001; Kolber et al., 2010b). Both of these well-characterized behaviors are easy to measure and can reveal information about age-dependent changes in central and peripheral nociceptive modulation. Previous studies have revealed conflicting changes in spontaneous formalin behavior in both mice and rats, but no study has yet investigated if age affects mechanical sensitivity following formalin injection (Gagliese and Melzack, 2000; King-Himmelreich et al., 2015).
Formalin-induced mechanical hypersensitivity is dependent on higher brain structures including the CeA (Carrasquillo and Gereau, 2007). Specifically, group 1 metabotropic glutamate receptor (mGluR1 and mGluR5) signaling in the CeA is necessary for this behavior (Carrasquillo and Gereau, 2008; Kolber et al., 2010b). Through downstream activation of extracellular signal-regulated kinases 1 and 2 (ERK1/2), mGluR1/5 mediate CeA excitability after injury (Carrasquillo and Gereau, 2007; Kolber et al., 2010b; Neugebauer et al., 2003). Interestingly, hemispheric lateralization of this signaling cascade has been noted in inflammatory pain; both mGluR5 and ERK1/2 phosphorylation in the right CeA are necessary for formalin-induced mechanical hypersensitivity in mice, and pharmacological activation of mGluR5 (Crock et al., 2012; Kolber et al., 2010b) or ERK1/2 (Carrasquillo and Gereau, 2008) in the right CeA is sufficient to induce bilateral pain-like behaviors in uninjured mice. Unknown however, are the effects that age has on this signaling pathway prior to and following inflammatory insult.
Overall, the goal of these studies was to identify age-sensitive proteins involved in supraspinal pain modulation. By assessing both formalin-induced changes in behavior and concomitant changes in CeA protein expression, we identified ERK1 in the right CeA as a potential age-dependent modulator of inflammatory pain.
2. Materials and methods
2.1 Animals
Male C57Bl/6 mice were used for all experiments. “Young” mice were 2 mo, “middle-aged” mice were 12 mo, and “old” mice were 22 mo, consistent with life history (Dutta and Sengupta, 2016). Animals were housed on a 12h light/dark schedule with ad libitum access to rodent chow and water. All protocols were in accordance with National Institutes of Health guidelines and were approved by the Animal Care and Use Committee at Duquesne University (Pittsburgh, PA; protocol #1410-14).
2.2 Behavior testing
Due to the nature of the following behavioral responses, different cohorts of animals were used for each pain test; spontaneous formalin responses were recorded through glass bottom cages while von Frey mechanical sensitivity testing was performed through wire mesh screen.
2.2.1 Spontaneous pain-like behaviors following formalin injection
Formalin testing was performed as previously described (Kolber et al., 2010b). Mice (n=6) were bred in-house and/or acclimated to in-house conditions for at least two weeks after receipt from the National Institutes of Aging breeding colony. Briefly, animals habituated in 10cm2 × 15 cm Plexiglas boxes situated over a Logitech Pro 9000 HD camera for 1.5 hr. Low dB white noise was played throughout the experiment to mitigate background sounds. The experimenter was present in the room for 30 min prior to start of testing to eliminate any sex-induced analgesic effects (Sorge et al., 2014). 10 μL of 4% formalin (in 0.9% saline vehicle) was injected into the rear right paw; total time for injection was less than 1 min per animal. Animals were returned to Plexiglas boxes as soon as injection was complete. Animal behavior was recorded for 60 min following injection then scored offline by an experimenter blinded to age. Pain-like behaviors including licking, shaking, and biting of the injected paw were summed in 5 min bins across the entire 60 min test. Responses obtained during the first 10 min were attributed to peripheral mechanisms and those obtained from 10–60 minutes were attributed to central mechanisms.
2.2.2 Paw mechanical sensitivity testing
Peripheral mechanical sensitivity was assessed using calibrated von Frey filaments with the up/down method (Chaplan, et al., 1994). Briefly, animals (n=8–10) habituated in 10cm2 × 15 cm Plexiglas boxes resting on a wire mesh grid for 1.5 hr. White noise was played throughout the experiment to mitigate background sounds. The experimenter was present in the room for 30 min prior to start of testing. Using calibrated von Frey filaments (0.02g–2.56g) and the up/down method, 50% withdrawal thresholds (the application forces that cause an animal to remove its paw during 50% of applications) were determined for the left and right paws of each animal by an experimenter blinded to age, as previously described (Chaplan et al., 1994). Each paw was assessed twice (>5 min between trials) then averaged to obtain a baseline withdrawal threshold. Paw mechanical sensitivity was then re-assessed 60, 120, and 180 min following 10 μL injection of 4% formalin in the right rear paw.
2.2.3 Motor sensory assessment
Animals (n=6) were placed through six different tests to assess age-dependent deficits in gross motor behavior and coordination. Each test was performed twice; scores were averaged across trials as previously described (Kolber et al., 2010a). Testing was done blinded to mouse age.
Walking initiation test
Mice were placed in center of 21cm2 square marked on a tabletop. The time for all four paws to exit square was recoded.
Vertical pole descent test
Mice were positioned head-up at the top of a vertical metal pole (8 cm diameter, 55 cm height). The time it took each animal to turn 180° and climb down to the base of the pole was recorded; animals that slid down pole or reached bottom without changing direction were given a score of 120 s.
Ledge crossing test
Mice were placed on one end of 0.75 cm wide × 51 cm long Plexiglas ledge. The time animal remained on ledge was recorded (60 s max). If animal traversed entire length of ledge and returned to starting point in less than 60 s, 60 s score was assigned.
Platform test
Mice were placed on 3 cm diameter Plexiglas platform at top of 47 cm vertical pole. The time animal remained on platform was recorded (60 s max).
60 and 90° inclined screen tests
Mice were positioned head-down at the bottom of a 47 cm high × 18 cm wide wire mesh screen inclined at either 60 or 90°. The time it took animal to re-orient and climb to top of screen and total time on screen (60 s max) were independently recorded.
Inverted screen hang
Mice were placed in center of 47 × 18 cm wire mesh screen oriented at 60°. The screen was then inverted to 180°so that mice were upside-down. The time animal hung onto screen was recorded (120 s max).
2.3 Western blotting
mGluR5 and pERK1/2 expression levels were assessed in the left and right CeA of naive 2, 12, and 22 month old animals and 3 hr following formalin injection in separate cohorts of 2, 12, and 22 month old animals. Mice were bred in-house and/or acclimated for at least two weeks after receipt from the National Institutes of Aging breeding colony or Hilltop Laboratories (Scottdale, PA). To obtain CeA tissue, animals were decapitated, brains were removed then segmented into 500 μm thick coronal sections using a stainless steel brain mold (Stoelting), and punches were taken from the left and right CeA using a 1mm diameter punch tool (Stoelting). Punches were homogenized in ice-cold buffer (20 mM Tris-HCl, pH 7.5, 1 mM EDTA, 1 mM Na4P2O7, 100 μM PMSF, 25 μg/mL aprotinin, 25 μg/mL leupeptin, Sigma Phosphatase Inhibitors II and III) then assessed for total protein content using the Pierce BCA protein assay kit (Thermo Scientific). 8 μg of protein were separated on a 4–15% gradient polyacrylamide gel then transferred to a nitrocellulose membrane for immunoblotting.
Membranes were blocked in Odyssey blocking buffer for 1 hr, then co-incubated with mouse anti-pERK1/2 (1:1,000, Cell Signaling) and rabbit anti-ERK1/2 primary antibodies (1:1,000, Cell Signaling; in 0.1% Tween 20 in Odyssey blocking buffer) for 1 hr. Blots were rinsed with 0.1% Tween 20 in TBS (TTBS) then incubated with goat anti-mouse Alexa 680 (1:20,000, Molecular Probes) and goat-anti rabbit IR 800 secondary antibodies (1:20,000, Rockland; in 0.1% Tween 20 in Odyssey blocking buffer) for 1 hr. Blots were rinsed in TTBS then scanned on an infrared Odyssey imager. Immediately following successful imaging, blots were incubated in nitrocellulose stripping buffer (LiCor) for 5 min. Following PBS rinse, blots were re-blocked in Odyssey blocking buffer for 5 min then co-incubated with mouse anti-β-tubulin (1:10,000, Sigma Aldrich) and rabbit anti-mGluR5 primary antibodies (1:500, Genemed Synthesis Inc.; in 0.1% Tween 20 in Odyssey blocking buffer). Blots were rinsed in TTBS then incubated with goat anti-mouse Alexa 680 (1:20,000, Molecular Probes) and goat-anti rabbit IR 800 secondary antibodies (1:20,000, Rockland; in 0.1% Tween 20 in Odyssey blocking buffer) for 1 hr. Following a final rinse in TTBS, blots were again scanned on an Odyssey imager. ImageStudio Lite software (LiCor, version 4) was used to assess band signal strength for ERK1, ERK2, pERK1, pERK2, mGluR5, and β-tubulin in each sample. pERK1/2 signals were normalized to ERK1/2 and mGluR5 was normalized to β-tubulin within a sample. Quantification was carried out in a blinded fashion.
2.4 Statistical analysis
Prism 6 software (GraphPad) was used to analyze all data. Results are expressed as means ± SEM. Two-way ANOVA with repeated measures was used to analyze data sets containing two independent variables and one-way ANOVA was used to analyze data sets containing one independent variable; Bonferroni’s post hoc tests were employed when significant main effects were found. A value of p≤0.05 was considered statistically significant for all comparisons.
3. Results
Acute peripheral inflammation is a common cause of pain throughout all stages of life. Pain variability and intensity during inflammation however, vary with age in human populations. In these experiments, both peripherally and centrally mediated acute pain-like behaviors were assessed in young (2 mo), middle-aged (12 mo), and old (22 mo) mice in order to compare preclinical modeling to human responses across the life span.
3.1 Spontaneous pain-like behaviors following peripheral inflammatory injury vary with age
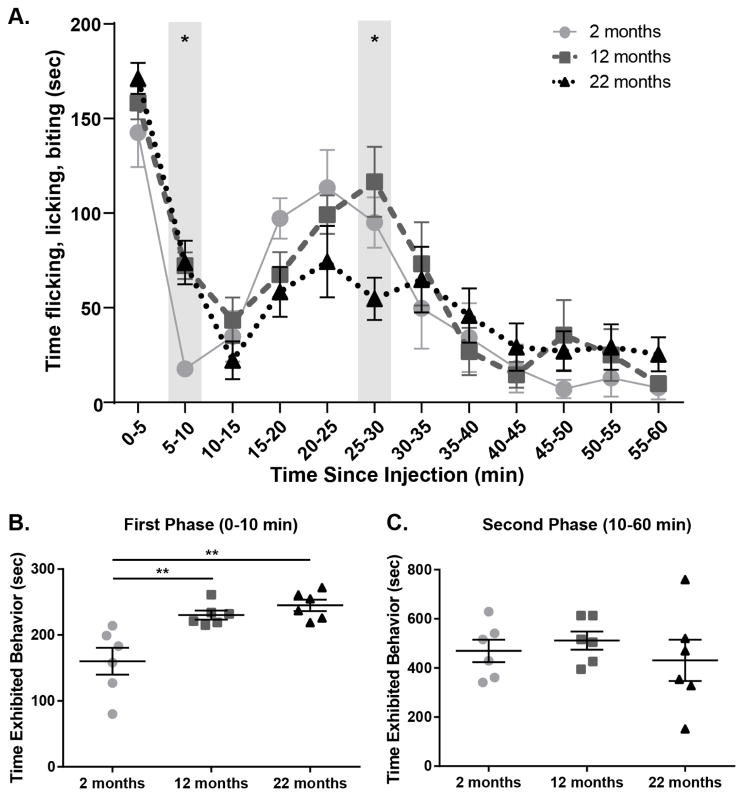
The formalin test was used to determine the effects of age on spontaneous nociceptive responses. To perform this assay, animals were injected with 4% formalin in the rear right paw then spontaneous pain-like behaviors (e.g. flicking, licking, biting) were recorded and tallied for the 60 min following injection. Typical bi-phasic response curves were observed in all age groups, however behavior times varied between ages during response progression (Figure 1A; Two-way ANOVA, effect of time, F(11, 165)=32.70, p<0.0001; time × age interaction, F(22, 165)=1.986, p=0.0082; n=6). When first (0–10 min) and second (10–60) phase responses were individually assessed, age-dependent differences were observed. During the peripherally mediated first phase, young animals spent significantly less time exhibiting pain-like behaviors than both middle-aged and old animals (Figure 1B; One-way ANOVA, F(2, 15)=11.53, p=0.0009; Bonferroni post-test, 2 mo vs. 12 mo p<0.01, 2 mo vs. 22 mo p<0.001). During the centrally mediated second phase however, all mice spent approximately the same total time exhibiting pain-like responses (Figure 1C; One-way ANOVA, F(2, 15)=0.4610, p=0.6393). Old animals were more variable in their responses during this period (Figure 1C), specifically demonstrating fewer pain-like behaviors than middle-aged animals 25–30 min following injection (Figure 1A; Bonferroni post-test, 12 mo vs. 22 mo at 25–30 min p<0.05). Overall, these results highlight age-dependent changes in peripheral processing of acute nociceptive stimuli, and suggest more complex age-dependent alterations in central nociceptive mechanisms.
Figure 1. Pain-like behaviors following peripheral inflammatory injury increase with age.
(a) All ages exhibited typical biphasic response to injury, however age-specific responses varied significantly across time (Two-way RM ANOVA, effect of time p<0.0001, time × age interaction p=0.0082; n=6). Specifically, young animals (2 mo) displayed significantly fewer pain-like behaviors than old animals (22 mo) during 5–10 min bin, and old animals displayed significantly fewer pain-like behaviors than middle age animals (12 mo) during 25–30 min bin (Bonferroni post-test 2 mo vs 22 mo at 5–10 min *p<0.05, 12 vs 22 mo at 25–30 min *p<0.05). (b) During the first phase of the test, middle age and older mice exhibited increased pain like behavior compared to young mice (One-way ANOVA, p=0.0009; Bonferroni post-test, 2 mo vs. 12 mo **p<0.01, 2 mo vs. 22 mo **p<0.01; n=6). (c) During the second phase of the test, mice exhibited overall equivalent pain like behavior compared across ages (One-way ANOVA, p=0.6393; n=6).
3.2 Hind paw mechanical sensitivity varies with age and injury state
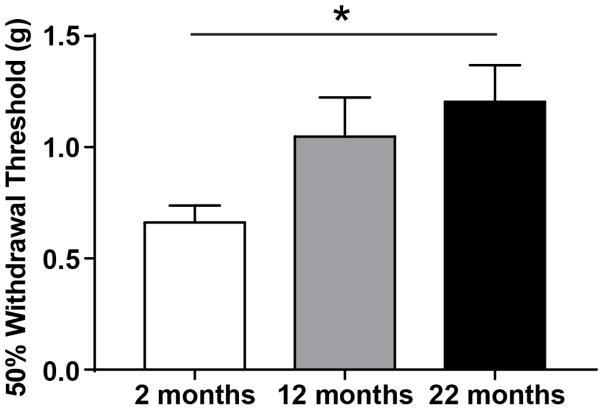
Age-dependent changes in both innocuous and noxious mechanical sensitivity have been reported in human somatosensory studies. To determine if C57Bl/6 naive male mice also exhibit age-dependent alterations in mechanical sensitivity, 50% hind paw withdrawal thresholds were determined in young, middle-aged, and old naive mice. Similar to the loss of sensitivity in aging humans, naive old mice also exhibited decreased mechanical sensitivity, withdrawing their paws at significantly higher thresholds than young animals (Figure 2; One-way ANOVA, F(2, 23)=4.315, p=0.0256; Bonferroni post-test 2 mo vs. 22 mo p<0.05; n=8–10).
Figure 2. Peripheral mechanical sensitivity decreases with age in naïve mice.
Mechanical sensitivity was assessed in young, middle-aged, and old naive male mice by probing both rear paws with calibrated von Frey filaments. 50% withdrawal thresholds increased with age; old animals have significantly higher withdrawal thresholds (i.e. reduced mechanical sensitivity) than young animals (One-way ANOVA p=0.0256; Bonferroni post-test, 2 mo vs 22 mo *p<0.05; n=8–10; L/R paw thresholds averaged for analysis).
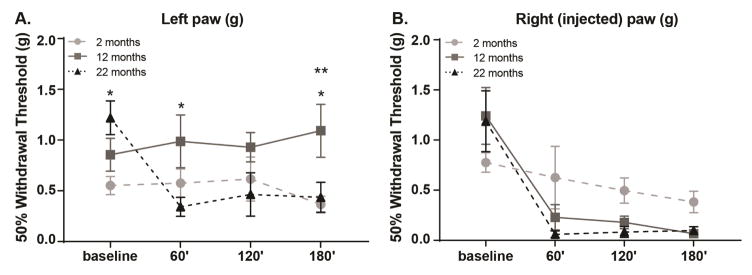
Mechanical sensitivity was reassessed during acute inflammation to determine if animal age also affected this measure. Withdrawal thresholds for both the left (non-injected) and right (injected) paws were calculated prior to and following formalin injection. Interesting age-dependent changes in mechanical sensitivity were observed in both the left (Figure 3A; Two-way ANOVA, time × age interaction, F(6, 69)=3.674, p=0.0032, main effect of age, F(2,23)=3.518, p=0.0464; n=8–10) and right paws (Figure 3B; Two-way ANOVA, time × age interaction, F(6, 66)=2.503, p=0.0309; n=8–10). As expected, significant decreases in withdrawal threshold were observed in the right (injected) paw; middle-aged and old mice developed dramatic mechanical allodynia that was maintained for 180 min following formalin injection (Figure 3B; Two-way ANOVA, effect of time, F(3, 66)=30.62, p<0.0001; Bonferroni post-test, 12 mo baseline vs. 60 or 120 min p<0.001, 12 mo baseline vs. 180 min p<0.0001, 22 mo baseline vs. 60, 120, or 180 min p<0.0001). Interestingly, mechanical allodynia also developed in the left (non-injected) paw of old mice and persisted for 180 min following formalin injection (Figure 3A; Two-way ANOVA, effect of time, F(3, 69)=2.125, p=0.0343; Bonferroni post-test, 22 mo baseline vs. 60 min p<0.001, 22 mo baseline vs. 120 or 180 min p<0.01).
Figure 3. Evaluation of mechanical hypersensitivity across age.
Formalin-induced mechanical hypersensitivity was evaluated 60, 120, and 180 min after right paw formalin injection. (a) Hypersensitivity development in the non-injected left paw varied with age (Two-way RM ANOVA, effect of age p=0.0464, time × age interaction p=0.0032; n=8–10). Old mice quickly developed robust hypersensitivity that was maintained for 180 min (Bonferroni post-test for 22 mo, baseline vs. 60 min ***p<0.001, baseline vs. 120 or 180 min **p<0.01). This old-aged specific hypersensitivity was significantly different than the responses of middle-aged mice 60′ and 180′ following injection (Bonferroni post-test at 60′ 12 vs. 22 mo *p<0.05, at 180′ 12 vs. 22 mo *p<0.05). Young mice also had significantly lower withdrawal thresholds 180′ following injection when compared to middle-aged mice, however these thresholds did not differ significantly from baseline thresholds for this age group (Bonferroni post-test at 180′ 2 vs. 12 mo **p<0.01, for 2 mo baseline vs. 60, 120, or 180 p>0.05). (b) Hypersensitivity development in the injected right paw varied between the ages across time (Two-way RM ANOVA, effect of time p<0.0001, time × age interaction p=0.0309; n=8–10). Both middle-aged and old mice exhibited significant hypersensitivity 60, 120, and 180 min following injection (Bonferroni post-test for 12 mo, baseline vs. 60 or 120 min ***p<0.001, baseline vs. 180 min ****p<0.0001; Bonferroni post-test for 22 mo baseline vs. 60, 120, or 180 min ****p<0.0001).
3.3 Age-dependent changes in motor function and coordination are not responsible for altered nociceptive responses
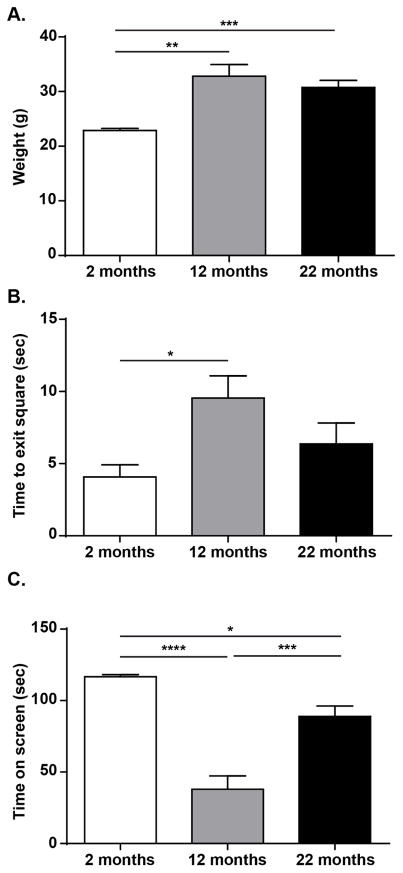
After observing increased pain-like behavior in old mice, we performed basic physiological assessments in all age groups to determine if impaired functioning led to the observed changes in behavior. Naive young, middle-aged, and old mice were placed through a motor sensory battery consisting of six different assays, weighed, and examined for gross anatomical variances. Both middle-aged and old mice weighed more than young mice (Figure 4A; One-way ANOVA, F(2, 15)=12.75, p=0.0006; Bonferroni post-test, 2 mo vs. 12 mo p<0.001, 2 mo vs. 22 mo p<0.01; n=6). The only motor/coordination assays that revealed age-specific differences were the walking initiation test and the inverted screen hang. Specifically, middle-aged animals took a longer time to exit a 21 cm2 square than young animals (Figure 4B; One-way ANOVA, F(2, 15)=4.381, p=0.0317; Bonferroni post-test 2 mo vs. 12 mo p<0.05; n=6). Likewise, middle-aged animals also failed to remain on the inverted screen as long as young or old animals; old animals performed only slightly better than middle-aged animals, still not hanging onto the screen as long as young mice (Figure 4C; One-way ANOVA, F(2, 15)=33.65, p<0.0001; Bonferroni post-test 2 mo vs. 12 mo p<0.001, 12 mo vs. 22 mo p<0.001, 2 mo vs. 22 mo p<0.05; n=6). Mice from all age groups had equal performances in the vertical pole descent, ledge crossing, platform balance, and 60° and 90° inclined screen tasks (data not shown). The only motor or coordination deficits suggested by these data are decreased walk initiation and ability to hold onto the inverted screen, both of which may have been related to increased weight gain in the older animals.
Figure 4. Age-dependent evaluation of weight and motor function.
(a) Middle-age and old mice weighed significantly more than young mice, however there was no weight difference between the older age groups (One-way ANOVA, p=0.0006; Bonferroni post-test, 2 mo vs. 12 mo ***p<0.001, 12 mo vs. 22 mo **p<0.01; n=6). (b) Middle aged mice took more time to exit a 21 cm2 square during the walking initiation test than young mice (One-way ANOVA, p=0.0317; Bonferroni post-test, 2 mo vs. 12 mo *p<0.05; n=6). (c) Additionally, middle-aged mice were not able to hang upside-down as long as young or old mice during the inverted screen hang test; old mice performed worse on this assay compared to young mice but better than middle-aged animals (One-way ANOVA, p<0.0001; Bonferroni post-test, 2 mo vs. 12 mo ***p<0.001, 12 mo vs. 22 mo ***p<0.001, 2 mo vs. 22 mo *p<0.05; n=6).
3.4 ERK1 activation in the right CeA occurs during peripheral inflammation in old mice
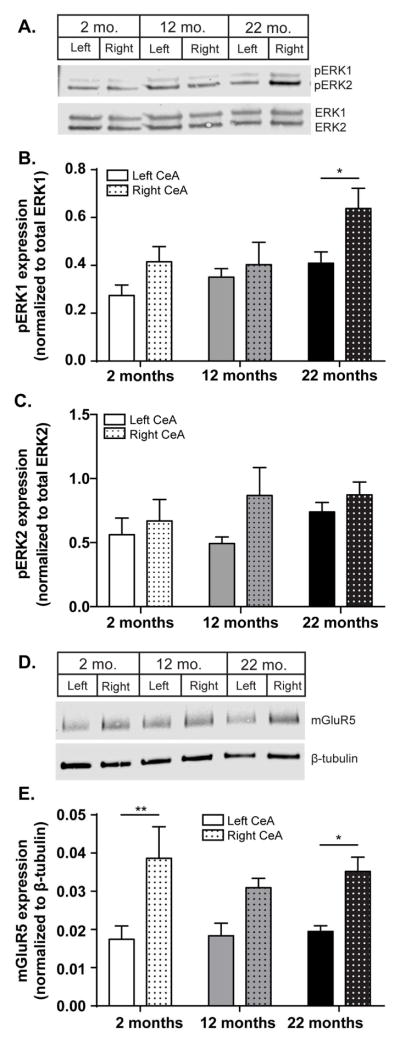
Formalin-induced mechanical allodynia is regulated, in part, by a specific signaling cascade within the right CeA (Carrasquillo and Gereau, 2007; Kolber et al., 2010b). To determine if the age-dependent changes observed in mechanical sensitivity (Figure 3A, 3B) matched age-dependent changes in this molecular pathway, pERK1/2 and mGluR5 expression were assessed 180 min after formalin injection. Age-dependent increases in pERK1 expression were observed following peripheral injury (Figure 5A, 5B). Specifically, old animals expressed more pERK1 in the right CeA than the left CeA (Figure 5B; Two-way ANOVA, effect of age, F(2, 45)= 4.114, p=0.0229, effect of side of brain, F(1, 45)= 5.639, p=0.0219; Bonferroni post-test, 22 mo left vs. right *p<0.05; n=6–9). ERK1 phosphorylation does not differ between age groups in naive conditions (young left CeA: 0.650±0.118, young right CeA: 0.794±0.146, middle-aged left: 0.641±0.068, middle-aged right: 0.712±0.150, old left: 0.805±0.196, old right: 0.574±0.060; two-way ANOVA, no main effect of age F(2, 14)=0.0393, p=0.9616, n=5–6). Additionally, ERK2 phosphorylation levels did not differ between sides of the brain or between different age groups following formalin injection (Figure 5C; Two-way ANOVA, no main effect of age F(2, 12)=0.8485, p=0.4476; n=6–9), or in naive conditions (young left: 3.271±0.326, young right: 3.275±0.219, middle-aged left: 3.205±0.214, middle-aged right: 3.411±0.532, old left: 3.233±0.495, old right: 3.062±0.216; two-way ANOVA, no main effect of age F(2, 14)=0.07949, p=0.9240, n=5–6).
Figure 5. Lateralized, age-dependent changes in CeA nociceptive signaling occur after peripheral formalin injection.
Peripheral injury is known to increase ERK activation partially through the signaling cascade downstream of mGluR5. Western analysis was used to evaluate these proteins 180 after formalin testing. (a) Representative blot of pERK and total ERK in the left and right CeA of young (2 mo), middle age (12 mo) and old (22 mo) mice. (b) Quantification of ERK1 activation showed significant age-dependent effects and that old animals express more pERK1 in the right CeA compared to the left CeA (Two-way ANOVA, effect of age p=0.0229, effect of side of brain p=0.0219; Bonferroni post-test, 22 mo left vs. right *p<0.05; n=6–9). (c) No age-dependent changes were noted in pERK2 expression following formalin injection (Two-way ANOVA, no main effect of age p=0.4476; n=6–9). (d) Representative blot of mGluR5 and loading control Beta-actin in the left and right CeA of young (2 mo), middle age (12 mo) and old (22 mo) mice. (e) Quantification revealed that following formalin injection, CeA mGluR5 expression is increased to approximately the same level across all age groups (Two-way ANOVA, effect of age p=0.7882; n=6). Notably, most of the mGluR5 expression changes appeared to occur in the right CeA; both young and old mice exhibit significantly more mGluR5 in the right CeA than the left CeA (effect of side of brain p<0.0001; Bonferroni post-test, 2 mo right vs. left ** p<0.01, 22 mo right vs. left *p<0.05).
mGluR5 is an upstream activator of ERK1/2 that is also required for the development of peripheral hypersensitivity. Based on the observed changes in pERK1, we hypothesized that age-dependent changes in mGluR5 expression would also coincide with age-dependent changes in mechanical sensitivity. We first assessed mGluR5 expression in naive mice as endogenous levels of this protein affect mechanical sensitivity in the absence of injury. Older naive mice trended towards decreased mGluR5 expression (mGluR5 expression 2 mo = 0.006 + 00012, 12 mo = 0.006 + 0.0004, 22 mo = 0.003 + 0.0006; One-way ANOVA, F(2, 18)= 0.3655, p=0.0507; n=6). 180 min following formalin injection however, CeA mGluR5 expression is increased to approximately the same level across all age groups, abolishing the age-dependent expression level trends that were suggested in naive animals (Figure 5D, 5E; Two-way ANOVA, effect of age, F(2, 15)= 0.2418, p=0.7882; n=6). Notably, the most robust mGluR5 expression changes appeared to occur in the right CeA; both young and old mice exhibit significantly more mGluR5 in the right CeA than the left CeA (Figure 5D; Two-way ANOVA, effect of side of brain, F(1, 15)= 35.48, p<0.0001; Bonferroni post-test, 2 mo right vs. left ** p<0.01, 22 mo right vs. left *p<0.05).
4. Discussion
In this study, we investigated the effects of animal age on common acute nociceptive measures and the molecular changes in the CeA that accompany these behaviors. Specifically, spontaneous pain-like behaviors and mechanical sensitivity were assessed in young, middle-aged, and old male C57Bl/6 mice following an intraplantar formalin injection. These behavioral assessments allowed for the dissection of age-dependent changes in both peripheral and central nociceptive systems, the latter of which has received very little attention in basic science research. Pain-signaling pathways in the CeA were assessed across age, ultimately revealing phosphorylated ERK1 as a potential modulator of increased pain sensitivity in old mice.
Although not the main focus of this paper, several peripherally mediated sensory changes were observed in these experiments. Prior to nociceptive insult, old mice exhibited low tactile sensitivity (i.e. increased mechanical withdrawal thresholds) compared to young mice. These data are consistent with previous reports in old C57Bl/6 mice (Garrison and Stucky, 2014) and geriatric populations, both of which show low tactile sensitivity (Wickremaratchi and Llewelyn, 2006). However other reports demonstrate the opposite trend in old mixed strain mice (Weyer et al., 2016) and rats (Kitagawa et al., 2005), or no effect of age on tactile sensitivity at all (Wang et al., 2006). After the initiation of acute inflammatory pain, the hyposensitivity in middle-aged and old mice was reversed as both ages exhibited significant pain-like behaviors during the first phase of the formalin test and robust ipsilateral mechanical allodynia for at least 180 min following injection. These data suggest gradual increases in peripheral nociceptive sensitivity that first manifest in mid-life and persist through old age. Similar changes in pain sensitivity are also observed in human populations; many chronic pain disorders are first diagnosed in mid-life however pain sensitivity continues to increase with age (Andersson et al., 1993; Bouhassira et al., 2008; Patel et al., 2013). The possible mechanisms responsible for altered peripheral nociceptive transmission are several fold; many others have demonstrated aging effects on peripheral afferent structure, channel protein expression, and resulting electrophysiological integrity (Garrison and Stucky, 2014; Wang et al., 2006; Weyer et al., 2016). Relevant to these studies however, expression of TrpA1, the cation channel responsible for formalin-induced pain, is similarly increased in dorsal root ganglia of young and old mice at acute time points following Complete Freud’s Adjuvant paw injections (Weyer et al., 2016). We expect that similar age-independent increases in TrpA1 occur following formalin injection, thus eliminating this target as the sole generator of age-dependent pain sensitivities. Additionally, the aging immune system cannot be ruled out as age is known to affect inflammatory responses and healing following injury (Arnardottir et al., 2014; Gagliese, 2009; Shaw et al., 2013).
Following formalin injection, old mice developed tactile hypersensitivity in the non-injected paw most likely as a result of altered central nociceptive systems. With efferent connections to key descending nociceptive regions including the periaqueductal gray and rostral ventromedial medulla, the CeA is well positioned to modulate both higher cortical components of pain and more rudimentary reflexive pain responses like the paw withdrawal behaviors measured in these experiments (Veinante et al., 2013). Previously, contralateral paw hypersensitivity was reported in young Swiss-Webster and C57Bl/6 male mice following formalin injection; these behaviors were credited to mGluR5 and ERK1/2 signaling in the right CeA (Carrasquillo and Gereau, 2007; Kolber et al., 2010b). In line with one of these reports, we too observed increases in ERK1 phosphorylation in the right CeA three hours following formalin injection (Kolber et al., 2010b). Although ERK1 phosphorylation generally increased with age, the most robust increases in activation of this protein were observed in the right CeA of old mice; significant increases in right-lateralized pERK1 expression were not observed in young or middle-aged mice. To definitively contribute altered age-dependent nociceptive behaviors to increases in ERK1 phosphorylation, functional blockade of MEK, the ERK1/2 kinase should be performed in young, middle, and old age mice. Unfortunately, these experiments were beyond the scope of the current study. No age or side-dependent increases in ERK2 phosphorylation were observed 180 min following formalin injection. This is in contrast with previous reports in which young mice (both Swiss Webster and C57Bl/6 males) developed contralateral hypersensitivity and expressed increased levels of pERK2 in the right CeA 180 min following formalin injection (Carrasquillo and Gereau, 2007; Kolber et al., 2010b). Result differences may be due to formalin concentration (4% dose used in these studies was validated in unpublished study from our lab), animal strain, or animal source among other variables. Nonetheless, the finding of significant age-dependent changes in pERK1, and not pERK2, highlights the fact that these proteins, while similar, do not show always perform parallel functions. Other functional differences between ERK1 and ERK2 have been found in peripheral nociceptive systems where ERK2 seems to play a larger role in modulating pain-like behavior and nociceptor development (Alter et al., 2010; O’Brien et al., 2015).
ERK phosphorylation in the CeA occurs downstream of multiple signaling cascades including group I metabotropic glutamate receptor (mGluR1/5) signaling pathways which can include protein kinase C (PKC) (Carrasquillo and Gereau, 2007; Cheng et al., 2011) or protein kinase A (PKA) (Fu et al., 2008). To determine if expression of mGluR5 was linked to age-dependent changes in ERK1 phosphorylation, and potentially nociceptive behaviors, mGluR5 levels were analyzed at baseline and 180 min following formalin injection. In the naive condition, older animals trend towards decreased mGluR5 in the CeA, providing one potential central mechanism for the decreased baseline mechanical sensitivity observed in these animals. 180 min following formalin injection, there was no significant difference in right CeA mGluR5 expression between young, middle age, or old mice, suggesting that other receptors and signaling systems are driving the changes in ERK1 activation.
To our knowledge, only two other papers have investigated the effects of age on signaling in the amygdala, a region that is not only important for acute pain modulation but also for the development and maintenance of chronic pain disorders which disproportionately affect older populations (Badowska-Szalewska et al., 2015; Hess et al., 1981; Veinante et al., 2013). These papers demonstrated age-dependent increases in expression of IL-β, an inflammatory cytokine, and opioid receptors in the amygdala, again suggesting that mGluR5 and MEK signaling pathways are not the exclusive modulators of age-dependent nociceptive behaviors (Badowska-Szalewska et al., 2015; Hess et al., 1981). Additional studies are required to determine if the observed behavioral and molecular changes are applicable across sexes and relative to chronic pain susceptibility and severity. Further studies are also needed to determine if changes in other central nervous system structures, especially those anatomically and functionally connected to the amygdala, contribute to age-dependent changes in pain sensitivity.
This basic science report, and others like it, is critical for understanding how aging affects central nociceptive systems. Overall, these data support the conclusion that gradual molecular changes in central regions like the CeA can lead to altered pain-related behavior in old animals.
Highlights.
Older mice exhibit more pain-like behaviors than young mice after injury.
Peripheral injury induces widespread mechanical sensitivity only in old mice.
Pain-related proteins are activated in the right CeA of old mice after injury.
Acknowledgments
This work was supported by the Duquesne University Aging Research and Teaching Consortium (Research Stimulator Grant to KES), the Duquesne University Chronic Pain Research Consortium (Research Stimulator Grant to JEC and BJK), and National Institutes of Complementary and Integrative Health (R15AT008060 to BJK). We would like to acknowledge technical assistance from Neil Lax and Jordan Waddell.
Abbreviations
- CeA
central nucleus of the amygdala
- ERK1/2
extracellular signal-regulated kinase 1/2
- mGluR5
metabotropic glutamate receptor 5
- pERK1/2
phosphorylated extracellular signal-regulated kinase 1/2
Footnotes
Author Contributions: KES, JEC, and BJK designed experiments. KES, NG, and BJK performed experiments. KES, JEC, and BJK analyzed data. KES and BJK wrote manuscript. KES, NG, JEC, and BJK edited manuscript.
Declaration of Conflicting Interests: The authors have no conflicts to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alter BJ, Zhao C, Karim F, Landreth GE, Gereau RW. Genetic targeting of ERK1 suggests a predominant role for ERK2 in murine pain models. J Neurosci. 2010;30:11537–47. doi: 10.1523/JNEUROSCI.6103-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson HI, Ejlertsson G, Leden I, Rosenberg C. Chronic pain in a geographically defined general population: studies of differences in age, gender, social class, and pain localization. Clin J Pain. 1993;9:174–182. doi: 10.1097/00002508-199309000-00004. [DOI] [PubMed] [Google Scholar]
- Arnardottir HH, Dalli J, Colas RA, Shinohara M, Serhan CN. Aging Delays Resolution of Acute Inflammation in Mice: Reprogramming the Host Response with Novel Nano-Proresolving Medicines. J Immunol. 2014;193:4235–4244. doi: 10.4049/jimmunol.1401313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badowska-Szalewska E, Ludkiewicz B, Spodnik JH, Krawczyk R, Moryś J. The influence of mild stressors on neurons containing interleukin-1β in the central (CeA) and medial (MeA) amygdala in the ageing process of rats. Acta Neurobiol Exp (Wars) 2015;75:279–92. [PubMed] [Google Scholar]
- Bouhassira D, Lanteri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136:380–387. doi: 10.1016/j.pain.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Carrasquillo Y, Gereau RW. Hemispheric lateralization of a molecular signal for pain modulation in the amygdala. Mol Pain. 2008;4:24. doi: 10.1186/1744-8069-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasquillo Y, Gereau RW. Activation of the extracellular signal-regulated kinase in the amygdala modulates pain perception. J Neurosci. 2007;27:1543–1551. doi: 10.1523/JNEUROSCI.3536-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Cheng SJ, Chen CC, Yang HW, Chang YT, Bai SW, Chen CC, Yen CT, Min MY. Role of Extracellular Signal-Regulated Kinase in Synaptic Transmission and Plasticity of a Nociceptive Input on Capsular Central Amygdaloid Neurons in Normal and Acid-Induced Muscle Pain Mice. J Neurosci. 2011;31:2258–2270. doi: 10.1523/JNEUROSCI.5564-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crock LW, Kolber BJ, Morgan CD, Sadler KE, Vogt SK, Bruchas MR, Gereau RW. Central amygdala metabotropic glutamate receptor 5 in the modulation of visceral pain. J Neurosci. 2012;32:14217–26. doi: 10.1523/JNEUROSCI.1473-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Sengupta P. Men and mice: Relating their ages. Life Sci. 2016;152:244–8. doi: 10.1016/j.lfs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- Fu KY, Light AR, Maixner W. Long-lasting inflammation and long-term hyperalgesia after subcutaneous formalin injection into the rat hindpaw. J Pain. 2001;2:2–11. doi: 10.1054/jpai.2001.9804. [DOI] [PubMed] [Google Scholar]
- Fu Y, Han J, Ishola T, Scerbo M, Adwanikar H, Ramsey C, Neugebauer V. PKA and ERK, but not PKC, in the amygdala contribute to pain-related synaptic plasticity and behavior. Mol Pain. 2008;4:26. doi: 10.1186/1744-8069-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliese L. Pain and aging: the emergence of a new subfield of pain research. J Pain. 2009;10:343–53. doi: 10.1016/j.jpain.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Gagliese L, Melzack R. Age differences in the response to the formalin test in rats. Neurobiol Aging. 2000;20:699–707. doi: 10.1016/s0197-4580(99)00061-5. [DOI] [PubMed] [Google Scholar]
- Garrison SR, Stucky CL. Contribution of transient receptor potential ankyrin 1 to chronic pain in aged mice with complete Freund’s adjuvant-induced arthritis. Arthritis Rheumatol (Hoboken, NJ) 2014;66:2380–90. doi: 10.1002/art.38724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess GD, Joseph JA, Roth GS, Forman LJ, Meites J, Rigter H, McGaugh JL. Effect of age on sensitivity to pain and brain opiate receptors. Neurobiol Aging. 1981;2:49–55. doi: 10.1016/0197-4580(81)90059-2. [DOI] [PubMed] [Google Scholar]
- King-Himmelreich TS, Moser CV, Wolters MC, Olbrich K, Geisslinger G, Niederberger E. Age-Dependent Changes in the Inflammatory Nociceptive Behavior of Mice. Int J Mol Sci. 2015;16:27508–19. doi: 10.3390/ijms161126041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa J, Kanda K, Sugiura M, Tsuboi Y, Ogawa A, Shimizu K, Koyama N, Kamo H, Watanabe T, Ren K, Iwata K. Effect of chronic inflammation on dorsal horn nociceptive neurons in aged rats. J Neurophysiol. 2005;93:3594–604. doi: 10.1152/jn.01075.2004. [DOI] [PubMed] [Google Scholar]
- Kolber BJ, Boyle MP, Wieczorek L, Kelley CL, Onwuzurike CC, Nettles SA, Vogt SK, Muglia LJ. Transient early-life forebrain corticotropin-releasing hormone elevation causes long-lasting anxiogenic and despair-like changes in mice. J Neurosci. 2010a;30:2571–81. doi: 10.1523/JNEUROSCI.4470-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolber BJ, Montana MC, Carrasquillo Y, Xu J, Heinemann SF, Muglia LJ, Gereau RW, Iv, Gereau R., Iv Activation of metabotropic glutamate receptor 5 in the amygdala modulates pain-like behavior 1/2 (ERK1/2) J Neurosci. 2010b Jun;16:8203–8213. doi: 10.1523/JNEUROSCI.1216-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C. The impact of age on a patient’s perception of pain and ways it can be managed. Pain Manag Nurs. 2000;1:2–7. doi: 10.1053/jpmn.2000.9760. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Bhave G, Gereau RW. Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5. J Neurosci. 2003;23:52–63. doi: 10.1523/JNEUROSCI.23-01-00052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien DE, Alter BJ, Satomoto M, Morgan CD, Davidson S, Vogt SK, Norman ME, Gereau GB, Demaro JA, Landreth GE, Golden JP, Gereau RW. ERK2 Alone Drives Inflammatory Pain But Cooperates with ERK1 in Sensory Neuron Survival. J Neurosci. 2015;35:9491–507. doi: 10.1523/JNEUROSCI.4404-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KV, Guralnik JM, Dansie EJ, Turk DC. Prevalence and Impact of Pain among Older Adults in the United States: Findings from the 2011 National Health and Aging Trends Study. Natl Institutes Heal. 2013;154:1–22. doi: 10.1016/j.pain.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13:875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, Leger P, Mapplebeck JCS, McPhail M, Delaney A, Wigerblad G, Schumann AP, Quinn T, Frasnelli J, Svensson CI, Sternberg WF, Mogil JS. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 2014;11:629–32. doi: 10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- Veinante P, Yalcin I, Barrot M. The amygdala between sensation and affect: a role in pain. J Mol psychiatry. 2013;1:9. doi: 10.1186/2049-9256-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Davis BM, Zwick M, Waxman SG, Albers KM. Reduced thermal sensitivity and Nav1.8 and TRPV1 channel expression in sensory neurons of aged mice. Neurobiol Aging. 2006;27:895–903. doi: 10.1016/j.neurobiolaging.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyer AD, Zappia KJ, Garrison SR, O’Hara CL, Dodge AK, Stucky CL. Nociceptor Sensitization Depends on Age and Pain Chronicity. eNeuro. 2016:3. doi: 10.1523/ENEURO.0115-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickremaratchi MM, Llewelyn JG. Effects of ageing on touch. Postgrad Med J. 2006;82:301–4. doi: 10.1136/pgmj.2005.039651. [DOI] [PMC free article] [PubMed] [Google Scholar]