Abstract
Clinical progressive disease (cPD) occurs during neoadjuvant chemotherapy (NAC) in 3%–5% of triple‐negative breast cancer (TNBC) patients. We aimed to identify the histopathological and immunohistochemical parameters that are correlated with the TNBC that showed cPD. We identified 22 TNBCs that showed cPD during NAC (cPD group) and 80 TNBCs that did not receive NAC (control group). Using surgically resected tumor specimens, we performed histopathologic examinations and immunohistochemical analysis of 11 molecules that appeared relevant to epithelial‐mesenchymal transition (EMT), and basal‐like, molecular apocrine and other features. Metaplastic carcinomas (MPCs) and high proliferation (≥50 mitoses per 10 high‐power fields or ≥50% Ki‐67 score) were more frequent in the cPD than in the control (41% vs 3%, P < 0.001, and 86% vs 50%, P = 0.0049, respectively). Positive cytokeratin 5/6, ZEB1, TWISTNB, vimentin, and HMGB1 expressions and negative androgen receptor were more frequent in the cPD than in the control. By an unsupervised hierarchical cluster analysis incorporating these 11 molecules, the 102 TNBCs were divided into two major clusters and seven subclusters that appeared to correspond to intrinsic subtype, cPD status, histological type, and clinical outcome. In 27% of cPD cases, the MPC component appeared only in the post‐NAC specimens. The combinations of high proliferation, metaplastic features, and immunohistochemical statuses of some EMT and basal‐like markers and androgen receptor appeared to be able to characterize the TNBCs that showed cPD after NAC.
Keywords: Basal‐like markers, clinical progressive disease, epithelial mesenchymal transition, neoadjuvant chemotherapy, triple negative breast cancer
Triple‐negative breast cancer (TNBC) is a subgroup of tumors that show negative expression of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), and accounts for 15%–20% of newly diagnosed breast cancer cases.1 This type of breast cancer tends to have a more aggressive nature compared with other breast cancer types.2, 3
Neoadjuvant chemotherapy (NAC) is a standard therapy for patients with early‐stage breast cancer,4, 5 and pathological complete response (pCR) after NAC is shown to be a surrogate marker for a better clinical outcome.6 Although 30%–40% of patients with TNBC can achieve pCR, residual TNBC has a greater risk of relapse and worse outcomes than other subtypes.7, 8 Especially, less than 5% of patients with primary breast cancer, mostly being TNBC, showed clinically progressive disease (cPD) during NAC, which leads the patients to inoperable stages, resistance to other chemotherapeutic regimens, and extremely poor clinical outcome.7, 8 Therefore, delineating features of TNBCs that showed cPD during NAC would be crucial for developing therapeutic strategies against these chemoresistant tumors.
Several parameters were already shown to be correlated with tumor progression during NAC, for example, T size, grade, and high Ki‐67 score before NAC.9 However, there were few studies that examined molecular markers characteristic of TNBCs that showed cPD. We conducted a retrospective study to identify histopathological and immunohistochemical features of TNBCs that showed cPD during NAC. Of the various candidate molecules, we focused on the basal‐like markers,10, 11, 12 epithelial–mesenchymal transition (EMT) markers,13, 14, 15, 16 inflammatory reaction markers,17, 18, 19, 20, 21 and the androgen receptor (AR).22, 23, 24
Materials and Methods
Study design and case selection
The study was approved by the institutional review board of the National Cancer Center (NCC), Tokyo (ID: 2011‐024), and complied with the ethical guidelines of the 1975 Declaration of Helsinki. At first, we retrospectively identified 22 patients with primary TNBC who consecutively received NAC but had cPD and subsequently received surgical therapy in the NCC Hospital (NCCH) between 1999 and 2011 (cPD group). Clinical progressive disease was defined as any increase in tumor size or new development of palpable lymphadenopathy according to the response evaluation criteria in solid tumors.25
In this study, we aimed at identifying characteristics of the TNBC that had shown cPD in comparison with untreated TNBC. Therefore, we chose the TNBC cases that did not receive NAC as the control group. When a 5% α error, a 10%–30% β error, and a 30% or more difference in the positivity of an immunohistochemical marker between the case and control groups were assumed, a 4‐fold number of control cases was sufficient to detect the difference statistically. At first, as the control group, we selected 110 patients who consecutively underwent surgical resection of primary TNBC without NAC at the NCCH between 1994 and 1998. This group represents pre‐NAC status of TNBCs, and <5% of these cases should result in cPD if treated with NAC.9
For these cPD and control cases, ER, PR and HER2 statuses were retested using current standardized methods with the antibodies listed in Table 1. We were able to confirm TNBC26, 27 and that a sufficient amount of cancer tissue was available for all 22 cPD tumors and 80 of the 110 tumors. Clinical and pathologic data including age and Tumor‐Node‐Metastasis28 were collected from clinical charts and pathology reports.
Table 1.
Antibodies used for immunohistochemistry in the present study
| Molecule | Antibody | Dilution | Localization | Control tissue | ||
|---|---|---|---|---|---|---|
| Type | Clone/code | Manufacturer | ||||
| Estrogen receptor | Mono (M) | 1D5 | Dako | ×100 | N | Mammary gland |
| Progesterone receptor | Mono (M) | PgR636 | Dako | ×100 | N | Mammary gland |
| HER2 | Poly (R) | HercepTest | Dako | Ready to use | Me | Breast cancer |
| CK5/6 | Mono (M) | D5/16 B4 | Dako | ×100 | C | Mammary gland |
| EGFR | Poly (R) | PharmDx | Dako | Ready to use | C, Me | Epidermis |
| BRCA1 | Mono (M) | MS110 | Calbiochem | ×100 | N | Mammary gland |
| ZEB1 | Poly (R) | ab87280 | Abcam | ×100 | N | MCF7, MDA‐MB‐231 |
| TWISTNB | Poly (R) | PAB21491 | Abnova | ×50 | N | Cerebellum |
| Vimentin | Mono (M) | V9 | Dako | ×100 | C | Fibroblast |
| E‐cadherin | Mono (M) | NCH‐38 | Dako | ×100 | Me | Epidermis |
| Snail‐2 | Poly (R) | PAB1923 | Abnova | ×200 | N | MCF7, MDA‐MB‐231 |
| HMGB1 | Mono (M) | 2F6 | Abnova | ×100 | C, N | Placenta |
| Androgen receptor | Mono (M) | AR441 | Dako | ×50 | N | Mammary gland |
C, cytoplasm; M, mouse; Me, membrane; N, nucleus; R, rabbit.
Chemotherapy
Chemotherapy for all patients in cPD group consisted of anthracycline‐based regimens followed by taxanes. The anthracycline‐based regimen used was either doxorubicin/cyclophosphamide (AC) or 5‐fluorouracil/epirubicin/cyclophosphamide (CEF), while the taxane used was either paclitaxel or docetaxel. Several previous studies showed that the effect of NAC was not different among these regimens.30, 31
As NAC for the cPD group, the anthracycline‐based regimens used were AC in 11 patients and CEF in the other 11, while the taxanes used were paclitaxel in 20 patients and docetaxel in two.
In the control group, all the 39 node‐positive cases received adjuvant chemotherapy and/or endocrine therapy, and 8 of 41 pN0 patients received adjuvant chemotherapy (Table S1).
Histological examinations and tissue microarray construction
Histological type, grade, mitotic counts, atypical medullary features, massive necrosis, and central acellular zone (massive infarction) were independently evaluated by two observers (Y.T. and H.T.)29 Tumor‐infiltrating lymphocytes (TILs) were assessed using a previously described method.21 For each tumor, two representative tissue cores 2 mm in diameter were enucleated from routinely processed formalin‐fixed paraffin‐embedded tumor tissue blocks and subjected to tissue microarray construction using a TMA arrayer (Azumaya, Tokyo, Japan).
Core needle biopsy specimens
In 22 cPD tumors, hematoxylin‐eosin (HE)‐stained slides of core needle biopsy specimens obtained before NAC were examined. For 12 of these, pre‐NAC tissue blocks were available for immunohistochemistry (IHC).
Immunohistochemistry
We examined Ki‐67, epidermal growth factor receptor (EGFR), cytokeratin 5/6 (CK5/6), breast cancer susceptibility gene 1 (BRCA1), high mobility group box 1 (HMGB1), vimentin, zinc finger E‐box binding homeobox 1 (ZEB1), TWIST neighbor (TWISTNB), E‐cadherin, Snail‐2 and AR. Primary antibodies are presented in Table 1, and for all these antibodies, antigens were retrieved in citrate buffer at 121°C for 10 min. Envision method was used for secondary antibody reaction, and diaminobenzidine tetrahydrochloride was used for peroxidase reaction.32 Ki‐67 labeling index (LI) was examined as described previously.32
For nuclear immunoreaction other than Ki‐67, the proportion of positive cells (score 0–5) and staining intensity (score 0–3) were considered,33 and the expression was regarded positive when the sum of these scores was >2. Cytoplasmic and/or membranous immunoreaction was regarded positive if ≥10% cells were stained regardress of intensity.11 For EGFR only, moderate to strong membranous/cytoplasmic staining (2+ or 3+) in ≥10% of tumor cells was regarded positive. IHC results were evaluated by two researchers (Y.T., H.T.) without knowledge of clinical characteristics. The basal‐like feature was defined as CK5/6 and/or EGFR expression.10
Statistical analysis
The cPD and control groups were compared by the χ2 test or Fisher's exact test, Student's t‐test, Kaplan–Meier survival curves and the log‐rank test. A hierarchical cluster analysis was conducted with the Ward method. These analyses were performed by using the SPSS software program, version 11.0.1 (SPSS Inc., Troy, NY, USA). In the unsupervised hierarchical cluster analysis, all patients of the cPD and control groups were included in order to visualize several subgroups classified by similarity of molecular expression patterns, relationship and distance among these subgroups, and their correlation with histological features and therapeutic effect. Differences were considered to be statistically significant for values of P < 0.05.
Results
Clinicopathological study results
The mean patient age was younger in the cPD group (42 years, range 25–62 years) than in the control group (55 years, range 25–80, P < 0.001) (Table 2). Most patients presented with stage II or III (100% in cPD group before NAC; 86% in control); but invasive T size of resected tumor was larger in the cPD than in the control (P = 0.0022). Axillary nodal metastasis was more frequent in the cPD group after NAC than in the control group (73% vs 48%; P = 0.041).
Table 2.
Comparison of clinicopathological features between the cPD and control groups
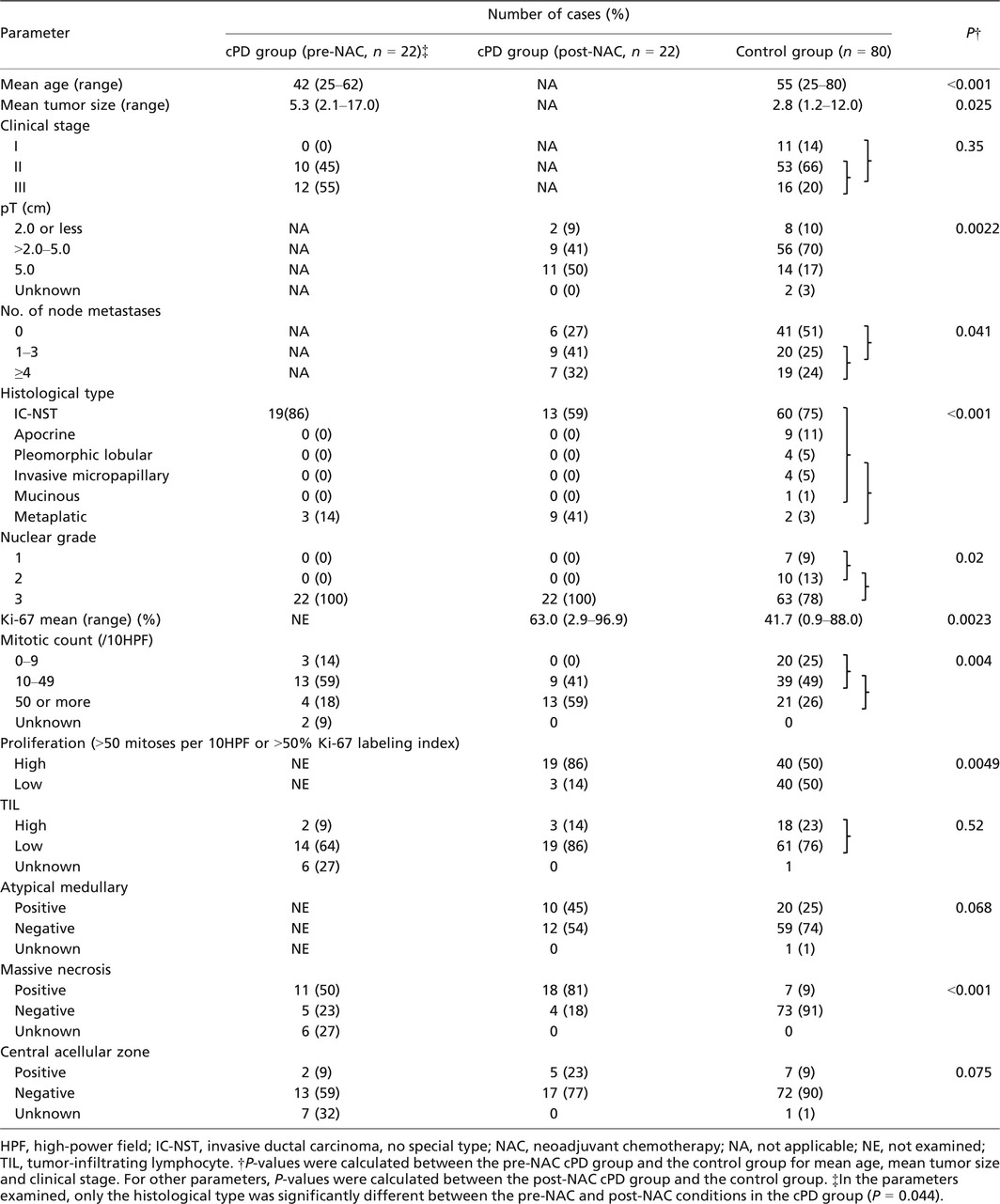
The histological type of the surgically resected cPD tumors included 13 (59%) invasive carcinomas of no special type (IC‐NST) and nine (41%) metaplastic carcinomas (MPCs), which included pure MPC (MPC component >90%), mixed MPC and IC‐NST (MPC component >50%–90%), and IC‐NST with MPC component (MPC component ≤50%). In these nine cases, the major metaplastic component was mesenchymal cell in four, spindle cell in three, squamous cell in one, and other in one (Fig. 1). In contrast, in the control group, 60 (75%) patients had IC‐NST, two had MPCs (3%), and 18 (22%) had other special types. MPCs was more frequent in the cPD group than in the control group (P < 0.001) (Table 2).
Figure 1.
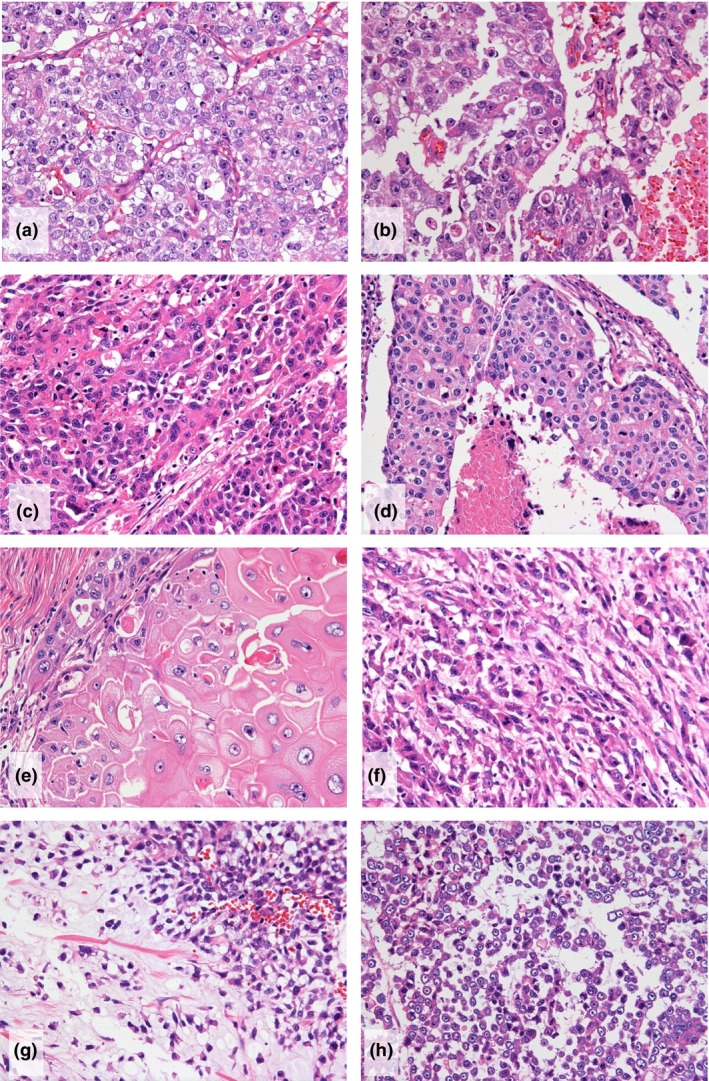
Histological presentation of triple‐negative breast cancers that showed clinical progressive disease after neoadjuvant chemotherapy (NAC). (a–d). Invasive carcinomas, no special type, of nuclear grade 3, with high mitotic figures and atypical medullary features. Necrosis (b,d) is also seen. (e–h). Metaplastic carcinomas (MPCs). MPC with (e) squamous cell, (f) spindle cell, and (g and h) mesenchymal differentiation (H & E × 200).
Nuclear grade was 3 in all 22 cPD tumors and in 77% (63/80) control tumors (P = 0.02). The mean Ki‐67 LI was higher in the cPD group (63.0%, range 2.9%–96.9%) than in the control group (41.7%, range 0.9%–88.0%) (P = 0.0023) (Fig. 2a). Mitotic counts per 10 high‐power fields (HPFs) were ≥50 in 59% of cPD tumors but were only in 26% of control tumors (P = 0.004). The high proliferation, defined as >50 mitoses per 10 HPFs or >50% Ki‐67 labeling index, was more frequent in the cPD group (86%) than in the control group (50%) (P = 0.0049).
Figure 2.
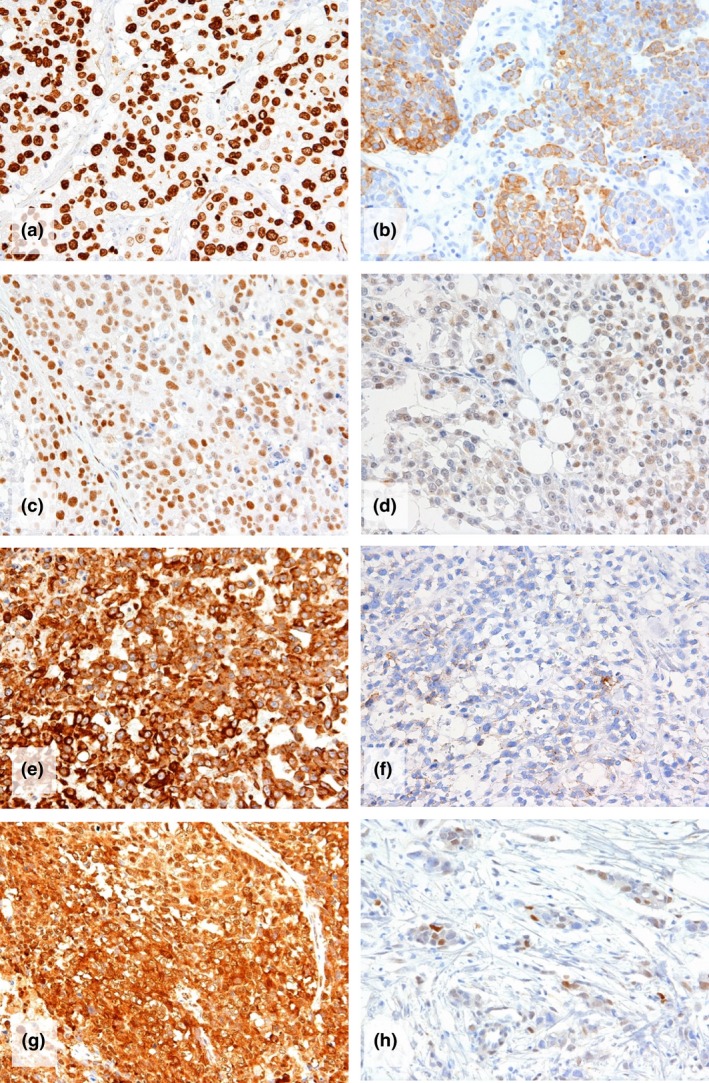
Immunohistochemical detection of (a) Ki‐67, (b) cytokeratin 5/6, (c) ZEB1, (d) TWISTNB, (e) vimentin, (f) E‐cadherin, (g) cytoplasmic HMGB1, and (h) androgen receptor. a to g are cPD tumors whereas H is a control tumor. (Immunoperoxidase stain, ×200).
Massive necrosis and atypical medullary features were significantly and marginally significantly more frequent in the cPD group than in the control group (P < 0.01 and =0 .068, respectively). High‐level TIL tended to be less frequent in the cPD group (14%) than in the control group (23%), but the difference was not significant (Table 2).
Immunohistochemical results
Clinical progressive disease cases were more frequently positive for CK5/6 (41%, 9/22) than control cases (5%, 4/80, P < 0.001), but the positivities of EGFR and BRCA1 did not differ between the two groups (Table 3, Fig. 2b).
Table 3.
Comparison of the immunohistochemical results in the surgically resected specimens between the clinical progressive disease (cPD) and control groups
| Molecule (localization) | Number of cases (%) | P a | ||
|---|---|---|---|---|
| Immunohistochemically positive | ||||
| cPD group (pre‐NAC, n = 12) | cPD group (post‐NAC, n = 22) | Control group (n = 80) | ||
| CK5/6 (cytoplasm) | 3 (25) | 9 (41) | 4 (5) | <0.001 |
| EGFR (membrane) | NE | 8 (36) | 24 (30) | 0.568 |
| BRCA1 loss (nucleus) | NE | 6 (27) | 29 (36) | 0.43 |
| Basal‐like (EGFR or CK5/6) | NE | 12 (55) | 25 (31) | 0.044 |
| ZEB1 (nucleus) | 2 (16) | 7 (32) | 10 (13) | 0.031 |
| TWISTNB (nucleus) | NE | 6 (27) | 2 (3) | 0.0011 |
| Vimentin (cytoplasm) | 9 (75) | 17 (77) | 43 (54) | 0.047 |
| E‐cadherin loss(cell membrane) | 5 (42) | 9 (41) | 26 (32) | 0.46 |
| Snail‐2 (nucleus) | 9 (41) | 13 (59) | 38 (48) | 0.33 |
| HMGB1 (cytoplasm) | 12 (100) | 19 (86) | 41 (51) | 0.0023 |
| AR (nucleus) | 1 (8) | 2 (9) | 23 (29) | 0.046 |
NE, not expamined.
There was no statistically significant difference between the pre‐NAC and post‐NAC marker positivity of the cPD group.
P‐values were calculated for the post‐NAC cPD and control groups.
The positivities of ZEB1, TWISTNB, and vimentin in cPD cases (32%, 27%, and 77%) were significantly higher than those in control cases (13%, 3%, and 54%), (P = 0.031, 0.0011, and 0.047, respectively) (Table 3, Fig. 2c–e). The expression rates of E‐cadherin, Snail‐2 and TWIST‐2 did not differ between these two groups (Fig. 2f).
Androgen receptor expression rate was significantly lower in the cPD group (9%, 2/22) than in the control group (23/80, 29%) (P = 0.046) (Fig. 2h). Cytoplasmic HMGB1 expression was more frequent in cPD cases (86%, 19/22) than in control cases (51%, 41/80), (P = 0.0023) (Fig. 2g).
Classification of TNBCs by unsupervised hierarchical cluster analysis
The statuses of proliferation, CK5/6, ZEB1, TWISTNB, vimentin, Snail‐2, AR, and HMGB1 significantly differed between the cPD and the control groups. In addition to these eight, three parameters that appeared important for subtype classification, E‐cadherin, EGFR, and BRCA1, were included as parameters for unsupervised hierarchical cluster analysis.
In the cluster analysis using 11 parameters, 102 TNBCs were largely classified into two clusters, A (n = 38) and B (n = 64), mainly according to proliferation and vimentin expression and further classified into seven subclusters, a to c in the A cluster and d to g in the B cluster (Fig. 3,Table S2). The number of tumors in subclusters a, b, c, d, e, f and g was 19, 11, 8, 9, 21, 12 and 22 cases, respectively. All but two cPD cases belonged to the subcluster d, f, or g in cluster B, that showed higher proliferation and vimentin expression. In terms of specific molecule expression and histology, b, c, d and f subclusters were distinct: b and c characterized by AR expression, lower proliferation, and negative vimentin showed low cPD rate and frequent apocrine or invasive lobular histology. The subcluster d was characterized by the EMT features including ZEB1, TWISTNB and Snail‐2 expressions and E‐cadherin loss, while the subcluster f was characterized by EGFR and CK5/6 expressions with or without E‐cadherin. These d and f showed the highest cPD and frequent metaplastic carcinoma histology. The a and e subclusters showed E‐cadherin loss, vimentin and/or Snail‐2, and the subcluster g showed vimentin and/or Snail‐2 expressions. These patterns in the subgroups a, e, and g suggested partial EMT features (Table S2).
Figure 3.
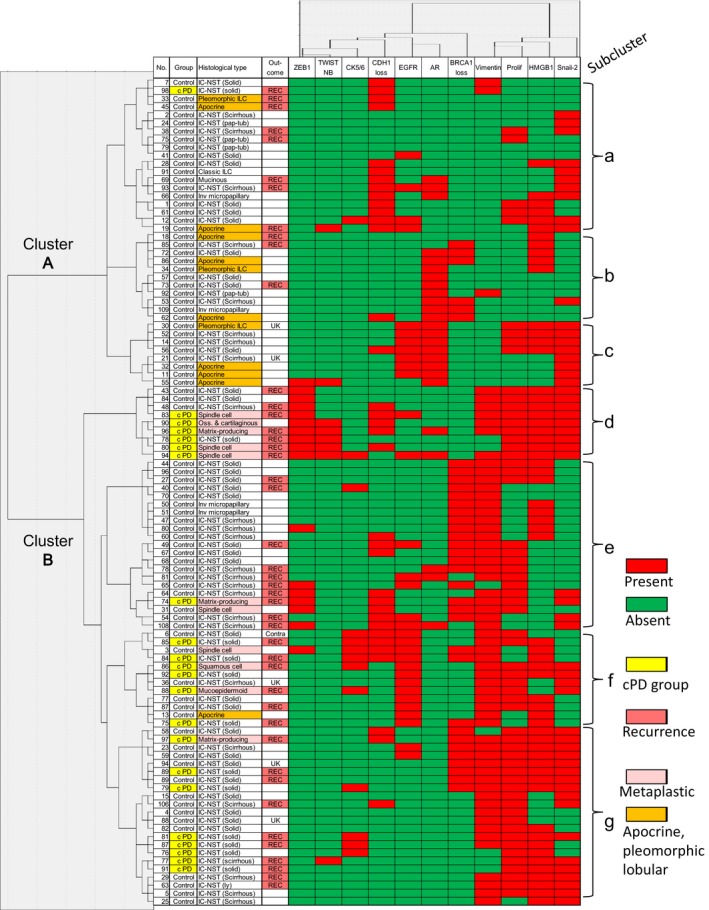
Dendrogram and heat map of the unsupervised hierarchical cluster analysis for 11 molecules in 102 triple‐negative breast cancers. Two clusters (A and B) and seven subclusters (a–g) are identified. According to the criteria described in Materials and Methods, for ZEB1, TWISTNB, CK5/6, EGFR, AR, vimentin, HGMB1, and Snail‐2 expressions, positive cases are indicated in red whereas negative cases are indicated in green. For E‐cadherin (CDH1) and BRCA1, cases with loss of expression are indicated in red whereas cases with expression are indicated in green. For proliferation, cases with high proliferation, defined as >50 mitotic counts per 10 high power fields or >50% Ki‐67 labeling index, are indicated in red, and otherwise the cases are indicated in green. As special histological types, metaplastic carcinomas are colored in pale pink, and apocrine carcinoma and pleomorphic lobular carcinoma are colored in orange. Cases that showed cPD and recurrence are also indicated in yellow and dense pink, respectively. UK, unknown; contra, metachronous contralateral breast.
Survival analysis
Disease‐free survival (DFS) curves for the cPD group and the control group differed siginificantly (P < 0.001) (Fig. 4a). DFS curves for clusters A and B were significantly different (P = 0.02), with 10‐year DFS rates being 75.0% and 46.3%, respectively (Fig. 4b). DFS curves for seven subclusters also showed difference, being better in the subclusters a, b, and c than in the d through g (P = 0.043, Fig. 4c). Ten‐year DFS rate was 100% in the subcluster c whereas 22.2% in the subcluster d.
Figure 4.
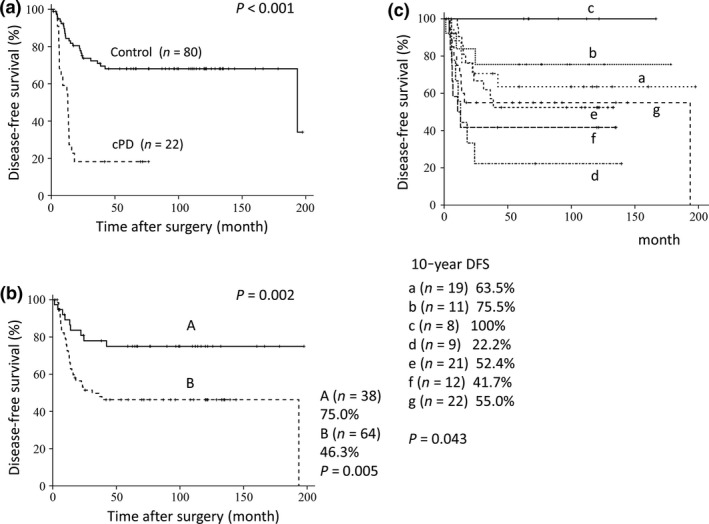
Disease‐free survival curves for patients with triple‐negative breast cancer. (a) Curves for the cPD group (n = 22) and the control group (n = 80). (b) Curves for the A (n = 38) and B (n = 64) groups determined by the cluster analysis. (c) Curves for seven subgroups (a–g) determined by the cluster analysis.
By univairiate analyses, age, pT, pN, metaplastic histology, proliferation, TWISTNB and CK5/6 were significantly correlated with DFS (Fig. [Link], [Link], [Link], [Link], [Link]a–i). Vimentin and ZEB1 were of marginal difference. The Cox multivariate analysis including all these parameters revealed that age and number of metastatic lymph nodes (pN) were independent prognostic factors (Table S3).
In the present study, we did not match age, invasive T size (pT) and pN between the cPD and control groups. These three parameters might have been influenced by the indication of NAC or by the tumor growth during NAC as selection bias. When these three factors were excluded from the multivariate analysis, CK5/6 only was of marginal significance (data not shown).
Comparison of pre‐ and post‐treatment in the PD group
Of the 22 pre‐NAC biopsy specimens, only three (14%) were MPCs, and the proportion was significantly lower than in that of post‐NAC tumors (9/22) (P = 0.044) (Table 2). In 27% (6/22) of cPD cases, histological type of the primary tumor changed from IC‐NST to MPCs. ZEB1, CK5/6, and Snail‐2 expressions also tended to be more frequent in post‐NAC tumors but difference was not significant (Tables 2 and 3).
Discussion
In the present study, we identified several histopathological and immunohistochemical markers that were significantly correlated with TNBCs that showed cPD. In addition to younger age, pT and pN, high proliferation, metaplastic histology, and massive necrosis were histological characteristics of the cPD group, and these factors except massive necrosis were correlated with DFS. TNBCs with extremely high proliferative activity were already shown to be indicators for chemotherapy resistance and worse clinical outcome,9, 34 and the present results are compatible with these observations.
MPC is reported to be 0.2%–5% in invasive breast carcinomas,35, 36 but in the present study, 41% of cPD cases had MPC components, and 9 (82%) of 11 MPCs were cPD cases (Table 2). Chen et al.37 mentioned that none of MPCs responded to anthracycline, vinorelbine, or cyclophosphamide‐based chemotherapy, and only 17.6% of MPCs showed partial response to taxane‐based chemotherapy. Hennessy et al.35, 36 described that both MPCs with squamous metaplasia and sarcomatoid metaplasia were highely resistant to anthracycline‐based NAC regimens. Frequent tumor necrosis in the cPD group might reflect very high proliferation rate of cancer cells. The findings of frequent atypical medullary features in the cPD group might be of clinical significance because it is sometimes difficult to differentiate typical and atypical medullary carcinomas and medullary carcinoma is shown to be better prognosis.38
TNBC has been classified into basal‐like, claudin‐low, and molecular apocrine subtypes39: vimentin and CK5/6 were basal‐like markers, vimentin, ZEB1, and TWISTNB were EMT markers related to basal‐like and claudin‐low subtypes, and AR was a molecular apocrine marker. In the present immunohistochemical study, expressions of CK5/6, ZEB1, TWISTNB, and vimentin were shown to be characteristics of the cPD group. These results were supported by the histological observations of frequent high proliferation and metaplastic histology in the cPD group. The expressions of some basal‐like and/or EMT markers and lack of apocrine features appeared to be commonly involved in the acquisition of the property of cPD in vivo.
Androgen receptor expression was identified in 20%–30% of the cases with TNBC,23, 24 and clinical outcome of AR‐positive cases was shown to be better than that of AR‐negative cases, irrespective of their ER status.22 The present results supported the inverse relationship between AR expression and cPD after NAC in TNBC.
HMGB1 is a novel proinflammatory cytokine and its cytoplasmic translocation under stressed condition activates innate immunity.40 Furthermore, anticancer agents including doxorubicin, cisplatin, and methotrexate each induced HMGB1 upregulation in human cancer cells, and HMGB1‐induced autophagy promotes resistance of cancer cells to chemotherapy.41, 42 In this study, cytoplasmic HMGB1 expression was more frequent in the cPD than in the control although its prognostic significance was absent. It may be possible to consider that cytoplasmic translocation of HMGB1 by chemotherapy might induce autophagy and promoted chemoresistance of TNBCs.
Disease‐free survival curves differed siginificantly between the cPD group and the control group (P < 0.001). In the present study, it was shown that high proliferation, metaplastic histology, and expressions of CK5/6, ZEB1, TWISTNB, and vimentin were significantly or marginally correlated with poorer clinical outcome. Some of these histological and immunohistochemical markers were shown to be correlated with worse prognosis in the patients with TNBC.43, 44, 45, 46, 47
From these results, we were able to demonstrate that most of the markers that were correlated with the acquisition of cPD properties by TNBCs were also indicators of worse patient prognosis. However, it was also evident that there was no overwhelming histological or immunohistochemical marker correlated with TNBCs that showed cPD after NAC.
In the present cluster analysis, the cohort was divided into two major clusters and seven subclusters. A majority of cPD cases were included in the subclusters d, f, and g, that were characterized by high proliferation and vimentin expression, with or without CK5/6, EGFR, ZEB1 and/or TWISTNB expressions frequent metaplastic histology. In contrast, the subclusters b and c, usually expressing AR with or without EGFR in association with lower proliferation and negative vimentin, CK5/6, or EMT markers, were characterized by the absence of cPD and frequent apocrine/pleomorphic lobular histology.
By Lehmann et al.,48 TNBCs were classified into six subtypes and, of these, basal‐like 1 (BL1) subtype had a high pCR rate, but basal‐like 2 (BL2) and luminal androgen receptor (LAR) subtypes had low pCR rates against taxane and anthracycline‐based chemotherapy, although the LAR had the best overall survival rate [48]. Plat et al. argued that this TNBC classification was based on three main groups (mesenchymal, basal‐like and LAR) in terms of response to drug therapies39 and that these three correspond well to claudin‐low, basal‐like, and luminal/HER2+ (or molecular apocrine) subtypes, respectively.39 The present subclusters b and c appear to correspond to LAR or luminal/HER2+, and the subclusers d and f appear to correspond to mesenchymal (M), mesenchymal stem‐like (MSL), or BL2, or claudin‐low subtypes (Table S2).
The molecular features characteristic of cPD tumors tended to be less frequent in the pre‐NAC primary tumors. In many cases, these features might have existed in minor or minimal clones prior to NAC and have become predominant through clonal selection.49, 50 Such clonal selection might have been accelerated due to NAC.
This study has several limitations. First, this was a retrospective study conducted in a small number of patients who received various chemotherapy regimens. Second, the number of molecules examined was small, and the measurement of expression was semi‐quantitative. Third, only a small number and amount of biopsy specimens were available for pretreatment evaluation in the cPD group. Nonetheless, the present approach is unique for screening molecules that showed cPD in TNBCs, based on both histopathological and immunohistochemical analyses. For the patients with TNBCs that showed cPD after NAC, establishment of reliable cPD markers can play a role for knowing the biological basis of these tumors and for the development of therapeutic strategy. A larger scale study and the development of the scoring system combining these immunohistochemical markers would be of clinical use for simple and reproducible characterization of the TNBCs that showed cPD by extrapolating Lehmann's six subtypes and three molecular subtypes of TNBC.
In summary, we found that high proliferation, metaplastic features, massive necrosis, EMT markers including ZEB1, TWISTNB and vimentin, part of basal‐like markers including CK5/6, cytoplasmic HMGB1, and absence of AR were indicators of the TNBCs that showed cPD during NAC.
Disclosure Statement
Yasuhiro Fujiwara received research grant from Nippon Kayaku Co. Ltd. The other authors have no conflicts of interest.
Supporting information
Fig. S1. Disease‐free survival curves for patients with triple‐negative breast cancer stratified by various clinicopathological and immunohistochemical parameters: (a) Age, (b) pN, (c) pT, (d) Proliferation, (e) Cytokeratin 5/6, (f) ZEB1, (g) TWISTNB, (h) Vimentin, (i) Metaplastic histology. Statistically significant or marginally significant parameters are shown.
Table S1. Adjuvant chemotherapy to 39 node‐positive patients and eight node‐negative patients in the control group.
Table S2. Molecular and histopathological characteristics of clusters and subclusters identified by the cluster analysis
Table S3. Multivariate analysis including all 94 informative cases
Acknowledgments
This work was supported in part by a Scientific Research Grant of the Ministry of Health, Labour and Welfare, by the National Cancer Center Research and Development Fund (23‐A‐17, 26‐A‐4), and by a Grant‐in‐Aid for Scientific Research from Japan Society for the Promotion of Science (KAKENHI 25430144). The authors acknowledge Ms. Sachiko Miura, Ms. Chizu Kina, Ms. Chimami Onuma, and Ms. Nao Nakamura for technical help, data collection and manuscript revisions.
Cancer Sci 108 (2017) 1520–1529
Funding Information
Scientific Research Grant of the Ministry of Health, Labour and Welfare, (Grant/Award Number: ‘2012200228’) National Cancer Center Research and Development Fund, (Grant/Award Number: ‘23‐A‐17, 26‐A‐4’) Grant‐in‐Aid for Scientific Research from Japan Society for the Promotion of Science, (Grant/Award Number: ‘KAKENHI 25430144’).
References
- 1. Iwase H, Kurebayashi J, Tsuda H et al Clinicopathological analyses of triple negative breast cancer using surveillance data from the Registration Committee of the Japanese Breast Cancer Society. Breast Cancer 2010; 17: 118–24. [DOI] [PubMed] [Google Scholar]
- 2. Haffty BG, Yang Q, Reiss M et al Locoregional relapse and distant metastasis in conservatively managed triple negative early‐stage breast cancer. J Clin Oncol 2006; 24: 5652–7. [DOI] [PubMed] [Google Scholar]
- 3. Carey LA, Dees EC, Sawyer L et al The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 2007; 13: 2329–34. [DOI] [PubMed] [Google Scholar]
- 4. Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine‐year results from National Surgical Adjuvant Breast and Bowel Project B‐18. J Natl Cancer Inst Monogr 2001; 30: 96–102. [DOI] [PubMed] [Google Scholar]
- 5. van der Hage JA, van de Velde CJ, Julien JP, Tubiana‐Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol 2001; 19: 4224–37. [DOI] [PubMed] [Google Scholar]
- 6. Cortazar P, Zhang L, Untch M et al Pathological complete response and long‐term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014; 384: 164–72. [DOI] [PubMed] [Google Scholar]
- 7. Rouzier R, Perou CM, Symmans WF et al Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res 2005; 11: 5678–85. [DOI] [PubMed] [Google Scholar]
- 8. Liedtke C, Mazouni C, Hess KR et al Response to neoadjuvant therapy and long‐term survival in patients with triple‐negative breast cancer. J Clin Oncol 2008; 26: 1275–81. [DOI] [PubMed] [Google Scholar]
- 9. Caudle AS, Gonzalez‐Angulo AM, Hunt KK et al Predictors of tumor progression during neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010; 28: 1821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abd El‐Rehim DM, Pinder SE, Paish CE et al Expression and co‐expression of the members of the epidermal growth factor receptor (EGFR) family in invasive breast carcinoma. Br J Cancer 2004; 91: 1532–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nielsen TO, Hsu FD, Jensen K et al Immunohistochemical and clinical characterization of the basal‐like subtype of invasive breast carcinomas. Clin Cancer Res 2004; 10: 5367–74. [DOI] [PubMed] [Google Scholar]
- 12. Foedermayr M, Sebesta M, Rudas M et al BRCA‐1 methylation and TP53 mutation in triple‐negative breast cancer patients without pathological complete response to taxane‐based neoadjuvant chemotherapy. Cancer Chemother Pharmacol 2014; 73: 771–8. [DOI] [PubMed] [Google Scholar]
- 13. Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 2009; 9(4): 265–73. [DOI] [PubMed] [Google Scholar]
- 14. Tomaskovic‐Crook E, Thompson EW, Thiery JP. Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res 2009; 11: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blick T, Hugo H, Widodo E et al Epithelial mesenchymal transition traits in human breast cancer cell lines parallel the CD44 (hi/) CD24 (lo/‐) stem cell phenotype in human breast cancer. J Mammary Gland Biol Neoplasia 2010; 15: 235–52. [DOI] [PubMed] [Google Scholar]
- 16. Moreno‐Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene 2008; 27: 6958–69. [DOI] [PubMed] [Google Scholar]
- 17. Dong Xda E, Ito N, Lotze MT et al High mobility group box I (HMGB1) release from tumor cells after treatment: implications for development of targeted chemoimmunotherapy. J Immunother 2007; 30: 596–606. [DOI] [PubMed] [Google Scholar]
- 18. Amornsupak K, Insawang T, Thuwajit P. O‐Charoenrat P, Eccles SA, Thuwajit C. Cancer‐associated fibroblasts induce high mobility group box 1 and contribute to resistance to doxorubicin in breast cancer cells. BMC Cancer 2014; 14: 955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kang R, Zhang Q, Zeh HJ 3rd, Lotze MT, Tang D. HMGB1 in cancer: good, bad, or both? Clin Cancer Res 2013; 19: 4046–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dieci MV, Criscitiello C, Goubar A et al Prognostic value of tumor‐infiltrating lymphocytes on residual disease after primary chemotherapy for triple‐negative breast cancer: a retrospective multicenter study. Ann Oncol 2014; 25: 611–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ono M, Tsuda H, Shimizu C et al Tumor‐infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple‐negative breast cancer. Breast Cancer Res Treat 2012; 132: 793–805. [DOI] [PubMed] [Google Scholar]
- 22. Vera‐Badillo FE, Templeton AJ, de Gouveia P et al Androgen receptor expression and outcomes in early breast cancer: a systematic review and meta‐analysis. J Natl Cancer Inst 2014; 106: djt319. [DOI] [PubMed] [Google Scholar]
- 23. He J, Peng R, Yuan Z et al Prognostic value of androgen receptor expression in operable triple‐negative breast. Med Oncol 2012; 29: 406–10. [DOI] [PubMed] [Google Scholar]
- 24. Loibl S, Muller BM, von Minckwitz G et al Androgen receptor expression in primary breast cancer and its predictive and prognostic value in patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat 2011; 130: 47–87. [DOI] [PubMed] [Google Scholar]
- 25. Eisenhauer EA, Therasse P, Bogaerts J et al New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 26. Hammond MEH, Hayes DF, Dowsett M et al American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010; 28: 2784–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wolff AC, Hammond EH, Hicks DC et al American Society of Clinical Oncology; College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline update. J Clin Oncol 2013; 31: 3997–4013. [DOI] [PubMed] [Google Scholar]
- 28. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17: 1471–4. [DOI] [PubMed] [Google Scholar]
- 29. Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. World Health Organization. WHO Classification of Tumours of the Breast. 4th edn Lyon: International Agency for Research on Cancer, 2012. [Google Scholar]
- 30. Sparano JA, Wang M, Martino S et al Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 2008; 358: 1663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peto R, Davies C , Godwin J et al Comparisons between different polychemotherapy regimens for early breast cancer: meta‐analyses of long‐term outcome among 100,000 women in 123 randomised trials. Lancet 2012; 379: 432–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ono M, Tsuda H, Yunokawa M et al Prognostic impact of Ki‐67 labeling indices with 3 different cutoff values, histological grade, and nuclear grade in hormone‐receptor‐positive, HER2‐negative, node‐negative invasive breast cancers. Breast Cancer 2015; 22: 141–52. [DOI] [PubMed] [Google Scholar]
- 33. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 1998; 11: 155–68. [PubMed] [Google Scholar]
- 34. Masuda H, Masuda N, Kodama Y et al Predictive factors for the effectiveness of neoadjuvant chemotherapy and prognosis in triple‐negative breast cancer patients. Cancer Chemother Pharmacol 2011; 67: 911–17. [DOI] [PubMed] [Google Scholar]
- 35. Hennessy BT, Giordano S, Broglio K et al Biphasic metaplastic sarcomatoid carcinoma of the breast. Ann Oncol 2006; 17: 605–13. [DOI] [PubMed] [Google Scholar]
- 36. Hennessy BT, Krishnamurthy S, Giordano S et al Squamous cell carcinoma of the breast. J Clin Oncol 2005; 23: 7827–35. [DOI] [PubMed] [Google Scholar]
- 37. Chen IC, Lin CH, Huang CS et al Lack of efficacy to systemic chemotherapy for treatment of metaplastic carcinoma of the breast in the modern era. Breast Cancer Res Treat 2011; 130: 345–51. [DOI] [PubMed] [Google Scholar]
- 38. Ridolfi RL, Rosen PP, Port A, Kine D, Miké V. Medullary carcinoma of the breast. A clinicopathologic study with 10 year follow‐up. Cancer 1977; 40: 1365–85. [DOI] [PubMed] [Google Scholar]
- 39. Prat A, Adamo B, Cheang MCU, Anders CK, Carey LA, Perou CM. Molecular characterization of basal‐like and non‐basal‐like triple‐negative breast cancer. Oncologist 2013; 18: 123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pistoia V, Pezzolo A. Involvement of HMGB1 in resistance to tumor vessel‐targeted, monoclonal antibody‐based immunotherapy. J Immunol Res 2016; 2016: 3142365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang J, Ni J, Liu K et al HMGB1 promotes drug resistance in osteosarcoma. Cancer Res 2012; 72: 230–8. [DOI] [PubMed] [Google Scholar]
- 42. Zhou J, Chen X, Gilvary DL et al HMGB1 induction of clusterin creates a chemoresistant niche in human prostate tumor cells. Sci Rep 2015; 5: 15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Keam B, Im SA, Lee KH et al Ki‐67 can be used for further classification of triple negative breast cancer into two subtypes with different response and prognosis. Breast Cancer Res 2011; 13: R22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Karihtala P, Auvinen P, Kauppila S, Haapasaari KM, Jukkola‐Vuorinen A, Soini Y. Vimentin, zeb1 and Sip1 are up‐regulated in triple‐negative and basal‐like breast cancers: association with an aggressive tumour phenotype. Breast Cancer Res Treat 2013; 138: 81–90. [DOI] [PubMed] [Google Scholar]
- 45. Yamashita N, Tokunaga E, Kitao H et al Vimentin as a poor prognostic factor for triple‐negative breast cancer. J Cancer Res Clin Oncol 2013; 139: 739–46. [DOI] [PubMed] [Google Scholar]
- 46. Jang MH, Kim HJ, Kim EJ, Chung YR, Park SY. Expression of epithelial‐mesenchymal transition‐related markers in triple‐negative breast cancer: ZEB1 as a potential biomarker for poor clinical outcome. Hum Pathol 2015; 46: 1267–74. [DOI] [PubMed] [Google Scholar]
- 47. Inanc M, Ozkan M, Karaca H et al Cytokeratin 5/6, c‐Met expressions, and PTEN loss prognostic indicators in triple‐negative breast cancer. Med Oncol 2014; 31: 801. [DOI] [PubMed] [Google Scholar]
- 48. Lehmann BD, Bauer JA, Chen X et al Identification of human triple‐negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011; 121: 2750–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Masuda H, Baggerly KA, Wang Y et al Differential response to neoadjuvant chemotherapy among 7 triple‐negative breast cancer molecular subtypes. Clin Cancer Res 2013; 19: 5533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang Y, Waters J, Leung ML et al Clonal evolution in breast cancer revealed by single nucleus genome sequencing. Nature 2014; 512: 155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Disease‐free survival curves for patients with triple‐negative breast cancer stratified by various clinicopathological and immunohistochemical parameters: (a) Age, (b) pN, (c) pT, (d) Proliferation, (e) Cytokeratin 5/6, (f) ZEB1, (g) TWISTNB, (h) Vimentin, (i) Metaplastic histology. Statistically significant or marginally significant parameters are shown.
Table S1. Adjuvant chemotherapy to 39 node‐positive patients and eight node‐negative patients in the control group.
Table S2. Molecular and histopathological characteristics of clusters and subclusters identified by the cluster analysis
Table S3. Multivariate analysis including all 94 informative cases


