Abstract
This study aimed to evaluate the clinical outcomes of patients with mucoepidermoid carcinomas in the head and neck treated with carbon‐ion radiotherapy. Data from 26 patients who underwent carbon‐ion radiotherapy in four facilities were analyzed in this multi‐institutional retrospective study: the Japan Carbon‐ion Radiation Oncology Study Group. The median follow‐up time was 34 months. One patient experienced local recurrence, and the 3‐year local control rate was 95%. One patient developed lymph node recurrence and five developed distant metastases. The 3‐year progression‐free survival rate was 73%. Five patients died, two of mucoepidermoid carcinoma and three of intercurrent disease. The 3‐year overall survival rate was 89%. Acute mucositis and dermatitis of grade 3 or higher were experienced by 19% and 8% of patients, respectively; these improved with conservative therapy. Late mucositis and osteonecrosis of jaw were observed in 12% and 23% of patients, respectively. The 3‐year cumulative rate of any late adverse event of grade 3 or higher was 14%. None of the patients died of the acute or late adverse events. Carbon‐ion radiotherapy was efficacious and safe for treating mucoepidermoid carcinoma in this multi‐institutional retrospective study (registration no. UMIN000024473). We are currently undertaking a prospective multicenter study.
Keywords: Carbon‐ion radiotherapy, head and neck tumors, mucoepidermoid carcinoma, non‐squamous cell carcinoma, charged particle therapy
Mucoepidermoid carcinoma is an uncommon malignancy of the salivary glands.1, 2 Of the major salivary glands, 84%, 13%, and 3% of these tumors occur in the parotid, submandibular, and sublingual glands, respectively.3 The palate is the most common site of minor salivary gland involvement.4 Histologically, this tumor is mainly composed of mucous, epidermoid, and intermediate cell types that form cysts. The clinical outcomes of patients with mucoepidermoid carcinoma have not been fully reported due to the rarity of the disease.2 Generally, surgery is considered the standard treatment because mucoepidermoid carcinoma is considered resistant to radiotherapy and chemotherapy.5 For patients with tumors that are inoperable – either because the tumor is bulky or the patient's clinical condition precludes surgery – treatment options are limited.
Carbon‐ion radiotherapy is a highly concentrated particle beam, and the dose to the surrounding normal tissues can be minimized because of the characteristics of the Bragg peak.6 Moreover, carbon‐ion radiotherapy has an excellent biological effect that overcomes radioresistant tumors. Given these characteristics, carbon‐ion radiotherapy is expected to be a promising treatment for patients with inoperable mucoepidermoid carcinoma. Indeed, carbon‐ion radiotherapy has shown excellent efficacy for other histologic types of salivary gland cancer, such as adenoid cystic carcinoma,7 adenocarcinoma,8 and basal cell adenocarcinoma.9 Therefore, we aimed to evaluate the efficacy and safety of carbon‐ion radiotherapy for mucoepidermoid carcinoma in a multi‐institutional retrospective study: the Japan Carbon‐ion Radiation Oncology Study Group (J‐CROS).
Materials and Methods
Patient and tumor characteristics
In May 2015, this multi‐institutional study (J‐CROS 1402 HN) retrospectively analyzed the data of patients with mucoepidermoid carcinoma who were treated with carbon‐ion radiotherapy between April 2004 and October 2014 (UMIN000024473). The inclusion criteria were: patients with histologically confirmed mucoepidermoid carcinoma, N0 or N1M0 status, inoperable tumors or refusal of surgical treatment, definitive radiotherapy, measurable tumors, and a performance status score of 0–2. Patients who had previously received irradiation to the head and neck were excluded. Twenty‐six patients with mucoepidermoid carcinomas were consecutively treated with carbon‐ion radiotherapy at Gunma University Heavy Ion Medical Center (Maebashi, Japan), Hospital of the National Institute of Radiological Sciences (Chiba, Japan), Hyogo Ion Beam Medical Center (Tatsuno, Japan), and Ion Beam Therapy Center, SAGA HIMAT Foundation (Tosu, Japan). The multi‐institutional retrospective study was approved by the Institutional Review Board of each participating institution. The protocol was carried out in accordance with the Declaration of Helsinki.
Carbon‐ion radiotherapy
The patients were positioned in customized cradles and immobilized using a low‐temperature thermoplastic shell. Magnetic resonance imaging and endoscopic findings were routinely carried out to determine the gross tumor volume. The clinical target volume included adjacent anatomic sites of the gross tumor volume. A shrinking‐field technique with boost was used to spare the organs at risk. The planning target volume had margins of 2–5 mm added around the clinical target volume. The target reference point was defined as the isocenter of the planning target volume. The median number of the treatment ports was 5. Selection of dose‐fractionation was dependent on institutions. The median radiation dose fractionation was 64.0 Gy (relative biological effectiveness [RBE]) in 16 fractions (n = 13, 50%). When tumors were close to skin or pharynx, 57.6 Gy (RBE) in 16 fractions was used (n = 6, 23%). Other dose fractionations were 65.0 Gy (RBE) in 26 fractions (n = 5, 19%), 70.2 Gy (RBE) in 26 fractions (n = 1, 4%), and 70.4 Gy (RBE) in 32 fractions (n = 1, 4%). All treatments were by carbon‐ion radiotherapy alone; concurrent or adjuvant chemotherapies were not used.
Follow‐up
Patients were seen every 2–3 months for the first 2 years and every 3–6 months thereafter. Follow‐up consisted of physical examination, laryngoscopy, computed tomography, MRI, and 18‐fluorodeoxyglucose‐positron emission tomography.
Statistical analysis
The local control, progression‐free survival (PFS), and overall survival (OS) rates and the cumulative rate of adverse events were calculated using the Kaplan–Meier method. Univariate analysis was undertaken and the log–rank test was used to compare subgroups, such as stage, radiation dose, performance status, age, operability, tumor location, and gross tumor volume. A P‐value <0.05 was considered statistically significant. All statistical analyses were carried out using IBM spss Statistics for Mac, version 23.0 (SPSS, Armonk, NY, USA). Acute and late adverse events were evaluated by Common Terminology Criteria for Adverse Events version 4.0.
Results
The clinical characteristics of the 26 patients are shown in Table 1. The median follow‐up time was 34 months (range, 6–75 months). One patient (4%) was lost to follow‐up within 1 year. A representative case of carbon‐ion radiotherapy is shown in Figure 1. One patient (4%) experienced local recurrence, and the 3‐year local control rate was 95% (Fig. 2, black line). The 3‐year local control rates of patients with T1–3 and T4 tumors were 100% and 90%, respectively, but the difference was not significant (P = 0.34). Other clinical characteristics were not significant factors for local control. There was one case (4%) of lymph node recurrence and five (19%) of distant metastases. The 3‐year PFS rate for all patients was 73% (Fig. 2, red line). The 3‐year PFS rates of patients with T1–3 and T4 tumors were 83% and 66%, respectively; those with T1–3 tumors showed a trend towards a better PFS than those with T4 tumors (P = 0.07). Other clinical and tumor characteristics were not significant factors for PFS. During follow‐up, two patients died of mucoepidermoid carcinoma and three died of intercurrent disease. The 3‐year OS rate for all patients was 89% (Fig. 3). The 3‐year OS rates of patients with T1−3 and T4 tumors were 86% and 93%, respectively (P = 0.35). No factors were significantly associated with OS.
Table 1.
Patient and tumor characteristics (n = 26)
| Characteristic | ||
|---|---|---|
| Age, years, median (range) | 61 (31−79) | |
| Sex, n (%) | Male | 10 (38) |
| Female | 16 (62) | |
| Performance status, n (%) | 0 | 19 (73) |
| 1 | 7 (27) | |
| Tumor site, n (%) | Parotid gland | 10 (38) |
| Oral cavity | 6 (23) | |
| Nasal cavity/paranasal sinus | 5 (19) | |
| Pharynx | 3 (12) | |
| Parapharyngeal space | 2 (8) | |
| Disease, n (%) | Primary tumor | 21 (81) |
| Postoperative recurrence | 5 (19) | |
| Operability, n (%) | Operable | 16 (62) |
| Inoperable | 10 (38) | |
| T stage, n (%) | T1 | 2 (8) |
| T2 | 4 (15) | |
| T3 | 4 (15) | |
| T4 | 16 (62) | |
| N stage, n (%) | N0 | 25 (96) |
| N1 | 1 (4) | |
| Gross tumor volume, cm3, median (range) | 21 (3−191) | |
| Radiation dose, n (%) | 64.0 Gy (RBE)/16 fractions | 13 (50) |
| 57.6 Gy (RBE)/16 fractions | 6 (23) | |
| 65.0 Gy (RBE)/26 fractions | 5 (19) | |
| 70.2 Gy (RBE)/26 fractions | 1 (4) | |
| 70.4 Gy (RBE)/32 fractions | 1 (4) | |
RBE, relative biological effectiveness.
Figure 1.

Representative case of mucoepidermoid carcinoma treated with carbon‐ion radiotherapy. (a) A 51‐year‐old female with T3N0M0 mucoepidermoid carcinoma. The MRI showed a 40‐mm gadolinium‐enhanced tumor located in the deep lobe of the parotid gland with extraparenchymal extension. The patient refused surgery and elected to receive carbon‐ion radiotherapy. (b) Carbon‐ion radiotherapy was carried out with 64 Gy (relative biological effectiveness) in 16 fractions. The gross tumor volume is represented by the red outline. (c) On repeat MRI performed 3 years after carbon‐ion radiotherapy, no enhancing lesions were detected. No distant or lymph node metastases were detected during follow‐up. There were no late adverse events of grade 2 or higher.
Figure 2.
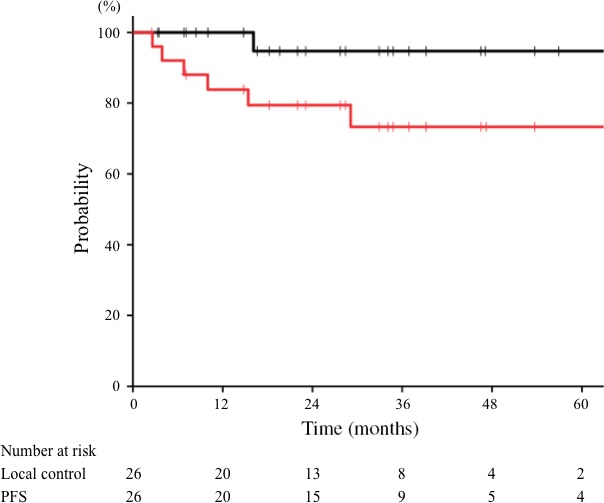
Local control and progression‐free survival (PFS) curves of mucoepidermoid carcinoma treated with carbon‐ion radiotherapy. The 3‐year local control (black line) and PFS (red line) rates for all patients were 95% and 73%, respectively.
Figure 3.
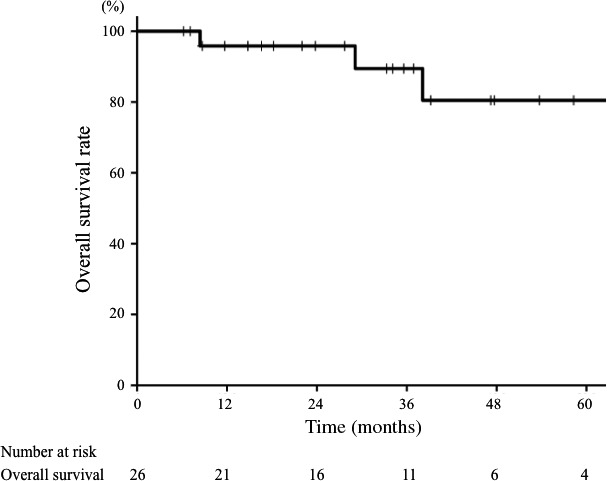
Overall survival curve of patients with mucoepidermoid carcinoma treated with carbon‐ion radiotherapy. The 3‐year overall survival rate for all patients was 89%.
Acute mucositis of grade 3 was observed in five patients (19%) and dermatitis of grade 3 was observed in two patients (8%). These acute symptoms were immediately improved by provision of conservative treatment. The late adverse events are summarized in Table 2. Regarding late adverse events, three patients (12%) experienced grade 2 or higher mucositis. Although one patient with postoperative skin flap recurrence at palate developed grade 4 oral ulceration with bleeding and flap necrosis, hyperbaric oxygen therapy improved the symptoms. Osteonecrosis of jaw was relatively common; six patients (23%) experienced the adverse event as grade 2 or higher. Two patients (8%) developed grade 2 tinnitus, one (4%) developed grade 3 cerebral abscess, one (4%) developed grade 2 glaucoma, and one (4%) developed grade 2 hearing impairment. None of the patients died of the acute or late adverse events. The 3‐year cumulative rate of any late adverse event of grade 3 or higher was 14% (Fig. 4). No factors were significantly associated with the severe late adverse events.
Table 2.
Late adverse events for all patients with mucoepidermoid carcinoma who received carbon‐ion radiotherapy (n = 26)
| Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|
| Mucositis | 2 (8) | 0 (0) | 1 (4) |
| Dermatitis | 0 (0) | 0 (0) | 0 (0) |
| Osteonecrosis of jaw | 4 (15) | 2 (8) | 0 (0) |
| Tinnitus | 2 (8) | 0 (0) | 0 (0) |
| Cerebral abscesss | 0 (0) | 1 (4) | 0 (0) |
| Glaucoma | 1 (4) | 0 (0) | 0 (0) |
| Hearing impairment | 1 (4) | 0 (0) | 0 (0) |
Data are shown as n (%).
Figure 4.
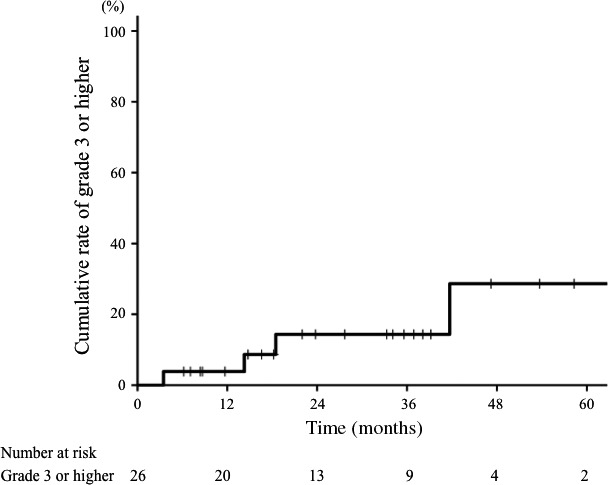
Cumulative curve of any late adverse events of grade 3 or higher in patients with mucoepidermoid carcinoma treated with carbon‐ion radiotherapy. The 3‐year cumulative rate for all patients was 14%.
Discussion
The major salivary glands are the most common site of mucoepidermoid carcinoma. Although several studies have reported the efficacy of surgery and/or radiotherapy for malignant salivary gland cancers, including various pathologic tumors,5 to our knowledge, there have been no reports on the use of radical radiotherapy for mucoepidermoid carcinoma only. The present study is the first to report the excellent efficacy of carbon‐ion radiotherapy for treating mucoepidermoid carcinoma: the 3‐year local control and OS rates were 95% and 89%, respectively.
There have been no prospective or randomized studies of radiotherapy for salivary gland cancers, including mucoepidermoid carcinoma, and a few retrospective studies have been reported.10, 11, 12 Terhaard et al.11 reported that postoperative radiotherapy significantly improved the 10‐year local control rate compared with surgery alone for locally advanced salivary gland cancers (84% vs 18%). Schoenfeld et al.12 reported that the 3‐year local control rate following postoperative intensity‐modulated radiation therapy (IMRT) was 92%. A few studies have shown clinical results with radical radiotherapy without surgery. Chen et al.13 reported that the 10‐year local control rate using radiotherapy alone was 31% for salivary gland cancers (57% of the cancers were mucoepidermoid carcinomas). Given these findings, surgery with postoperative radiotherapy is considered standard therapy, indicating better outcomes than either radiotherapy or surgery alone.
Fast neutron therapy, a high linear energy transfer beam, has been shown to achieve favorable local control rates. In a phase III study for salivary gland cancers, Laramore et al.14 reported that fast neutron therapy improved local control rates compared with photon therapy (56% vs 17%; P = 0.009). However, severe late adverse events were frequently observed in neutron therapy, and the OS rate was inferior to that of photon therapy. Another retrospective study reported similar findings; they concluded that further improvement might be achieved by using another high linear energy transfer beam with a superior dose profile, such as carbon‐ion radiotherapy.15
Jensen et al.16 reported the clinical outcomes of IMRT plus an additional boost of carbon‐ion radiotherapy for salivary gland cancers; 45% of the salivary gland cancers included in their study were mucoepidermoid carcinomas. The median radiation doses of IMRT and carbon‐ion radiotherapy were 50 Gy and 24 Gy (RBE), respectively. The 3‐year local control and OS rates were 81.5% and 72.8%, without an increased rate of late adverse events. Although 43% of patients included in their study underwent surgery plus postoperative radiotherapy (microscopic disease), the outcomes were relatively worse than those observed in the present study using definitive carbon‐ion radiotherapy. Although it is difficult to compare these results due to different treatment regimens used, the discrepant results might relate to the combination of photon therapy and carbon‐ion radiotherapy.
Carbon‐ion radiotherapy has a sharp dose distribution; however, it is difficult to avoid adverse events when tumors are located close to important organs. Generally, acute adverse events are improved by conservative therapy, whereas late adverse events are indolent and refractory. In the present study, the 3‐year cumulative rate of grade 3 or higher adverse events was 14%. Recently, the dose constraints of several organs at risk have been reported,17, 18, 19, 20 and safer treatment planning is required to minimize the severe adverse events based on these constraints. Furthermore, meticulous follow‐up to timeously detect late adverse events is needed, and conservative therapy should be considered to avoid decreases in the patients' quality of life.
In the present study, five patients (19%) developed distant metastases and required systemic therapy. However, there have been few reports of efficacious chemotherapy for metastatic or recurrent mucoepidermoid carcinomas.5 A phase II trials of paclitaxel showed a partial response rate of 21% (n = 3/14) and a 3‐year OS rate of 11% for mucoepidermoid carcinoma.21 Promising genetic and molecular markers offer new outlooks for diagnosis and treatment because the molecular biology approach has been extended to several signaling pathways.22, 23 For salivary gland cancers, these markers are expected to be therapeutic targets for molecular targeting drugs.24 Several phase II trials have been undertaken using these drugs, including trials of gefitinib,25 lapatinib,26 cetuximab,27 and trastuzumab.28 However, these studies included only two to three patients with mucoepidermoid carcinomas, and objective response rates to the drugs were low (0%–33%). Efficacious systemic therapies, including chemotherapy and molecular targeting drugs, are required for treatment of recurrent or metastatic mucoepidermoid carcinoma in the future.
The present study has some limitations. First, it was a retrospective study with a small sample size. However, the clinical outcomes of carbon‐ion radiotherapy for mucoepidermoid carcinoma reported herein are considered useful due to the extreme rarity of the disease. Further follow‐up will be necessary to confirm the long‐term efficacy and late toxicities. Second, the radiation dose was not uniform as the various participating institutions used different regimens. However, in the present study, the radiation dose was not a prognostic factor for clinical outcomes. We are currently carrying out a multicenter prospective study using a standardized treatment regimen. Third, information on histologic grading of the tumors, considered a prognostic factor for mucoepidermoid carcinoma, was absent in this study. However, recent reports have shown that the traditional grading systems are controversial.29 Further study is warranted to evaluate the impact of histologic grading on the outcomes of patients with mucoepidermoid carcinoma treated with carbon‐ion radiotherapy.
In conclusion, carbon‐ion radiotherapy showed excellent local control and survival rates in patients with mucoepidermoid carcinomas of the head and neck. Further follow‐up is required to evaluate treatment efficacy over a longer period. In addition, determining the dose constraints of organs at risk when using carbon‐ion radiotherapy is necessary to establish safer treatment planning.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
We would like to thank Dr. Atsushi Musha and Dr. Takanori Abe for their useful discussions.
Cancer Sci 108 (2017) 1447–1451
Funding Information
None declared.
References
- 1. Speight PM, Barrett AW. Salivary gland tumours. Oral Dis 2002; 8: 229–40. [DOI] [PubMed] [Google Scholar]
- 2. Coca‐Pelaz A, Rodrigo JP, Triantafyllou A et al Salivary mucoepidermoid carcinoma revisited. Eur Arch Otorhinolaryngol 2015; 272: 799–819. [DOI] [PubMed] [Google Scholar]
- 3. Goode RK, Auclair PL, Ellis GL. Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer 1998; 82: 1217–24. [DOI] [PubMed] [Google Scholar]
- 4. Eversole LR. Mucoepidermoid carcinoma: review of 815 reported cases. J Oral Surg 1970; 28: 490–4. [PubMed] [Google Scholar]
- 5. Adelstein DJ, Koyfman SA, El‐Naggar AK, Hanna EY. Biology and management of salivary gland cancers. Semin Radiat Oncol 2012; 22: 245–53. [DOI] [PubMed] [Google Scholar]
- 6. Kanai T, Endo M, Minohara S et al Biophysical characteristics of HIMAC clinical irradiation system for heavy‐ion radiation therapy. Int J Radiat Oncol Biol Phys 1999; 44: 201–10. [DOI] [PubMed] [Google Scholar]
- 7. Jensen AD, Poulakis M, Nikoghosyan AV et al High‐LET radiotherapy for adenoid cystic carcinoma of the head and neck: 15 years' experience with raster‐scanned carbon ion therapy. Radiother Oncol 2016; 118: 272–80. [DOI] [PubMed] [Google Scholar]
- 8. Koto M, Hasegawa A, Takagi R et al Feasibility of carbon ion radiotherapy for locally advanced sinonasal adenocarcinoma. Radiother Oncol 2014; 113: 60–5. [DOI] [PubMed] [Google Scholar]
- 9. Jingu K, Hasegawa A, Mizo JE et al Carbon ion radiotherapy for basal cell adenocarcinoma of the head and neck: preliminary report of six cases and review of the literature. Radiat Oncol 2010; 5: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cerda T, Sun XS, Vignot S et al A rationale for chemoradiation (vs radiotherapy) in salivary gland cancers? On behalf of the REFCOR (French rare head and neck cancer network). Crit Rev Oncol Hematol 2014; 91: 142–58. [DOI] [PubMed] [Google Scholar]
- 11. Terhaard CH, Lubsen H, Rasch CR et al The role of radiotherapy in the treatment of malignant salivary gland tumors. Int J Radiat Oncol Biol Phys 2005; 61: 103–11. [DOI] [PubMed] [Google Scholar]
- 12. Schoenfeld JD, Sher DJ, Norris CM Jr et al Salivary gland tumors treated with adjuvant intensity‐modulated radiotherapy with or without concurrent chemotherapy. Int J Radiat Oncol Biol Phys 2012; 82: 308–14. [DOI] [PubMed] [Google Scholar]
- 13. Chen AM, Bucci MK, Quivey JM, Garcia J, Eisele DW, Fu KK. Long‐term outcome of patients treated by radiation therapy alone for salivary gland carcinomas. Int J Radiat Oncol Biol Phys 2006; 66: 1044–50. [DOI] [PubMed] [Google Scholar]
- 14. Laramore GE, Krall JM, Griffin TW et al Neutron versus photon irradiation for unresectable salivary gland tumors: final report of an RTOG‐MRC randomized clinical trial. Radiation Therapy Oncology Group. Medical Research Council. Int J Radiat Oncol Biol Phys 1993; 27: 235–40. [DOI] [PubMed] [Google Scholar]
- 15. Stannard C, Vernimmen F, Carrara H et al Malignant salivary gland tumours: can fast neutron therapy results point the way to carbon ion therapy? Radiother Oncol 2013; 109: 262–8. [DOI] [PubMed] [Google Scholar]
- 16. Jensen AD, Poulakis M, Vanoni V et al Carbon ion therapy (C12) for high‐grade malignant salivary gland tumors (MSGTs) of the head and neck: do non‐ACCs profit from dose escalation? Radiat Oncol 2016; 11: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koto M, Hasegawa A, Takagi R et al Risk factors for brain injury after carbon ion radiotherapy for skull base tumors. Radiother Oncol 2014; 111: 25–9. [DOI] [PubMed] [Google Scholar]
- 18. Hasegawa A, Mizoe JE, Mizota A, Tsujii H. Outcomes of visual acuity in carbon ion radiotherapy: analysis of dose‐volume histograms and prognostic factors. Int J Radiat Oncol Biol Phys 2006; 64: 396–401. [DOI] [PubMed] [Google Scholar]
- 19. Musha A, Shimada H, Shirai K et al Prediction of acute radiation mucositis using an oral mucosal dose surface model in carbon ion radiotherapy for head and neck tumors. PLoS One 2015; 10: e0141734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sasahara G, Koto M, Ikawa H et al Effects of the dose‐volume relationship on and risk factors for maxillary osteoradionecrosis after carbon ion radiotherapy. Radiat Oncol 2014; 9: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gilbert J, Li Y, Pinto HA. Phase II trial of taxol in salivary gland malignancies (E1394): a trial of the Eastern Cooperative Oncology Group. Head Neck 2006; 28: 197–204. [DOI] [PubMed] [Google Scholar]
- 22. Yin LX, Ha PK. Genetic alterations in salivary gland cancers. Cancer 2016; 122: 1822–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stenman G, Persson F, Andersson MK. Diagnostic and therapeutic implications of new molecular biomarkers in salivary gland cancers. Oral Oncol 2014; 50: 683–90. [DOI] [PubMed] [Google Scholar]
- 24. Lagha A, Chraiet N, Ayadi M et al Systemic therapy in the management of metastatic or advanced salivary gland cancers. Head Neck Oncol 2012; 4: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jakob JA, Kies MS, Glisson BS et al Phase II study of gefitinib in patients with advanced salivary gland cancers. Head Neck 2015; 37: 644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Agulnik M, Cohen EW, Cohen RB et al Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non adenoid cystic carcinoma malignant tumors of the salivary glands. J Clin Oncol 2007; 25: 3978–84. [DOI] [PubMed] [Google Scholar]
- 27. Locati LD, Bossi P, Perrone F et al Cetuximab in recurrent and/or metastatic salivary gland carcinomas: a phase II study. Oral Oncol 2009; 45: 574–8. [DOI] [PubMed] [Google Scholar]
- 28. Haddad R, Colevas AD, Krane JF et al Herceptin in patients with advanced or metastatic salivary gland carcinomas. A phase II study. Oral Oncol 2003; 39: 724–7. [DOI] [PubMed] [Google Scholar]
- 29. Katabi N, Ghossein R, Ali S, Dogan S, Klimstra D, Ganly I. Prognostic features in mucoepidermoid carcinoma of major salivary glands with emphasis on tumour histologic grading. Histopathology 2014; 65: 793–804. [DOI] [PubMed] [Google Scholar]


