Abstract
Tumor growth depends on the formation of blood vessels that provide the supply of nutrients and oxygen. Previous data have shown that glioblastoma stem cells are able to give rise to vascular cells to constitute the functional vessels in tumor tissues. However, which kinds of vascular cells are generated from glioblastoma stem cells is largely debated. In addition, there is little evidence showing that the stem cells from other kinds of tumors can produce vascular cells to constitute the functional blood vessels in tumor tissues. Here we show that cancer stem cells of human colorectal carcinomas (CoCSC) can give rise to vascular endothelial cells and compose the vasculatures in cancer tissues. The human‐cell‐specific nuclear antigen NuMA + vascular endothelial cells were detected in the blood vessels in xenografts derived from CoCSC. NuMA + endothelial cells incorporated into functional blood vessels. Our data indicate that the cancer stem cells derived from human colorectal carcinomas have the capacity to generate functional blood vessels and provide a new mechanism for tumor vasculogenesis in carcinoma.
Keywords: Cancer stem cell, CD31, colon carcinoma, endothelial cells, tumor vasculogenesis
Colorectal cancer, one of the most common cancers worldwide, has high incidence and mortality rates.1 In China, colorectal carcinoma is the most common malignant tumor in the lower digestive tract. With the improvements in therapeutic approaches, the survival rate of patients with colorectal carcinomas has greatly improved.2 However, recurrence and metastasis of the carcinomas are inevitable and are the major cause of death of patients with colorectal carcinoma, suggesting that a fraction of the cancer cells are resistant to therapy. In recent ten years, published data have demonstrated that the main reason for the therapeutic resistance of colorectal carcinoma is the existence of colorectal cancer stem cells (CoCSC).3, 4, 5, 6, 7
Cancer stem cells (CSC) represent a population of cancer cells that are capable of self‐renewal, carry differentiation potential and have the ability to generate the heterogeneity tumor. CSC populations have been demonstrated in several types of solid tumors, including brain tumors,8 breast cancer,9 melanomas,10 pancreas adenocarcinomas,11 gastric cancer12 and colorectal cancer.4, 7, 13, 14 Over the past ten years, several studies have reported that CoCSC are enriched in the population of CD133+,14 ALDH1+( 15 ) or CD44+CD54+.7 The identification of the characteristics of CSC can help us to further understand cancer biology and to improve therapeutic approaches to cure cancer.
Tumor angiogenesis and vasculogenesis are essential for solid tumor growth, which depends on the formation of vessels to gain the necessary supply of nutrients and oxygen. Angiogenesis refers to the process by which new blood vessels sprout from the existing vessels, while vasculogenesis involves de novo production of endothelial cells from bone marrow‐derived endothelial progenitor cells.16 The importance of tumor vasculature has led to the development of anti‐angiogenic agents for the treatment of colorectal cancer. The addition of bevacizumab, a monoclonal antibody against vascular endothelial growth factor (VEGF), to chemotherapy in patients with metastatic colon cancer has demonstrated improved overall survival, progression‐free survival and response rate compared with chemotherapy alone.17 In contrast, single‐agent use of bevacizumab has not led to meaningful beneficial activity in many cases.18 Additional studies has provided preclinical evidence that anti‐angiogenic therapy causes cancer cells to become more malignant.18, 19 Thus, the mechanisms of tumor angiogenesis and vasculogenesis and their involvement in the vascularization in cancer tissues are more complicated than previously considered. Several studies have reported that glioblastoma stem cells can give rise to tumor vascular endothelial cells (EC)20, 21, 22 and vascular pericytes23 to constitute functional blood vessels in tumor tissues. The tumor‐generated vascular cells may play essential roles in the resistance to anti‐VEGF therapy. However, which kinds of vascular cells are generated from glioblastoma stem cells is largely debated. In addition, there is little evidence that the stem cells from other kinds of tumors, including carcinomas, can produce vascular cells to constitute functional blood vessels in tumor tissues. Here, we demonstrate that CoCSC are able to generate EC that constitute functional vessels in tumor tissues. Our data indicate that the cancer stem cells derived from human colorectal carcinomas have the capability to generate functional blood vessels and provide a new mechanism for tumor vasculogenesis in carcinoma.
Materials and Methods
Isolation of cancer stem cells of human colorectal carcinomas from colon tumor tissues and lentiviral infection
Cancer stem cells of human colorectal carcinomas were derived from tumor tissues obtained from consenting patients who underwent colon resection for primary colon adenocarcinoma at the Department of Gastrointestinal Surgery, West China Hospital, Sichuan University, as previously described.7 Briefly, tumor tissues were finely minced with scissors on ice and dissociated in DMEM/F12 (HyClone, Logan, UT, USA) containing collagenase (Sigma, St. Louis, MO, USA) by incubation for 1 h at 37°C. After mechanical and enzymatic dissociation and filtration through a 70‐μm pore filter (BD, Franklin Lakes, NJ, USA), the dissociated tumor cells were cultured in stem cell medium (DMEM/F12 supplemented with 20 ng/mL EGF and 10 ng/mL bFGF) on Ultra Low Attachment plates (Corning, Lowell, MA, USA).
The lentiviral vector expressing red fluorescent protein (RFP) under (EF) human elongation factor‐1 alpha promoter and the corresponding viruses were from Genepharma (Shanghai, China). CoCSC infection was performed as previously described.24
In vivo xenotransplantation of cancer cells
Studies involving nude mice were approved by the Sichuan University Institutional Animal Care and Use Committee. For subcutaneous xenografts, 1 × 105 cells from CoCSC spheres and RFP‐labeled CoCSC spheres were resuspended in 0.05 mL PBS, mixed with an equal volume of BD Matrigel (356230, BD Biosciences, Franklin Lakes, NJ, USA) at 4°C and injected into the flanks of nude mice using a 1‐mL syringe. Male or female nude mice (BALB/c strain), 4–6‐weeks old, were purchased from the Beijing Experimental Animal Center of the Chinese Academy of Sciences. Mice were sacrificed when the xenograft was approximately 10 mm in diameter. Xenografts were harvested for the next experiment. No randomization or blinding techniques were applied in this study. Injected mice were killed when the established criteria for end‐stage disease were reached.
Immunofluorescence
For detection with fluorescence, the CoCSC xenografts, RFP‐labeled CoCSC xenografts and tumorspheres were embedded in OCT (Sakura, Tokyo, Japan) and cut into 8‐μm frozen section using a sliding microtome (Thermo Fisher Scientific, Boston, MA, USA) at −20°C. Then we processed the sections for standard IF staining. The frozen sections were fixed with 4% paraformaldehyde for 15 min at room temperature and washed twice in 1× PBS, followed by incubation in blocking buffer (5% BSA [Sigma] in 1× PBST [1× PBS supplemented with 0.1% Tween‐20] supplemented with 0.25% Triton X‐100 [Sigma]) for 1 h. Then the sections were incubated in primary antibodies diluted in blocking buffer overnight at 4°C and washed in PBST thoroughly three times for approximately 30 min, followed by incubation in respective secondary antibodies for 1 h at room temperature. Sections were washed in PBST twice and counterstained with 0.5 μg/mL nuclear dye DAPI (Sigma) for 2 min, followed by three washes in PBS. Section were covered with slides using Fluoromount‐G (eBioscience, San Diego, CA, USA) and allowed to air‐dry. The slides were stored at 4°C. Fluorescence images were acquired with a fluorescence microscope (Zeiss Axio Scope A1).
The following primary antibodies were used: rat anti‐CD31 (1:500 550274, BD Biosciences); rabbit anti‐CD144 (1:500 D87F2, Cell Signaling Technology, Beverly, MA, USA); rabbit anti‐VEGFR2 (1:500 55B11, Cell Signaling Technology); goat anti‐NuMA (1:200 sc‐18557, Santa Cruz Biotechnology, Santa Cruz, CA, USA); rabbit anti‐NuMA (1:500 ab84680, Abcam, Cambridge, UK); rabbit anti‐CK20 (1:1000 ab76126, Abcam); and mouse anti‐CDX2 (1:1000 TA802667, Origene, Beijing, China).
The following secondary antibodies were used: Alexa Fluor 488‐conjugated donkey anti‐rabbit or rat or goat IgG (1:500 Thermo Fisher Scientific); Alexa Fluor 555‐conjugated donkey anti‐rabbit or goat IgG (1:500 Thermo Fisher Scientific); Alexa Fluor 647‐conjugated donkey anti‐goat IgG (1:500 Thermo Fisher Scientific).
All immunofluorescent staining results of in vitro cultured cells shown in this article were replicated more than five times. Immunofluorescent staining results of frozen sections of xenografts were replicated three times.
Vessel function assays
1 × 105 CoCSC or RFP‐labeled CoCSC were resuspended in 1× PBS with Matrigel and injected subcutaneously into the flanks of nude mice. Tumor size was monitored. When the tumor was approximately 10 mm in diameter, a mixture (100 μL) of 50 μg of Biotinylated Euonymus Europaeus Lectin (EEL) (B‐1335, Vector Laboratories) in 50 μL volume along with 50 μg of Biotinylated Lycopersicon Esculentum Lectin (LEL) (B‐1175, Vector Laboratories) in 50 μL volume was injected into the tail veins of the mice using an insulin syringe. After 15 min, we removed the tumor. The frozen sections of the tumor were stained with APC‐streptavidin (17‐4317‐82, eBioscience).
Capillary‐like tubes formation assay
The basement membrane extract used for this assay is growth factor reduced Matrigel matrix (356230, BD Biosciences). The Matrigel was removed from a freezer set at −20°C and its temperature was increased to 4°C before use. Then 20 μL of Matrigel was loaded per well of a 24‐well plate. The 24‐well plate was transferred to an incubator and incubated at 37°C for 30 min to allow the Matrigel to gel. The cells were suspended in respective media and seeded on Matrigel. Cells were periodically observed with a microscope.
RNA extraction and RT‐PCR
Total RNA were isolated from CoCSC, Co‐EC and CoCSC derivative grown in 10% FBS condition using a TriPure Reagent Kit (Roche), and then subjected to RT‐PCR analyses. cDNA was synthesized using an RT Reagent Kit (Takara). The primers for EC markers were: CD31 (forward: 5′ AGC TAA CAG TCA TTA CGG TCA 3′, reverse: 5′ TCC GTT CTA GAG TAT CTG CT 3′); CD144 (forward: 5′ AAA GAA TCC ATT GTG CAA GTC C 3′, reverse: 5′ CCT TGG TAT GCT CCC GGT CA 3′); TIE2 (forward: 5′ CAG CTT CTA TCG GAC TCC C 3′, reverse: 5′ TGA CTC TAG CTC GGA CCA C 3′); VEGFR2 (forward: 5′ GGC ACG AAA TAT CCT CTT ATC GG 3′, reverse: 5′ ATC TTT ACC CCA GGA TAT GGA G 3′); GAPDH (forward: 5′ CCT TCA TTG ACC TCA ACT ACA TGG T 3′, reverse: 5′ GCC TTC TCC ATG GTG GTG AAG AC 3′). The conditions were as follows: 4 min at 94°C for denaturation, followed by 30 s at 94°C, 30 s at 60°C and 30 s at 72°C for 38 cycles and 5 min at 72°C. The products were analyzed in 1% agarose gel.
Results
Endothelial cells generated from colorectal cancer stem cells constitute blood vessels
The cancer stem cell (CSC) model possesses self‐renewal and multilineage differentiation potential for tumorigenesis.25, 26 Colorectal cancer stem cells (CoCSC), with self‐renewal capacity, multipotency, tumor formation and drug resistance ability were previously isolated from human colorectal adenocarcinoma tissues.7 To investigate the tumor vasculature in vivo, we subcutaneously injected the CoCSC into the nude mice. The xenografts were removed and prepared for frozen sections. The immunofluorescence of the sections was then carried out using the antigens targeting platelet endothelial cell adhesion molecule‐1 (CD31), vascular endothelial cadherin (CD144) and vascular endothelial growth factor receptor 2 (VEGFR2) as markers of vascular endothelial cells (EC) and the human‐cell‐specific nuclear antigen‐nuclear mitotic apparatus protein (NuMA) as a marker of cells derived from human CoCSC. All of the tumor cells expressed NuMA and the EC expressed CD31 (Fig. 1a). In the xenografts, the CD31+ EC of blood vessels were detected. Some blood vessels were composed entirely of NuMA− EC (Fig. 1b), in which the CD31+ cells were completely distinct from the NuMA+ cells. Some vessels with typical vessel morphology were composed of both NuMA− and NuMA+ EC (Fig. 1d). In some vessels, the majority of cells were CD31+ cells with NuMA staining; only a few of CD31+ cells were NuMA− (Fig. 1e). The results indicate that the endothelial transdifferentiation of CoCSC contributes to the formation of vessels. We identified that the majority of CoCSC‐derived EC (Co‐EC) formed vessels together with murine EC. However, a few of vessels were composed of only NuMA+ EC (Fig. 1c), indicating that the CoCSC are able to differentiate into EC to constitute the blood vessels without murine EC in tumor tissues. The majority of vessels containing NuMA+ EC are in the inner portion of the tumor. We also detected blood vessels composed of human and mouse CD31+ EC located between the mouse tissues and the tumor tissues (Fig. 1f). We analyzed the blood vessels and discovered that 15–23% of EC were NuMA+ (Table 1).
Figure 1.
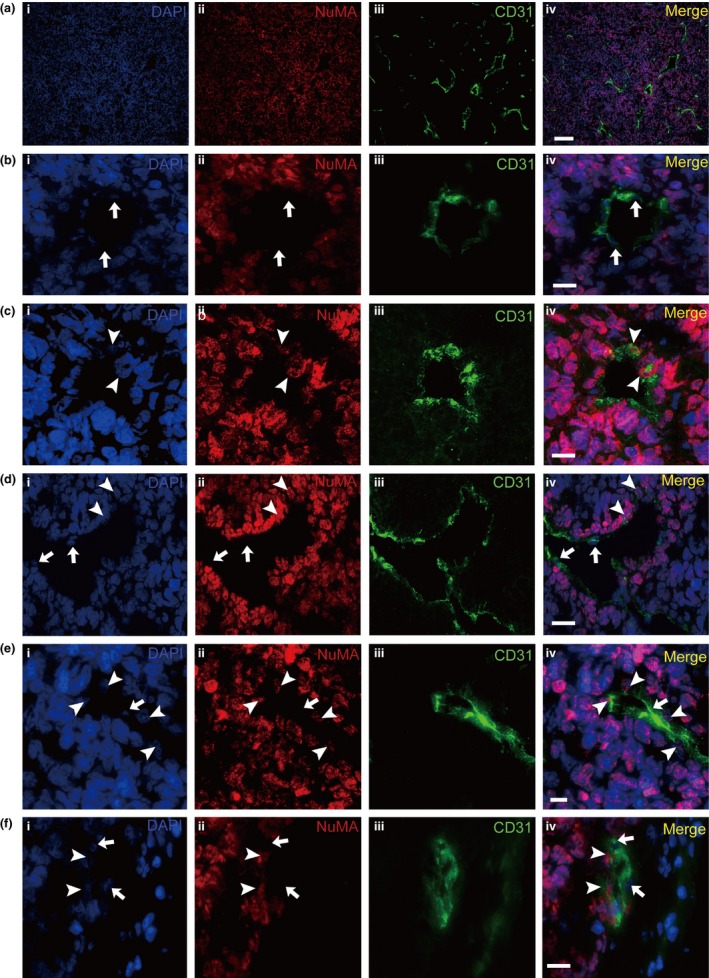
Cancer stem cells of human colorectal carcinomas (CoCSC) contribute to tumor vasculogenesis in xenograft tumor. Representative immunofluorescence micrographs of tumor xenograft sections were shown. DAPI was used as the nuclear marker (a–f(i)). The endothelial cells (EC) expressed CD31 (a–f(iii)) and the cells originating from CoCSC expressed NuMA (a–f(ii)). The images were incorporated (a–f(iv)). Arrowheads indicate NuMA + EC and arrows indicate NuMA − EC. Scale bars: 100 μm (a) and 20 μm (b–f).
Table 1.
Frequency of NuMA+ EC in the tumor
| Tumor | NuMA+ EC number/total EC number (%)a |
|---|---|
| 1 | 35/215 (16) |
| 2 | 30/196 (15) |
| 3 | 55/240 (23) |
| 4 | 28/187 (15) |
Each result was obtained by counting cells in six images representing an area (870 × 652 μm) of xenograft frozen sections. EC, endothelial cells.
We next subcutaneously injected RFP‐labeled CoCSC into the nude mice. The presence of Co‐EC was confirmed by labeling frozen sections of tumor xenografts with the anti‐CD31, anti‐RFP and anti‐NuMA antibodies. Accordingly, Figure 2a shows a vessel consisting of only RFP+/NuMA+/CD31+ EC. In addition, some RFP+/NuMA+/CD31+ EC formed vessels with RFP−/NuMA−/CD31+ EC (Fig. 2b), while some vessels were consisted of only RFP−/NuMA−/CD31+ EC (Fig. 2c).
Figure 2.
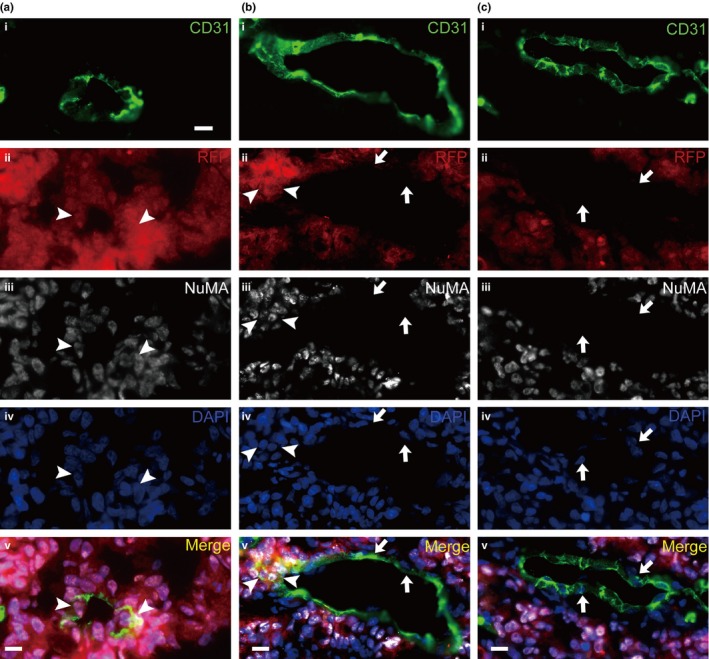
Red fluorescent protein (RFP)‐labeled cancer stem cells of human colorectal carcinomas (CoCSC) generate RFP+ endothelial cells (EC) in xenograft tumors. Representative immunofluorescence micrographs of tumor xenograft sections are shown. The EC expressed CD31 (a–c(i)) and the cells originating from CoCSC‐expressed RFP (a–c(ii)) and NuMA (a–c(iii)). DAPI was used as the nuclear marker (a–c(iv)). The images were incorporated (a–c(v)). Arrowheads indicate NuMA +/RFP + EC and arrows indicate NuMA −/RFP − ECs. Scale bars: 20 μm.
Immunofluorescence with the endothelial antigen VEGFR2 and CD144 also showed that many cells with endothelial markers carried NuMA antigens (Fig. 3a,b). However, further observations identified that many of the tumor cells also expressed CD144 (Fig. 3d). In the xenografts, some of NuMA+ tumor cells without typical vascular morphology were stained for CD144, suggesting that CD144 is a marker that lacks specificity for EC in xenografts generated from CoCSC. In contrast, both CD31 and VEGFR2 stained only EC but not tumor cells (data not shown). Moreover, triple staining with CD31, VEGFR2 and NuMA demonstrated that the NuMA+ EC co‐expressed CD31 and VEGFR2. This result supports our finding that CoCSC‐derived EC are involved in the formation of blood vessels in vivo (Fig. 3b). We validated the results in five xenografts produced from the CoCSC isolated from two different patients who underwent colon resection for primary colorectal adenocarcinoma. Taken together, these data demonstrate that CoCSC have the capacity to differentiate into EC and contribute to tumor vasculogenesis in xenografts.
Figure 3.
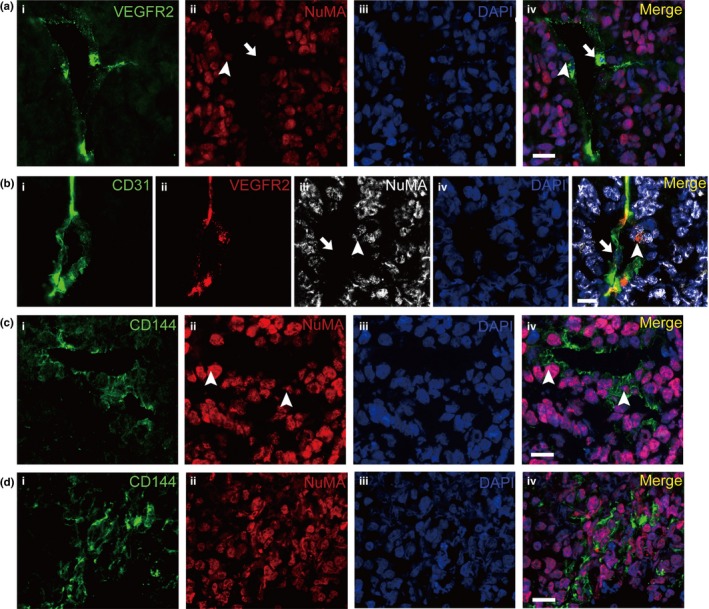
Cancer stem cells of human colorectal carcinomas (CoCSC) generate endothelial cells (EC) expressing the endothelial antigen VEGFR2 and CD144 in xenograft tumors. (a) Representative immunofluorescence micrographs of tumor xenograft sections labeled with VEGFR2 (i) and NuMA (ii) showed that some EC expressed the markers of EC and NuMA. (b) Tripe staining with CD31 (i), VEGFR2 (ii) and NuMA (iii) show that some EC co‐expressing CD31 and VEGFR2 are NuMA +. Arrowheads indicate NuMA + EC and arrows indicate NuMA − EC. (c) Representative immunofluorescence micrographs of tumor xenograft sections labeled with CD144 (i) and NuMA (ii) showed that some EC expressed the markers of EC and NuMA. (d) IF staining of CD144 and NuMA showed that some tumor cells expressed CD144. Arrowheads indicate NuMA + EC. Scale bars: 20 μm.
Blood vessels originated from colorectal cancer stem cells are functional
To investigate whether blood flow ran through the blood vessels constituted by the Co‐EC, we injected biotinylated lectin into the tail veins of tumor‐bearing mice 15 min before euthanasia. If the blood went through the vessels composed of the Co‐EC, the lectin would bind to EC from human and mouse sources. The xenografts were removed and the frozen sections of the xenografts were prepared and stained with CD31 and fluorescence‐labeled streptavidin that binds to biotinylated lectin. As shown in Figures 4 and 5b, lectin‐bound NuMA+ EC were detected in blood vessels with typical blood vessel morphology, indicating that the blood flow went through the vessels constituted by the Co‐EC. Further examination showed that the NuMA− vessels were bound to lectin (Fig. 5a). We further analyzed the frozen sections and found that in total 19–29% of lectin+ EC were NuMA+ (Table 2). The frozen sections of xenograft generated by RFP‐labeled CoCSC were stained with CD31, RFP and fluorescence‐labeled streptavidin. The fact that some CD31+ EC co‐labeled with RFP and lectin demonstrated that the vessels were functional (Fig. 5c) and some CD31+/lectin+ EC were RFP− (Fig. 5d). There were also nonfunctional Co‐EC‐forming vessels in which the EC were RFP+ and lectin− (Fig. 5e). Lectin−/RFP− vessels were also observed (Fig. 5f), indicating that in tumor xenografts, some of the blood vessels from the host were also nonfunctional. These assays demonstrate that the Co‐EC‐constituted blood vessels are functional.
Figure 4.
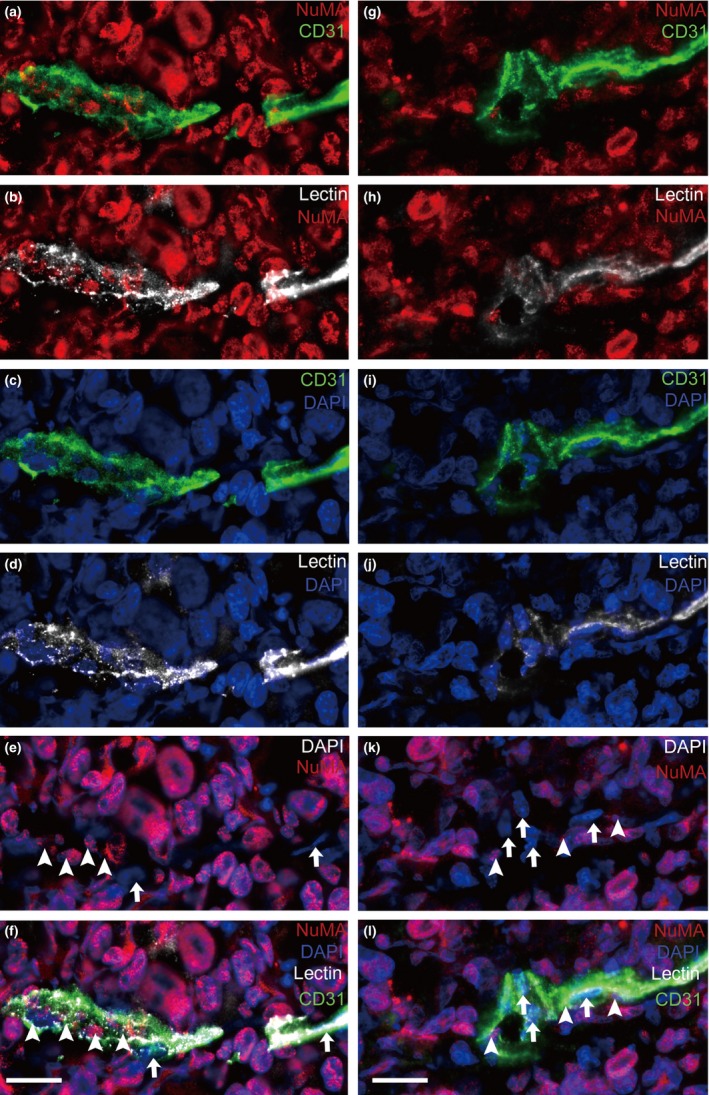
The vessels containing CoCSC‐derived EC (Co‐EC) are functional. Representative immunofluorescence micrographs of tumor xenograft sections labeled with NuMA (red), CD31 (green) and lectin (white) showed that the NuMA + CD31+ endothelial cells (EC) were bound with lectin. Arrowheads indicate NuMA + EC and arrows indicate NuMA − EC. Scale bars: 20 μm.
Figure 5.
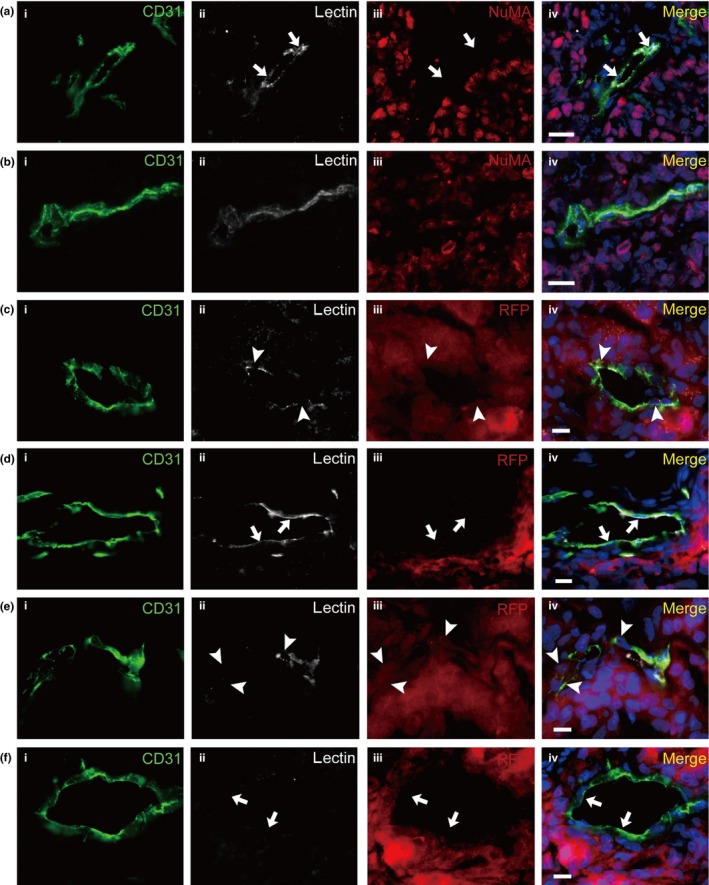
Blood flow in the vessels of cancer stem cells of human colorectal carcinomas (CoCSC) xenograft. Lectin which can bind to endothelial cells (EC) was injected into the tail veins of the mice. Representative immunofluorescence micrographs showed that a vessel constituted by NuMA − endothelial cells (EC) (a) or RFP − EC (d), a vessel constituted by RFP + EC (c) and a vessel containing several NuMA + EC (b) were functional. (e) Nonfunctional RFP + EC‐forming vessels were also observed. (f) Nonfunctional RFP − vessels were also observed. Arrowheads indicate NuMA + or RFP + EC and arrows indicate NuMA − or RFP − EC. Scale bars: 20 μm.
Table 2.
Frequency of NuMA+ lectin+ EC in the xenograft
| Xenograft | NuMA+ lectin+ EC number/total lectin+ EC number (%)a |
|---|---|
| 1 | 20/70 (29) |
| 2 | 18/96 (19) |
| 3 | 30/108 (28) |
Each result was obtained by counting cells in six images representing an area (870 × 652 μm) of xenograft frozen sections.
Colorectal cancer stem cells directly generate endothelial cells in vitro
Because CoCSC can transdifferentiate into functional EC in vivo, we next attempted to induce CoCSC differentiation in vitro. We cultured CoCSC in DMEM medium supplemented with 10% FBS (DFS) and endothelial growth medium (EGM2). At first, single CoCSC were small and rounded. After 4 days, the cells cultured in DFS aggregated into clusters of polygonal cells and exhibited epithelial‐like cell morphology, whereas the cells cultured in EGM2 were spindle and exhibited endothelial‐like cell morphology (Fig. 6a). Because endothelial cells have the capability to undergo angiogenesis and to organize into capillary‐like tubes on Matrigel‐based coverclips in vitro, we seeded the epithelial‐like cells and the endothelial‐like cells onto the Matrigel‐based coverclips, respectively. After 24 h, we found that the endothelial‐like cells formed capillary‐like tubes and the epithelial‐like cells did not (Fig. 6b). We next analyzed the endothelial‐cell related gene expression of the different differentiated cells and the undifferentiated cell spheres of CoCSC. RT‐PCR of key endothelial markers (CD31, CD144, VEGFR2 and tyrosine kinase receptors 2 [TIE2]) confirmed that the endothelial‐like cells expressed CD144, CD31, VEGFR2 and TIE2, while the epithelial‐like cells expressed CD144 but not CD31, VEGFR2 and TIE2 (Fig. 6c). Immunofluorescent staining demonstrated partial CD144 expression but complete absence of CD31 and VEGFR2 both in the spheres of CoCSC (Fig. 7a) and the epithelial‐like cells differentiated in DFS (Fig. 7b). However, the endothelial‐like cells differentiated in EGM2 were positive for CD144, CD31 and VEGFR2 (Fig. 7c). All of these cells expressed NuMA or caudal‐type homeobox transcription factor 2 (CDX2), indicating that these cells are derived from CoCSC. These results demonstrate that the endothelial‐like cells cultured in EGM2 are EC.
Figure 6.
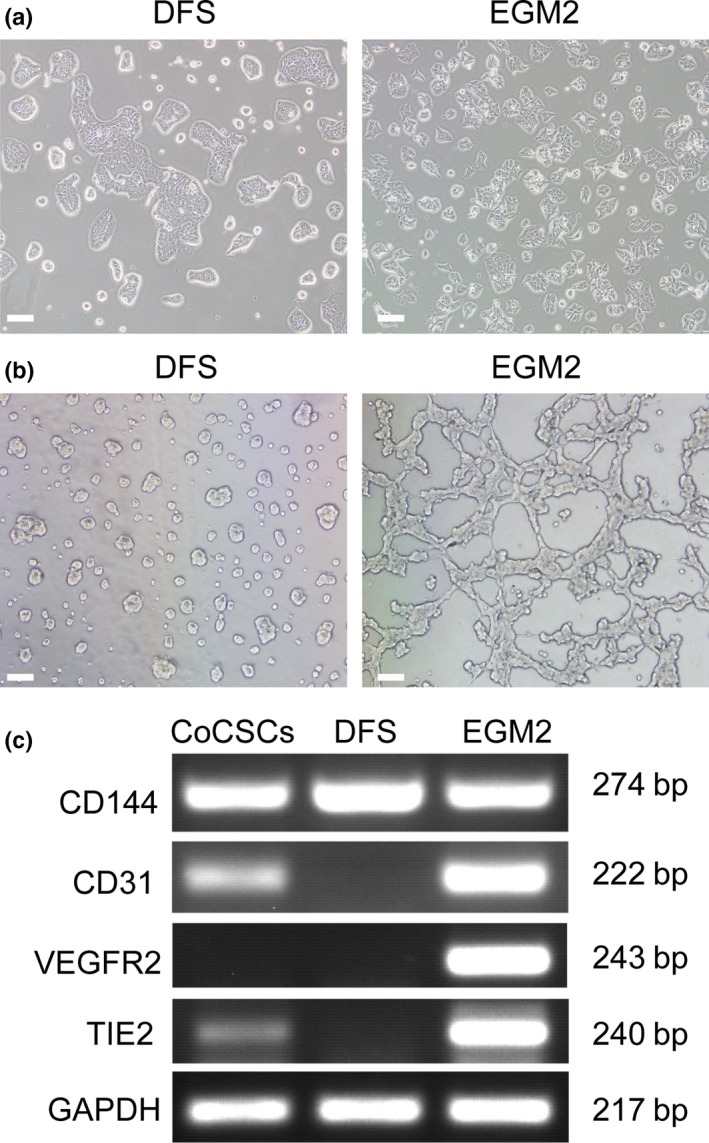
Inducing differentiation of cancer stem cells of human colorectal carcinomas (CoCSC) with DFS and EGM2. (a) Morphological changes of CoCSC cultured in DFS and EGM2. (b) Capillary‐like tube formation assay. CoCSC cultured in DFS and EGM2 were seeded on Matrigel in respective medium. (c) RT‐PCR analysis of endothelial marker expression in CoCSC, CoCSC cultured in DFS and CoCSC cultured in EGM2. Scale bars: 50 μm.
Figure 7.
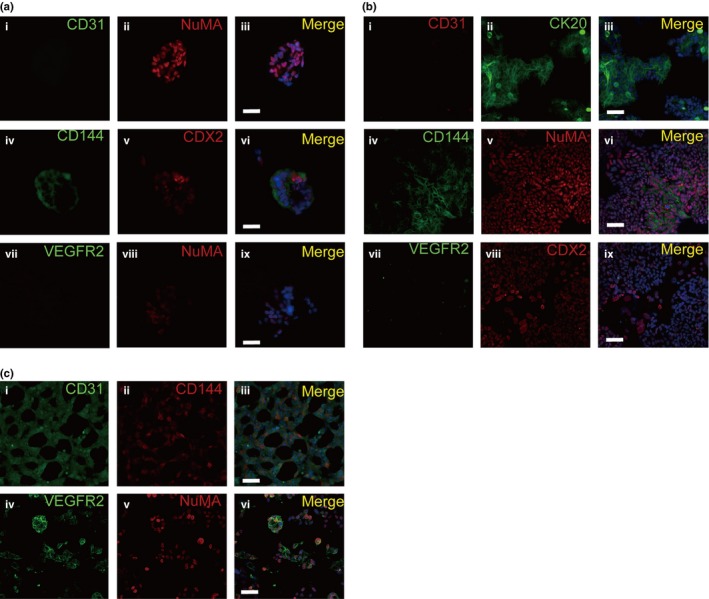
Endothelial differentiation of cancer stem cells of human colorectal carcinomas (CoCSC) in vitro. (a) Representative immunofluorescence micrographs of CoCSC spheres sections labeled with CD31 and NuMA (i–iii), CD144 and CDX2 (iv–vi) or VEGFR2 and NuMA (vii–ix) showed that CD31 and VEGFR2 were negative but CD144, NuMA and CDX2 were positive in CoCSC sphere. (b) Representative immunofluorescence micrographs of CoCSC cultured in DFS showed that the CD31 (i) and VEGFR2 (vii) were negative but the CD144 (iv), CDX2 (viii), CK20 (ii) and NuMA (v) were positive. (c) Representative immunofluorescence micrographs of CoCSC cultured in EGM2 showed that the CD31 (i), CD144 (ii) and VEGFR2 (iv) were positive. Scale bars: 50 μm.
In this study, we showed the EC formed by CoCSC in human CoCSC xenografts, as demonstrated by detection of NuMA expression in CD31+, CD144+ or VEGFR2+ EC, to constitute the functional blood vessels in cancer tissues. In conclusion, our studies demonstrate that CoCSC not only generate tumor cells but also contribute to tumor vasculogenesis by transdifferentiating into Co‐EC.
Discussion
Solid tumor growth depends on the formation of vessels. Tumor blood vessels are thought to be derived from sprouting of preexisting vessels and cells recruited from the bone marrow.27 To date, it is becoming clear that blood vessels can also originate from cancer stem cells. The possibility of endothelial differentiation of cancer stem cells has been suggested in glioblastoma, breast carcinomas,28 renal carcinomas29 and multiple myeloma.30 In addition, the data suggest that tumor stem cells from glioblastoma are able to generate vascular pericytes. The vascular cells generated from glioblastoma stem cells have been reported to constitute the functional blood vessels in tumor tissues.
In this study, we show the presence of EC formed by CoCSC in human CoCSC xenografts, as demonstrated by detection of human‐specific protein NuMA expression in CD31+, CD144+, or VEGFR2+ EC. The widely‐accepted vascularization mechanisms in cancer suggest that the tumors promote their own vascularization by inducing the formation of new capillary buds from preexisting host tissue capillaries. As described previously, the glioblastoma stem cell‐derived vessels are mainly composed of human CD34+, CD144+ or VEGFR2+ EC in glioblastoma xenografts.22 In this study, we identified that both Co‐EC and murine EC can participate in forming the luminal surface of functional blood vessels in CoCSC xenografts. In some tumor blood vessels, the majority of EC are Co‐EC. Blood vessels mainly composed of murine EC were also detected. We also find that some blood vessels constituted by murine EC and Co‐EC were functional in tumor tissues.
Vascular endothelial growth factor is a crucial EC growth factor in the tumor vasculature and regulates the formation and survival of tumor blood vessels.31 VEGF can bind to three different receptors, known as VEGFR1, VEGFR2 and VEGFR3. VEGFR1 regulates the migration of monocyte and macrophage, and VEGFR3 is important for the function of lymphoendothelial cells, whereas the function of VEGFR2 has been implicated in all aspects of normal and pathological vascular‐endothelial‐cell biology.32 In the present study, Co‐EC expressed VEGFR2, but the CoCSC did not, suggesting that VEGFR2‐mediated differentiation may not be the first process to trigger endothelial differentiation of CoCSC. The recent studies on breast carcinoma and glioblastoma indicated that CSC are able to differentiate into EC by a VEGF‐independent mechanism.21, 28 Therefore, molecular triggers and the role of VEGF during the processes of the endothelial differentiation of CoCSC are still to be addressed.
By RT‐PCR, we detected very low levels of CD31 mRNA in the spheres of CoCSC in comparison to EC transdifferentiated from CoCSC. However, the protein of CD31 was not detected by immunofluorescent staining in CoCSC. This suggests that CD31 transcription was slightly activated in cells in CoCSC spheres. The fact that CD31 expression was not detected by immunofluorescent staining is probably due to the low sensitivity of IF or post‐transcriptional silencing of CD31.
A previous study demonstrated that mouse bone marrow cells can fuse spontaneously with other cells and subsequently adopt the phenotype of the recipient cells.33 The results of endothelial differentiation in vitro excluded the possibility. The CoCSC all expressed the colon‐cell‐specific nuclear antigen CDX2, but not CD31 and VEGFR2, indicating that CoCSC did not contain any EC. Our data show that CoCSC give rise to Co‐EC by differentiation but not cell fusion.
Vasculogenic mimicry (VM) has been reported in melanomas34 and other tumors.35, 36 The tumor cells are not EC. The channels are also not vessels and just mimic the function of vessels. “Mosaic” vessels have been reported, in which both endothelial cells and tumor cells form the luminal surface in the xenograft of the LS174T human colon adenocarcinoma cell line.37 The tumor cells in the two aberrant tumor vessels do not express EC markers. We found that the CoCSC generated Co‐EC directly participated in tumor vasculogenesis. The phenotypes might represent a novel underlying mechanism for vasculogenesis in tumor tissues.
In conclusion, our results demonstrate that CoCSC not only generate tumors in the mouse models but also contribute to tumor vasculogenesis by transdifferentiating into Co‐EC.
Disclosure Statement
The authors have no conflict of interest to declare.
Acknowledgments
The institutional Ethics Committees of West China hospital approved this study. We thank the participants and their families for their kind cooperation, generosity, and patience. We appreciate Prof. Dr. Anming Meng for suggestion and advice. This work was supported by the National High‐tech R&D Program of China (863 Program), No.2015AA020306.
Cancer Sci 108 (2017) 1357–1367
Funding Information
National High‐tech R&D Program (863 Program) (Grant/Award Number: 2015AA020306).
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Cunningham D, Atkin W, Lenz HJ et al Colorectal cancer. Lancet 2010; 375: 1030–47. [DOI] [PubMed] [Google Scholar]
- 3. Ricci‐Vitiani L, Fabrizi E, Palio E, De Maria R. Colon cancer stem cells. J Mol Med (Berl) 2009; 87: 1097–104. [DOI] [PubMed] [Google Scholar]
- 4. Todaro M, Alea MP, Di Stefano AB et al Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin‐4. Cell Stem Cell 2007; 1: 389–402. [DOI] [PubMed] [Google Scholar]
- 5. Pang R, Law WL, Chu AC et al A subpopulation of CD26 + cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell 2010; 6: 603–15. [DOI] [PubMed] [Google Scholar]
- 6. Anderson EC, Hessman C, Levin TG, Monroe MM, Wong MH. The role of colorectal cancer stem cells in metastatic disease and therapeutic response. Cancers 2011; 3: 319–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fan CW, Chen T, Shang YN et al Cancer‐initiating cells derived from human rectal adenocarcinoma tissues carry mesenchymal phenotypes and resist drug therapies. Cell Death Dis 2013; 4: e828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singh SK, Hawkins C, Clarke ID et al Identification of human brain tumour initiating cells. Nature 2004; 432: 396–401. [DOI] [PubMed] [Google Scholar]
- 9. Al‐Hajj M, Wicha MS, Benito‐Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003; 100: 3983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fang D, Nguyen TK, Leishear K et al A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res 2005; 65: 9328–37. [DOI] [PubMed] [Google Scholar]
- 11. Dembinski JL, Krauss S. Characterization and functional analysis of a slow cycling stem cell‐like subpopulation in pancreas adenocarcinoma. Clin Exp Metastas 2009; 26: 611–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen T, Yang K, Yu J et al Identification and expansion of cancer stem cells in tumor tissues and peripheral blood derived from gastric adenocarcinoma patients. Cell Res 2011; 22: 248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007; 445: 106–10. [DOI] [PubMed] [Google Scholar]
- 14. Ricci‐Vitiani L, Lombardi DG, Pilozzi E et al Identification and expansion of human colon‐cancer‐initiating cells. Nature 2007; 445: 111–5. [DOI] [PubMed] [Google Scholar]
- 15. Carpentino JE, Hynes MJ, Appelman HD et al Aldehyde dehydrogenase‐expressing colon stem cells contribute to tumorigenesis in the transition from colitis to cancer. Cancer Res 2009; 69: 8208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lyden D, Hattori K, Dias S et al Impaired recruitment of bone‐marrow‐derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 2001; 7: 1194–201. [DOI] [PubMed] [Google Scholar]
- 17. Hurwitz H, Fehrenbacher L, Novotny W et al Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350: 2335–42. [DOI] [PubMed] [Google Scholar]
- 18. Ebos JM, Lee CR, Cruz‐Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short‐term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 2009; 15: 232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paez‐Ribes M, Allen E, Hudock J et al Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 2009; 15: 220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang R, Chadalavada K, Wilshire J et al Glioblastoma stem‐like cells give rise to tumour endothelium. Nature 2010; 468: 829–33. [DOI] [PubMed] [Google Scholar]
- 21. Soda Y, Marumoto T, Friedmann‐Morvinski D et al Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc Natl Acad Sci USA 2011; 108: 4274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ricci‐Vitiani L, Pallini R, Biffoni M et al Tumour vascularization via endothelial differentiation of glioblastoma stem‐like cells. Nature 2010; 468: 824–8. [DOI] [PubMed] [Google Scholar]
- 23. Cheng L, Huang Z, Zhou W et al Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell 2013; 153: 139–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu R, Fan C, Shangguan W et al Neurons generated from carcinoma stem cells support cancer progression. Signal Trans Target Ther 2017; 2: 16036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell 2012; 10: 717–28. [DOI] [PubMed] [Google Scholar]
- 26. Vermeulen L, Sprick MR, Kemper K, Stassi G, Medema JP. Cancer stem cells–old concepts, new insights. Cell Death Differ 2008; 15: 947–58. [DOI] [PubMed] [Google Scholar]
- 27. Ahn GO, Brown JM. Matrix metalloproteinase‐9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow‐derived myelomonocytic cells. Cancer Cell 2008; 13: 193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bussolati B, Grange C, Sapino A, Camussi G. Endothelial cell differentiation of human breast tumour stem/progenitor cells. J Cell Mol Med 2009; 13: 309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bruno S, Bussolati B, Grange C et al CD133 + renal progenitor cells contribute to tumor angiogenesis. Am J Pathol 2006; 169: 2223–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rigolin GM, Fraulini C, Ciccone M et al Neoplastic circulating endothelial cells in multiple myeloma with 13q14 deletion. Blood 2006; 107: 2531–5. [DOI] [PubMed] [Google Scholar]
- 31. Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med 2011; 17: 1359–70. [DOI] [PubMed] [Google Scholar]
- 32. Olsson AK, Dimberg A, Kreuger J, Claesson‐Welsh L. VEGF receptor signalling ‐ In control of vascular function. Nat Rev Mol Cell Biol 2006; 7: 359–71. [DOI] [PubMed] [Google Scholar]
- 33. Terada N, Hamazaki T, Oka M et al Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 2002; 416: 542–5. [DOI] [PubMed] [Google Scholar]
- 34. Maniotis AJ, Folberg R, Hess A et al Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol 1999; 155: 739–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. El Hallani S, Boisselier B, Peglion F et al A new alternative mechanism in glioblastoma vascularization: tubular vasculogenic mimicry. Brain 2010; 133: 973–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sood AK, Seftor EA, Fletcher MS et al Molecular determinants of ovarian cancer plasticity. Am J Pathol 2001; 158: 1279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chang YS, di Tomaso E, McDonald DM, Jones R, Jain RK, Munn LL. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci USA 2000; 97: 14608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]


