Abstract
Biobanks have become an important component of the routine practice of pathology. At the 2016 meeting of the Association of Pathology Chairs, a series of presentations covered several important aspects of biobanking. An often overlooked aspect of biobanking is the fiscal considerations. A biobank budget must address the costs of consenting, procuring, processing, and preserving high-quality biospecimens. Multiple revenue streams will frequently be necessary to create a sustainable biobank; partnering with other key stakeholders has been shown to be successful at academic institutions which may serve as a model. Biobanking needs to be a deeply science-driven and innovating process so that specimens help transform patient-centered clinical and basic research (ie, fulfill the promise of precision medicine). Pathology’s role must be at the center of the biobanking process. This ensures that optimal research samples are collected while guaranteeing that clinical diagnostics are never impaired. Biobanks will continue to grow as important components in the mission of pathology, especially in the era of precision medicine.
Keywords: biorepository, informed consent, precision medicine, The Cancer Genome Atlas, the Cancer Human Biobank
Biobanking Budget Considerations
The value that a high-quality biobank adds to an academic medical center cannot be underestimated. Consistent, high-quality biospecimens coupled with immediate specimen availability will accelerate clinical trials. Progress made on the discovery for potential biomarkers involved in disease advances with a trained, expert team dedicated to obtaining, maintaining, and distributing high-quality specimens.1 More money from grants can be used to fund the science of research, rather than individual investigators attempting to consent, collect, process, and store the required specimens. However, the creation of a high-quality biobank takes a significant amount of work, capital funding, and infrastructure.2 The multiple concepts to consider when deciding to operate a biorepository are listed in Table 1.
Table 1.
Biobank Considerations.*
| What specimens will be banked (only tissue destined for discard or blood, saliva, etc)? |
| Will the biobank offer additional services (ie, histology)? |
| What services should be offered? |
| How will the data be managed? |
| Who are the institutional stakeholders? |
| What are the ethical implications for the patients involved? |
*There are many questions that should be considered prior to starting a biobank. The list above is far from comprehensive.
One of the first issues to address is whether the biobank will be financially sustainable. A business plan can be used to determine the feasibility of the proposed biobank with an examination of the available resources, personnel, space, equipment, infrastructure, and a scope of work.2,3
Collecting biospecimens from hospital-based patients has historically been the purview of pathology departments and independent research scientists. In recent years, there has been documented concern about both the quality of collected biospecimens and the lack of diversity among the source patients.4,5 The Biorepositories and Biospecimen Research Branch (BBRB) at the National Cancer Institute (NCI) has issued best practice guidelines that provide excellent recommendations and templates for the collection, storage, and distribution of high-quality biospecimens.6 Additionally, the BBRB web site has a Biobank Economic Modeling Tool available to help scientists and institutions develop financial planning and an understanding of the numerous resource costs. Table 2 provides a list of abbreviations that are frequently used in the biobanking literature. Furthermore, the best practices document recommends the development of a business plan that provides justification for institutional commitment and quantification of start-up and sustainability costs, including a formal continuity plan and emergency response (disaster) planning.6 Finally, as a functioning core or center: quality, safety, service, customer satisfaction, and a sustainable revenue cycle should all be part of a biobank’s mission.
Table 2.
List of Abbreviations Commonly Used in Biobanking.
| Biorepositories and Biospecimen Research Branch (BBRB) |
| Cancer Human Biobank (caHUB) |
| Congressionally Directed Medical Research Program (CDMRP) |
| Cooperative Human Tissue Network (CHTN) |
| Clinical Proteomic Tumor Analysis Consortium (CPTAC) |
| Data Use Agreement (DUA) |
| Formalin fixation and paraffin embedding (FFPE) |
| Good laboratory practices (GLPs) |
| Informed consent forms (ICFs) |
| Institutional review board (IRB) |
| Lung Cancer Biospecimen Resource Network (LCBRN) |
| Material Transfer Agreement (MTA) |
| National Cancer Institute (NCI) |
| Quality control (QC) |
| Quality improvement (QI) |
| Quality management systems (QMS) |
| Standard operating procedures (SOPs) |
| Statement of Work (SOW) |
| The Cancer Genome Atlas (TCGA) |
It is well documented that these activities, critical to a functioning biobank, add a significant cost-burden to participants in the biobanking process. Collection of high-quality biospecimens usually requires extramural and intramural funding, both formal and creative, to fully allow a private organization or academic department to recover costs.7,8 This section describes the biobanking business experience of a midsized pathology department in an academic medical center (Boston University School of Medicine and Boston Medical Center) that serves an underrepresented patient population at a private, nonprofit, safety net hospital.
Although the fundamental costs of establishing a successful biobank have been extensively described,9 it is important to emphasize that the scope of the business plan should include the start-up phase, the operational phase, and the plans for cost recovery in addition to legacy planning. Considerable administrative effort must be invested in preparation of a business plan or Statement (scope) of Work. Additionally, the costs of hiring and training staff, identifying and engaging participating faculty (surgeons, nurses, pathologists, and information technology [IT] personnel), purchasing equipment and supplies, developing IT infrastructure to maintain data, and identifying appropriate space should all be considered. Operational start-up costs include but are not limited to preparing and submitting an institutional review board protocol(s) with informed consent forms, possibly in more than one language; writing Material Transfer Agreement and Data Use Agreement documents; and building appropriate space with equipment and personnel space. Running a high-quality biobank requires maintaining and monitoring proficiency, similar to other functions in the laboratory. One method for monitoring proficiency is to develop a quality management system (QMS), if one is not already in place. A well-developed QMS includes standard operating procedures (SOPs), document control processes, emergency response and recovery plans, security of physical and virtual data, staff competencies, equipment maintenance and supply purchase records, and good laboratory practice (GLP) guidelines. A corrective action/preventative action system should be an integral part of the QMS, as well as setting up a system of internal and external audits to ensure the overall quality of the biobank. Accreditation in the College of American Pathologists (CAP) Biorepository Program10 is highly suggested but adds another expense. Developing or buying the appropriate software to manage all data is an additional but necessary step in setting up a high-quality biobank. Elements of the IT system should allow for storage management, specimen annotation, and data security. Appropriate management of staff, including safety training, compliance and competency training, and maintenance of staff certifications would be required. The largest portion of the initial start-up will be in capital planning, followed by salaries and fringe benefits.2,4,7,11,12
In the operational phase of a biorepository, the single largest cost will be salary for personnel. Hiring well-qualified staff who can perform more than one task is highly beneficial and a good investment. Biospecimen collection activities can be divided into the preanalytic, analytic, and postanalytic phases traditionally used in laboratory medicine. In the preanalytic phase, patients are screened and entered into the study. Significant time may be required to identify appropriate patients who have consented to donate biospecimens. Annotation of clinical data could occur in the preanalytical stage or in the postanalytical stage. During the analytic phase, biospecimens such as blood or urine may be collected before and after surgery, which could be used to extract either nucleic acid (DNA or RNA) or relevant biomarkers. Tissues need to be collected after surgery with optimized ischemic times, so working closely with the surgical staff and nursing is essential. Samples should be dissected by a board-certified pathologist or a pathologists’ assistant and selected biobank samples ultimately preserved by freezing or formalin fixation and paraffin embedding (FFPE). The samples may also be processed for cell or tissue culture depending on the scientific requirements. Quality control (QC) examination of tissues is essential to ensure that tumor and/or normal adjacent tissue has been properly sampled by having a board-certified pathologist review the slides. Postanalytic responsibilities include sample storage either in freezers or in the vapor phase of liquid nitrogen (LN2), distribution and shipping of samples, as well as maintenance of databases with annotated demographic and clinical history. Another critical aspect may be the need to change the annotation of the sample in the event that the original diagnosis changes. A biobank may also wish to consider accepting data from investigators back into the biobank to expand the utility of the biospecimen. Significant resources should be budgeted to ensure data security to make sure adherence to the Health Insurance Portability and Accountability Act of 1996 for protection of human patients. One of the most important roles of the biobank is to act as an honest broker by being the link between clinical and research activities. Expanding the role of the IT software to allow internal clinical systems to speak with the biobank’s database and allow for automatic deidentification in fulfillment of the honest broker’s role is an important aspect to consider. Continual management of the data through this IT system will not be static and will need frequent updates to stay current with the clinical systems as well as to update data definitions as those evolve.13,14 Ongoing administrative duties might also include regular report preparation, presentations on project progress, managing site visits, and accreditation inspections. Time and money should be spent on internal audits to assure the quality of the specimens as well as to assess the overall usage of the biobank.3 One of the key aspects to consider in setting up a biobank is that the goal should not be how many specimens can be collected, but what are the needs of the institution so that there is a high turnover of samples.3 Additional requirements when collecting data include considering the ethical and legal processes of sample collection. In collaboration with the University of New Mexico, University of Pittsburgh, Emory University, and the NCI biospecimen preanalytic variables (BPV) study, the Boston Medical Center developed an ethical, legal, and social implications questionnaire that was subsequently implemented.4,15 This allowed biospecimen donors to have a voice in the process of biospecimen collection by completing the questionnaire.
In terms of assessing expense, the time required for a single patient to go through the process (informed consent, donation, and responding to a follow-up questionnaire) required about 3 weeks from consent to final assessment. The final assessment was used to determine whether the specimen met the required collection needs. Securing funding for technicians, biobank managers, quality managers, quality directors, pathologists, IT personnel, and of course the principal investigator is essential in this portion of the biobank’s life cycle. As with other aspects in pathology, a QMS and formal quality improvement (QI) plan should serve as the foundation for a successful program. Strategic budget planning for replacing major equipment must be in place to avoid just-in-time emergency requests. A 7- to 10-year depreciation plan should be considered with a view to replacing large equipment such as freezers and fridges, LN2 storage tanks, temperature monitoring and alarm systems, centrifuges, and key histology equipment. Contingency emergency response planning for major equipment failure must also be considered; a written plan for rapid relocation of biospecimens should a freezer fail is essential to avoid confusion and loss of valuable specimens. Ideally, a backup LN2 storage tank and −80ºC freezer that has capacity should be maintained near to the core biobank.
Cost recovery should be the final part of the business plan to ensure the biobank will be a sustainable resource for the institution. Initial funds will typically come from donors and substantial institutional support. This will generally sustain the biobank through the first 3 years, which has been shown to be the typical start-up phase.2,12 After this phase, other means of support will need to be found. Grant funding is one source, but grants are not always a consistently reliable revenue stream for core facilities.12 Contracts with governmental agencies or other tissue repositories tend to provide a more reliable source of funds, but adherence to procurement protocols will necessitate more administrative initiatives to assure compliance.16 Finally, a fee-for-service schedule can be generated; however, extensive thought should be given to determine: (1) What will internal researchers be willing to pay? (2) Will there be a different pricing schedule for an internal versus external customer? (3) How will the different services be priced? (4) Can training and education be another service offered? These are among some of the questions that would need answers.16 Most likely, a combination of the 3 types of funding will be needed to sustain a successful, high-quality biobank, but with an initial plan in place and yearly review of the plan, a sustainable biobank should be attainable.
Identifying other revenue streams to support the biobanking mission including developing a histopathology core, tissue microarray, nucleic acid preparation, or immunohistochemistry services offers funding opportunities. Digital scanning of slides may also be offered on a fee-for-service basis. The Moffitt Cancer Center, Tampa, Florida offers an excellent model of Total Cancer Care that has greatly benefitted patients, research partnerships, and the biobanking core. Partnerships with the pharmaceutical industry and industry-sponsored clinical trials support the mission of the Moffitt Biobank and leverages biomarker assay development for improved care. Collaborations with epidemiologic or public health studies can also lead to nontraditional funding streams. Finally, major philanthropic gifts can be solicited with institutional support. Focusing on the connectivity between collecting high-quality biospecimens and translational research science often captures the attention of donors, particularly if they or a family member have had a particular disease.
As mentioned previously, legacy planning is a key strategic concept to bear in mind should extramural funding decline. Establishing an approved core service at an academic institution may provide bridge funding as support. Importantly, senior leaders at the organization should be educated by biobanking leadership as to the costs and resources required to run the service well in advance of any request for funding. Intramural funding may also be available in small grants. Demonstrating and documenting a track record of excellent service that supports other investigators will improve the likelihood of institutional support. If the biobank is department based, appropriate budgeting can keep a biobank stable at low cost. Commercial entities and “tissue brokers” as well as industry clinical trials can provide steady revenue though an increase in administrative burden should be anticipated.
Budget Considerations in Consortium Settings
As already discussed, biobanking is an important, but expensive, research infrastructure at academic medical centers. Covering the expense of biobanking and allied processing/analytic services usually is multifaceted. While charging fees to investigators is fairly common, realistic fee structures that investigators are willing or able to pay are seldom sufficient to cover the costs of required biorepository personnel and equipment. Thus, institutional subsidies are generally required to cover costs of biorepository functions not covered by user fees. Some of these costs may be covered by funding as “core facilities” for extramurally funded research projects, typically either as part of large research center grants or as part of multi-investigator consortia groups. Indeed, having documented biorepository facilities are often prerequisites for such large grants, hence it is in an institution’s best interests to provide sufficient funding to ensure the presence of robust biobanking infrastructure.
Another strategy to leverage existing biorepository infrastructure, and to utilize extra capacity that may be present in biospecimen resources, is to pursue funding targeted for biospecimen-specific activities. Access to high-quality human biospecimens is recognized as a national research need that is often not met by local resources. To address this issue, national research funding agencies have occasionally created programs that are specifically designed to lower the barriers to obtain human biospecimens for the general research community or have searched for partners to provide biospecimens for specific research programs. Some examples of this are The Cancer Genome Atlas (TCGA),17 Biospecimen Preanalytic Variables (BPV) program,18 and the Office of Clinical Proteomic Tumor Analysis Consortium.19
The experience at the University of Virginia (UVA) School of Medicine serves as an example of how to work in a consortium. Biorepository functions reside in a core facility named the Biorepository and Tissue Research Facility (BTRF). The BTRF and allied biospecimen programs employ 10 full-time employees (2 faculty-level managers and 8 technicians) with an annual budget of approximately 1 million dollars. The BTRF covers approximately 24% of its budget from local user fees, receives 18% of its budget for its support of Cancer Center activities and receives a subsidy from the School of Medicine of approximately 13% of its budget. The remaining 45% of its budget consists of support obtained from its extramural activities.
Two major grants make up the bulk of the BTRF extramural activity. The first is a competitive cooperative agreement (UM1) grant from the NCI to serve as the Mid-Atlantic division of the Cooperative Human Tissue Network (CHTN). The CHTN consists of 6 divisions that work together to fulfill research requests for human tissues and biofluids and acts primarily as a prospective procurement service. Although the CHTN maintains uniform SOPs that govern specimen collection and QC, unique procurement services are being utilized at each site, so that the labeling, packaging, and associated services will vary to a degree. In addition to biospecimen procurement, the BTRF is the major manufacturer of tissue microarrays for this consortium.
The second major extramural grant that the BTRF supports is a competitive award from the Congressionally Directed Medical Research Program of the Department of Defense to host the Lung Cancer Biospecimen Resource Network (LCBRN).20 The LCBRN is a consortium of 3 institutions that recruit lung cancer patients undergoing cancer resections to donate tissue, bronchial lavage fluid, blood, saliva, and urine samples. Biofluids are collected preoperatively and at intervals postoperatively. Patients are followed continually to obtain clinical follow-up data as well as serial biofluid samples. The UVA serves as the coordinating center of the LCBRN and provides collection kits to all collection sites, so all samples are collected under uniform SOPs and are uniformly labeled and packaged. All biospecimens are sent to UVA for central histology, QC, and storage. Investigators interact with the coordinating center at UVA to submit applications to receive biospecimens and annotated clinical data.
Although such extramural funding is not specifically designed to support local biorepository efforts, the local efforts are bolstered by the ability to cost share personnel, informatics infrastructure, and equipment with such programs. An intangible benefit is the additional expertise that local biorepository personnel develop by interacting with an expanded number of scientific investigators and with biorepository personnel at institutions within such national networks. As with any academic endeavor, the ability to compare and discuss different approaches to procurement techniques, quality measures, and regulatory requirements leads to the exchange of ideas that can improve local practices and stimulate creative problem solving.
Pathology-Centered Biobank
There are several advantages to establishing a biobank managed by pathologists. First, all specimens are in a single location, allowing investigators a single source of biospecimens. Second, cost savings may occur through centralization. Finally, and perhaps most importantly, a pathology-centered biobank ensures that the tissue necessary for diagnostic work has the highest priority so that no patient is harmed. Such a biobank was established at Duke University in 2012. The Biospecimen Repository and Processing Core (BRPC) and its broad consent protocol were established in the Department of Pathology at Duke University with investment from the Duke Cancer Institute and the School of Medicine. Under the universal consent protocol at Duke, thousands of patients have now given permission for the storage and future use of their excess clinical specimens annotated with their clinical information. Most of these participants also opted to donate an additional blood sample.
The concept of “governance” in biobanking not only describes the responsible custodianship of the biospecimen collection within the physical biorepository but also includes oversight of specimen utilization and contingency planning for collection maintenance if funding loss requires infrastructure decommissioning. The novel disease-based group (DBG) paradigm for governance has been vital to BRPC’s success by increasing investigator buy-in and engagement. In the DBG model, governance is shared between the direction and oversight responsibilities of the DBG and assigned physical custodianship of the BRPC. A multidisciplinary subcommittee within each DBG is responsible for approving the distribution of limited samples (eg, frozen tissues). In addition, it is clear that disease-specific biospecimen collections (having been created through shared and central investment) do not leave the institution if a single prominent investigator departs. In the event of defunding or decommission of the biorepository, the disease-specific collection would be transferred to the custody of DBG leadership.
In partnership with BRPC, the DBGs donate the effort of their clinical research staff to recruit and enroll patients onto the BRPC’s broad consent protocol. These research nurses and coordinators become key personnel on the protocol and often coconsent in combination with appropriate clinical trial(s). Since 2012, there have been over 90 clinical research staffs across multiple disease groups trained to administer broad consent. Combined with the efforts of 2 dedicated consenting staff members within BRPC, this model has allowed considerable accrual. Upcoming endeavors at Duke, including e-consent, are expected to further expand participation.
Because the principal investigator for the broad consent protocol is the pathologist-director of the BRPC, the BRPC takes responsibility for training all Duke clinical research staff who administer the broad consent. Consent training sessions include an hour of in-person didactic teaching as well as multiple observed consent events. Training includes references to biobanking publications geared toward laypersons such as the NCI’s brochure “How you can help medical research: donating your blood, tissue, and other samples.”21 Staff are taught that excess tissue procurement occurs only in partnership with the pathologist in the surgical pathology suite. This understanding helps consenting staff reassure patients that their medical care will not change if they elect to participate.
Some may question the value of procuring fresh and frozen tissue samples at a time when more and more molecular tests can be performed on archival formalin-fixed paraffin-embedded tissues.22-24 However at Duke, one of the most valuable BRPC activities is the distribution of fresh tissue aliquots to research laboratories whose work absolutely requires fresh tissue. This includes laboratories for immune cell profiling, cell culture, and patient-derived xenograft creation. In these cases, the resulting models and data are all united by a single BRPC ID, allowing them to be used together in research representative of a single patient’s disease.
Financial independence and sustainability of biorepositories is a lofty, and many say unattainable, goal.25,26 Indeed, the BRPC receives yearly subvention from both Duke Cancer Institute and Duke University School of Medicine. Additionally, the BRPC has developed mechanisms for improving cost recovery aimed at earlier engagement of disease groups in clinical trial preparation (to allow for proper budgeting) and improved remuneration for pathologist and administrative time when processing archival tissue requests. The BRPC also received some cost recovery by providing samples to TCGA.17 Currently, 48 federal and industry-sponsored clinical trials are receiving services through BRPC at different rates of cost recovery as required. As noted in section “Biobanking Budget Considerations”, true cost recovery is attainable in the support of industry-initiated clinical trials.
Finally, it should be mentioned that the BRPC received biorepository accreditation from the CAP in 2013 and maintains this high standard of safety, quality, and reproducibility in all operations. The biorepository accreditation program by the CAP continues to grow in popularity, with 47 US biorepositories now accredited.27 A large portion of the CAP requirements were recently incorporated into the NCI’s best practices for biorepositories.28 At Duke, CAP accreditation allows BRPC to work in partnership with Duke Clinical Laboratories and Anatomic Pathology for tissue procurement, discard blood procurement, and archival specimen retrieval. The leadership BRPC and Pathology have shown in education, quality, and investigator engagement have established the Department of Pathology as the “home of biobanking” at Duke.
Science-Driven Biobanking
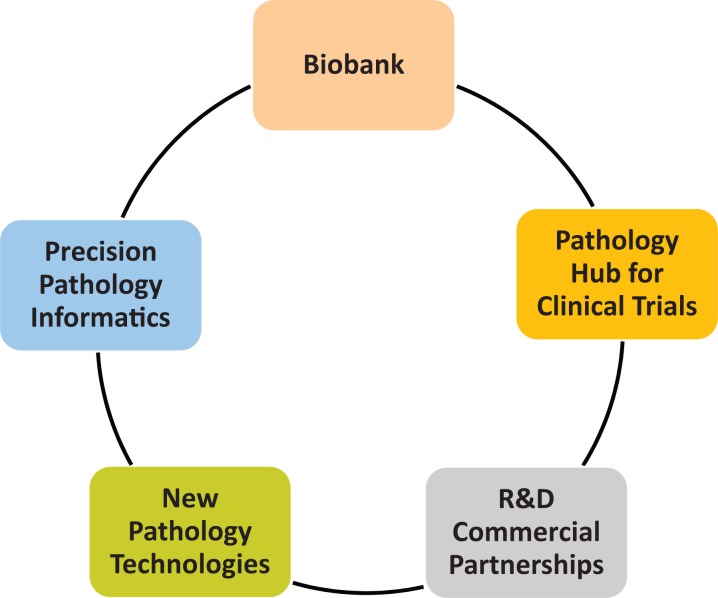
High-quality human samples for research are key for personalized precision health care of the future. The initiative has taken on increased importance with the President’s declaration of the Cancer Moon Shot29 in 2016, and several organizations are rising to the challenge. Some groups have already put in place the appropriate infrastructure to provide high quality biospecimens. In 2015, a major new initiative at Memorial Sloan Kettering Cancer Center was launched by creating the Precision Pathology Biobanking Center (PPBC), which is being built around 5 highly interconnected pillars (Figure 1).
Figure 1.
The 5 pillars of activity within the Precision Pathology Biobanking Center at Memorial Sloan Kettering Cancer Center.
When designing the PPBC’s specimen acquisition, preservation, storage, and distribution workflows, the concept of “future proofing” was front and center: all samples (tissues, bloods, and other liquid samples) are rapidly procured (time to freezing <15 minutes) and are uniformly held in vapor-phase LN2 rather than −80°C freezers. It has been convincingly shown that some of the most interesting components of the pathophysiome (RNA, posttranslational modifications of proteins, and small metabolites) degrade unpredictably even at −80°C, while they remain stable in vapor phase LN2 (−180°C).28 The PPBC banks biospecimens from approximately 7000 new patients with cancer per year, including surgical resections, interventional radiology biopsies, and companion blood and body fluid collections. Diseased and matched normal tissues are frozen in LN2 without further additives and spatially indexed FFPE blocks are prepared that match each sampling location of a corresponding frozen vial. The availability of carefully matched FFPE blocks has numerous advantages, including (1) morphologic quality assessment of each mirrored frozen tissue (without need for freeze-thaw cycling); (2) availability for research (independent of frozen samples) for sequencing, immunohistochemistry, Fluorescent In Situ Hybridization (FISH), or other assays; (3) dedicated “clinical trial blocks” and “tissue microarray blocks”; and (4) source of routine hematoxylin and eosin (H&E) slides that are whole-slide scanned prospectively as a digital representation of each sample in tissue biobank (“digital biorepository”). Highest quality for the research FFPE blocks is maintained by consistent annotation of warm and cold ischemia times, fixation chemistry and time, and block processing protocols. The FFPE processing can be separated or embedded in existing clinical [Clinical Laboratory Improvement Amendment (CLIA)-grade] workflows depending on volumes and institutional preference. We prefer to include research FFPE processing into the existing clinical stream because it makes the blocks a priori CLIA-compliant (advantageous for regulatory submission of research results, such as FDA), and it affords cost savings (no need for duplicate FFPE processing equipment line). Blood (obtained both pretreatment and posttreatment) is processed into frozen serum, plasma [double centrifuged for use as source of cell free DNA (cfDNA)], and buffy coat aliquots. More than 30 000 specimen units are created annually. There are more than 1600 units of frozen samples and 1000 units of FFPE material available for immediate research. Additionally, a rapidly growing portion of PPBC activities (about 1700 fresh samples) involves “living” biobanking (ie, the creation of organoids, mouse xenografts, primary cell lines, etc). The PPBC’s biobank has developed innovative QI metrics and processes, including RNA integrity monitoring in sentinel samples and participation in international proficiency testing programs (eg, International Society for Biological and Environmental Repositories Integrated BioBank of Luxembourg Proficiency Testing Program).30
Informatics may provide an important component of future biobanks since a physical repository of biospecimens is only as good as the level of annotation and knowledge that can be associated with each and every specimen in the bank. Pathology as a discipline will increasingly evolve into the medical specialty of dynamic data management and big data integration to drive patient care (“theranostics”), rather than the status quo of “just” providing static diagnoses. Translated to biobanking, this means that we need to create tools that cross-reference physical samples in real time to all other data that may be known about a patient (eg, clinical status, therapeutic status, imaging results, clinical trial participation, molecular features of the disease, etc). Building a longitudinal representation of every patient (from diagnosis through stages of treatment to follow up/recurrence, etc) in which all physical samples are mapped onto a common time line together with all other observational or interventional medical events creates this approach. For example, the question may be asked: how many frozen research samples (containing primary, metastatic, and/or normal) does the bank hold from patients born after 1960 with a diagnosis of KRAS-mutated colon cancer? These searches have become instrumental tools for feasibility arguments in grant submissions and hypothesis generation for numerous biomarker studies, as highlighted in the section on working with consortiums.
Delivery of health care is currently at the beginning of a wave of major new disruptive technologies that will become part of our diagnostic and theranostic tool set. With next generation sequencing reaching technological maturity in clinical laboratories, we have already seen new technologies (such as mass spectrometry-based deep proteomics, functional assessment of pathway activities, metabolomics, highly multiplexed immunofluorescence, ex vivo living models of drug response, etc.) that hold the promise of changing the way we will assess and monitor disease.31 For example, mass spectrometry is currently being assessed as a highly quantitative and multiplexed tool (assessing several thousand proteins in tissue in parallel) that can complement or even replace conventional immunohistochemistry (no antibody requirements, mutations, and posttranslational activation states such as phosphorylation are directly detectable, etc). Importantly, many of these new technologies require highest quality frozen biobanked or fresh living samples while conventional FFPE-based clinical archives are simply not usable or highly suboptimal.
Pathology has historically not been a driver discipline in clinical trials or clinical drug development, with its role often limited to providing slide review for patient enrollment or sending FFPE material to third-party trial sponsors. In the era of what may be called “specimen-centered, molecularly driven” clinical trials (eg, basket trials like NCI Molecular Analysis for Therapy Choice32), our discipline is becoming an active player in clinical trials and drug development. The PPBC trials division provides a dedicated platform for pathology’s representation already at the earliest stages of new trials (including design, protocol writing, budgeting, direct discussions with sponsoring pharma, specimen acquisition, companion diagnostic development, etc). For example, at Memorial Sloan Kettering Cancer Center, a dedicated phase 1 biobank for patients on first-in-man clinical trials has been created that provides a priceless resource for research.
The combination of comprehensive biobanking and new technologies provides a natural and externally visible infrastructure that now allows pathologists to engage directly with the biotechnology and pharma sector to carry out joint R&D projects, such as development of new companion diagnostics, evaluation of biomarkers, or the use of new instrumentation. Such projects frequently also hold the opportunity for intellectual property generation. In addition, funding raised through research and commercialization can represent a major contribution to the long-term sustainability of research biobanking.
Conclusion
Biobanking offers opportunities for pathologists to actively engage in multiple new research and diagnostic initiatives. A strong case can be made that biobanking should be housed within pathology to ensure optimal specimen procurement without compromising the patient’s diagnostic material. There are several important fiscal considerations that must be considered in creating a sustainable biobank that serves the needs of our patients and fellow investigators.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by NIH grants AA022122 (DGR), AI112887 (DGR), NCI contracts BPV HHSN261200800001E (CDA), and CPTAC HHSN261201600011I (CDA).
References
- 1. Rogers J, Carolin T, Vaught J, Compton C. Biobankonomics: a taxonomy for evaluating the economic benefits of standardized centralized human biobanking for translational research. J Natl Cancer Inst Monogr. 2011;2011:32–38. [DOI] [PubMed] [Google Scholar]
- 2. Vaught J, Rogers J, Carolin T, Compton C. Biobankonomics: developing a sustainable business model approach for the formation of a human tissue biobank. J Natl Cancer Inst Monogr. 2011;2011:24–31. [DOI] [PubMed] [Google Scholar]
- 3. Matharoo-Ball B, Thomson BJ. Nottingham Health Science Biobank: a sustainable bioresource. Biopreserv Biobank. 2014;12:312–316. [DOI] [PubMed] [Google Scholar]
- 4. Vaught J, Rogers J, Myers K, et al. An NCI perspective on creating sustainable biospecimen resources. J Natl Cancer Inst Monogr. 2011;2011:1–7. [DOI] [PubMed] [Google Scholar]
- 5. Robb JA, Gulley ML, Fitzgibbons PL, et al. A call to standardize preanalytic data elements for biospecimens. Arch Pathol Lab Med. 2014;138:526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Branch Biorepositories and Biospecimen Research. NCI Best Practices for Biospecimen Resources. 2016. https://biospecimens.cancer.gov/bestpractices/2016-NCIBestPractices.pdf. Accessed November 30, 2016.
- 7. Albert M, Bartlett J, Johnston RN, Schacter B, Watson P. Biobank bootstrapping: is biobank sustainability possible through cost recovery? Biopreserv Biobank. 2014;12:374–380. [DOI] [PubMed] [Google Scholar]
- 8. Clément B, Yuille M, Zaltoukal K, et al. ; EU-US Expert Group on cost recovery in biobanks. Public biobanks: calculation and recovery of costs. Sci Transl Med. 2014;6:261fs45. [DOI] [PubMed] [Google Scholar]
- 9. Hansell D, Jewell SD. Developing and Organizing an Institutional Biospecimen Repository. Biorepository Accreditation Program. Chicago, IL: College of American Pathologists; 2014. [Google Scholar]
- 10. Pathologists College of American. Biorepository Accreditation Program. http://www.cap.org/web/home/lab/accreditation/biorepository-accreditation-program. Accessed November 22, 2016.
- 11. Warth R, Perren A. Construction of a business model to assure financial sustainability of biobanks. Biopreserv Biobank. 2014;12:389–394. [DOI] [PubMed] [Google Scholar]
- 12. Wilson GD, D’Angelo K, Pruetz BL, Geddes TJ, Larson DM, Akervall J. The challenge of sustaining a hospital-based biobank and core molecular laboratory: the Beaumont experience. Biopreserv Biobank. 2014;12:306–311. [DOI] [PubMed] [Google Scholar]
- 13. Boyd AD, Hosner C, Hunscher DA, Athey BD, Clauw DJ, Green LA. An ‘Honest Broker’ mechanism to maintain privacy for patient care and academic medical research. Int J Med Inform. 2007;76:407–411. [DOI] [PubMed] [Google Scholar]
- 14. Dhir R, Patel AA, Winters S, et al. A multidisciplinary approach to honest broker services for tissue banks and clinical data: a pragmatic and practical model. Cancer. 2008;113:1705–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bromley RL. Financial stability in biobanking: unique challenges for disease-focused foundations and patient advocacy organizations. Biopreserv Biobank. 2014;12:294–299. [DOI] [PubMed] [Google Scholar]
- 16. Barnes RO, Schacter B, Kodeeswaran S, Watson PH; CTRNet Management Committee. Funding sources for Canadian biorepositories: the role of user fees and strategies to help fill the gap. Biopreserv Biobank. 2014;12:300–305. [DOI] [PubMed] [Google Scholar]
- 17. National Cancer Institute. The Cancer Genome Atlas. https://cancergenome.nih.gov/. Accessed November 30, 2016.
- 18. National Cancer Institute. Cancer Human Biobank (caHUB). https://biospecimens.cancer.gov/programs/cahub/. Accessed November 22, 2016.
- 19. National Cancer Institute. Clinical Proteomic Tumor Analysis Consortium CPTAC. https://proteomics.cancer.gov/programs/cptacnetwork. Accessed November 30, 2016.
- 20. Network Lung Cancer Biospecimen Resource. Lung Cancer Biospecimen Resource Network. http://lungbio.sites.virginia.edu/. Accessed November 22, 2016.
- 21. National Cancer Institute. How you can help medical research. https://www.cancer.gov/publications/patient-education/help-research-donate-tissue. Accessed November 22, 2016.
- 22. Hester SD, Bhat V, Chorley BN, et al. Dose-response analysis of RNA-Seq profiles in archival formalin-fixed paraffin-embedded samples. Toxicol Sci. 2016;154:202–213. [DOI] [PubMed] [Google Scholar]
- 23. Kakimoto Y, Tanaka M, Kamiguchi H, Ochiai E, Osawa M. MicroRNA stability in FFPE tissue samples: dependence on GC content. PLoS One. 2016;11:e0163125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ly A, Buck A, Balluff B, et al. High-mass-resolution MALDI mass spectrometry imaging of metabolites from formalin-fixed paraffin-embedded tissue. Nat Protoc. 2016;11:1428–1443. [DOI] [PubMed] [Google Scholar]
- 25. Campos AH, Schreeder M, Parry-Jones A, et al. Addressing the challenge of financial sustainability in biobanking. Biopreserv Biobank. 2015;13:387–395. [DOI] [PubMed] [Google Scholar]
- 26. Seiler CY, Eschbacher J, Bowser R, LaBaer J. Sustainability in a hospital-based biobank and university-based DNA biorepository: strategic roadmaps. Biopreserv Biobank. 2015;13:401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. College of American Pathologists (CAP). Accredited Laboratory Directory. 2016. http://www.cap.org/web/home/lab/accreditation/accredited-laboratory-biorepository-directory. Accessed November 22, 2016.
- 28. National Cancer Institute. NCI Best Practices for Biospecimen Resources. https://biospecimens.cancer.gov/bestpractices/2016-NCIBestPractices.pdf. Accessed November 22, 2016.
- 29. Lowy D, Singer D, DePinho R, Simon GC, Soon-Shiong P. Cancer moonshot countdown. Nat Biotechnol. 2016;34:596–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. ISBER. ISBER Biospecimen Proficiency Testing. http://www.isber.org/?page=PTGI. Accessed November 30, 2016.
- 31. Huang L, Holtzinger A, Jagan I, et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat Med. 2015;21:1364–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. National Cancer Institute. NCI-Molecular Analysis for Therapy Choices (NCI_MATCH) Trial. https://www.cancer.gov/about-cancer/treatment/clinical-trials/nci-supported/nci-match. Accessed November 23, 2016.