Abstract
Project Santa Fe was established both to provide thought leadership and to help develop the evidence base for the valuation of clinical laboratory services in the next era of American healthcare. The participants in Project Santa Fe represent major regional health systems that can operationalize laboratory-driven innovations and test their valuation in diverse regional marketplaces in the United States. We provide recommendations from the inaugural March 2016 meeting of Project Santa Fe. Specifically, in the transition from volume-based to value-based health care, clinical laboratories are called upon to provide programmatic leadership in reducing total cost of care through optimization of time-to-diagnosis and time-to-effective therapeutics, optimization of care coordination, and programmatic support of wellness care, screening, and monitoring. This call to action is more than working with industry stakeholders on the basis of our expertise; it is providing leadership in creating the programs that accomplish these objectives. In so doing, clinical laboratories can be effectors in identifying patients at risk for escalation in care, closing gaps in care, and optimizing outcomes of health care innovation. We also hope that, through such activities, the evidence base will be created for the new value propositions of integrated laboratory networks. In the very simplest sense, this effort to create “Clinical Lab 2.0” will establish the impact of laboratory diagnostics on the full 100% spend in American healthcare, not just the 2.5% spend attributed to in vitro diagnostics. In so doing, our aim is to empower regional and local laboratories to thrive under new models of payment in the next era of American health care delivery.
Keywords: disruption, innovation, laboratory medicine, pathology, value
Introduction
In vitro diagnostics, the healthcare industry term for clinical laboratory services, represents US$73 billion of the US$3 trillion spend on US healthcare—about 2.5%. And yet, this sector of healthcare informs the majority of health care management, estimated at “up to 70% of decisions.”1,2 To date, very few laboratories or pathologists are actively engaged in providing leadership for optimizing integration of laboratory diagnostics into clinical workflows and population management. Building the evidence base for the positive outcomes that arise from acting upon laboratory diagnostic information is actually a difficult task. But upon such evidence rest decisions about the role that the laboratory plays in the coming era of risk-based health care. The question is: are laboratory services strictly a commodity or do laboratory services have a higher valuation that can drive better outcomes for patients, providers, and financial stakeholders alike?
Project Santa Fe was established to test the latter hypothesis. Project Santa Fe is a coalition of like-minded major regional laboratories, coming together to create and help drive the new frontiers that will define the future economic valuation and placement of laboratory diagnostic services in American healthcare. Both through “think tank” efforts and through the building of a rigorous evidence base, the members will pursue a disruptive “value” paradigm. Our collective goal is to be trailblazers in the role of laboratory leadership in reengineering health care delivery and the practice of medicine. This is a first report from Project Santa Fe, articulating foundational premises for moving laboratory services from its current posture as “Clinical Lab 1.0” to “Clinical Lab 2.0” in the next era of health care.
Opportunities for Laboratory Services
Health care expenditures currently represent 17.5% of the US gross domestic product (GDP).3 Virtually all analysts agree that, without major reform, health care’s share of GDP will continue to rise rapidly, potentially reaching 28% in 2030 and 34% in 2040.4 Escalating health care costs are due in part to system inefficiencies:
spending a substantial amount on high-cost, low-value treatments,
patients obtaining too little of certain types of care that are effective and of high value,
patients not receiving care in the most cost-effective setting,
extensive variation in the quality of care provided to patients,
preventable medical errors that lead to worse outcomes and higher costs, and
system complexity that adds high administrative costs.
We believe that laboratory professionals must provide strategic programmatic leadership in reducing these inefficiencies and in promoting better health care delivery. Moreover, we do not feel that we have the luxury of even single years to accomplish these ends.5 The evidence base for laboratory valuation must be established in a proximate time frame, including bringing institutional demonstration projects forward in the peer-review literature.
In the inpatient setting, information generated by the clinical laboratory can inform the severity of illness that the patient has, provide real-time risk stratification of the evolving acuity of care needed, and track the patient’s progress through the episode of care. In the ambulatory setting, laboratory services can constitute a driver for continuity in care, both through the longitudinal continuity of laboratory testing performed on patients with chronic diseases and through informing providers about evolving risk conditions and potential gaps in care. In both instances, the laboratory can inform real-time, targeted intervention for populations of patients with risk conditions. Moreover, in both inpatient and ambulatory settings, the increasing use of esoteric testing brings to health care the leading edge of medical science and the promise of “precision medicine” for individual patients. Laboratory professionals play a central role in driving appropriate test utilization and in guiding the clinical action based on these tests.
While laboratory testing necessarily informs the diagnoses rendered under the disease-related groups model of payment for inpatient health care, only recently has ambulatory health care come under a risk-based form of healthcare payment. The alternative payment models for US health care require documentation of risk conditions that describe the severity of acute and chronic illness for covered beneficiaries, in order to guide payment for such care.6 Similar to inpatient care, the diagnosis of disease conditions in the ambulatory setting is also substantially informed by laboratory services. Hence, in both settings now, laboratory test data underpin the valuation of care given by health-care providers. It stands to reason that laboratory professionals can provide leadership in optimizing this use of laboratory data as well.
Collectively, these opportunities are summarized in Table 1. This optimistic view of the opportunities contrasts with the premise that laboratory diagnostics are movable and are unlinked to the local delivery of care except as dictated by turnaround time obligations and the provision of test result data. Such depersonalization of laboratory diagnostics threatens the premise that “health care is local” and disempowers the ability of local health care providers to work with laboratory subject matter experts. In addition, time delays in test results due to this commodity mentality risk creating clinician behavioral changes such as massive lab order sets to avoid any potential delay in diagnoses. The logical, stepwise approach to clinical diagnosis is supplanted by a “shotgun” approach with the ensuing information chaos and loss of clarity.
Table 1.
Opportunities for Laboratory Services Under Alternative Payment Models.
| Organizing principles of alternative payment models |
| Transition from volume based to value based reimbursement |
| Transition from cost per unit to total episodic costs |
| Transition from fee-for-service transactions to bundled payments |
| Leadership activities for laboratory services |
| Establishment of institution-wide laboratory test formularies |
| Documentation and Education of Provider test utilization patterns |
| Laboratory Utilization Management of expensive and esoteric testing: inpatient, ambulatory |
| Real-time risk stratification of covered populations (eg, in managed care products) |
| Predictive modelling of chronic disease states in those covered populations |
| Provision of analytical services to reduce physician burden in quality measurement and reporting (HEDIS, MIPS, P4P, ACO metrics) |
| Closing of “care gaps” |
| Provision of real-time laboratory data to providers at the point of care |
| Working with health systems and civic authorities to identify patients in-need |
| Provision to physicians and provider groups of information on utilization and cost of laboratory testing, including peer-to-peer benchmark comparative reports |
| Reduction of out-of-network leakage of laboratory testing, both as a cost-savings exercise and as part of attaining comprehensive laboratory data on covered populations |
| Assisting providers in identifying, monitoring, and following up on patients with chronic and costly conditions, as through Disease Registries |
| Working with payers and ACOs to identify and manage patients enrolled in disease management and care management programs |
| Using point-of-care laboratory testing to improve patient access and for effective patient engagement and chronic care management (including testing at patients’ homes) |
| Integration of laboratory testing with telemedicine care delivery models |
Abbreviations: ACO, Accountable Care Organization; HEDIS, Healthcare Effectiveness Data and Information Set; MIPS, Merit-Based Incentive Payment System; P4P, Pay-for-Performance.
An article of faith for laboratory professionals is that pathology and laboratory medicine touch the virtual entirety of the human condition, in a high-impact “patient-centered” fashion. Since laboratory testing is part of wellness and preventive care as well as treatment for disease, under the best of circumstances clinical laboratories touch the lives of almost everyone. In so doing, laboratory diagnostics constitute an extraordinarily broad front to help bring the promise of health care innovations to fruition for the entire population. This promise can be realized at the time of the diagnostic assessment and at the time of health care decision-making.
The question is, who carries responsibility for delivering on that promise? Under the “commoditization of laboratory services” model, it is other sectors of the health-care industry that will compile the massive sources of information emanating from the clinical laboratory, to extract value and put that value into play. The clinical laboratory simply remains a source of transactional data, without significant input into either the demand for that data (test ordering) or its interpretation. We argue that this displacement of interpretive effort is not commutative—that something is lost by separating the laboratory from data analysis and interpretation. The basis for such argument is that laboratory professionals have unique expertise (Table 2). For the technical professionals of the laboratory workforce, their expertise lies in maximizing the accuracy of laboratory diagnostics, optimizing the efficiency of their delivery and helping to ensure that innovations from medical science are successfully deployed at this front line of health care delivery. For the medical professionals—pathologists, clinical doctoral scientists, and informaticists alike—a primary career responsibility is knowledge of the impact of diagnostic testing and interpretations on patient prognosis, treatment, and outcomes. It is precisely this expertise that enables pathologists, clinical scientists, and informaticists to inform decision-making regarding the most effective ways to deliver outstanding patient care. With medical care becoming increasingly specialized, the laboratory testing that goes with it is also increasingly specialized. It is becoming exceedingly difficult for providers to stay abreast of best evidence regarding laboratory medicine, a factor which may help to explain the wide variation in ordering patterns. Pathologists can be of enormous value in workup of specific clinical disorders.7,8
Table 2.
The Unique Attributes of Laboratory Professionals.
|
Our specialty requires us to understand the scientific basis of all of human disease. We must be lead adapters for advances in the medical science of diagnosis. We must understand the impact of treatment and intervention on the entirety of the human condition, not just the disease being treated (owing to the impact of such treatments on the host). To be effective consultants to providers, we must understand the impact of our test information on medical decision-making. We live-and-breath Quality and Safety. We have sight lines to virtually every sector of health care. We practice “system management” as a core expertise. Our innovations can be rapidly promulgated throughout a health system and can be quickly emulated by other health systems (scalability and replicability). Our innovations don’t cost much, but can have great impact. We have data streams on the entire population. We see the diagnostic test data first. |
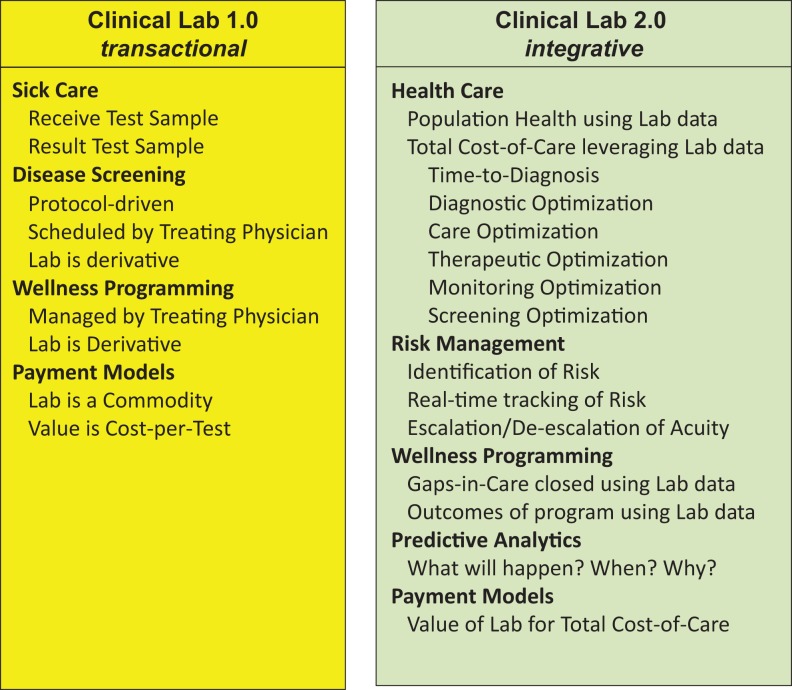
In sum, we believe that laboratory professionals are well-positioned to play a key role in the transition of US health care from “sick care” to “health care.” We are proposing that this be accomplished via the clinical laboratory business model’s evolution from “Clinical Lab 1.0” (transactional) to “Clinical Lab 2.0” (integrative), the attributes of which are depicted in Figure 1. This effort can be driven through such population-based activities as given in Table 3.
Figure 1.
Proposed Transition of Pathology and Laboratory Services from a Transactional to an Integrative Model: “Clinical Lab 2.0”.
Table 3.
Population Health activities of the Laboratory.
|
Reducing time to diagnosis and time to intervention Chronic disease management Gaps in care: alerts, notifications, improvements in patient access, tracking of outcomes Registries: for risk assessment and intervention and for actuarial planning Wellness care: screening; early intervention High-acuity care: real-time risk escalation and intervention Transitions in care Continuity in problem lists; support of coordinated care across multiple care sites Advance notifications to downstream sites Laboratory: pharmacy reconciliation Antibiotic stewardship Chronic disease reconciliation and compliance Real-time risk stratification and assessment Unmasking of at-risk populations through real-time analytics Assessing the disease burden of populations Tracking disease progression (or not) through time Identifying actionable subgroups of patients Populating actuarial risk models with real clinical data Assessing the real-time actuarial value of laboratory-generated information Accelerating (decreasing) the cycle time for identifying risk acquisition by cost-bearing stakeholders Quality tracking and reporting Providing quality measures for health systems and providers Building the evidence base for innovation Precision medicine Pharmacogenomics Clinical outcomes of interventions at the population level |
Leadership by Clinical Laboratories
A specific challenge thus emerges: what leadership can and should laboratory professionals provide? The downside concern is that, absent such leadership, the threats to the laboratory industry will continue unabated. The upside opportunity is that such laboratory leadership will improve the delivery of health care more rapidly, with greater realized benefit, than could otherwise be realized. With those polar opposites in mind, programmatic examples of laboratory leadership are given in Table 4. Unfortunately, barriers to providing such leadership are many (Table 5) and will have to be overcome in order to realize the benefit that the laboratory can provide in the abbreviated time frames now required for successful improvement of the US health care system.
Table 4.
New Opportunities for Leadership by Laboratory Professionals.
|
Promoting better patient access to health care services, to include: Identification of care gaps and their root causes Enhancing access of patients to ambulatory laboratory services Supporting provider use of cost effective and rational choices for diagnostic testing Linking laboratory diagnostics to patient outcomes, to help maximize utility of laboratory testing Linking laboratory diagnostics to population outcomes, to help guide coordinated care programming Linking laboratory data to risk stratification, to include: Identification of patients at risk for adverse health outcomes Tracking of HCCs in covered patient populations Linking laboratory data to claims and total cost-of-care Empowering health systems to optimize revenue recovery in the provision of episodes-of-care Empowering health systems to optimize the total coordination of care Knowledge of health IT architecture, utilization, and analytics Acting as subject matter experts on data sourcing and interpretation Knowledge of APM, to include: Understanding of metrics for quality performance that depend on laboratory test data Understanding total costs of care (including claims data) and its relationship to laboratory test data Providing leadership for optimization of health system revenue performance under APM Engagement in managed care contracting processes of health system to help ensure effective implementation of pay-for-performance outcomes measures Engagement with providers, care management organizations, and payers in effective design and delivery of disease and care management programs |
Abbreviations: APM, alternative payment models; IT, information technology; HCC, hierarchical condition categories.
Table 5.
Barriers to Laboratory Leadership in Health Care Innovation.
|
Lack of a common language with providers, health systems, payers Lack of models for comparisons and benchmarking Lack of integrative information management technologies Lack of outcomes-based evidence for laboratory-led innovation Lack of aligned incentives Inadequate leveraging of laboratory data into actionable information Lack of access to capital for in-system laboratories, that is available to the for-profit sector of laboratory industry Lack of access to new required new skill sets Inadequate engagement with senior leadership (“C-suite”) of health systems Lack of playbook for providing leadership |
Examples of Laboratory Leadership to Date
In the traditional paradigm of medicine, laboratory services are a transactional event: a provider orders a test based on her/his clinical impression of a patient’s ailment at a given point in time and interprets the individual test results at the time of receiving the test results. The value of this activity is thus finite and time limited. However, in generating a test result, the laboratory creates a specific and indelible record of the state of health of a patient at a given point of time and often over an extended spectrum of disease progression. Laboratory data (unlike clinical data) are often very structured, quantifiable, and classifiable, ergo, the data are amenable to multiple retrievable and analytical methods. Thus, it is not surprising that the very field of clinical informatics found a firm footing in the clinical laboratory, in the form of laboratory information systems, representing the first home of the electronic health record.9
In this new era in which pathologists and clinical laboratory professionals are working with clinical stakeholders to better leverage the immense value of information emanating from the clinical laboratory, there are superb examples in the recent literature. Appendix A presents a compilation of such recent activities. In turn, the member laboratories of Project Santa Fe have embarked upon demonstration projects in support of the recommendations of the March 2016 inaugural meeting (Table 6).
Table 6.
2016-2017 Demonstration Projects by Project Santa Fe Membership.
|
Gaps in care: identification of and tracking of pregnancies in a Medicaid population (TriCore) Gaps in care: identification of acute kidney injury (AKI) during hospital admission (Northwell) Gaps in care: latency in laboratory test data, not acted upon clinically (Kaiser-Permanente) Patient experience: structured quality monitors for anatomic pathology turnaround time (Geisinger) Utilization management: laboratory test formulary (Henry Ford) |
The Scientific Method
Comment must be made regarding the scientific method. Laboratory-led innovations will be implemented in the setting of real-time health care delivery, with almost countless variables that may have impact on measured outcomes. Attributing quantitative outcomes causally to the programmatic leadership of the clinical laboratory will not fall into the paradigm of randomized controlled clinical trials because laboratory services cannot be withdrawn from patient care to test a hypothesis (placebo group). Instead, the established formulation for testing the utility of laboratory services is worth recalling10:
Did the (new) laboratory testing cause harm?
Was the laboratory testing able to distinguish between patients who had disease versus those who did not?
Was the innovation in laboratory testing able to provide better patient diagnostic information than previous options for such testing?
Did patients benefit from such testing having been done?
We propose a new formulation to meet the scientific aims of these Project Santa Fe recommendations:
Do innovations introduced by laboratory leadership cause harm to patients or populations?
Are populations of individuals subjected to such innovations measurably different from populations not subject to innovation?
Are the differences in a favorable direction?
Did patients (or populations) benefit from such innovation having been introduced?
Through this scientific formulation, we aim to evaluate the hypothesis that leadership emanating from the clinical laboratory can benefit individual patients, patient populations, and the health systems that provide for their care. Conversely, we must be attentive to the null hypothesis that leadership and interventions initiated by laboratories have no identifiable effect on health care outcomes and the total costs of delivering care.
Conclusion
The recommendations brought forth in this report constitute a call to action for creation of the evidence base in support of the role of clinical laboratories in the next era of American health care. This is to be achieved through innovative programmatic leadership by laboratory professionals, drawing upon their unique expertise in understanding the potential impact of information coming from laboratory diagnostics. We recommend that laboratory professionals do more than work with clinical and institutional stakeholders in leveraging such information. We feel that programmatic leadership by laboratory professionals is also required, including the design and execution of health care delivery programs. In so doing, the clinical laboratory can itself become an efferent arm in advancing innovation, for the betterment of the populations we serve.
Supplementary Material
Acknowledgments
The authors express their appreciation to the participants of the first convening of Project Santa Fe, in Santa Fe, NM on March 21-23, 2016, who collectively helped shape the ideas presented in this paper. As delegations from the participating health systems, these were from Geisinger Health Laboratories, Jordan Olson and Jeffrey Pritchard; from Henry Ford Health Laboratories, Gaurav Sharma; from Kaiser-Permanente North Laboratories, Thomas Lorey and Richard Motta; from Northwell Health Laboratories, Dwayne Breining, Tylis Chang, James Crawford, Joseph Schulman, and Robert Stallone; and from TriCore Laboratories, Doug Clark, Michael Crossey, Nancy Fisher, Christina Goleman, and Khosrow Shotorbani. The authors also thank our invited speakers and industry observers from ARUP Family Health Clinic, Peter Weir; from Avalon Health Care, Michael Snyder; from CAP Today, Robert McGonnagle; from Dark Report, Robert Michel; from MIT Sloan School of Management, Mark Trusheim; from Presbyterian Healthcare Services, Jason Mitchell; from Roche, Alan Wright; from the University of New Mexico College of Pharmacy, Larry Georgopoulos; from Verisk Analytics, James Colbert and Richard Wheeler; and TriCore Consultant Bruce Greenstein. Not in attendance but members of the planning committee also were from Geisinger Health Laboratories, Conrad Schuerch and Myra Wilkerson; and from Henry Ford Health Laboratories, John Waugh and Richard Zarbo.
Appendix A
A Record of Laboratory Leadership
While pathology and laboratory medicine have contributed to advances in medicine since the middle of the 19th century, the current era of health care requires a higher level of effort to integrate the science of diagnostic testing into the coordinated care of individual patients and of populations. In this latter era, leading the list is a 30-year effort to drive the highest levels of laboratory performance, safety, and quality11-13 (Supplementary Table 1). The clinical laboratory must also ensure that clinical practioners are notified when laboratory test results signify immediate threat to the life of the patient—“panic values” when first described in 1981,14 or what are now known as “critical values.”15 The laboratory has had to verify that test results sent to the electronic health record actually arrive accurately.16 There are excellent example of programs to ensure that laboratory testing actually has beneficial impact on clinical management of patients17 and is utilized appropriately,18-21 including use of point-of-care testing.22
Examples are emerging of pathology providing leadership in managing patients with chronic diseases such as diabetes23 and in management of patients with complex disorders of high clinical acuity.8 Stunning examples of leveraging new technologies in infectious disease diagnostics for rapid treatment of patients with sepsis or infections by antibiotic-resistant organisms have been given.24-26 Laboratory leadership has also played a major role in management of pandemic outbreaks—real27 or in the performance of mock drills.28 Most recently, pathology is providing extensive leadership in the fields of genomics, computational pathology, and machine learning,29-32 with necessary collateral leadership in biobanking.33
Thus, there is much to be proud of in the recent efforts of pathology and laboratory medicine to drive improvement in American health care. However, under evolving value-based care models, the laboratory will increasingly have to influence the numerator and the denominator of the value equation as defined by Porter and Tiesberg—health outcomes achieved per dollar spent.34 For the most part, laboratory professionals have focused on improving quality and outcomes35-41—the numerator in the value equation. But transformation from volume to value will require meaningful quantification of costs—the denominator. The challenge for the laboratory is to demonstrate both the utility and the cost-effectiveness of diagnostic testing.42,43 This is especially important for expensive genomic testing which will be a lynchpin for precision medicine.44 It will also be imperative to effectively communicate the medical and nonmedical value of diagnostic testing.45
Alternative payment models will shift the conversation from charges to cost of care delivery, and understanding of cost and outcome data will be essential. Variability in laboratory test ordering contributes to significant waste, costs of care, and patient harm.46-48 An important driver of this variability is lack of adherence to evidence-based care guidelines. One of the greatest opportunities that laboratories have is to understand and document the underlying drivers of cost and outcome variability.47 Aggregating and analyzing postanalytic laboratory data will be crucial to perform peer-to-peer comparison on laboratory test utilization and enable targeted interventions.49 Laboratory data are the one of the most discrete, structured, and objective data types in the electronic health record. Secondary use of laboratory data has a huge potential and remains an untapped opportunity especially in the evolving big data space. Lastly, it is increasingly recognized that in order to drive meaningful population health outcomes, laboratory professionals have to understand how laboratory data relate to claims data and total costs of care across the entire patient continuum.50 This can then be used to identify and manage high-risk and high-cost patients51 and develop novel predictive models for proactive disease identification and management.52–54
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: The online supplementary table is available at http://journals.sagepub.com/doi/suppl/10.1177/2374289517701067.
References
- 1. Hallworth MJ. The ‘70% claim’: what is the evidence base? Ann Clin Biochem. 2011;48:487–488. [DOI] [PubMed] [Google Scholar]
- 2. Badrich T. Evidence-based laboratory medicine. Clin Biochem Rev. 2013;34:43–46. [PMC free article] [PubMed] [Google Scholar]
- 3. National Health Expenditure data. Centers for Medicare & Medicaid Services. 2014. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/nationalhealthexpenddata/nhe-fact-sheet.html. Accessed October 1, 2016.
- 4. Executive Office of the President Council of Economic Advisors. The Economic Case for Health Care Reform. 2009. http://www.politico.com/pdf/PPM138_economic_case.pdf. Accessed April 12, 2017.
- 5. Louis DN, Gerber GK, Baron JM, et al. Computational pathology: an emerging definition. Arch Pathol Lab Med. 2014;138:1133–1138. [DOI] [PubMed] [Google Scholar]
- 6. Centers for Medicare & Medicaid Services. March 31, 2016, HHS-Operated Risk Adjustment Methodology Meeting. Discussion Paper 2016. https://www.cms.gov/CCIIO/Resources/Forms-Reports-and-Other-Resources/Downloads/RA-March-31-White-Paper-032416.pdf. Accessed October 1, 2016. [PubMed]
- 7. Laposata ME, Laposata M, Van Cott EM, Buchner DS, Kashalo MS, Dighe AS. Physician survey of a laboratory medicine interpretive service and evaluation of the influence of interpretations on laboratory test ordering. Arch Pathol Lab Med. 2004;128:1424–1427. [DOI] [PubMed] [Google Scholar]
- 8. Seegmiller AC, Kim AS, Mosse CA, et al. Optimizing personalized bone marrow testing using an evidence-based, interdisciplinary team approach. Am J Clin Pathol. 2013;140:643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Evans AS. Development of a computer program for the public health laboratory. Public Health Rep. 1967;82:169–179. PMID: 4959926. [PMC free article] [PubMed] [Google Scholar]
- 10. Price CP. Evidence-based laboratory medicine: is it working in practice? Clin Biochem Rev. 2012;33:13–19. [PMC free article] [PubMed] [Google Scholar]
- 11. Dighe AS, Laposata M. Making the laboratory a partner in patient safety. Clin Leadersh Manag Rev. 2004;18:356–360. [PubMed] [Google Scholar]
- 12. Becich MJ, Gilbertson JR, Gupta D, Patel A, Grzybicki DM, Raab SS. Pathology and patient safety: the critical role of pathology informatics in error reduction and quality initiatives. Clin Lab Med. 2004;24:913–943. [DOI] [PubMed] [Google Scholar]
- 13. Howanitz PJ, Perrotta PL, Bashleben CP, et al. Twenty-five years of accomplishments of the College of American Pathologists Q-probes program for clinical pathology. Arch Pathol Lab Med. 2014;138:1141–1149. [DOI] [PubMed] [Google Scholar]
- 14. Lundberg GD. Acting on significant laboratory results. J Am Med Assoc. 1981;245:1762–1763. [DOI] [PubMed] [Google Scholar]
- 15. Doering TA, Plapp F, Crawford JM. Establishing an evidence base for critical laboratory value thresholds. Am J Clin Pathol. 2014;142:617–628. [DOI] [PubMed] [Google Scholar]
- 16. Perrotta PL, Karcher DS. Validating laboratory results in electronic health records: a College of American Pathologists Q-Probes study. Arch Pathol Lab Med. 2016;140:926–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schuerch C, Selna M, Jones J. Laboratory clinical effectiveness: pathologists improving clinical outcomes. Clin Lab Med. 2008;28:223–244, vi. [DOI] [PubMed] [Google Scholar]
- 18. Rao GG, Crook M, Tillyer ML. Pathology tests: is the time for demand management ripe at last? J Clin Pathol. 2003;56:243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fryer AA, Smellie WS. Managing demand for laboratory tests: a laboratory toolkit. J Clin Pathol. 2013;66:62–72. [DOI] [PubMed] [Google Scholar]
- 20. Baron JM, Dighe AS. The role of informatics and decision support in utilization management. Clin Chim Acta. 2014;427:196–201. [DOI] [PubMed] [Google Scholar]
- 21. He R, Wiktor AE, Hanson CA, et al. Conventional karyotyping and fluorescence in situ hybridization: an effective utilization strategy in diagnostic adult acute myeloid leukemia. Am J Clin Pathol. 2015;143:873–878. [DOI] [PubMed] [Google Scholar]
- 22. Jones JB. Information management of point-of-care testing: strategies for improving outcomes in point-of-care testing performance improvement and evidence-based outcomes In: Nichols J, ed. Point-of-Care Testing. New York, NY: Marcel Dekker; 2003:59–100. [Google Scholar]
- 23. Bevis CC, Nogle JM, Forges B, et al. Diabetes wellness care: a successful employer-endorsed program for employees. J Occup Environ Med. 2014;56:1052–1061. [DOI] [PubMed] [Google Scholar]
- 24. Huang AM, Newton D, Kunapuli A, et al. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis. 2013;57:1237–1245. [DOI] [PubMed] [Google Scholar]
- 25. Nagel JL, Huang AM, Kunapuli A, et al. Impact of antimicrobial stewardship intervention on coagulate-negative Staphylococcus blood cultures in conjunction with rapid diagnostic testing. J Clin Microbiol. 2014;52:2849–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lockwood AM, Perez KK, Musick WL, et al. Integrating rapid diagnostics and antimicrobial stewardship in two community hospitals improved process measures and antibiotic adjustment time. Inect Control Hosp Epidemiol. 2016;37:425–432. [DOI] [PubMed] [Google Scholar]
- 27. Crawford JM, Stallone R, Zhang F, et al. Laboratory surge response to the novel influenza A H1N1 outbreak in the New York City metropolitan area. Emerg Infect Dis. 2010;16:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Olsen RJ, Fittipaldi N, Kachroo P, et al. Clinical laboratory response to a mock outbreak of invasive bacterial infections: a preparedness study. J Clin Microbiol. 2014;52:4210–4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sinard JH, Morrow JS. Informatics and anatomic pathology: meeting challenges and charting the future. Human Pathol. 2001;32:144–148. [DOI] [PubMed] [Google Scholar]
- 30. Louis DN, Feldman M, Carter AB, et al. Computational pathology: a path ahead. Arch Pathol Lab Med. 2016;140:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jones R, O’Connor J. Information management and informatics: need for a modern pathology service. Ann Clin Biochem. 2004;41:183–191. [DOI] [PubMed] [Google Scholar]
- 32. Luo Y, Szolovits P, Dighe AS, Baron JM. Using machine learning to predict laboratory test results. Am J Clin Pathol. 2016;145:778–788. [DOI] [PubMed] [Google Scholar]
- 33. Vaught J, Rogers J, Myers K, et al. An NCI perspective on creating sustainable biospecimen resources. J Natl Cancer Inst Monogr. 2011;2011:1–7. doi:10.1093/jncimonographs/lgr006. [DOI] [PubMed] [Google Scholar]
- 34. Porter ME. What is value in health care? N Engl J Med. 2010;363:2477–2481. doi:10.1056/NEJMp1011024 PMID: 21142528. [DOI] [PubMed] [Google Scholar]
- 35. Price CP, John AS, Christenson R, et al. Leveraging the real value of laboratory medicine with the value proposition. Clin Chim Acta. 2016;462:183–186. doi:10.1016/j.cca.2016.09.006 pii: S0009-8981(16)30379-5 PMID: 27649855 [DOI] [PubMed] [Google Scholar]
- 36. Rohr UP, Binder C, Dieterle T, et al. The value of in vitro diagnostic testing in medical practice: a status report. PLoS One. 2016;11:e0149856 doi:10.1371/journal.pone.0149856. Erratum in: PLoS One 2016;11(4): e0154008 PMID: 26942417, PMCID: PMC4778800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. St John A, Edwards G, Fisher S, Badrick T, Callahan J, Crothers J. A call for a value based approach to laboratory medicine funding. Clin Biochem. 2015;48:823–826. doi:10.1016/j.clinbiochem.2015.07.024. PMID: 26210846. [DOI] [PubMed] [Google Scholar]
- 38. Hallworth MJ, Epner PL, Ebert C, et al. ; IFCC Task Force on the Impact of Laboratory Medicine on Clinical Management and Outcomes. Current evidence and future perspectives on the effective practice of patient-centered laboratory medicine. Clin Chem. 2015;61:589–599. Review doi:10.1373/clinchem.2014.232629 PMID: 25646214. [DOI] [PubMed] [Google Scholar]
- 39. Price CP, St John A. Anatomy of a value proposition for laboratory medicine. Clin Chim Acta. 2014;436:104–111. Review doi:10.1016/j.cca.2014.05.017. PMID: 24880041. [DOI] [PubMed] [Google Scholar]
- 40. Price CP, St John A. Innovation in healthcare. The challenge for laboratory medicine. Clin Chim Acta. 2014;427:71–78. Review doi:10.1016/j.cca.2013.09.043. PMID: 24113488. [DOI] [PubMed] [Google Scholar]
- 41. Lee VS, Kawamoto K, Hess R, et al. Implementation of a value-driven outcomes program to identify high variability in clinical costs and outcomes and association with reduced cost and improved quality. JAMA. 2016;316:1061–1072. doi:10.1001/jama.2016.12226. PMID: 27623461. [DOI] [PubMed] [Google Scholar]
- 42. Fang C, Otero HJ, Greenberg D, Neumann PJ. Cost-utility analyses of diagnostic laboratory tests: a systematic review. Value Health. 2011;14:1010–1018. doi:10.1016/j.jval.2011.05.044. Review. PMID: 22152169. [DOI] [PubMed] [Google Scholar]
- 43. Melton LD, Bradley K, Fu PL, Armata R, Parr JB. Reference-based pricing: an evidence-based solution for lab services shopping. Am J Manag Care. 2014;20:1033–1040. PMID: 25526391. [PubMed] [Google Scholar]
- 44. Dickerson JA, Cole B, Conta JH, et al. Improving the value of costly genetic reference laboratory testing with active utilization management. Arch Pathol Lab Med. 2014;138:110–113. doi:10.5858/arpa.2012-0726-OA. PMID: 24377818. [DOI] [PubMed] [Google Scholar]
- 45. Lee DW, Neumann PJ, Rizzo JA. Understanding the medical and nonmedical value of diagnostic testing. Value Health. 2010;13:310–314. doi:10.1111/j.1524-4733.2009.00597.x. Review. PMID: 19744295. [DOI] [PubMed] [Google Scholar]
- 46. Donofrio JJ, Horeczko T, Kaji A, Santillanes G, Claudius I. Most routine laboratory testing of pediatric psychiatric patients in the emergency department is not medically necessary. Health Aff (Millwood). 2015;34:812–818. doi:10.1377/hlthaff.2014.1309. PMID: 25941283. [DOI] [PubMed] [Google Scholar]
- 47. Zhi M, Ding EL, Theisen-Toupal J, Whelan J, Arnaout R. The landscape of inappropriate laboratory testing: a 15-year meta-analysis. PLoS One. 2013;8:e78962 . Review doi:10.1371/journal.pone.0078962. PMID: 24260139, PMCID: PMC3829815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Epner PL, Gans JE, Graber ML. When diagnostic testing leads to harm: a new outcomes-based approach for laboratory medicine. BMJ Qual Saf. 2013;22:ii6–ii10. Review. No abstract available doi:10.1136/bmjqs-2012-001621. PMID: 23955467, PMCID: PMC3786651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Minerowicz C, Abel N, Hunter K, Behling KC, Cerceo E, Bierl C. Impact of weekly feedback on test ordering patterns. Am J Manag Care. 2015;21:763–768. PMID: 26633250. [PubMed] [Google Scholar]
- 50. Tu K, Mitiku T, Lee DS, Guo H, Tu JV. Validation of physician billing and hospitalization data to identify patients with ischemic heart disease using data from the Electronic Medical Record Administrative data Linked Database (EMRALD). Can J Cardiol. 2010;26:e225–e228. PMID: 20847968, PMCID: PMC2950731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bates DW, Saria S, Ohno-Machado L, Shah A, Escobar G. Big data in health care: using analytics to identify and manage high-risk and high-cost patients. Health Aff (Millwood). 2014;33:1123–1131. doi:10.1377/hlthaff.2014.0041 PMID: 25006137. [DOI] [PubMed] [Google Scholar]
- 52. Steinberg GB, Church BW, McCall CJ, Scott AB, Kalis BP. Novel predictive models for metabolic syndrome risk: a “big data” analytic approach. Am J Manag Care. 2014;20:e221–e228. PMID: 25180505. [PubMed] [Google Scholar]
- 53. Tu K, Mitiku TF, Ivers NM, et al. Evaluation of Electronic Medical Record Administrative Data Linked Database (EMRALD). Am J Manag Care. 2014;20:e15–e21. PMID: 24669409. [PubMed] [Google Scholar]
- 54. Georgiou A, Williamson M, Westbrook JI, Ray S. The impact of computerised physician order entry systems on pathology services: a systematic review. Int J Med Inform. 2007;76:514–529. Review. PMID: 16567121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.