Abstract
Recent advances in secondary stroke prevention include new evidence in hypertension, nutrition, anticoagulation, antiplatelet therapy, intracranial stenosis, percutaneous closure of patent foramen ovale, and lipid-lowering therapy. Individualized therapy for hypertension based on phenotyping with plasma renin and aldosterone markedly improves blood pressure control in patients with resistant hypertension. A Mediterranean diet can reduce the risk of stroke by nearly half. The diagnosis and treatment of metabolic vitamin B12 deficiency, and B vitamins to lower homocysteine, can reduce the risk of stroke by approximately 30%. There are problems with clopidogrel that can be overcome by using ticagrelor, and new anticoagulant drugs markedly improve anticoagulation for stroke prevention, particularly in atrial fibrillation. There are pharmacokinetic problems with dabigatran that deserve attention. Intensive medical therapy is better than stenting for intracranial stenosis, and new therapies directed at proprotein convertase subtilisin–kexin type 9 (PCSK9) will revolutionize lipid-lowering therapy. In the past, it was estimated that rational therapy could reduce recurrent stroke by about 80%. With recent advances, we should be able to do even better.
Keywords: stroke recurrence, hypertension, diet, B12 deficiency, anticoagulation, antiplatelet, intracranial stenosis, LDL cholesterol
Introduction
In 2013, I reviewed intensive medical therapy for secondary stroke prevention 1. Since then, there have been a number of important advances, which are reviewed in this update. These include advances in hypertension, nutrition, anticoagulation, antiplatelet therapy, intracranial stenosis, percutaneous closure of patent foramen ovale, and lipid-lowering therapy.
Hypertension
A major preventable cause of stroke is hypertension, which rose to the top of the list of causes of death and disability in the world in 2012 2. It is a particular problem in Africa and among African-Americans. In Sub-Saharan Africa and in the US, strokes among Africans and African-Americans are disproportionately due to hypertensive small vessel disease (hypertensive lacunar infarctions and intracranial hemorrhage). African-American adults have among the highest prevalence of hypertension in the world: 45% in men and 46% in women 3. They also have the highest risk of hemorrhagic stroke 4. African-Americans are more likely to have their hypertension diagnosed, treated, and treated more intensively but less likely to have it controlled 5.
Salt and water retention may have a survival advantage in conditions of heat and salt and water privation in sub-Saharan Africa 6. Grim et al. 7, 8 hypothesized that a further advantage of salt and water retention was conferred on slaves who survived the Atlantic crossing between decks on slave ships and aided their survival for the first few years of working on plantations in the Southern US. Reasons for the excess of hypertension and poorer control of hypertension in blacks may have some of their origin in socioeconomic conditions but also clearly are based on genetic factors causing salt and water retention 6. These are of two types: an increase in primary aldosteronism due to bilateral adrenocortical hyperplasia, and mutations affecting the retention of salt and water via the renal tubular epithelial sodium channel (ENac) 9. The former includes variants of CYP11B2 (aldosterone synthase); the latter includes mutations of ENac itself ( SCNN1B) and mutations of other genes affecting the function of ENac 10, including NEDD4L, GRK4, UMOD, NPPA, and CYP4A11 11. Among patients with uncontrolled hypertension in Africa, many variants of these genes were present, and some of the variants were present in all participants studied 10.
The potential for the reduction of hemorrhagic stroke by the control of hypertension was illustrated in a community-based experience in London, Canada, between 1978 and 1983 and in the North American Symptomatic Carotid Endarterectomy Trial (NASCET). A major push to improve blood pressure control initiated by the Department of Family Medicine in London 12, Canada, between 1978 and 1983 resulted in the detection of hypertension in 94% of the community, treatment in 92%, and control in 72% 13. This was aided by the initiation in 1977 of a hypertension clinic basing the management of resistant hypertension on levels of stimulated plasma renin activity. By 1983, strokes in London were reduced by half; the strokes that were prevented were entirely those due to hypertensive small vessel disease 14. At Victoria Hospital in London, Canada, the number of patients per year with hypertensive intracerebral hemorrhage declined from 200/year to almost none.
In the NASCET trial 15, investigators routinely received a stiff letter reminding them to follow the protocol whenever a participant’s blood pressure at a follow-up visit was above the specified target (140/90) and antihypertensive medication was not increased. In the medical arm of the study, this approach virtually eliminated therapeutic inertia and resulted in virtual elimination of hemorrhagic strokes: they were reduced to 0.5% of strokes at a time when approximately 20% of strokes in North America were hemorrhagic, and this included lobar strokes and subarachnoid strokes, which are not due to hypertensive small vessel disease; therefore, therapeutic inertia can be overcome. A remaining important cause of uncontrolled hypertension is “diagnostic inertia”—failure to ask what the underlying cause of the hypertension is when blood pressure is not controlled with usual therapy 16, 17.
In 2006, I hypothesized 18 that much of the stroke disparity among African-Americans could be eliminated by physiologically individualized therapy (PhysRx) based on phenotyping by plasma renin and aldosterone. That hypothesis has now been supported by a clinical trial in Africa.
Patients with uncontrolled hypertension attending clinics in Nigeria, Kenya, and South Africa were allocated to usual care based on the guidelines in place in each country versus physiologically individualized therapy based on phenotyping by plasma renin and aldosterone 19. The study was based on observations among descendants of escaped slaves who made their way to Canada via the Underground Railroad to North Buxton, Ontario, one of four such settlements established in Canada in the 19th century 20. The algorithm used to guide therapy is shown in Table 1. Patients with low renin/high aldosterone (primary aldosteronism phenotype) are best treated with aldosterone antagonists, those with low renin/low aldosterone (Liddle phenotype) are best treated with amiloride (a specific antagonist of ENac), and those with high renin/high aldosterone (secondary hyperaldosteronism, a renal phenotype) are best treated with angiotensin receptor blockers (ARBs).
Table 1. Physiologically individualized therapy * based on renin/aldosterone profile.
| Primary hyper-aldosteronism | Liddle’s syndrome and variants
(renal Na + channel mutations) |
Renal/renovascular | |
|---|---|---|---|
| Renin | Low ** | Low | High |
| Aldosterone | High ** | Low | High |
| Primary
treatment |
Aldosterone antagonist
(spironolactone or eplerenone) Amiloride for men where eplerenone is not available (rarely surgery) |
Amiloride | Angiotensin receptor
blocker or renin inhibitor *** (rarely revascularization) |
*It should be stressed that this approach is suitable for tailoring medical therapy in resistant hypertensives; further investigation would be required to justify adrenalectomy or renal revascularization
**Levels of plasma renin and aldosterone must be interpreted in the light of the medication the patient is taking at the time of sampling. In a patient taking an angiotensin receptor blocker (which would elevate renin and lower aldosterone), a plasma renin level that is in the low normal range for that laboratory with a plasma aldosterone level in the high normal range probably represents primary hyperaldosteronism for the purposes of adjusting medical therapy.
***Angiotensin converting enzyme (ACE) inhibitors are less effective because of aldosterone escape via non-ACE pathways such as chymase and cathepsin.
Reproduced by permission of Elsevier from 9.
In the study, there was no effect of the algorithm at the site in Kenya, where low socioeconomic status and perhaps cultural factors limiting adherence resulted in no difference between usual care (UC) and PhysRx. The results of the study were as follows 19: “in the overall study population, control of both systolic and diastolic pressures was obtained in 11.1% of UC vs. 50.0% of PhysRx (p = 0.0001). Systolic control was achieved in 14% of UC vs. 60% of PhysRx ( P = 0.0001); diastolic control in 36% of UC vs. 67% of PhysRx ( P = 0.003). Control was significantly worse in Kenya. When only the sites in Nigeria and South Africa were considered, systolic control was obtained in 15% of UC vs. 79% of PhysRx ( P < 0.0001), diastolic control in 45% vs. 71% ( P = 0.04), and control of both in 15% vs. 67% ( P = 0.0001). If only the Nigerian site (where patients were randomized to the two treatment strategies, and conditions were more similar to those in North America) is considered, systolic control was obtained in 15% of UC vs. 85% of PhysRx ( P = 0.0001), diastolic control in 45% vs. 75% ( P = 0.11), and control of both systolic and diastolic pressure in 15% vs. 75% ( P < 0.0001) even though the renal function was worse at that site”. Thus, it is clear that if diagnostic inertia can be overcome, there is a marked improvement in blood pressure control. The cost of the two blood tests (around $50) is very low compared with the cost of high doses of the wrong medication and the cost of events such as stroke, renal failure, and heart failure from uncontrolled hypertension. This approach should be tested in a randomized trial in the US.
Nutrition
Mediterranean diet
Diet is far more important than most physicians (and the public) suppose. In the Lyon Diet Heart Study 21, there was a greater than 60% reduction of stroke and myocardial infarction over 4 years in secondary prevention. This was approximately twice the effect of simvastatin in the contemporaneous Scandinavian Simvastatin Survival Study 22 (a 40% reduction of recurrent myocardial infarction in 6 years). In the US, the worst of the lifestyle and risk factor issues is diet: only 0.1% of Americans consume a healthy diet, and only 8.3% consume even a moderately healthy diet 3.
Adherence to a Mediterranean diet reduces the risk of ischemic stroke 23, adherence to a healthy diet and other lifestyle factors reduced myocardial infarction in Swedish men by 80% 24, and a healthy diet and other lifestyle factors reduced stroke in Swedish women by 62% 25. In 2013, it was already clear that, compared to a low-fat diet, a Mediterranean diet significantly reduced stroke in primary prevention: there was a 47% reduction of stroke in the Mediterranean arm of the study fortified with mixed nuts 26. Since then, it has been reported that the Mediterranean diet improved the metabolic syndrome 27 and reduced age-related cognitive decline 28.
B vitamin therapy to lower homocysteine
In 2004, the Vitamin Intervention in Stroke (VISP) trial 29 reported no benefit of folic acid 2.5 mg, pyridoxine 25mg, and cyanocobalamin 400 μg daily compared with low-dose vitamins. In 2006, when the Norwegian Vitamin Trial (NORVIT) 30 and the Heart Outcomes Prevention Evaluation (HOPE-2) trial 31 were published, the cant was “homocysteine is dead”. Loscalzo hypothesized 32 that harm from unmetabolized folic acid may have accounted for the null results. However, in NORVIT, there was harm from B vitamins in the arm of the study that included cyanocobalamin, and in HOPE-2 there was actually a 23% reduction of stroke with B vitamins. In the French SU.FOL.OM3 trial 33, there was also a 43% reduction of stroke in a study in which renal function was better than in earlier studies: mean serum creatinine in SU.FOL.OM3 was 78 mmol/L compared with 99.9 mmol/L in VISP, 91 mmol/L in NORVIT, and 88.4 mmol/L in HOPE-2. Some meta-analyses also indicated a reduction of stroke with B vitamins 34, 35.
Importantly, the form of vitamin B12 used in all of these studies was cyanocobalamin, a form that contains cyanide, which must be decyanated to become active. Cyanide is converted to thiocyanate before renal elimination occurs, consuming hydrogen sulfide (H 2S), a gasotransmitter 36 that is an endothelium-derived relaxing factor, in the process, and thiocyanate is a powerful oxidant, accelerating the oxidation of LDL cholesterol (LDL-C) 37. It accumulates in renal failure 38. In the Western Norwegian study (WENBIT) 39, cyanocobalamin lowered total homocysteine (tHcy) but not asymmetric dimethylarginine (ADMA), a nitric oxide antagonist that is elevated in the presence of elevated levels of tHcy. In 2010, Koyama found that in patients with renal failure, methylcobalamin lowered both tHcy and ADMA 40.
In 2011, Spence and Stampfer hypothesized 41 that it may have been harm from cyanocobalamin among participants with impaired renal function that accounted for the null result in VISP. That was based on two studies: a subgroup analysis of the VISP trial excluding participants with renal impairment and a study in patients with diabetic nephropathy, the Diabetic Intervention with Vitamins in Diabetic Nephropathy (DIVINe) study. The VISP subgroup analysis was undertaken because in VISP there were several factors that mitigated against an effect of B vitamin therapy. The first was that folate fortification of the grain supply in North America coincided with the initiation of the trial, thereby limiting the benefit of folic acid. The second was that participants in the low-dose arm of the study received the recommended daily allowance of vitamin B12. The third was the decision to give injections of cyanocobalamin to all participants with low B12 levels at baseline, in both the high-dose and the low-dose arms of the study, thus negating the benefit of B12 in the very participants who stood to benefit most. In the subgroup analysis 42, we excluded participants who received B12 injections and, for the wrong reason, also excluded participants in the lowest 10% of estimated glomerular filtration rate (eGFR), which was <46 mL/minute/1.73 m² (based on an earlier study 43, we thought participants with renal failure would not respond as well to B vitamins; we never dreamed they would be harmed by high-dose B vitamins). To assess the adequacy of B12 absorption, the study population was stratified by the median serum B12 of 322 pmol/L. As shown in Figure 1, there was a 34% reduction of stroke/myocardial infarction/vascular death among participants with a serum B12 level above the median receiving high-dose B vitamins versus those with a serum B12 level below the median receiving low-dose B vitamins.
Figure 1. Kaplan-Meier survival free of stroke/myocardial infarction/vascular death in the Vitamin Intervention for Stroke Prevention (VISP) trial excluding patients who received vitamin B12 injections outside the randomized therapy and those with impaired renal function (estimated glomerular filtration rate <46 mL/minute/1.73 m²).
Participants were stratified by the median baseline serum B12 level (to characterize the adequacy of B12 absorption) and by randomized allocation to low-dose B vitamins versus high-dose B vitamins (B6 25 mg, B12 400 μg, and folic acid 2.5 mg versus B6 200 μg, B12 6 μg, and folic acid 20 μg daily). The red line represents low-dose vitamins/B12 <median, the green line is high-dose vitamins/B12 above the median, the blue line is low-dose vitamins/B12 above the median, and the black line is high-dose B vitamins/B12 below the median. There was a 34% reduction of the composite endpoint of stroke/myocardial infarction/vascular death in the group with adequate B12 absorption receiving high-dose B vitamins (green line) and the group with less adequate B12 absorption receiving low-dose vitamins. The log-rank p value for all four groups was 0.02. CI, confidence interval; HR, hazard ratio. Reproduced by permission of Wolters Kluver from 42.
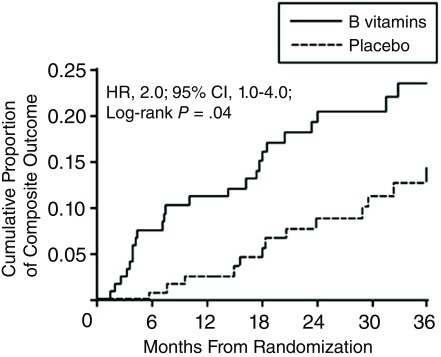
Then, in 2010, we found in the DIVINe trial 44 that high-dose B vitamins were harmful in patients with impaired renal function. As shown in Figure 2, there was a doubling of stroke, myocardial infarction, and mortality among participants receiving folic acid 5 mg, vitamin B6 25 mg, and vitamin B12 1,000 μg daily compared with those receiving placebo. All of the events occurred among participants with a GFR of <50 mL/minute/1.73 m² 45.
Figure 2. Cumulative proportion of myocardial infarction, stroke, revascularization, and all-cause mortality with high-dose B vitamins in patients with diabetic nephropathy.
Diabetic nephropathy patients receiving folic acid 5 mg, B6 25 mg and B12 1,000 μg daily had twice the risk of events compared to patients receiving placebo. All of the events occurred in patients with a glomerular filtration rate of <50 mL/minute/1.73 m² 45. Reproduced by permission of the American Medical Association from 44.
The final piece of the puzzle fell into place when the China Stroke Primary Prevention Trial (CSPPT) reported in 2015 46 a significant 25% reduction of stroke with folic acid versus placebo in hypertensive patients receiving the ACE inhibitor enalapril. Among participants with LDL-C above 2 mmol/L, the benefit of folic acid was greater: a 31% reduction in stroke 47. Importantly, folic acid was beneficial in participants with impaired renal function 48. A meta-analysis stratified by renal function and dose of cyanocobalamin supports the hypothesis that the reason the early trials of B vitamin therapy for stroke prevention were null was due to harm from cyanocobalamin among participants with impaired renal function 49, cancelling out benefit among those with good renal function. The implication of all of this is that we should be using methylcobalamin, not cyanocobalamin, for stroke prevention.
Metabolic B12 deficiency
In 2015, I reviewed the evidence that metabolic B12 deficiency is very common and usually undiagnosed 50. It is usually undiagnosed because physicians tend to think that a serum B12 level in the reference range (about 160–600 pmol/L) is adequate; however only a small and variable proportion of total serum B12 is active (about 6–20%). In the low end of the normal range of serum B12, further testing is required to assess the adequacy of functional B12. It is necessary to measure holotranscobalamin, or the metabolites that become elevated in B12 deficiency: methylmalonic acid (MMA), the specific metabolite, or tHcy, which in patients who are folate replete is a reasonable surrogate for MMA. It is difficult to measure MMA, and quantification is not routinely available. Since folate fortification of the grain supply in North America has made folate deficiency rare, tHcy is usually assessed for this purpose 51. In the National Health and Nutrition study, the serum B12 level below which tHcy began to rise was approximately 400 pmol/L 52; in the Hordaland study, a serum B12 level below 400 pmol/L was also the level below which levels of MMA began to increase 53. Thus, to be clearly adequate, the serum B12 level needs to be above 400 pmol/L; as shown in Figure 3, only 26% of patients attending a stroke prevention clinic have such levels of serum B12 50. Among patients attending a stroke prevention clinic, metabolic B12 deficiency was present in 10% of patients below the age of 50 and 30% above the age of 70 54.
Figure 3. Serum B12 levels among patients attending a stroke prevention clinic.
Among 3,025 patients attending a stroke prevention clinic, only 26% had a clearly adequate level of serum B12 >400 pmol/L; of those, many (as seen by the very high levels) were taking B12 supplements. Reproduced by permission of Elsevier from 30.
Why does this matter? Besides causing neuropathy, myelopathy, and dementia, B12 deficiency elevates levels of tHcy, a clotting factor that is increased in patients with carotid microemboli 55 and increased in patients with paradoxical embolism 56 and quadruples the risk of stroke in atrial fibrillation 57. There is a perfect storm in the aging of the population—the much higher prevalence of atrial fibrillation in the elderly and the very high prevalence of elevated tHcy in the elderly; 40% of vascular patients above the age of 80 had tHcy levels >14μmol/L 58 ( Figure 4).
Figure 4. Plasma total homocysteine by age group in vascular patients.
Among 2,372 patients referred to our stroke prevention clinic or vascular prevention clinic, the proportion with concentrations of plasma total homocysteine of 14 μmol/L or more increased significantly with age. Reproduced by permission of Elsevier from 58.
Anticoagulation
In recent years, oral anticoagulants have become available to replace warfarin. This is a great development because warfarin is impossible to use well. Even in carefully controlled clinical trials, the international normalized ratio (INR) is in the therapeutic range only around 60% of the time. Reasons for this include genetic variation in both the metabolism of warfarin and the susceptibility to a given level of warfarin 59 as well as a plethora of interactions with warfarin 60. New oral anticoagulants include dabigatran, rivaroxaban, apixaban, and edoxaban (in order of appearance on the market). An important advantage of these drugs is that frequent blood tests are not needed. Unfortunately, all of these drugs are renally excreted, so there are problems with patients with impaired renal function, which includes the elderly 61. Among the new oral anticoagulants (NOACs) available, the one with the greatest renal excretion is dabigatran. Furthermore, dabigatran is also the one with the lowest bioavailability (only 6%). This means that drug interactions or a change in absorption would have a much bigger effect on levels of dabigatran than on the other NOACs. There is evidence that dabigatran levels should probably be monitored 62, thus eliminating for dabigatran one of the main advantages of NOACs.
Antiplatelet therapy
An important issue for clopidogrel is that it is an inactive prodrug that requires metabolism via CYP2C19 to the active metabolite. Among Chinese people, 59% are carriers of a loss-of-function allele that results in lack of efficacy of clopidogrel 63. Ticagrelor, an antiplatelet agent that acts by the same mechanism (inhibition of the platelet ADP receptor P2Y12), avoids this problem because the parent drug is active 64. Although in the main trial ticagrelor did not significantly reduce stroke, it has been shown that ticagrelor reduced stroke among Asian participants 65 and also that ticagrelor reduced recurrent stroke among study participants with large artery disease 66. As a clinical pharmacologist, I expect that ticagrelor will replace clopidogrel for secondary stroke prevention. It is also likely that dual antiplatelet therapy will be better than monotherapy for high-risk patients; this has not yet been tested with aspirin and ticagrelor.
Percutaneous closure of patent foramen ovale
There have been important recent advances in percutaneous closure of patent foramen ovale (PFO). This issue has been difficult because 25% of the population have a PFO, but only approximately 5.5% of strokes are due to paradoxical embolism. This means that, in most patients with stroke and a PFO, the PFO is incidental, and there is a problem with statistical power in randomized trials.
Although the early studies of PFO closure did not show significant benefit individually in intent-to-treat analyses, a pooled analysis of individual data from three trials did show significant benefit of closure 67. A manuscript is in preparation reporting the benefit of closure in a more recent trial of two new devices. There are occasional serious complications of percutaneous closure, and there is an increased risk of atrial fibrillation following closure, so the selection of patients is important.
There are ways to identify which patients are more likely to benefit from closure: there are clinical clues to paradoxical embolism, and a large shunt on transcranial Doppler (TCD) is predictive of recurrent stroke. Clinical clues include previous pulmonary embolism, deep vein thrombosis or varicose veins, waking up with stroke, a history of migraine, prolonged sitting, a history of sleep apnea, and a Valsalva maneuver at the onset of stroke 56. Saline studies with TCD are more sensitive to detect PFO than is trans-esophageal echocardiography (TEE), which missed 15% of right–left shunts (among which over 40% were large shunts) 68. Large shunts on TCD were more predictive of recurrent events than was the presence of a shunt or atrial septal mobility on TEE 68.
Intracranial stenosis
Intracranial stenosis is more common in Asian patients and in diabetics 69, 70. The Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial 71 reported in 2014 that intensive medical therapy that included lifestyle modification was more efficacious than stenting for intracranial stenosis. In addition to statins and blood pressure treatment, initial dual antiplatelet therapy and intensive lifestyle modification throughout the study were key elements of successful medical therapy.
Lipid-lowering therapy
Therapy to lower levels of LDL-C clearly reduces the risk of recurrent stroke, particularly in patients with large artery disease 72. However, there are important problems with persistence with statins, in part due to adverse effects. After 2 years, less than half of patients prescribed statins are still taking them. The truly causal adverse effects of statins, myopathy and a small increase in the risk of diabetes, are probably due largely to the depletion of ubiquinone (CoQ10) 73, 74.
A major recent development in lipid-lowering therapy has been in therapies directed at proprotein convertase subtilisin–kexin type 9 (PCSK9), a protein that results in higher levels of LDL-C by reducing the number of active LDL receptors. Injection of antibodies to PCSK9 and RNA-silencing therapy reduce levels of LDL-C by more than half. Evolocumab, one of the antibody therapies, reduced the composite endpoint of stroke, myocardial infarction, and cardiovascular death by 20% in only 2 years 75; lifetime reduction of events would be expected to be much greater. In a post hoc analysis, there was a 48% reduction in 78 weeks, of a composite endpoint of death from coronary heart disease, non-fatal myocardial infarction, fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization with alirocumab 76. Tall has suggested 77 that the marked reduction of vascular risk observed in persons with loss-of-function mutations of PCSK9 (and therefore lifelong reduction of LDL-C to the same extent as therapy with statins) means that we should be using lipid-lowering therapy earlier in life among persons at risk of vascular disease. As pointed out by Wilkins 78, this essentially means all North Americans who aspire to attain old age.
In 2003, in our prevention clinics we initiated a strategy of “treating arteries instead of treating risk factors”; the goal of therapy is to stop the progression of atherosclerosis or achieve regression. Among patients with asymptomatic carotid stenosis, this strategy reduced the risk of myocardial infarction and stroke by more than 80%. We recently observed that some patients have “resistant atherosclerosis”, requiring very low levels of LDL-C to stop progression, and there was no correlation between LDL-C and the progression or regression of carotid plaque burden 79 ( Figure 5). Both age and renal impairment increased the resistance of atherosclerosis to LDL-lowering therapy. The effect of age may in part be due to impaired renal function; by the age of 80, the average estimated glomerular filtration rate is <60 mL/minute/1.73 m 2 61. Uremic toxins that would aggravate atherosclerosis include tHcy, ADMA, thiocyanate, and products of the intestinal microbiome, including trimethylamine n-oxide (TMAO, produced from carnitine in red meat and phosphatidylcholine in egg yolk 80– 82), p-cresyl sulfate, and other toxins produced from amino acids 61. I now have over 100 patients with LDL-C <0.5 mmol/L (19 mg/dL) and a handful with LDL-C <0.3 mmol/L (12 mg/dL) because that’s what it took to stop plaque progression.
Figure 5. Correlation of low-density lipoprotein cholesterol (LDL-C) (mmol/L), change in LDL-C, and change in total plaque area (mm 2).
Among 4,512 patients followed in a stroke prevention clinic, there was no correlation between achieved LDL-C with lipid-lowering therapy and change in carotid burden over 1 year (panel A). Similarly, there was no correlation between change in LDL-C from baseline to follow-up and change in plaque burden (panel B).
There is no reason to be concerned about low levels of LDL-C. Adverse effects of statins are not due to low levels of LDL-C, and many of the putative adverse effects of statins are myths: statins probably do not cause hepatotoxicity, renal impairment, intracerebral hemorrhage 83– 85, or cognitive impairment 73. In the pooled analysis of trials with alirocumab 86, there were no intracerebral hemorrhages reported, even with LDL-C <15 mg/dL, and in the evolocumab trial 75 there was no significant difference in hemorrhagic stroke between evolocumab and placebo. Likewise, very low levels of LDL-C from therapies directed at PCSK9 do not appear to cause myopathy, diabetes, or cognitive impairment 75, 86.
Conclusion
In 2007, we estimated 87 that about 80% of recurrent strokes could be prevented by a combination of lifestyle changes and appropriate medication. Recently, I reviewed rational medical therapy for stroke prevention 88. With these recent advances in hypertension, nutrition, anticoagulation, antiplatelet therapy, intracranial stenosis, and lipid-lowering therapy, it is likely that recurrent stroke could be reduced even more. The key to preventing recurrent stroke is to identify the cause of the initial event so that therapy can be individualized to the patient. While the vascular risk factors should be treated in all patients, anticoagulation and PFO closure would be appropriate in only some patients.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Tatjana Rundek, University of Miami, Miami, FL, USA
Cheryl Bushnell, Wake Forest Baptist Health, Winston Salem, NC, USA
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Spence JD: Intensive risk factor control in stroke prevention. F1000Prime Rep. 2013;5:42. 10.12703/P5-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lim SS, Vos T, Flaxman AD, et al. : A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–60. 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Mozaffarian D, Benjamin EJ, Go AS, et al. : Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. 10.1161/CIR.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 4. Feigin VL, Krishnamurthi RV, Parmar P, et al. : Update on the Global Burden of Ischemic and Hemorrhagic Stroke in 1990–2013: The GBD 2013 Study. Neuroepidemiology. 2015;45(3):161–76. 10.1159/000441085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Howard G, Prineas R, Moy C, et al. : Racial and geographic differences in awareness, treatment, and control of hypertension: the REasons for Geographic And Racial Differences in Stroke study. Stroke. 2006;37(5):1171–8. 10.1161/01.STR.0000217222.09978.ce [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Rayner BL, Spence JD: Hypertension in blacks: insights from Africa. J Hypertens. 2017;35(2):234–9. 10.1097/HJH.0000000000001171 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Wilson TW, Grim CE: Biohistory of slavery and blood pressure differences in blacks today. A hypothesis. Hypertension. 1991;17(1 Suppl):I122–8. 10.1161/01.HYP.17.1_Suppl.I122 [DOI] [PubMed] [Google Scholar]
- 8. Grim CE, Robinson M: Salt, slavery and survival-hypertension in the African diaspora. Epidemiology. 2003;14(1):120–2; discussion 124–6. [DOI] [PubMed] [Google Scholar]
- 9. Spence JD: Lessons from Africa: the importance of measuring plasma renin and aldosterone in resistant hypertension. Can J Cardiol. 2012;28(3):254–7. 10.1016/j.cjca.2011.11.010 [DOI] [PubMed] [Google Scholar]
- 10. Jones ES, David Spence J, Mcintyre AD, et al. : High Frequency of Variants of Candidate Genes in Black Africans with Low Renin-Resistant Hypertension. Am J Hypertens. 2017;30(5):478–83. 10.1093/ajh/hpw167 [DOI] [PubMed] [Google Scholar]
- 11. Laffer CL, Elijovich F, Eckert GJ, et al. : Genetic variation in CYP4A11 and blood pressure response to mineralocorticoid receptor antagonism or ENaC inhibition: an exploratory pilot study in African Americans. J Am Soc Hypertens. 2014;8(7):475–80. 10.1016/j.jash.2014.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bass MJ, McWhinney IR, Donner A: Do family physicians need medical assistants to detect and manage hypertension? CMAJ. 1986;134(11):1247–55. [PMC free article] [PubMed] [Google Scholar]
- 13. Birkett NJ, Donner AP, Maynard M: Prevalence and control of hypertension in an Ontario county. Can Med Assoc J. 1985;132(9):1019–24. [PMC free article] [PubMed] [Google Scholar]
- 14. Spence JD: Antihypertensive drugs and prevention of atherosclerotic stroke. Stroke. 1986;17(5):808–10. 10.1161/01.STR.17.5.808 [DOI] [PubMed] [Google Scholar]
- 15. Barnett HJ, Taylor DW, Eliasziw M, et al. : Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339(20):1415–25. 10.1056/NEJM199811123392002 [DOI] [PubMed] [Google Scholar]
- 16. Spence JD, Rayner BL: J Curve and Cuff Artefact, and Diagnostic Inertia in Resistant Hypertension. Hypertension. 2016;67(1):32–3. 10.1161/HYPERTENSIONAHA.115.06562 [DOI] [PubMed] [Google Scholar]
- 17. Spence JD: Blood pressure control in Canada: the view from a stroke prevention clinic. Can J Cardiol. 2015;31(5):593–5. 10.1016/j.cjca.2015.03.005 [DOI] [PubMed] [Google Scholar]
- 18. Spence JD: Individualized therapy for hypertension. Hypertension. 2006;47(3):e11. 10.1161/01.HYP.0000203771.24792.bf [DOI] [PubMed] [Google Scholar]
- 19. Akintunde A, Nondi J, Gogo K, et al. : Physiological Phenotyping for Personalized Therapy of Uncontrolled Hypertension in Africa. Am J Hypertens. 2017. 10.1093/ajh/hpx066 [DOI] [PubMed] [Google Scholar]
- 20. Spence JD: Physiologic tailoring of therapy for resistant hypertension: 20 years' experience with stimulated renin profiling. Am J Hypertens. 1999;12(11 Pt 1):1077–83. 10.1016/S0895-7061(99)00154-5 [DOI] [PubMed] [Google Scholar]
- 21. de Lorgeril M, Salen P, Martin JL, et al. : Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99(6):779–85. 10.1161/01.CIR.99.6.779 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. 4S Study Group: Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383–1389. 10.1016/S0140-6736(94)90566-5 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Tsivgoulis G, Psaltopoulou T, Wadley VG, et al. : Adherence to a Mediterranean diet and prediction of incident stroke. Stroke. 2015;46(3):780–5. 10.1161/STROKEAHA.114.007894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Akesson A, Larsson SC, Discacciati A, et al. : Low-risk diet and lifestyle habits in the primary prevention of myocardial infarction in men: a population-based prospective cohort study. J Am Coll Cardiol. 2014;64(13):1299–306. 10.1016/j.jacc.2014.06.1190 [DOI] [PubMed] [Google Scholar]
- 25. Larsson SC, Akesson A, Wolk A: Healthy diet and lifestyle and risk of stroke in a prospective cohort of women. Neurology. 2014;83(19):1699–704. 10.1212/WNL.0000000000000954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Estruch R, Ros E, Salas-Salvadó J, et al. : Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279–90. 10.1056/NEJMoa1200303 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Babio N, Toledo E, Estruch R, et al. : Mediterranean diets and metabolic syndrome status in the PREDIMED randomized trial. CMAJ. 2014;186(17):E649–57. 10.1503/cmaj.140764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Valls-Pedret C, Sala-Vila A, Serra-Mir M, et al. : Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern Med. 2015;175(7):1094–103. 10.1001/jamainternmed.2015.1668 [DOI] [PubMed] [Google Scholar]
- 29. Toole JF, Malinow MR, Chambless LE, et al. : Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291(15):565–75. 10.1001/jama.291.5.565 [DOI] [PubMed] [Google Scholar]
- 30. Bønaa KH: Norvit: Randomised trial of homocysteine-lowering with b-vitamins for secondary prevention of cardiovascular disease after acute myocardial infarction. European Society of Cardiology. Stockholm. Sept 5, 2005. 2005. [Google Scholar]
- 31. Lonn E, Yusuf S, Arnold MJ, et al. : Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354(15):1567–77. 10.1056/NEJMoa060900 [DOI] [PubMed] [Google Scholar]
- 32. Loscalzo J: Homocysteine trials--clear outcomes for complex reasons. N Engl J Med. 2006;354(15):1629–32. 10.1056/NEJMe068060 [DOI] [PubMed] [Google Scholar]
- 33. Galan P, Kesse-Guyot E, Czernichow S, et al. : Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ. 2010;341:c6273. 10.1136/bmj.c6273 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Dong H, Pi F, Ding Z, et al. : Efficacy of Supplementation with B Vitamins for Stroke Prevention: A Network Meta-Analysis of Randomized Controlled Trials. PLoS One. 2015;10(9):e0137533. 10.1371/journal.pone.0137533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ji Y, Tan S, Xu Y, et al. : Vitamin B supplementation, homocysteine levels, and the risk of cerebrovascular disease: a meta-analysis. Neurology. 2013;81(15):1298–307. 10.1212/WNL.0b013e3182a823cc [DOI] [PubMed] [Google Scholar]
- 36. Wang R: Is H 2S a stinky remedy for atherosclerosis? Arterioscler Thromb Vasc Biol. 2009;29(2):156–7. 10.1161/ATVBAHA.108.180190 [DOI] [PubMed] [Google Scholar]
- 37. Rader DJ, Ischiropoulos H: 'Multipurpose oxidase' in atherogenesis. Nat Med. 2007;13(10):1146–7. 10.1038/nm1007-1146b [DOI] [PubMed] [Google Scholar]
- 38. Koyama K, Yoshida A, Takeda A, et al. : Abnormal cyanide metabolism in uraemic patients. Nephrol Dial Transplant. 1997;12(8):1622–8. 10.1093/ndt/12.8.1622 [DOI] [PubMed] [Google Scholar]
- 39. Løland KH, Bleie O, Borgeraas H, et al. : The association between progression of atherosclerosis and the methylated amino acids asymmetric dimethylarginine and trimethyllysine. PLoS One. 2013;8(5):e64774. 10.1371/journal.pone.0064774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Koyama K, Ito A, Yamamoto J, et al. : Randomized controlled trial of the effect of short-term coadministration of methylcobalamin and folate on serum ADMA concentration in patients receiving long-term hemodialysis. Am J Kidney Dis. 2010;55(6):1069–78. 10.1053/j.ajkd.2009.12.035 [DOI] [PubMed] [Google Scholar]
- 41. Spence JD, Stampfer MJ: Understanding the complexity of homocysteine lowering with vitamins: the potential role of subgroup analyses. JAMA. 2011;306(23):2610–1. 10.1001/jama.2011.1834 [DOI] [PubMed] [Google Scholar]
- 42. Spence JD, Bang H, Chambless LE, et al. : Vitamin Intervention For Stroke Prevention trial: an efficacy analysis. Stroke. 2005;36(11):2404–9. 10.1161/01.STR.0000185929.38534.f3 [DOI] [PubMed] [Google Scholar]
- 43. Spence JD, Cordy P, Kortas C, et al. : Effect of usual doses of folate supplementation on elevated plasma homocyst(e)ine in hemodialysis patients: no difference between 1 and 5 mg daily. Am J Nephrol. 1999;19(3):405–10. 10.1159/000013486 [DOI] [PubMed] [Google Scholar]
- 44. House AA, Eliasziw M, Cattran DC, et al. : Effect of B-vitamin therapy on progression of diabetic nephropathy: a randomized controlled trial. JAMA. 2010;303(16):1603–9. 10.1001/jama.2010.490 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Spence JD, House AA, Eliasziw M: B-vitamin therapy for diabetic nephropathy: Reply. JAMA. 2010;304(6):636–637. 10.1001/jama.2010.1106 [DOI] [PubMed] [Google Scholar]
- 46. Huo Y, Li J, Qin X, et al. : Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313(13):1325–35. 10.1001/jama.2015.2274 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Qin X, Li J, Spence JD, et al. : Folic Acid Therapy Reduces the First Stroke Risk Associated With Hypercholesterolemia Among Hypertensive Patients. Stroke. 2016;47(11):2805–12. 10.1161/STROKEAHA.116.014578 [DOI] [PubMed] [Google Scholar]
- 48. Xu X, Qin X, Li Y, et al. : Efficacy of Folic Acid Therapy on the Progression of Chronic Kidney Disease: The Renal Substudy of the China Stroke Primary Prevention Trial. JAMA Intern Med. 2016;176(10):1443–50. 10.1001/jamainternmed.2016.4687 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Spence JD, Yi Q, Hankey G: B vitamins in stroke prevention: Time to reconsider Lancet Neurology.2017; in press. [DOI] [PubMed] [Google Scholar]
- 50. Spence JD: Metabolic vitamin B12 deficiency: a missed opportunity to prevent dementia and stroke. Nutr Res. 2016;36(2):109–16. 10.1016/j.nutres.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 51. Robertson J, Iemolo F, Stabler SP, et al. : Vitamin B 12, homocysteine and carotid plaque in the era of folic acid fortification of enriched cereal grain products. CMAJ. 2005;172(12):1569–73. 10.1503/cmaj.045055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bang H, Mazumdar M, Spence D: Tutorial in biostatistics: Analyzing associations between total plasma homocysteine and B vitamins using optimal categorization and segmented regression. Neuroepidemiology. 2006;27(4):188–200. 10.1159/000096149 [DOI] [PubMed] [Google Scholar]
- 53. Vogiatzoglou A, Oulhaj A, Smith AD, et al. : Determinants of plasma methylmalonic acid in a large population: implications for assessment of vitamin B 12 status. Clin Chem. 2009;55(12):2198–206. 10.1373/clinchem.2009.128678 [DOI] [PubMed] [Google Scholar]
- 54. Spence JD: Nutrition and stroke prevention. Stroke. 2006;37(9):2430–5. 10.1161/01.STR.0000236633.40160.ee [DOI] [PubMed] [Google Scholar]
- 55. Spence JD, Tamayo A, Lownie SP, et al. : Absence of microemboli on transcranial Doppler identifies low-risk patients with asymptomatic carotid stenosis. Stroke. 2005;36(11):2373–8. 10.1161/01.STR.0000185922.49809.46 [DOI] [PubMed] [Google Scholar]
- 56. Ozdemir AO, Tamayo A, Munoz C, et al. : Cryptogenic stroke and patent foramen ovale: clinical clues to paradoxical embolism. J Neurol Sci. 2008;275(1–2):121–7. 10.1016/j.jns.2008.08.018 [DOI] [PubMed] [Google Scholar]
- 57. Poli D, Antonucci E, Cecchi E, et al. : Culprit factors for the failure of well-conducted warfarin therapy to prevent ischemic events in patients with atrial fibrillation: the role of homocysteine. Stroke. 2005;36(10):2159–63. 10.1161/01.STR.0000183620.06179.7b [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Spence D: Mechanisms of thrombogenesis in atrial fibrillation. Lancet. 2009;373(9668):1006;author reply 1006–7. 10.1016/S0140-6736(09)60604-8 [DOI] [PubMed] [Google Scholar]
- 59. Schwarz UI, Ritchie MD, Bradford Y, et al. : Genetic determinants of response to warfarin during initial anticoagulation. N Engl J Med. 2008;358(10):999–1008. 10.1056/NEJMoa0708078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Spence JD: Atrial fibrillation and stroke prevention: is warfarin still an option? Yes: Debate at the Controversies in Neurology Congress, Beijing, October 2011. J Neural Transm (Vienna). 2013;120(10):1447–51. 10.1007/s00702-012-0941-5 [DOI] [PubMed] [Google Scholar]
- 61. Spence JD, Urquhart BL, Bang H: Effect of renal impairment on atherosclerosis: only partially mediated by homocysteine. Nephrol Dial Transplant. 2016;31(6):937–44. 10.1093/ndt/gfv380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Reilly PA, Lehr T, Haertter S, et al. : The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy). J Am Coll Cardiol. 2014;63(4):321–8. 10.1016/j.jacc.2013.07.104 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Wang Y, Zhao X, Lin J, et al. : Association Between CYP2C19 Loss-of-Function Allele Status and Efficacy of Clopidogrel for Risk Reduction Among Patients With Minor Stroke or Transient Ischemic Attack. JAMA. 2016;316(1):70–8. 10.1001/jama.2016.8662 [DOI] [PubMed] [Google Scholar]
- 64. Schömig A: Ticagrelor--is there need for a new player in the antiplatelet-therapy field? N Engl J Med. 2009;361(11):1108–11. 10.1056/NEJMe0906549 [DOI] [PubMed] [Google Scholar]
- 65. Wang Y, Minematsu K, Wong KS, et al. : Ticagrelor in Acute Stroke or Transient Ischemic Attack in Asian Patients: From the SOCRATES Trial (Acute Stroke or Transient Ischemic Attack Treated With Aspirin or Ticagrelor and Patient Outcomes). Stroke. 2017;48(1):167–73. 10.1161/STROKEAHA.116.014891 [DOI] [PubMed] [Google Scholar]
- 66. Amarenco P, Albers GW, Denison H, et al. : Efficacy and safety of ticagrelor versus aspirin in acute stroke or transient ischaemic attack of atherosclerotic origin: a subgroup analysis of SOCRATES, a randomised, double-blind, controlled trial. Lancet Neurol. 2017;16(4):301–10. 10.1016/S1474-4422(17)30038-8 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Kent DM, Dahabreh IJ, Ruthazer R, et al. : Device Closure of Patent Foramen Ovale After Stroke: Pooled Analysis of Completed Randomized Trials. J Am Coll Cardiol. 2016;67(8):907–17. 10.1016/j.jacc.2015.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Tobe J, Bogiatzi C, Munoz C, et al. : Transcranial Doppler is Complementary to Echocardiography for Detection and Risk Stratification of Patent Foramen Ovale. Can J Cardiol. 2016;32(8):986.e9–986.e16. 10.1016/j.cjca.2015.12.009 [DOI] [PubMed] [Google Scholar]
- 69. Nilanont Y, Poungvarin N: Management and prevention of intracranial atherosclerotic disease.In: Spence JD, Barnett HJ, eds. Stroke prevention, treatment and rehabilitation. New York: McGraw-Hill Medical;2012;139–151. [Google Scholar]
- 70. Lam TD, Lammers S, Munoz C, et al. : Diabetes, intracranial stenosis and microemboli in asymptomatic carotid stenosis. Can J Neurol Sci. 2013;40(2):177–81. 10.1017/S031716710001369X [DOI] [PubMed] [Google Scholar]
- 71. Derdeyn CP, Chimowitz MI, Lynn MJ, et al. : Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. 2014;383(9914):333–41. 10.1016/S0140-6736(13)62038-3 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Sillesen H, Amarenco P, Hennerici MG, et al. : Atorvastatin reduces the risk of cardiovascular events in patients with carotid atherosclerosis: a secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke. 2008;39(12):3297–302. 10.1161/STROKEAHA.108.516450 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Spence JD, Dresser GK: Overcoming Challenges With Statin Therapy. J Am Heart Assoc. 2016;5(1): pii: e002497. 10.1161/JAHA.115.002497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rundek T, Naini A, Sacco R, et al. : Atorvastatin decreases the coenzyme Q10 level in the blood of patients at risk for cardiovascular disease and stroke. Arch Neurol. 2004;61(6):889–92. 10.1001/archneur.61.6.889 [DOI] [PubMed] [Google Scholar]
- 75. Sabatine MS, Giugliano RP, Keech AC, et al. : Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376(18):1713–22. 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Robinson JG, Farnier M, Krempf M, et al. : Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489–99. 10.1056/NEJMoa1501031 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 77. Tall AR: Protease variants, LDL, and coronary heart disease. N Engl J Med. 2006;354(12):1310–2. 10.1056/NEJMe068026 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Wilkins JT, Ning H, Berry J, et al. : Lifetime risk and years lived free of total cardiovascular disease. JAMA. 2012;308(17):1795–801. 10.1001/jama.2012.14312 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 79. Spence JD, Solo K: Resistant Atherosclerosis: The Need for Monitoring of Plaque Burden. Stroke. 2017;48(6):1624–9. 10.1161/STROKEAHA.117.017392 [DOI] [PubMed] [Google Scholar]
- 80. Wang Z, Klipfell E, Bennett BJ, et al. : Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. 10.1038/nature09922 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 81. Koeth RA, Wang Z, Levison BS, et al. : Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85. 10.1038/nm.3145 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Tang WH, Wang Z, Levison BS, et al. : Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–84. 10.1056/NEJMoa1109400 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 83. Spence JD: Statins do not cause intracerebral hemorrhage. Neurology. 2012;79(11):1076–7. 10.1212/WNL.0b013e3182608caf [DOI] [PubMed] [Google Scholar]
- 84. Hackam DG, Woodward M, Newby LK, et al. : Statins and intracerebral hemorrhage: collaborative systematic review and meta-analysis. Circulation. 2011;124(20):2233–42. 10.1161/CIRCULATIONAHA.111.055269 [DOI] [PubMed] [Google Scholar]
- 85. Hackam DG, Austin PC, Huang A, et al. : Statins and intracerebral hemorrhage: a retrospective cohort study. Arch Neurol. 2012;69(1):39–45. 10.1001/archneurol.2011.228 [DOI] [PubMed] [Google Scholar]
- 86. Robinson JG, Rosenson RS, Farnier M, et al. : Safety of Very Low Low-Density Lipoprotein Cholesterol Levels With Alirocumab: Pooled Data From Randomized Trials. J Am Coll Cardiol. 2017;69(5):471–82. 10.1016/j.jacc.2016.11.037 [DOI] [PubMed] [Google Scholar]
- 87. Hackam DG, Spence JD: Combining multiple approaches for the secondary prevention of vascular events after stroke: a quantitative modeling study. Stroke. 2007;38(6):1881–5. 10.1161/STROKEAHA.106.475525 [DOI] [PubMed] [Google Scholar]
- 88. Spence JD: Rational Medical Therapy Is the Key to Effective Cardiovascular Disease Prevention. Can J Cardiol. 2017;33(5):626–34. 10.1016/j.cjca.2017.01.003 [DOI] [PubMed] [Google Scholar]