Abstract
Metastasis is a serious risk that may occur during the treatment of hepatocellular carcinoma (HCC), preventing many patients from being surgical candidates and contributing to poor prognosis. Hypoxia has been proved an important factor of metastasis through the epithelial–mesenchymal transition (EMT) pathway. Acetyl‐CoA synthase 2 (ACSS2) provides an acetyl group for the acetylation of hypoxia‐inducible factor (HIF)‐2α, and this epigenetic modification affects the activity of HIF‐2α and the subsequent EMT process. Here, we showed that ACSS2 expression was negatively correlated with HCC malignancy. Knockdown of ACSS2 increased the invasion and migration ability of HCC cells and promoted EMT without increasing the total protein level of HIF‐2α, even in hypoxic conditions. The immunoprecipitation assay revealed downregulated acetylation levels of HIF‐2α after ACSS2 knockdown in hypoxic conditions, which resulted in enhanced HIF‐2α activity. Finally, decreased expression of ACSS2 was found to be related to advanced stage and poor overall survival and disease‐free survival rates in a cohort of patients with HCC. In conclusion, ACSS2 plays an important role in the acetylation process of HIF‐2α, which effectively modifies the activity of HIF‐2α under hypoxic conditions and greatly impacts on the prognosis of patients with HCC.
Keywords: Acetylation, ACSS2, hepatocellular carcinoma, HIF‐2α, metastasis
Hepatocellular carcinoma (HCC) is one of the most malignant cancers and has high morbidity and mortality rates in China.1 Surgery is considered one of the best treatment options for patients with this disease,2, 3 although metastasis greatly limits the number of patients who are surgical candidates. Not only do many patients diagnosed with HCC show metastasis, but many postoperative patients also suffer from metastasis after undergoing curative resection.4 Therefore, metastasis is a major issue in the treatment of HCC, and elucidating its underlying mechanism is a key research topic.
Hypoxia is a common phenomenon that occurs in solid tumors, especially after tumor growth.5 Hypoxia can result in many changes in the genomic expression of tumor cells, allowing cells to adapt to harsh environments that may be associated with metastasis and poor prognosis.6, 7, 8, 9 According to many reports, a series of changes caused by hypoxia is regulated by hypoxia‐inducible factors (HIFs), namely, HIF‐1α and HIF‐2α. Hypoxia‐inducible facotr‐2α is more common in chronic hypoxia than HIF‐1α and is more specifically expressed in endothelial cells and hepatocytes.10 It has been reported that both the expression level and acetylation status of HIF‐2α are precisely adjusted in hypoxia.11 We previously reported that HIF‐2α can regulate CDCP1(CUB domain containing protein 1) to promote PKCδ(protein kinase Cδ)‐mediated migration in HCC.12
Acetyl‐CoA synthase 2 (ACSS2) is an enzyme that converts acetate to acetyl‐CoA, which is an important intermediate metabolite.13, 14 Acetyl‐CoA synthase 2 also plays a key role in the acetylation of HIF‐2α, which can provide the acetyl group for acetylation.15, 16 Recent studies on ACSS2 have been carried out for a variety of cancers, and there are is controversy regarding the effect of ACSS2 on HCC and other cancers.17, 18, 19
Materials and Methods
Cell culture and transfection
The MHCC97H, MHCC97L, and HCCLM3 cell lines were obtained from the Liver Cancer Institute of Fudan University (Shanghai, China), and the other HCC cell lines were purchased from ATCC (American Type Culture Collection Manassas, VA, USA). The LO2 cell line was obtained from the Chinese Academy of Sciences, Shanghai, China. ACSS2 gene expression was silenced with ACSS2 siRNA, purchased from Suzhou GenePharma China (Suzhou, China) and synthesized using the following probes: ACSS2‐Homo‐1791,5′‐CAGGAUGGCUAUUACUGGATT‐3′ (sense) and 5′‐UCCAGUAAUAGCCAUCCUGTT‐3′ (antisense); and ACSS2‐Homo‐2176,5′‐CAUCUGUCAUCAGUCACCUTT‐3′ (sense) and 5′‐ AGGUGACUGAUGACAGAUGTT‐3′ (antisense). The ACSS2 siRNA (50 pmol) was incorporated into MHCC97H cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Transfected cells were cultured for 48 h after siRNA treatment for further experiments. MHCC97H‐NC and MHCC97H‐siACSS2 cells were obtained by transfecting MHCC97H‐wt cells with siRNA. All cells were maintained in DMEM (Gibco BRL, Gaithersburg, MD, USA) supplemented with 10% FBS (Gibco) and cultured in an incubator with 5% CO2 and 95% air. The hypoxic environment was created by adding CoCl2 (400 μM) to the DMEM.
Patients and specimens
The HCC tissue specimens for IHC were acquired from 110 patients who underwent surgery at Tianjin Medical University Cancer Institute and Hospital (Tianjin, China; 2010–2015). We chose paraffin blocks that were well fixed and embedded and that had complete clinical pathology and follow‐up data. The clinical stage of the HCC patients was judged based on the TNM classification system of the International Union Against Cancer. Ethical approval was obtained from the Tianjin Medical University Cancer Institute and Hospital research ethics committee. The protocol for the research project conforms to the provisions of the Declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013). The other cases used for Western blot (WB) detection and RT‐PCR were from patients who received surgery during the 2015. Carcinoma (C), non‐carcinoma (N), precancerous (PR), and portal vein tumor thrombus (PVTT) tissues were collected from these patients to compare the ACSS2 expression levels.
Transwell invasion assays
Transwell chambers (Corning, Corning, NY, USA) with 8‐μm pores and Matrigel (Corning, Bedford, MA, USA) were used for the Transwell invasion experiments. Fifty microliters of Matrigel diluted with DMEM (1:4) was added to the Transwell chambers, and the chambers were then incubated at 37°C in an incubator with 5% CO2 for 1 h. Next, the MHCC97H cells were diluted to a concentration of 1 × 105 cells/mL in DMEM only, and 200 μL cell suspension was plated into the upper chamber. Then 600 μL DMEM containing EGF (epidermal growth factor) (10 ng/mL) was added to the lower chamber. After 48 h, the cells that successfully invaded the lower membrane were fixed with 4% paraformaldehyde and stained. We used a light microscope to observe and photograph the cells at ×10 magnification. We chose three random fields for counting and took the average of the three fields as the result of the Transwell invasion experiment.
Scratch test
Cells were plated at a concentration of 5 × 105 cells per well into 6‐well plates. After transfection, we allowed the cells to become confluent and then replaced the culture medium with DMEM containing 0.5% serum to begin the experiment. A 10‐μL tip was used to induce scratches with equal widths. The cells were then maintained at 37°C in a 5% CO2 air incubator, and the distances between both sides of the scratch at 0, 3, 6, 9, 12, and 24 h after scratching were recorded. We chose three random sites to calculate the mean and standard deviation. Images were collected at 0 and 24 h.
Western blot analysis and antibodies
Protein from human tissue samples and cells was quantified by the BCA method (Thermo Fisher Scientific, Waltham, MA, USA) and separated by SDS‐PAGE. The protein was then transferred to membranes (Millipore, Billerica, MA, USA). After blocking with 5% skimmed milk powder, the membrane was incubated with anti‐ACSS2 rabbit polyclonal antibody (1:1000, ab66038; Abcam, HongKong, China), anti‐HIF‐2α rabbit polyclonal antibody (1:1000, NB100‐122; Novus Biologicals, Littleton, Colorado, USA), anti‐transforming growth factor‐α (TGFα) rabbit mAb (1:1000, ab208156; Abcam), anti‐cyclinD1 rabbit mAb (1:5000, ab134175; Abcam), anti‐E‐cadherin rabbit polyclonal antibody (1:1000, ab15148; Abcam), anti‐N‐cadherin rabbit polyclonal antibody (1:500, ab18203; Abcam), anti‐Snail rabbit mAb (1:1000, #3876; Cell Signaling Technology, Danvers, MA, USA), anti‐vimentin rabbit mAb (1:1000, ab92547; Abcam), and anti‐β‐actin mouse mAb (1:1000, sc‐47778; Santa Cruz Biotechnology, CA, USA). After incubating overnight at 4°C, the membrane was further probed with anti‐mouse (1:4000, sc2005; Santa Cruz Biotechnology) or anti‐rabbit IgG (1:4000, sc2004; Santa Cruz Biotechnology).
Real‐time PCR
RNA from human tissue samples and cells was extracted with TRIzol (Invitrogen). Then 2 μg total RNA was reverse transcribed into cDNA using a transcriptor First Strand cDNA Synthesis Kit (Invitrogen, Paisley, UK) according to the manufacturer's instructions. Next, quantitative real‐time PCR using a Bio‐Rad (Hercules, CA, USA) Laboratories CFX96 system was carried out to analyze the amount of cDNA produced in the above transcription step. The Power SYBR Green Master Mix used in the RT‐PCR was purchased from Roche (Basel, Switzerland). We determined the expression in a sample using three separate experiments with triplicate wells per experiment. The primer sequences used were as follows: ACSS2 forward, 5′‐GGATTCCAGCTGCAGTCTTC‐3′; ACSS2 reverse, 5′‐CATGCCACCACAAGTCAATC‐3′; phosphoglycerate kinase 1 PGK1 (Phosphoglycerate kinase 1) forward, 5′‐GACTGTGTAGGCCCAGAAGT‐3′; PGK1 reverse, 5′‐CTGGCTCGGCTTTAACCTTG‐3′; vascular endothelial growth factor VEGF (Vascular endothelial growth factor) forward, 5′‐GGCCAGCACATAGGAGAGAT‐3′; VEGF reverse, 5′‐ACGCTCCAGGACTTATACCG‐3′; glucose transporter 1 GLUT1 (Glucose transporter 1) forward, 5′‐GGCCATCTTTTCTGTTGGGG‐3′; GLUT1 reverse, 5′‐CCAGCAGGTTCATCATCAGC‐3′; TGFα forward, 5′‐CGCTCTGGGTATTGTGTTGG‐3′; TGFα reverse, 5′‐TGGGAATCTGGGCAGTCATT‐3′; cyclinD1 forward, 5′‐GCATGTTCGTGGCCTCTAAG‐3′; and cyclinD1 reverse, 5′‐TTCAATGAAATCGTGCGGGG‐3′.
Immunohistochemistry
All HCC patient paraffin blocks used for this assay were approved by the Tianjin Medical University Cancer Institute and Hospital and were confirmed histologically by HE staining. After blocking with 3% BSA, the tissues were incubated with anti‐ACSS2 antibody (1:100, ab66038; Abcam), anti‐HIF‐2α antibody (1:250, NB100‐122; Novus Biologicals) and anti‐TGFα antibody (1:1000, ab208156; Abcam) for 30 min and then transferred to 4°C overnight. The next day, after incubating with a goat anti‐rabbit secondary antibody PV‐6000 Kit (Zhongshan Golden Bridge Biotechnology, Beijing, China) for 1 h at 37°C, the tissues were stained with DAB and hematoxylin and then dehydrated and dried before observing. Slides with the primary antibodies omitted were used as negative controls. The staining results were estimated by one researcher who was blind to the clinicopathologic staging and outcomes of all patients during observation. Five fields were selected for an average.
Immunoprecipitation
Hypoxia‐inducible factor‐2α protein was immunoprecipitated from a 35‐mm plate of HCC cells. Whole‐cell extracts were incubated with protein A beads (16‐156; Millipore) and normal rabbit IgG (2729S; Cell Signaling Technology) to clear non‐specific binding. We then incubated the supernatants with 2 μL anti‐HIF‐2α (NB100‐122; Novus Biologicals) antibody or normal rabbit IgG (2729S; Cell Signaling Technology) for 3 h before mixing with protein A beads. After binding overnight, the supernatant was removed by centrifugation, and the immunoprecipitated proteins were eluted and detected by WB.
Statistical analysis
The results and data were analyzed by spss 18.0 software (SPSS, Chicago, IL, USA) and expressed as mean ± SD. Differences between two groups were evaluated with t‐tests, and P < 0.05 was considered statistically significant. Some of the WB images were used for grayscale analysis, and the resulting histograms represent the average of three replicates. The χ2‐test was used to explore the relationship between two variables. Survival curves (overall survival [OS] and disease‐free survival [DFS]) of 110 patients were calculated by Kaplan–Meier analysis.
Results
Acetyl‐CoA synthase 2 expression in HCC cell lines and patients
To elucidate the effect of ACSS2 on HCC, four HCC cell lines and the LO2 cell line were selected for the detection of ACSS2 expression. We initiated the culture of the five cell lines at the same time and harvested the cells when they were in the logarithmic growth phase. Western blot results revealed that the MHCC97L and Hep3B cells showed higher ACSS2 expression than the more malignant MHCC97H and LM3 cell lines (Fig. S1).20, 21, 22, 23, 24 The LO2 cell line, which was derived from normal liver tissue,25 also showed high ACSS2 expression (Fig. 1a). We then detected ACSS2 expression in C and NC tissues of 25 patients by WB and RT‐PCR; patient background characteristics are shown in Table 1. The WB data showed that NC tissues exhibited higher ACSS2 expression (Fig. 1b). Similar results were found in the RT‐PCR data of another 13 pairs of human HCC samples (Fig. 1c). These results indicate that ACSS2 may be negatively correlated with the malignancy of HCC.
Figure 1.
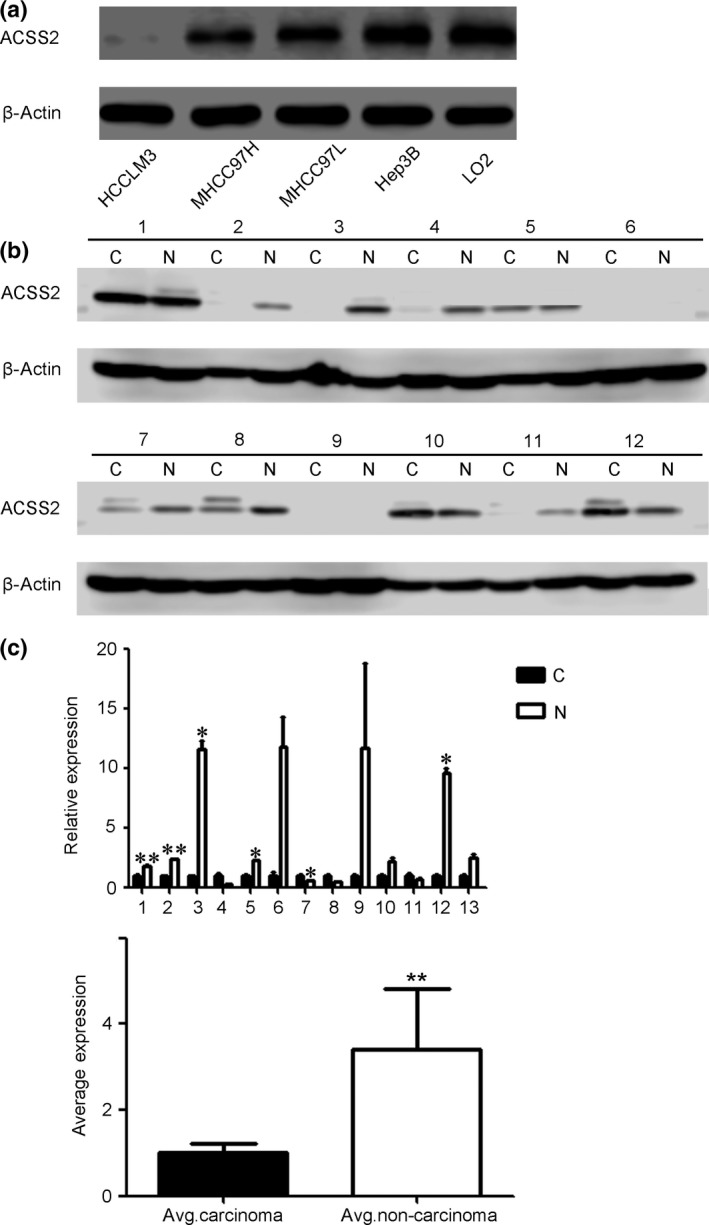
Acetyl‐CoA synthase 2 (ACSS2) expression in hepatocellular carcinoma (HCC) cell lines and patients. (a) Expression of ACSS2 in four HCC cell lines was detected by Western blot analysis. The data showed that a higher degree of malignancy was associated with lower ACSS2 protein expression in the HCC cell lines. The LO2 cell line, which was derived from normal liver tissue, was used as a control. The LM3 and MHCC97H cell lines are highly metastatic compared with the MHCC97L cell line, whereas the Hep3B cell line shows almost no metastatic ability. All cell lines were maintained in normoxic conditions. (b) ACSS2 expression was also detected in 12 pairs of human HCC carcinoma (c) and non‐carcinoma (N) tissues by Western blot analysis. Non‐carcinoma tissues showed higher expression. (c) Another 13 pairs of human HCC and non‐carcinoma tissues were assessed by RT‐PCR, and a similar result was obtained. Average (Avg.) expression of ACSS2 mRNA in the carcinoma and non‐carcinoma tissues is shown. Data are shown as the mean ± SD of three independent experiments. Error bars represent SD. *P < 0.05; **P < 0.01.
Table 1.
Background characteristics of patients with hepatocellular carcinoma
| Parameter | Yes | No |
|---|---|---|
| Age, ≥50 years | 19 | 6 |
| Gender, male | 23 | 2 |
| Tumor size, ≥5 cm | 12 | 13 |
| Tumor number, multiple | 7 | 18 |
| Satellite lesions | 11 | 14 |
| Portal vein invasion | 10 | 15 |
| Microvascular invasion | 17 | 8 |
| Cirrhosis | 20 | 5 |
| Clinical stage, advanced | 11 | 14 |
| Histological grade, poor | 8 | 17 |
| HBV infection | 21 | 4 |
| HCV infection | 0 | 25 |
HBV, hepatitis B virus; HCV, hepatitis C virus.
Acetyl‐CoA synthase 2 knockdown promoted the invasion and migration ability of HCC cells under hypoxic conditions
We used siRNA technology to knock down ACSS2 gene expression and explored its influence on the invasion and migration ability of MHCC97H cells. Knockdown was verified (Fig. 2a), and Transwell experiments were then carried out to detect the invasion ability of the cells. A hypoxic environment was induced by adding CoCl2 (400 μmol/mL) to the medium.26 The results revealed that the siACSS2 group showed higher invasion ability in the hypoxic environment than the control group (Fig. 2b). The migration test showed that knockdown of ACSS2 enhanced the migration ability of MHCC97H cells under hypoxic conditions (Fig. 2c). Taken together, these results suggest that downregulation of ACSS2 expression may result in increased metastases under hypoxia.
Figure 2.
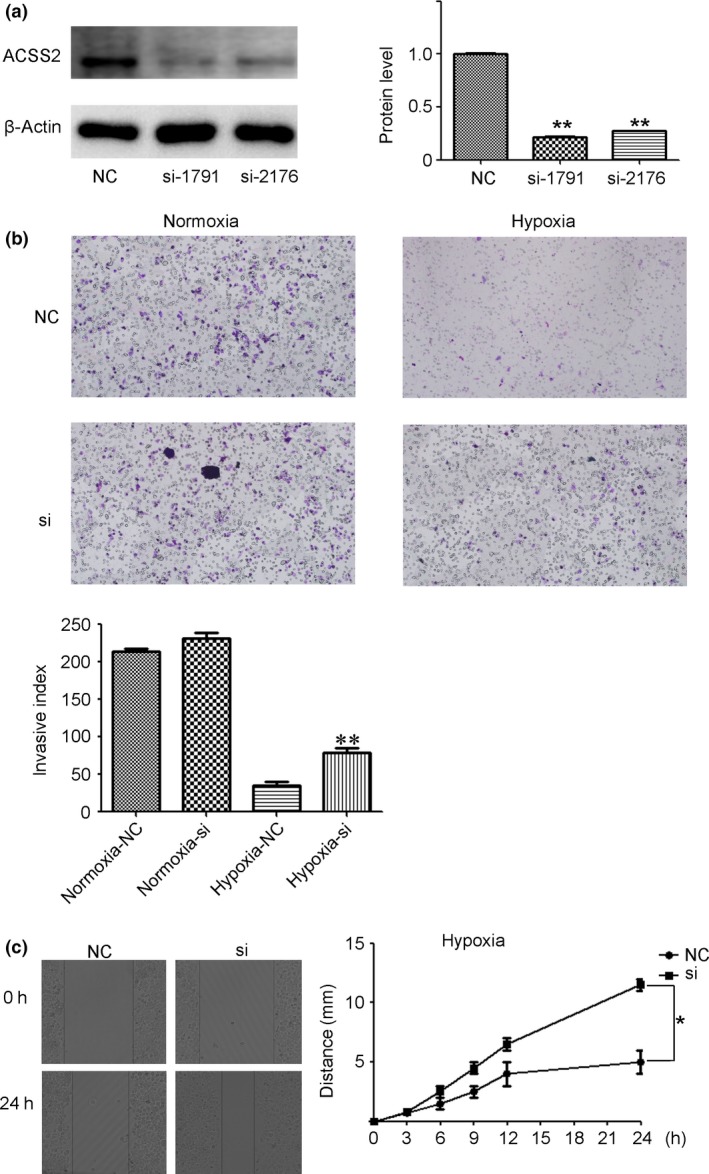
Downregulation of acetyl‐CoA synthase 2 (ACSS2) increased the invasion and migration of hepatocellular carcinoma (HCC) cells in hypoxic conditions. (a) siRNA knockdown was verified by Western blot analysis in MHCC97H cells. Grayscale analysis of the three replicates is shown as a histogram. ACSS2 expression was normalized to β‐actin. (b,c) Transwell invasion experiment and scratch test showed that downregulation of ACSS2 resulted in increased invasiveness and migration of HCC cells in hypoxic conditions. Data are shown as mean ± SD of three independent experiments. Error bars represent SD. *P < 0.05; **P < 0.01. NC, normal control.
Knockdown of ACSS2 promoted epithelial–mesenchymal transition of HCC cells without HIF‐2α elevation under hypoxia
Epithelial–mesenchymal transition (EMT) is described as the process by which epithelial cells convert into individual migratory cells. The EMT phenomenon is also considered an important step in cancer metastasis.27, 28 Our siRNA experiments found that more spindle‐shaped cells were recognized in the hypoxia‐si group under hypoxic conditions than in other groups (Fig. 3a). We then examined the relevant indicators of EMT in siACSS2 cells and negative control (NC) cells. The results showed that there were more N‐cadherin, vimentin, and Snail and less E‐cadherin expression in siACSS2 cells compared to NC cells during hypoxia, which indicated that EMT was promoted in siACSS2 cells under hypoxic conditions (Fig. 3b). Notably, this phenomenon did not occur in normoxic conditions (Fig. 3b). Due to the different results obtained between the hypoxic and normoxic conditions, we speculated that some hypoxia‐induced molecules might lead to these differences. We next detected HIF‐2α expression in siACSS2 cells and NC cells under hypoxia and normoxia. The data showed that HIF‐2α expression was markedly higher under hypoxic conditions. However, there was no difference in HIF‐2α protein expression levels between the siACSS2 group and the NC group (Fig. 3c), which indicated that the total amount of HIF‐2α protein was not the driving factor affecting the EMT process after ACSS2 knockdown.
Figure 3.
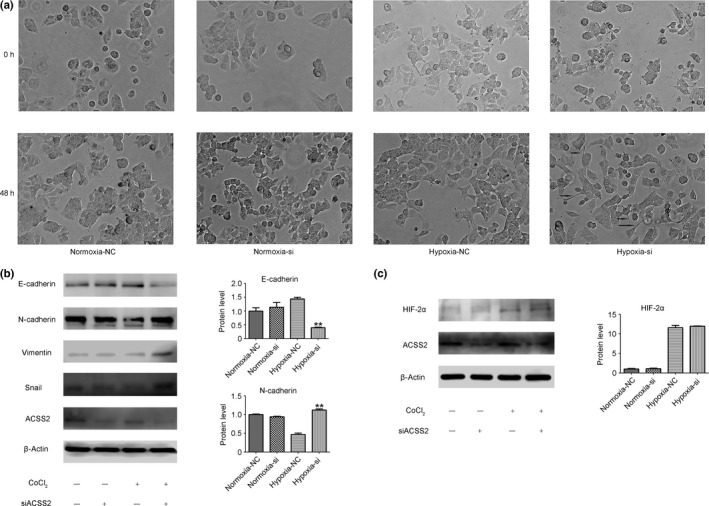
Knockdown of acetyl‐CoA synthase 2 (ACSS2) promoted epithelial–mesenchymal transition of hepatocellular carcinoma cells without increasing hypoxia‐inducible factor‐2α (HIF‐2α). (a) Morphology of MHCC97H after siRNA interference in normoxic and hypoxic conditions. (b) Western blot images show that there were more N‐cadherin, vimentin, and Snail, and less E‐cadherin expression in the siACSS2 cells compared to the normal control (NC) cells in hypoxic conditions. Grayscale analysis of N‐cadherin and E‐cadherin is shown as histograms, which represent three replicates. Data in the histograms are normalized to β‐actin. (c) There was no difference in HIF‐2α expression between the siACSS2 group and the NC group. Grayscale analysis of HIF‐2α is also shown. Error bars represent SD. *P < 0.05; **P < 0.01.
Acetylation of HIF‐2α affected the EMT process
Epigenetic modifications, including methylation and acetylation, have great impact on protein function.29, 30 Hypoxia‐inducible factor‐2α also undergoes these post‐translational modifications. Acetylation and deacetylation modifications of HIF‐2α even surpass the role of expression regulation, which in turn affects the expression of the downstream molecules of HIF‐2α activity. We examined the downstream molecules of HIF‐2α by RT‐PCR. The result revealed increased expression levels of some molecules downstream of HIF‐2α, including TGFα and cyclinD1, after ACSS2 knockdown, during hypoxia (Fig. 4a). However, these changes did not occur under normoxia. We also detected some molecules that were regulated by HIF‐1α, or both HIF‐1α and HIF‐2α, and there were no differences between the hypoxic and normoxic conditions after ACSS2 knockdown (Fig. 4a). Next, we selected TGFα for further verification by WB, and the data revealed a similar result (Fig. 4b). Then we used immunohistochemistry (IHC) to investigate the relationship between ACSS2 and TGFα and HIF‐2α in HCC samples, which indicated that the correlation between ACSS2 expression and TGFα expression had statistical significance (Table 2, Fig. S2). However, there was no similar statistical significance between the expression of ACSS2 and HIF‐2α (Table 2, Fig. S2). Moreover, to ensure the acetylation status of HIF‐2α, we undertook immunoprecipitation on the NC and ACSS2‐knockdown groups. The data indicated significant downregulation of the acetylation levels of HIF‐2α protein in the ACSS2‐knockdown group (Fig. 4c), which confirmed the influence of ACSS2 on HIF‐2α acetylation in other studies.15, 16 Taken together, these results suggest that deacetylation of HIF‐2α occurred under hypoxia after ACSS2 knockdown, which enhanced the activity of HIF‐2α and promoted the EMT process.
Figure 4.
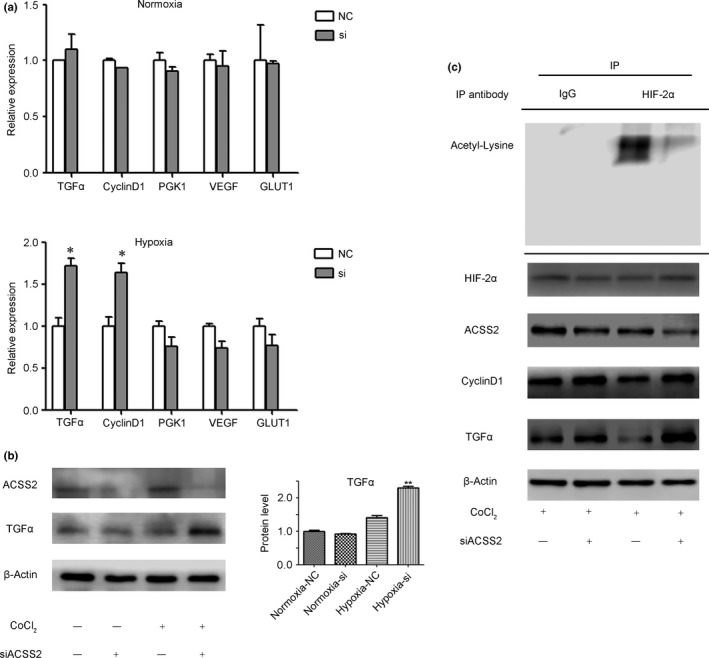
Knockdown of acetyl‐CoA synthase 2 (ACSS2) induced deacetylation of hypoxia‐inducible factor‐2α (HIF‐2α) and enhanced the activity of HIF‐2α in hypoxic conditions. (a,b) RT‐PCR and Western blot results revealed that ACSS2 knockdown enhanced the expression of some HIF‐2α downstream molecules in hypoxic conditions. Grayscale analysis of transforming growth factor α (TGFα) and ACSS2 is shown as histograms. Data are normalized to β‐actin and are shown as mean ± SD of three independent experiments. (c) Immunoprecipitation (IP) showed that ACSS2 knockdown led to higher deacetylated HIF‐2α expression under hypoxic conditions. Error bars represent SD. *P < 0.05; **P < 0.01. GLUT1, glucose transporter 1; PGK1, phosphoglycerate kinase 1; VEGF, vascular endothelial growth factor.
Table 2.
Clinical characteristics and laboratory parameters of patients with hepatocellular carcinoma according to ACSS2 expression level
| Parameter | n | ACSS2 low expression, n = 56 | ACSS2 high expression, n = 54 | P‐value |
|---|---|---|---|---|
| Age, years | ||||
| <50 | 35 | 21 | 14 | 0.193 |
| ≥50 | 75 | 35 | 40 | |
| Gender | ||||
| Male | 98 | 48 | 50 | 0.247 |
| Female | 12 | 8 | 4 | |
| Tumor size, cm | ||||
| <5 | 52 | 25 | 27 | 0.574 |
| ≥5 | 58 | 31 | 27 | |
| Tumor number | ||||
| Single | 76 | 35 | 41 | 0.128 |
| Multiple | 34 | 21 | 13 | |
| Satellite lesions | ||||
| No | 59 | 21 | 38 | 0.001* |
| Yes | 51 | 35 | 16 | |
| Portal vein invasion | ||||
| No | 58 | 21 | 37 | 0.001* |
| Yes | 52 | 35 | 17 | |
| Microvascular invasion | ||||
| No | 35 | 10 | 25 | 0.001* |
| Yes | 75 | 46 | 29 | |
| Cirrhosis | ||||
| No | 22 | 5 | 17 | 0.003* |
| Yes | 88 | 51 | 37 | |
| Clinical stage | ||||
| Stage I and II | 63 | 22 | 41 | <0.001* |
| Stage III and IV | 47 | 34 | 13 | |
| Histological grade | ||||
| Well | 82 | 38 | 44 | 0.101 |
| Poor | 28 | 18 | 10 | |
| HIF‐2α expression | ||||
| Low expression | 60 | 27 | 33 | 0.174 |
| High expression | 50 | 29 | 21 | |
| TGFα expression | ||||
| Low expression | 65 | 28 | 37 | 0.048* |
| High expression | 45 | 28 | 17 | |
HIF‐2α, hypoxia‐inducible factor‐2α; TGFα, transforming growth factor α. *P ≤ 0.05.
Low expression of ACSS2 is associated with poor prognosis and progression in HCC patients
The cell biology experiments above showed that the metastatic potential of HCC cells increased following knockdown of ACSS2 expression. Next, we undertook IHC assays using a cohort of 110 HCC patients (2010–2015). Staining intensity was divided into four levels, as shown in Figure 5(a) : negative, weak, moderate, and strong staining. We defined the first two images as the low‐expression group and the other two images as the high‐expression group (Fig. 5a). We analyzed the clinical pathology characteristics of 110 patients by ACSS2 expression level (Table 2) and found that ACSS2 expression was related to satellite lesions, cirrhosis, vascular invasion, and poor clinical stage. The correlation of ACSS2 expression and clinical stage was shown intuitively with a histogram (Fig. 5b). We then undertook survival analysis of the cases. Kaplan–Meier analysis revealed that low ACSS2 expression was associated with poor prognosis, which was reflected in both OS and DFS (Fig. 5c). The difference between high expression and low expression groups was statistically significant (Table 3). We recently obtained three pairs of special specimens: two specimens of PR and their corresponding N; the other specimen included C, PVTT, and N tissue. We detected ACSS2 expression in these specimens and found that the PR tissue showed lower ACSS2 expression than the corresponding non‐carcinoma tissue (Fig. 5d). Moreover, the PVTT showed low ACSS2 expression, which was similar to the level in the primary tumor (Fig. 5d). All of the results above suggested that low expression of ACSS2 is not only an important feature in the development of HCC, but also a significant factor that impacts HCC metastasis.
Figure 5.
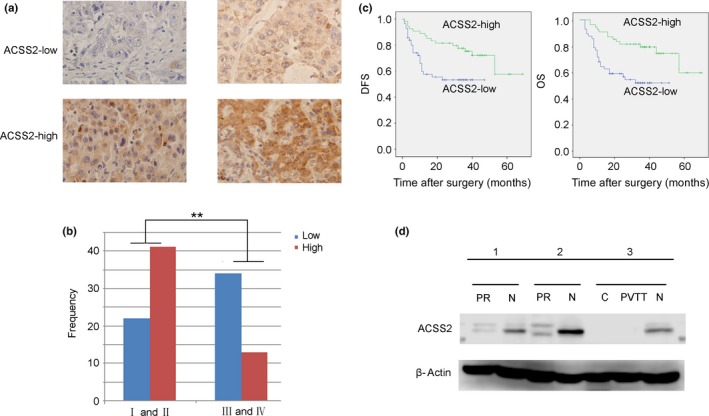
Low acetyl‐CoA synthase 2 (ACSS2) expression was associated with poor prognosis and progression in hepatocellular carcinoma (HCC) patients. (a) Representative images of ACSS2 expression in HCC tumors were obtained by immunohistochemical staining. (b) The relationship between ACSS2 immunohistochemical staining intensity and clinical stage is presented as histograms. Data indicate that more advanced HCC tumors (stage III and IV) show much less intense staining of ACSS2 protein than early stage tumors (stage I and II). The Y‐axis indicates the frequency of patients in different groups. (c) Decreased expression of ACSS2 was related to poor prognosis (P < 0.05). DFS, disease‐free survival; OS, overall survival. (d) Precancerous tissue (PR) also showed less ACSS2 expression than corresponding non‐carcinoma tissue (N). Furthermore, vascular invasion tissue (portal vein tumor thrombus, PVTT) showed lower ACSS2 expression than non‐carcinoma tissue (N), which was similar to its primary tumor (C).
Table 3.
Analysis of prognostic factors influencing overall survival (OS) and disease‐free survival (DFS) in patients with hepatocellular carcinoma
| OS | DFS | |||||
|---|---|---|---|---|---|---|
| n | χ2‐test | P‐value | n | χ2‐test | P‐value | |
| Age, years | ||||||
| <50 | 35 | 3.494 | 0.062 | 35 | 1.502 | 0.220 |
| ≥50 | 75 | 75 | ||||
| Gender | ||||||
| Male | 98 | 0.660 | 0.416 | 98 | 0.637 | 0.425 |
| Female | 12 | 12 | ||||
| Tumor size, cm | ||||||
| <5 | 52 | 4.621 | 0.032* | 52 | 1.304 | 0.253 |
| ≥ 5 | 58 | 58 | ||||
| Tumor number | ||||||
| Single | 76 | 11.437 | 0.001* | 76 | 11.948 | 0.001* |
| Multiple | 34 | 34 | ||||
| Satellite lesions | ||||||
| No | 59 | 20.775 | <0.001* | 59 | 17.669 | <0.001* |
| Yes | 51 | 51 | ||||
| Portal vein invasion | ||||||
| No | 58 | 12.616 | <0.001* | 58 | 14.445 | <0.001* |
| Yes | 52 | 52 | ||||
| Microvascular invasion | ||||||
| No | 35 | 13.368 | <0.001* | 35 | 10.825 | 0.010* |
| Yes | 75 | 75 | ||||
| Cirrhosis | ||||||
| No | 22 | 2.578 | 0.108 | 22 | 6.321 | 0.012* |
| Yes | 88 | 88 | ||||
| Clinal stage | ||||||
| Stage I and II | 63 | 41.424 | <0.001* | 63 | 26.147 | <0.001* |
| Stage III and IV | 47 | 47 | ||||
| Histological grade | ||||||
| Well | 82 | 0.535 | 0.465 | 82 | 0.062 | 0.804 |
| Poor | 28 | 28 | ||||
| HIF‐2α expression | ||||||
| Low expression | 60 | 7.063 | 0.008* | 60 | 1.998 | 0.158 |
| High expression | 50 | 50 | ||||
| TGFα expression | ||||||
| Low expression | 65 | 4.294 | 0.038* | 65 | 0.841 | 0.359 |
| High expression | 45 | 45 | ||||
| ACSS2 expression | ||||||
| Low expression | 56 | 9.980 | 0.002* | 56 | 7.048 | 0.008* |
| High expression | 54 | 54 | ||||
HIF‐2α, hypoxia‐inducible factor‐2α; TGFα, transforming growth factor α. *P ≤ 0.05.
Discussion
Acetyl‐CoA synthase 2 is one isoform of the acetyl‐CoA synthetase family, which consists of ACSS1, ACSS2, and ACSS3. Acetyl‐CoA synthase 2 is localized in cytoplasm and nuclei, whereas the other two isoforms are located in mitochondria.31 Acetyl‐CoA synthase 2 has been a key research topic in recent years with emphases on two areas. The first area is the relationship between ACSS2 and energy metabolism. It has been reported that acetate is an important source of energy for many tumors, and ACSS2 plays a key role in this energy supply process.13, 14, 31, 32 In addition, ACSS2 is indispensable for the acetylation of certain molecules,33 such as HIF‐2α.15, 16 However, there are some controversies surrounding the effect of ACSS2 in tumors. Some scientists have recognized ACSS2 as a cancer‐promoting factor,13, 14, 32 whereas others have reported opposing results, in that the loss of ACSS2 expression predicts poor prognosis in patients,34, 35 especially in cancer of the digestive system. The theory of ACSS2 promoting tumors is mainly found in breast cancer and glioma, which emphasizes the role of ACSS2 in producing acetyl‐CoA; the opposite opinion on ACSS2 in digestive system cancer is mainly from the IHC of clinical samples. Our data indicate that decreased expression of ACSS2 may promote HCC metastasis and predict poor prognosis. In contrast to mammary glands, our data indicate that normal liver tissue and normal liver cells show high ACSS2 expression, and the gradual loss of ACSS2 indicates the occurrence and progression of HCC.
Our invasion and migration experiments showed that there were significant changes after ACSS2 knockdown under hypoxia. We thus speculated that the impact of ACSS2 on invasion and metastasis might be executed through the HIF family, which is considered to play an important role in inducing EMT and metastasis.36, 37, 38 Previous studies have reported that the acetate/ACSS2 switch could regulate HIF‐2α stress signaling15 and that HIF‐2α is more common in chronic hypoxia.10 Therefore, we focused on both the amount and the activity of HIF‐2α. We found that ACSS2 knockdown reduced the acetylation levels of HIF‐2α and enhanced the expression levels of its downstream molecules. However, ACSS2 knockdown had no effect on the amount of HIF‐2α protein under hypoxia. We then identified changes in EMT status after ACSS2 knockdown and HIF‐2α deacetylation. All the results reported above indicate that ACSS2 knockdown deacetylates HIF‐2α, which enhances the activity of HIF‐2α and induces EMT. Our IHC experiment of clinical specimens also proved that low expression of ACSS2 was related to many metastasis indicators, such as satellite lesions and vascular invasion.
Taken together, our study revealed that ACSS2 plays an important role in the acetylation process of HIF‐2α protein and therefore effectively modulates the activity of HIF‐2α under hypoxic conditions, which has great impacts on the prognosis of HCC patients.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. Expression of N‐cadherin and E‐cadherin in hepatocellular carcinoma cell lines.
Fig. S2. Representative images of hypoxia‐inducible factor‐2α (HIF‐2α) and transforming growth factor α (TGFα) expression in a cohort of 110 human hepatocellular carcinoma samples.
Acknowledgments
We thank Professor Ning Zhang, Vice President of Tianjin Medical University, and his group for technical support. This work was supported by the National Natural Science Foundation of China (Grant Nos. 81372635, 81572434,81572858, and 81672884) and the Major Program of the Natural Science Foundation of Tianjin (Grant No. 11JCZDJC18800).
Cancer Sci 108 (2017) 1338–1346
Funding Information
National Natural Science Foundation of China; Natural Science Foundation of Tianjin
Contributor Information
Tianqiang Song, Email: tjchi@hotmail.com.
Ti Zhang, Email: zhangti@tjmuch.com.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Zhong JH, Ke Y, Gong WF et al Hepatic resection associated with good survival for selected patients with intermediate and advanced‐stage hepatocellular carcinoma. Ann Surg 2014; 260: 329–40. [DOI] [PubMed] [Google Scholar]
- 3. Kokudo T, Hasegawa K, Matsuyama Y et al Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol 2016; 65: 938–43. [DOI] [PubMed] [Google Scholar]
- 4. Singal AG, Marrero JA. Recent advances in the treatment of hepatocellular carcinoma. Curr Opin Gastroenterol 2010; 26: 189–95. [DOI] [PubMed] [Google Scholar]
- 5. Nath B, Szabo G. Hypoxia and hypoxia inducible factors: diverse roles in liver diseases. Hepatology 2012; 55: 622–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruan K, Song G, Ouyang G. Role of hypoxia in the hallmarks of human cancer. J Cell Biochem 2009; 107: 1053–62. [DOI] [PubMed] [Google Scholar]
- 7. Jiang J, Tang YL, Liang XH. EMT: a new vision of hypoxia promoting cancer progression. Cancer Biol Ther 2011; 11: 714–23. [DOI] [PubMed] [Google Scholar]
- 8. Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer 2011; 12: 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koukourakis MI, Giatromanolaki A, Skarlatos J et al Hypoxia inducible factor (HIF‐1a and HIF‐2a) expression in early esophageal cancer and response to photodynamic therapy and radiotherapy. Can Res 2001; 61: 1830–2. [PubMed] [Google Scholar]
- 10. Lofstedt T, Fredlund E, Holmquist‐Mengelbier L et al Hypoxia inducible factor‐2alpha in cancer. Cell Cycle 2007; 6: 919–26. [DOI] [PubMed] [Google Scholar]
- 11. Dioum EM, Chen R, Alexander MS et al Regulation of hypoxia‐inducible factor 2alpha signaling by the stress‐responsive deacetylase sirtuin 1. Science 2009; 324: 1289–93. [DOI] [PubMed] [Google Scholar]
- 12. Cao M, Gao J, Zhou H et al HIF‐2alpha regulates CDCP1 to promote PKCdelta‐mediated migration in hepatocellular carcinoma. Tumour Biol 2016; 37: 1651–62. [DOI] [PubMed] [Google Scholar]
- 13. Schug ZT, Peck B, Jones DT et al Acetyl‐CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell 2015; 27: 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Comerford SA, Huang Z, Du X et al Acetate dependence of tumors. Cell 2014; 159: 1591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen R, Xu M, Nagati JS et al The acetate/ACSS2 switch regulates HIF‐2 stress signaling in the tumor cell microenvironment. PLoS ONE 2015; 10: e0116515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu M, Nagati JS, Xie J et al An acetate switch regulates stress erythropoiesis. Nat Med 2014; 20: 1018–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bjornson E, Mukhopadhyay B, Asplund A et al Stratification of hepatocellular carcinoma patients based on acetate utilization. Cell Rep 2015; 13: 2014–26. [DOI] [PubMed] [Google Scholar]
- 18. Lakhter AJ, Hamilton J, Konger RL, Brustovetsky N, Broxmeyer HE, Naidu SR. Glucose‐independent acetate metabolism promotes melanoma cell survival and tumor growth. J Biol Chem 2016; 291: 21869–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoshii Y, Furukawa T, Yoshii H et al Cytosolic acetyl‐CoA synthetase affected tumor cell survival under hypoxia: the possible function in tumor acetyl‐CoA/acetate metabolism. Cancer Sci 2009; 100: 821–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li Y, Tang Y, Ye L et al Establishment of a hepatocellular carcinoma cell line with unique metastatic characteristics through in vivo selection and screening for metastasis‐related genes through cDNA microarray. J Cancer Res Clin Oncol 2003; 129: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Y, Tang ZY, Ye SL et al Establishment of cell clones with different metastatic potential from the metastatic hepatocellular carcinoma cell line MHCC97. World J Gastroenterol 2001; 7: 630–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tian J, Tang Z, Ye S. Establishment of a human hepatocellular carcinoma (HCC) cell line with high metastatic potential (MHCC97) and its biological characteristics. Zhonghua Zhong liu za zhi [Chinese Journal of Oncology] 1998; 20: 405–7. [PubMed] [Google Scholar]
- 23. Tian J, Tang ZY, Ye SL et al New human hepatocellular carcinoma (HCC) cell line with highly metastatic potential (MHCC97) and its expressions of the factors associated with metastasis. Br J Cancer 1999; 81: 814–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guo K, Kang NX, Li Y et al Regulation of HSP27 on NF‐kappaB pathway activation may be involved in metastatic hepatocellular carcinoma cells apoptosis. BMC Cancer 2009; 9: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ye XZ, Zhu DH, Shen DW. Ultrastructures of LO2 normal adult hepatocytes over successive in vitrocultures. Acta Biol Exp Sin 1980; 13: 3612–41. [Google Scholar]
- 26. Befani C, Mylonis I, Gkotinakou IM et al Cobalt stimulates HIF‐1‐dependent but inhibits HIF‐2‐dependent gene expression in liver cancer cells. Int J Biochem Cell Biol 2013; 45: 2359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Diepenbruck M, Christofori G. Epithelial‐mesenchymal transition (EMT) and metastasis: yes, no, maybe? Curr Opin Cell Biol 2016; 43: 7–13. [DOI] [PubMed] [Google Scholar]
- 28. Gupta GP, Massague J. Cancer metastasis: building a framework. Cell 2006; 127: 679–95. [DOI] [PubMed] [Google Scholar]
- 29. Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell 2012; 150: 12–27. [DOI] [PubMed] [Google Scholar]
- 30. Ferreira WA, Pinheiro Ddo R, Costa Junior CA et al An update on the epigenetics of glioblastomas. Epigenomics 2016; 8: 1289–305. [DOI] [PubMed] [Google Scholar]
- 31. Lyssiotis CA, Cantley LC. Acetate fuels the cancer engine. Cell 2014; 159: 1492–4. [DOI] [PubMed] [Google Scholar]
- 32. Mashimo T, Pichumani K, Vemireddy V et al Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell 2014; 159: 1603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gao X, Lin SH, Ren F et al Acetate functions as an epigenetic metabolite to promote lipid synthesis under hypoxia. Nat Commun 2016; 7: 11960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hur H, Kim YB, Ham IH, Lee D. Loss of ACSS2 expression predicts poor prognosis in patients with gastric cancer. J Surg Oncol 2015; 112: 585–91. [DOI] [PubMed] [Google Scholar]
- 35. Bae JM, Kim JH, Oh HJ et al Downregulation of acetyl‐CoA synthetase 2 is a metabolic hallmark of tumor progression and aggressiveness in colorectal carcinoma. Mod Pathol 2016; 30: 267–277. [DOI] [PubMed] [Google Scholar]
- 36. Chen Y, Shi Y, Dai G. [Role of HIF‐induced EMT in invasion andmetastasis of tumor]. Zhong nan da xue xue bao Yi xue ban = Journal of Central South University Medical Sciences 2016; 41: 872–8. [DOI] [PubMed] [Google Scholar]
- 37. Guo Q, Qin W. DKK3 blocked translocation of beta‐catenin/EMT induced by hypoxia and improved gemcitabine therapeutic effect in pancreatic cancer Bxpc‐3 cell. J Cell Mol Med 2015; 19: 2832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou C, Liu J, Tang Y et al Coexpression of hypoxia‐inducible factor‐2alpha, TWIST2, and SIP1 may correlate with invasion and metastasis of salivary adenoid cystic carcinoma. J Oral Pathol Med 2012; 41: 424–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Expression of N‐cadherin and E‐cadherin in hepatocellular carcinoma cell lines.
Fig. S2. Representative images of hypoxia‐inducible factor‐2α (HIF‐2α) and transforming growth factor α (TGFα) expression in a cohort of 110 human hepatocellular carcinoma samples.


