Abstract
From 2009 to 2015, the laboratories of the 19-hospital North Shore-LIJ Health System experienced 5 threatened interruptions in service and supported 2 regional health-care providers with threatened interruptions in their laboratory service. We report our strategies to maintain laboratory performance during these events, drawing upon the strengths of our integrated laboratory service line. Established in 2009, the laboratory service line has unified medical and administrative leadership and system-wide divisional structure, quality management, and standardization of operations and procedures. Among many benefits, this governance structure enabled the laboratories to respond to a series of unexpected events. Specifically, at our various service sites, the laboratories dealt with pandemic (2009), 2 floods (2010, 2012), 2 fires (2010, 2015), and laboratory floor subsidence (2013). We were also asked to provide support for a regional physician network facing abrupt loss of testing services from closure of another regional clinical laboratory (2010) and to intervene for a non-health system hospital threatened with closure owing to noncompliance of laboratory operations (2012). In all but a single instance, patient care was served without interruption in service. In the last instance, fire interrupted laboratory services for 30 minutes. We conclude that in a large integrated health system, threats to continuous laboratory operations are not infrequent when measured on an annual basis. While most threats are from external physical circumstances, some emanate from unexpected administrative events. A strong laboratory governance mechanism that includes unified medical and administrative leadership across the entirety of the laboratory service line enables successful responses to these threats.
Keywords: laboratory service line, integration, fire, flood, disaster, pandemic
Introduction
Laboratory services are an integral component of a health-care system and are necessary for maintaining successful licensure of hospital organizations. Interruptions in laboratory services are not compatible with ongoing hospital operations and would have immediate negative impact on the high-acuity clinical services that are definitional for an acute care hospital. The vast majority of laboratory management effort is directed toward providing timely, accurate, and reliable laboratory testing for every patient, in full compliance with all regulatory requirements for laboratory operations.1,2 However, intercurrent events may impose potentially catastrophic conditions on a given laboratory practice site. Laboratory leadership and management must be prepared for responding to threatened interruptions in service. Failure to mount a successful response may result in diversion of services from a hospital site or, in the worst circumstance, hospital closure.
The Northwell Health system is the largest integrated health system in New York State, and at the current time is the 14th largest health-care system in the United States (note 1).3 Its service area encompasses more than 7 million people in the greater New York metropolitan area. The Northwell Health Laboratories are a service line encompassing 11 hospital-based clinical laboratories in the health system, a central in-system reference laboratory, and the Department of Pathology and Laboratory Medicine of the Northwell Health Physician Partners and Hofstra Northwell School of Medicine.4
In this report, we describe the foundational architecture of the Northwell Health Laboratory Service Line, and how this architecture supported our successful responses to seemingly annual threats to service interruption at one of our service sites. The multiplicity of events enables us both to affirm strengths of the service line structure and to identify points of weakness needing correction or enhancement.
Northwell Health Laboratory Service Line
Environment
Northwell Health is one of the nation’s largest nonprofit, secular health systems, with 19 hospitals representing more than 6400 hospital and long-term care beds, 3 skilled nursing facilities, over 450 ambulatory practice sites, and over 60 000 employees. The system serves a market area that includes the 5 boroughs of New York City, Westchester County, and Nassau and Suffolk Counties in Long Island. In 2014, there were 147 731 ambulatory surgeries, 276 495 hospital discharges, and over 4 million unique patient contacts. The Northwell Health Laboratories, and the department of pathology and laboratory medicine that oversees those laboratories, are integrated across the length and breadth of the health system. Both through health system growth and growth in the laboratory “outreach” program, the annualized growth rate of system-wide laboratory test volumes has exceeded 20% per year for the past 7 years. Including hospital-based clinical laboratory budgets, the annual gross operations for the laboratory service line have grown from US$231 million in 2009 to US$391 million in 2015.
Governance
The laboratory service line was formally created in January 2009 in order to achieve the goals delineated in Table 1. The governance architecture is shown in Figure 1. First, laboratory services leadership are unified under 1 pathologist “system chair” who also serves as Senior Vice President and Executive Director of laboratory services for the health system. The system chair presides over a Senior Leadership Group of laboratory Medical Directors, which meets monthly and serves as a “committee of the whole” for oversight of health system laboratory services (see Supplemental Table 1). Second, the administrative management of all health system clinical laboratories is unified under a Vice President of Laboratory Services who presides over a System Managers group consisting of the senior managers of health system laboratories, meeting bimonthly (see Supplemental Table 2). The governance activities of these 2 groups are closely aligned, owing to pairing of each hospital-based laboratory site Medical Director and Administrative Director as a working team. Third, a smaller Executive Committee drawn from both the Senior Leadership and System Managers groups meets weekly and is responsible for daily oversight of system laboratory operations. Fourth, 6 service line divisions based on key American Board of Pathology-certified subspecialties (Hematopathology, Cytopathology, Pediatric Pathology, Blood Banking & Transfusion Medicine, Infectious Diseases Diagnostics, and Cytogenetics and Molecular Pathology) coordinate their respective services at all hospital sites across the health system. One additional division oversees Point-of-Care Testing, given that such testing constitutes approximately 10% of all testing events in the health system. Autopsy Services are a unified service, with clustering of autopsy performance at 3 of the 19 system hospitals.
Table 1.
Strategic Objectives, Northwell Health Laboratory Service Line.
| Coordination of patient care across the entire health system |
| Achieving best practices at multiple institutions and sites, at the lowest cost |
| Attaining depth-of-service that is unattainable by individual sites |
| Coordinated distribution of services to maximize efficiencies and capacity |
| Redundant internal backup capabilities, both in people and in technologies |
| Information management for better delivery of health care and better patient outcomes |
Figure 1.
Laboratory service line governance. Under the leadership of the system chair of the department of pathology and laboratory medicine (who is also the system Senior Vice President [SVP] and Executive Director [ED] of laboratory services) and the system vice president (VP) of laboratory services, the Senior Leadership Group and a tandem group of System Managers oversee all health system laboratory operations. This includes oversight of the 15 hospital-based clinical laboratories by the respective Medical Director and Administrative Director pairs; system integration of subspecialty service operations through 7 subspecialty divisions; and standardization of laboratory operations, procedures, and technologies through 16 joint standards committees. The Core Laboratory provides key support services for system laboratory operations. A system Laboratory Process Improvement Coordinating Group (PICG) monitors laboratory performance across all sites, intervening when appropriate to ensure consistent high-quality delivery of laboratory services. BB/TM indicates blood banking and transfusion medicine; LIS, laboratory information system; Path Assistants, pathologists’ assistants; Transfusion Med, transfusion medicine.
Joint Standards Committees
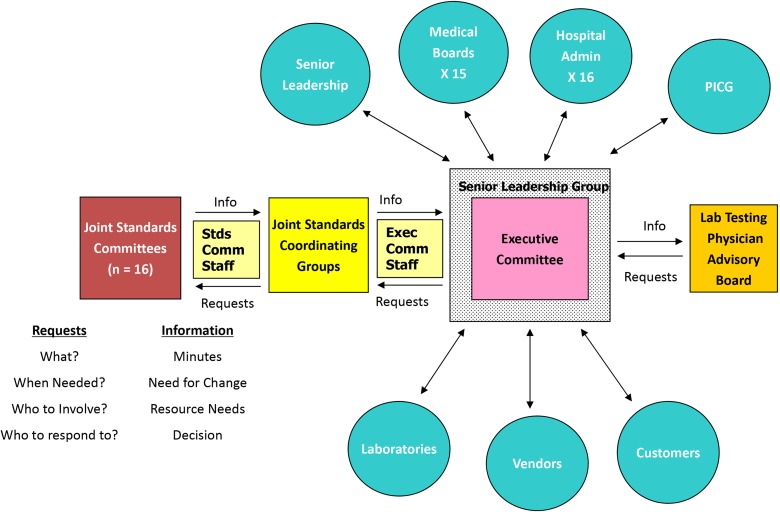
Sixteen “joint standards committees” (Figure 1) span the breadth of laboratory diagnostics and are composed of subject matter experts drawn from the medical and managerial ranks across all sites of the laboratory service line. The chairs of each committee are knit into “joint standards coordinating groups” (within broad laboratory areas such Anatomic Pathology, Laboratory Medicine, Operations and Logistics, and Information Technology), which report up to the Senior Leadership Group (Figure 2). Each joint standards committee drives their own agenda, both for standardization of technologies and operating procedures across all laboratory sites and for innovations and implementing new technologies. Incoming requests from the Senior Leadership Group for change management, including inquiries about new equipment and technologies, are referred to the respective committees for due diligence. In all instances, recommendations from the joint standards committees are under the oversight of the joint standards coordinating groups. Major actions of the joint standards committees are advanced to the Senior Leadership Group for review and approval through their function as a committee of the whole. This structure empowers the Medical Directors to be the “owners” of technologies and procedures deployed at their local site laboratories and empowers the Administrative Directors to manage the laboratory workforce using these technologies and procedures. These joint standards committees have assigned administrative staff for clusters of related committees and full-time administrative leadership positions for oversight of the entire joint standards system. Such staffing ensures that committee productivity is steady and sustained.
Figure 2.
Joint Standards Committees, Northwell Health Laboratory Service Line. System architecture for laboratory Joint Standards Committees, reporting up through Joint Standards Coordinating Groups and thence to the Laboratory Service Line Senior Leadership Group. This Senior Leadership Group acts as a committee-of-the-whole to review and approve recommendations from the Joint Standards Committees. In turn, this empowers the site Medical Directors in the Senior Leadership Group to be fully informed in overseeing their own laboratories and to represent laboratory operations and performance to their site Medical Boards, Hospital Leadership, and Process Improvement Committee Governance (PICG). This structure also provides centralized governance for incoming requests and suggestions from customers (including physician stakeholders) and industry vendors. A system Physician Advisory Group helps advise laboratory leadership.
Laboratory Performance Improvement Coordination Group
The quality performance of all health system laboratories is unified under a system-wide Laboratory Performance Improvement Coordinating Group (PICG), under the aegis of the service line Quality Department. The latter sets guidelines to assess and improve performance of clinical, organizational, and support processes (see Supplemental Table 3). The program also delineates the quality management process for ensuring safe, appropriate, and consistent care delivered to all patients in all facilities. All clinical laboratory sites are responsible for implementing standardized operating procedures and staff competencies and then for reporting up to the laboratory PICG. These metrics are monitored continually and compiled monthly. Follow-up is required for metrics not meeting predefined targets. If needed, outliers are addressed through task groups, up to and including high-priority on-site work teams if needed. This process also supports a state of continual inspection readiness for all clinical laboratory sites. The laboratory Quality Department submits metrics reports up to the health system’s Quality Department, and the hospital laboratories’ own PICG subcommittees reported to their respective hospital PICGs.
Relevant to this report, during threatening events, the Quality Department monitors the event impact on the delivery of all laboratory services to patients, physicians, and the public. Each threatening event—including routine events such as winter storms—is viewed as an opportunity to further improve the quality process system wide. Over the years, service line Quality has been able to identify opportunities for improvement processes such as the development of a comprehensive Escalation Policy, Business Continuity Plan, and Alternative Service Policy. Moreover, for each specific event, quality metrics constitute lead indicators for potential degradation in laboratory performance and help guide corrective action. Both through our laboratory PICG and through the joint standards committees, lessons learned from deficiencies identified through our many inspections can also be propagated quickly through the entire health system.
Budgets and Finance
Finally, the budgets of all site laboratories are coordinated under laboratory service line governance. The system hospitals retain their direct authority for setting hospital clinical laboratory budgets and tracking local financial performance of the laboratories. However, by integrating under laboratory service line governance “virtual” financial coordination of the 15 hospital-based clinical laboratories (serving the 19 hospitals, some of which share a common campus), the Core Laboratory (described below), and the “department of pathology and laboratory medicine” budgets, numerous efficiencies can be realized (Table 2).
Table 2.
Advantages Achieved Through Integrated Budgetary Oversight, Northwell Health Laboratory Service Line.
| Optimize utilization of resources, to include: |
| Optimization of test formularies at hospital sites |
| Optimized utilization of distributed laboratory capacity |
| Optimal deployment of personnel |
| Optimization of laboratory efficiencies per site |
| Leverage size of health system for procurement |
| Standardized hospital laboratory budgeting, to include: |
| standardized negotiations with local hospitals for Part A support |
| standardized methodologies for charge-back to hospitals for in-system reference testing |
| Pan-system implementation of best practices in clinical laboratories |
| Pan-system propogation of information gained from regulatory inspections |
| Centralization of technologies in Core Laboratory, to include: |
| capitalization of “big ticket” items |
| centralization of Microbiology, Cytogenetics, Molecular Pathology |
| centralization of laboratory testing for system ambulatory network |
| Core Laboratory support of system laboratory operations* |
| Resourcing of Joint Standards Committees and their recommended actions |
| Governance of laboratory service line |
| Laboratory service line accountability to senior health system leadership |
| Laboratory service line accountability to hospital site administrations |
*See Figure 1.
The Core Laboratory
The above system architecture for the laboratory service line was formalized in 2009. But this was built upon a laboratory architecture dating back to 1992, when the “North Shore Health System” was first created by merging of the North Shore University Hospital (NSUH; Manhasset, New York) and Glen Cove Hospital (Glen Cove, New York) and in turn their respective clinical laboratories. The collaboration of these 2 laboratories gave rise to the proposal to create an intrasystem “Core Laboratory,” impelled further by the merging in 1996 of Long Island Jewish Medical Center (LIJMC; New Hyde Park, New York) into what then became the “North Shore-LIJ Health System” (ultimately renamed “Northwell Health” in January, 2016; note 1). The Core Laboratory was commissioned in 1998 at an off-site location, located approximately midway between NSUH and LIJMC in Lake Success, New York, where it has operated continuously ever since.
The initial inspiration of the Core Laboratory was to consolidate “non-stat testing” from system hospitals so as to attain cost efficiencies and better enable acquisition of new technologies. However, from the outset, “outreach” was part of the mission of the Core Laboratory, serving as a powerful financial driver for reinvestment in the laboratory service line as the health system grew to 15 hospitals in 2009 and 19 hospitals in 2015. The Core Laboratory grew at an annualized rate in excess of 20%, both through intrinsic growth of the health system and through an even greater growth of Core Laboratory outreach for unrelated business over this same time period. The Core Laboratory is thus able to serve as both the operational and the financial “engine” for the laboratory service line. System functions supported by the Core Laboratory are shown in Figure 1 and in greater detail in Supplemental Table 4.
Laboratory Information Systems
An essential component of system integration of clinical laboratories is their using a single integrated Laboratory Information System (LIS), which is Cerner Millenium for the Core Laboratory, and by 2015, 11 of our 15 hospital laboratories. When new hospitals are acquired by the health system, their clinical laboratory LIS are put onto the strategic calendar for conversion to Cerner Millenium. This calendar is scheduled through 2019. As will be seen with the 2012 Hurricane Sandy event, when laboratories remain on a separate legacy LISs, a major barrier is encountered when test volumes need to be diverted on an emergency basis.
Taken collectively, the pan-system governance and operations, and the centralized support of the Core Laboratory, constitute a highly integrated laboratory network. Very importantly, for the workforce of the clinical laboratories, it is a way of life to be working with laboratory colleagues across the breadth of the health system on a continual and daily basis. It was this integrated system that was tested during a series of unusual events.
Threatened Interruptions in Service
A crisis is defined as “a difficult or dangerous situation that needs serious attention.”5 In almost every calendar year from 2009 to 2015, the laboratory service line experienced unusual events that challenged the ability of individual sites to operate successfully (Table 3). Through the coordinated effort of the laboratory service line, timely and accurate test results could continue to be provided to patients and physicians.
Table 3.
Unusual Events, Northwell Health Laboratory Service Line.*
| Date | Event | Leadership | Communication | Quality | Validation | Safety | Logistics | Laboratory Information Services | Education | Workforce Re-deployment | Capacity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| April-June 2009 | Novel H1N1 Influenza Virus pandemic | • | • | • | • | • | • | • | • | • | • |
| March 2010 | Franklin Hospital: flood | • | • | • | • | • | • | • | • | • | |
| March 2010 | 5-day support of regional ambulatory Physician Network, with fire | • | • | • | • | • | • | • | • | • | |
| December 2011 -September 2014 | Multiyear support of St. John’s Episcopal Hospital clinical laboratories | • | • | • | • | • | • | • | • | • | • |
| October 2012 | Hurricane Sandy: flood of Staten Island University Hospital offsite lab | • | • | • | • | • | • | • | • | • | • |
| August 2013 | Southside Hospital: threatened laboratory floor subsidence | • | • | • | • | • | ◦ | ◦ | ◦ | ||
| January 2015 | North Shore University Hospital: fire | • | • | ◦ | ◦ | • | ◦ | ◦ | ◦ |
*Key contributions from the laboratory service line are shown in the columns at right; • denotes contribution that was utilized; ◦ denotes a contribution that was available if needed but was not required. “Capacity” refers to redistribution of test volumes to a different site that had available capacity.
2009 Novel Influenza A (H1N1) Pandemic: April to June 2009
On Friday April 24, 2009, beginning at about 3:00 pm and upon recommendation of their local school officials, 20 high school students from a private school in Queens, New York, some of whom had previously traveled to Mexico for their senior trip and now had symptoms of respiratory illness, began arriving at the Emergency Department of the North Shore-LIJ Health System’s children’s hospital. More students were seen at the Emergency Department through the weekend, and by Monday, April 27, it was clear that an unusual event was occurring. In what was ultimately to be declared a pandemic, the novel influenza A(H1N1) virus (Swine-like) became a major event in our health system, owing to the influx of both pediatric and adult patients with genuine signs of infectious illness, and Emergency Department visits by the “worried well.” Our health system response to the influenza A(H1N1) outbreak is documented elsewhere.6,7
The laboratory response to the massive influx of test samples involved emergent construction of an additional 250 sqft virology laboratory, conversion of virology testing from routine screening tests and viral culture to high-capacity molecular assays for respiratory viruses, building LIS interfaces, increasing the client service workforce, and development of same-day epidemiologic reports, all in a 7-day period.6 Laboratory leadership communicated daily with system leadership, especially with Infection Control. Frequent surveillance reports and testing guidance documents were sent from laboratory leadership to civic authorities and community physicians to keep them abreast of the latest changes in the status of the pandemic. In serving as a “sentinel laboratory,” the Core Laboratory also provided critical support to regional Departments of Health.
Management of workload surge capacity as the crisis unfolded over the first 3 weeks is shown in Figure 3. During this period, the molecular “respiratory virus panel” (RVP) test was not yet online, and the virology laboratory was still relying on screening viral assays and viral culture. The lowest 2 colors on the stacked histogram represent the preexisting technologists of the viral technologist workforce denoted as “regular hours” and “overtime hours.” Over the first week of the real crisis (April 27-May 1 and into the weekend of May 2-3), the overtime hours were unsustainable. The key point of this previously unpublished histogram is the redeployment from elsewhere in the health system of appropriately licensed laboratory technologists and other personnel (including administrative, information technology, client services, data analysists, and a rotating schedule of “professional” Medical Directors to oversee operations on a 24/7 basis) to address the crisis. By mid-May, the first wave of the crisis had passed; a second wave lasting into mid-June was dealt with in a similar fashion (data not shown), taking advantage of the now-routine high-capacity molecular RVP testing panel.8
Figure 3.
Workload crisis management, novel influenza A (H1N1) virus pandemic. Virology workforce hours are shown for the first phase of the crisis, segmented by workforce contribution. The second and third weeks of April 2009 represented the tail end of the annual winter influenza season, and workforce effort was under normal operations. On Friday, April 24, the first influx of testing from patients with what was found to be the novel influenza A (H1N1) virus began, continuing over the weekend. On Monday, April 27, it was clear that a crisis was occurring. In addition to overtime hours for the regular technologists, personnel redeployment from elsewhere in the laboratory service line began. By the beginning of the third week of the crisis (May 12, 2009), overtime by the regular technologists had been brought back under control, and a sustainable state of workforce hours had been achieved. Nevertheless, for the duration of the crisis (through early June, not shown), the increased work hours of an expanded workforce were required. Abbreviations: IT, Information Technology; Non-Tech, non-technical; OT, overtime; Reg, regular; Tech, technical.
Success was achieved through deployment of outstanding, dedicated personnel, each member vital to completing specific, delineated tasks that demanded extended service hours. Importantly, operative performance metrics of the remainder of the Core Laboratory and system hospital laboratories were maintained (data not shown). The virology laboratory was recognized by the health system’s 2010 “President’s Award.”9
Franklin Hospital Flood: March 2010
Franklin Hospital is one of the 9 community hospitals in the Northwell Health system, located in Valley Stream, New York. A severe nor’easter storm moved through the northeastern United States on March 12 to 15, 2010. On March 14, 2010, Franklin Hospital’s North Wing ground floor began showing signs of flooding, with water seeping onto the floor. An emergency team of surveyors and engineers determined that the water table was rising rapidly with little sign of receding. The underlying cause was progressive rising of the water table in that region of southern Long Island to more than 9 feet above prior year levels.10
The Franklin Hospital clinical laboratories were located on the ground floor. The laboratory staff continued to work with up to 2 inches of water surrounding their feet, despite frequent sessions of removing water by wet vacuums and air purifiers running constantly to reduce buildup of mildew. Large quantities of rubber boots and earplugs were ordered to make these conditions somewhat bearable. Meanwhile, intense preparations were made for relocation of the clinical laboratories to the former Labor-and Delivery Operating Rooms on a higher floor, representing a 75% reduction in assigned space. The relocated laboratory had to meet all regulatory requirements and also function at the same performance standard as their prior laboratory.
On April 7, 2010, 25 days after the first drop of water, the laboratory relocated to the second floor. Each instrument had to undergo revalidation, calibration, and quality control monitoring prior to running patient samples. There was no downtime and no service interruption during this implementation. With a previously scheduled state Department of Health (DOH) inspection looming, staff and management worked together to organize mock audits, revise manuals, and perform checklist overviews in their new space. As measured by deficiencies, the state inspection in June 2010 was the most successful inspection to date. One year to the month after the emergency relocation, the clinical laboratory returned to newly renovated ground floor space, “hardened” against the future intrusion of ground water.
While primary credit goes to the dedicated staff of the Franklin Hospital clinical laboratories, the role of the laboratory service line was as follows. First, service line Quality personnel were immediately on-site to work alongside Franklin laboratory personnel both to help protect their own safety (as from electrical hazard) and to assist in maintaining continuous laboratory operations—as all quality personnel are themselves licensed laboratory technologists. Second, while the frontline laboratory personnel were coping with the flooded work conditions, the service line helped staff the planning and execution of the laboratory relocation and worked alongside the frontline personnel for instrument validation and verification so that laboratory operations could remain in continuous compliance with regulatory requirements. Third, there was selected diversion of non-stat testing to the Core Laboratory. Fourth, service line management assisted in planning the redesign of what was ultimately a much-better laboratory footprint for the renovated laboratory. Finally, the service line served as valuable backup, should laboratory services have genuinely been interrupted.
Serving as a Regional Resource for Disruption in Service Elsewhere—Ambulatory Services: March 2010
In the metropolitan New York market, a major insurer worked closely with one of the major regional multisite multispecialty physician ambulatory networks, with that insurer’s managed care product line constituting most of the patient volumes for that network. A dedicated centralized clinical laboratory supported the laboratory diagnostics for the insurer’s covered patients. In March 2010—the same month as the “Franklin flood”—the reference laboratory abruptly closed. Although a competitor in the market was the contractually obligated fallback clinical laboratory, that competitor was not ready for taking on the testing load, owing to the lack of LIS interfaces with the physician practice. With 24 hours’ notice, the North Shore-LIJ Core Laboratory was asked if we could support this physician network until the competitor laboratory prepared and validated its interfaces.
Based on regional rumors, the closure of the dedicated reference laboratory was not unexpected. Since our Core Laboratory was already the provider of laboratory diagnostics for the small remainder of that physician network’s managed care products, we had functioning interfaces for all test orders. So in the 72 hours before being asked to provide assistance, we had already implemented planning for that possibility. That being said, we had 24 hours’ formal notice for a 30% surge in test volumes handled by our Core Laboratory.
On Thursday, March 11, 2010, the test volumes came. Through Monday evening, March 15, the surge continued—constituting 3 “business days” for the physician network (Thursday, Friday, and Monday) plus Saturday clinic hours. Technical personnel in the Core Laboratory were able to meet the surge requirements, with strategic cross-support from administrative personnel drawn from throughout the service line sites, for 24/7 oversight of the surge. On Tuesday, the competitor laboratory had built the necessary LIS interfaces, and our surge stood down.
Complicating this 5-day sequence was the aforementioned March 2010 nor’easter (and the beginning of the “Franklin flood”), 2 hours of unscheduled LIS downtime during maximal testing volumes on Friday night March 12, owing to the failure of our LIS vendor’s off-site “central node,” and a fire. The fire occurred Monday afternoon March 15 and was localized to a plumbing closet. It occurred due to inadequate fire protection against torch work in sweating pipe joints by a plumbing contractor. Although the fire was ultimately deemed minor, our Core Laboratory was emptied of all personnel for 45 minutes while a hook-and-ladder Fire Department task force helped achieve containment. During this remarkable 5-day surge sequence, the operative performance of the entire Core Laboratory was actually better than our usual metrics, as measured by turnaround time, defects per million events, and, not unexpectedly, productivity (data not shown). Although the Core Laboratory largely withstood this event on the basis of its own personnel, the value of a laboratory service line in supporting the ability of other regional providers to maintain continuous operations was demonstrated.
Serving as a Regional Resource for Disruption in Service Elsewhere—Threatened Hospital Closure: December 2011
At 9:00 pm Friday evening, December 16, 2011, the North Shore-LIJ Health System Chief Executive Officer received an emergent call from the New York State Commissioner of Health, asking whether our laboratories had the ability to assist a regional community hospital. Earlier that day, the New York State DOH had suspended that hospital’s laboratory permit for blood transfusion and related testing.11 This action was taken after a DOH inspection found that the hospital failed to meet accepted standards for blood transfusion and blood bank services, which put patient safety at risk. Without immediate corrective action, hospital closure was inevitable and continuous quality patient care was in immediate jeopardy. The impact to the surrounding community served by the hospital would be devastating, as it was the only full-service hospital aiding that particular geographic service area, including its 60 000 patients per year Emergency Department. During the proposed 30-day suspension, the hospital could continue to perform blood transfusions only if it used an outside laboratory to handle pretransfusion blood testing and demonstrated to DOH that proper protocols and procedures were in place for protecting patient safety.
With the strong support of that hospital’s senior leadership, a 12-person Northwell Health Laboratories quality team arrived at 7:00 am Saturday morning December 17, with obligation to report on planned corrective action in a scheduled 2:00 pm conference call with the state Commissioner of Health and his staff. This was done, with a correction plan encompassing all laboratory operations across all testing categories. Implementing this plan required extensive use of North Shore-LIJ Laboratories resources and personnel (see Supplemental Table 5). The immediate needs of keeping the hospital laboratories (and hospital) open were met. North Shore-LIJ Laboratories managers took semipermanent reassignment to that hospital, and we hired both the pathologist Medical Director for the site (DS) and a second staff pathologist.
Per clear request of the state, that hospital’s clinical laboratories were to become a permanent part of the North Shore-LIJ Laboratory Service Line, with adherence to all safety and quality requirements of same. This was done, an arrangement that continues to this day. Although that hospital’s clinical laboratories remained open for the duration, blood bank testing was performed at LIJMC in our health system until September 2014, when the state gave final approval for return of all blood product testing to the local site. In the meantime, an emergency blood supply was kept at that hospital. The hospital laboratories were not taken completely off the DOH “technical watch” list until December 2015.
The importance of keeping that hospital open was made even more clear when a nearby community hospital was closed by the state in April 2012 after its clinical laboratory failed state inspection and was shut down.12 Beyond the daily care given to the citizens and visitors of that region by the hospital that remained open, the importance of so doing was made even more clear when Hurricane Sandy blasted through the Northeast in the final days of October 2012. It was the Emergency Department of this hospital which was able to provide massive blood transfusions to save the lives of 4 patients—3 trauma and 1 obstetric—at the height of the storm. For the purposes of this report, the value of a regional integrated laboratory service network was again demonstrated, receiving commendation from the state for helping other laboratories—and hospitals—in crisis.
Hurricane Sandy: October 2012
The 13-day chronicle of the devastating East Coast storm and its aftermath that killed more than 100 people, destroyed whole communities in coastal New York and New Jersey, left tens of thousands homeless, crippled mass transit, triggered paralyzing gasoline shortages, inflicted billions of dollars in infrastructure damage, and cut power to more than 8 million homes, some of which remained dark for weeks is well documented.13 As in so many natural disasters elsewhere in the country and the world, health-care providers provided extraordinary service to others even as, for some, their own homes were being destroyed and their own family members made homeless. For this report, the laboratory natural disaster was the flooding on Monday, October 29, of the off-site clinical laboratories of the Staten Island University Hospital (SIUH). This off-site reference laboratory was at ground level in an industrial building—formerly the “Pouch Marine Terminal” on the northeast shore of Staten Island, 160 feet from the waters of New York Harbor. This laboratory provided full-service Microbiology, molecular diagnostics, ambulatory clinical chemistry, and the professional component of anatomic pathology for the 2 SIUH hospitals (North and South) on Staten Island, New York, and their associated physician practices serving over half of the resident population on Staten Island.
Despite sea walls, ground floor elevation was only 8 feet above sea level. Four feet of brackish water filled the entirety of the Pouch Laboratory and the adjacent data center for SIUH.14 Put simply, the laboratory and data center were total losses.15 On an immediate basis, the North site of SIUH accommodated a 50% surge in clinical chemistry test volumes into space wholly inadequate to the task. Office space at SIUH/North was repurposed for Cytopathology technical processing. The pathologist workforce for Anatomic Pathology was squeezed into any available office space near to the SIUH/North laboratories. Molecular virology testing was diverted to a regional competitor laboratory.
The problem was Microbiology. Ramping up what was already a mature courier logistics system for specimen transport, the North Shore-LIJ Core Laboratory immediately assumed responsibility for all Microbiology testing previously performed at the Pouch Laboratory. Within 2 weeks, it was apparent that the surge in Microbiology test volumes was putting the Core Laboratory Microbiology unit into a state of performance failure. For the first and only time in the 16-year history of the Core Laboratory, work volume was diverted and sent to a regional competitor laboratory.
Repatriating microbiology testing to the North Shore-LIJ Core Laboratory took until August 2014, requiring an exceedingly difficult 20-month microbiology test order-and-resulting interface build between the Meditech LIS (Westwood, Massachusetts) of the SIUH Laboratories, and the Cerner Millenium LIS of the Northwell Health Core Laboratory, and just-in-time recruitment of the technical workforce to accommodate the increase in microbiology test volumes from the two SIUH hospitals and the Staten Island and Brooklyn ambulatory physician practices served by the SIUH laboratories. Against prior volumes, SIUH Microbiology constituted a 25% increase in microbiology test volumes processed by the Core Laboratory, and microbiology personnel from the SIUH laboratories were not obliged to, and did not, relocate to the Long Island-based Core Laboratory.
Upon return of SIUH Microbiology testing to the health system, accuracy of Core Laboratory microbiology testing was not a point of issue with SIUH leadership and clinical providers. Turnaround time (TAT) was. Relabeling of SIUH Meditech-registered samples with barcoded Cerner labels and courier time from SIUH-North to the Core Laboratory in Long Island added on average an additional 3.1 hours. By way of reassurance to clinical stakeholders at SIUH, the Core Laboratory was able to demonstrate that final resulting of “positive” blood culture tests back to SIUH occurred within 50 to 100 hours after ordering of the culture at SIUH—inclusive of all preanalytical time, primary culture, secondary subculture, and further subculture to determine antibiotic sensitivities. This met clinical care needs, particularly given that Core Laboratory personnel telephoned all intermediate positive findings in as critical values. Furthermore, the Core Laboratory also has deployed matrix-assisted laser-desorption-and-ionization time-of-flight mass spectrometry and is poised to deploy multiplex film array for early diagnosis of positive blood cultures in mid-2016, potentially reducing “final” TAT by up to 48 hours for over 90% of all positive blood cultures.16
At the very least, the laboratory service line was ultimately able to service the SIUH requirements for microbiology diagnostics. But in this instance, 2 limitations were encountered: the disadvantage of in-system laboratories being on differing legacy LISs, substantially delaying redistribution of test volumes, and the geographic inability to relocate the workforce for the highly labor-oriented laboratory work that is microbiology. It was 21 months before the Core Laboratory could fully overcome these limitations. This example demonstrates that there are limits to the surge capacity of even a highly functional integrated laboratory network.
It took even longer for a scaled-down version of the Pouch Laboratory to be reopened in April 2015 on an upper floor of the Pouch Marine Terminal Building, housing (once again) Molecular Virology testing, Cytopathology, and professional services for Anatomic Pathology. SIUH Microbiology remains permanently with the North Shore-LIJ Core Laboratory. In all, 2.5 years were required to recover full functionality following Hurricane Sandy.
Hospital Laboratory Floor Subsidence: August 2013
The clinical laboratory of Southside Hospital, in Bayshore, New York, is on the first floor of Southside Hospital (owing again to the high water table of South Shore communities in Long Island, there was no basement floor), with an understory of 4-feet interval height. An upgrade of automated clinical laboratory equipment for Southside Hospital was scheduled for August 2013 and was reviewed at all levels of system procurement for engineering requirements prior to approval. The plan was to bring one of the new automated chemistry processors into the clinical laboratory in a location adjacent to the preexisting automated processors, validate the new machine, swap out one of the legacy processors, and repeat the process with the second new processor.
The problem was that in the first 3 days of operation of the new machine, it could not be leveled. An emergency assessment was made that the floor of the main clinical laboratory was at risk of subsidence and potential collapse. One of the legacy chemistry processors was immediately moved into space adjacent to the main laboratory, the new chemistry processor was removed from the site, and the central 800 sqft of the clinical laboratory roped off and closed.
The laboratory service line was alerted immediately, and logistics support was immediately activated for potential complete diversion of chemistry laboratory testing to the Core Laboratory, 45 minutes away. Simultaneously, the Core Laboratory activated an emergent plan for standing up Point-of-Care Testing to ensure continuous on-site capabilities for stat testing (which is used to obtain immediate results).
Fortunately, the relocated legacy chemistry processer was successfully validated and state approval quickly obtained for its emergent use in the adjacent “nonlaboratory” location. But for 3 weeks of engineering consultations and assessment of building integrity, the main Southside clinical laboratory floor remained closed, and the Core Laboratory remained on alert as a “safety net” for activation of emergency diversion. In the end, the floor was deemed stable but with the strong recommendation that no more than 2 chemistry processors be placed on it at the same time. The chemistry processors were replaced successfully following this restriction.
Fire: January 2015
The final interruption in service was the only event that placed laboratory personnel at immediate personal risk. The mean outside temperature in Manhasset, New York, in January was 29.9°F, the fifth coldest January in the past 20 years. Owing to problems with pipes freezing in a sub-basement of NSUH, at 1:15 pm and under supervision, an internal facilities workman lit a blow torch to thaw a piping coil. The flame was not properly shielded from flammable insulation material, which quickly caught fire. The workman immediately used a fire extinguisher on the flames but the burning matrix got disbursed, not extinguished. An estimated total of 10 minutes was required to extinguish the burning material. Unfortunately, the entire work area had filled with smoke and fumes. What the 2-man team did not know was that a substantial burden of smoke and fumes had gotten into the air handling system of the hospital.
This sub-basement was directly under the NSUH blood bank clinical laboratory located in the basement. The blood bank laboratory, and to a lesser extent the adjacent main laboratory, began to fill rapidly with acrid, dense smoke. Laboratory personnel had no knowledge of the evolving event and had to make instantaneous decisions regarding their own safety, let alone patient safety. Their decisive actions are given in Table 4. In brief, all laboratory personnel were evacuated to the hospital cafeteria 300 feet away on the same floor, and patients in the Pheresis Center immediately adjacent to the blood bank were emergently discharged by the blood bank Medical Director. Immediately following, within 10 minutes of initiating the laboratory evacuation and under the watchful eye of the blood bank Medical Director, blood bank personnel returned to the blood bank wearing protective equipment and gathered approximately 40 units of O-negative blood. These units were delivered immediately to refrigerated storage cabinets of the second floor Operating Rooms, which were under temporary suspension of activities. Upon arrival of blood—approximately 15 minutes after the initial evacuation, active Operating Room procedures resumed.
Table 4.
Fire, North Shore University Hospital: Chronology of the Fire Event, January 9, 2015.
| Time | Event |
|---|---|
| 1:15 pm | Sub-basement flame torch work, ignition of flammable materials |
| 1:18 pm | Rapid entry of smoke and fumes into NSUH blood bank and main laboratory |
| 1:20 pm | Beginning of evacuation of all NSUH clinical laboratory personnel |
| 1:25 pm | Completion of evacuation of all NSUH clinical laboratory personnel |
| 1:25 pm | Completion of emergency discharge of patients from adjacent Pheresis unit |
| 1:25 pm | Reentry of 4 blood bank personnel, to obtain 40 units of O-negative blood |
| 1:27 pm | Verification by laboratory Administrative Director of complete evacuation |
| 1:30 pm | Delivery of O-negative blood to second floor Operating Rooms |
| 1:45 pm | Reentry of selected main laboratory personnel to perform Stat testing |
| 3:15 pm | Fire Department declares “All Clear,” all laboratory personnel return |
| 5:30 pm | Escalation to hospital leadership of continued threat to blood bank operations |
| 7:15 pm | Hospital air handling systems returned to normal |
Abbreviation: NSUH, North Shore University Hospital.
Table 4 also gives the chronology of laboratory events. The fire event had been rapidly escalated to hospital leadership, and a hospital command center immediately activated. In the midst of minute-by-minute reporting, the perimeter of the fire event was defined and attentions given to patient and personnel safety for the Emergency Department, the other occupant of that basement floor of NSUH. However, a detail was not sent to the clinical laboratories, and in these critical early minutes, the laboratory was unaware of the extent of the fire event. It was approximately 12 minutes before the Administrative Director of the clinical laboratories had verified evacuation of the blood bank and main clinical laboratory, so that he was then able to report to the hospital command center and gain knowledge of the event, including the fact that the flames had been extinguished.
With knowledge that the fire event had been contained, in 30 minutes into the event selected laboratory personnel were permitted back into the main laboratory wearing protective gear so as to resume and be available for stat testing from the Emergency Department, which had remained under continual operation. Air handlers were obtained from the local Fire Department (which was now very much on-site), to clear out the laboratory air. At 3:15 pm—2 hours after the initial event—the “all clear” was given, and all laboratory personnel were permitted to return to their workstations.
All was not yet well. The laboratory air remained acrid and pungent. The Fire Department had left the premises, and hospital engineering was focusing its efforts on cleanup of the sub-basement. System air handling for the blood bank had been shut down, and blood bank ambient temperature was rising past 76°F toward a “shut-down” temperature of 80°F. The air remained acrid and pungent, affecting pharyngeal mucus membranes and the eyes of laboratory personnel. At 5:30 pm, the Senior Vice President for Laboratory Services (J.M.C.), who was on-site, escalated to hospital leadership the need for further decontamination of laboratory air. Through the ensuing 2 hours, additional portable air handlers were deployed, new system air filters installed, and the building air handling system rebalanced, bringing the laboratory environment back toward a state of tolerability. It was some days before the pungent smell of a fire had dissipated.
The actions of laboratory personnel in the midst of a true emergency were stellar. Personnel who reentered the blood bank at the 10-minute point to obtain units of O-negative blood put themselves at risk, not knowing the status of the fire event. All laboratory personnel showed fierce determination to provide continual care to the patients they served. While obeying the strict dictates of evacuation, they were glad to reenter the work space to do their jobs, even given the pungent air.
But there were lessons to be learned from this fire event (Table 5). First, laboratory leadership made strong recommendations to hospital administration about notifications and management of a fire event, including recommendation that advance knowledge be given to hospital units when an active flame was being used in an adjacent location. Second, laboratory service line leadership were not alerted until 30 minutes into the event. Although no patient harm came from this lack of notification, should NSUH laboratory services have been denied for more than 15 minutes of the actual event, precious time had been lost in activating collateral support for NSUH hospital operations. Third, “evacuation of the blood bank” was now to include procurement of previously placed units of O-negative blood and placement into a cooler for transport, an action that would take only seconds if properly planned in advance.
Table 5.
Fire, North Shore University Hospital: After Analysis of Fire Event, January 9, 2015.
| Recommendations to Hospital | Immediate inclusion of Laboratory in EOC |
| Immediate EOC dispatch of detail team to adjacent units | |
| Immediate notification to affected unit leadership of fire containment status | |
| Prior notification to adjacent units of active flame work | |
| Opportunities for improvement: Laboratory | Immediate escalation to laboratory service line leadership for activation of laboratory EOC |
| Activation of service line “workforce alert” in the event of injury or disability of site laboratory workforce | |
| Proactive monitoring and mitigation of after-action consequences of a denial-of-service event, including continued assessment of environmental conditions, equipment performance, workplace safety, and workforce status | |
| State-of-readiness for emergent blood bank procurement of O-negative blood |
Abbreviation: EOC, Emergency Operations Center.
The hospital drew from this experience to increase even further their preparedness for disaster. This now includes immediate dispatch from the Emergency Operations Center of detail teams to units adjacent to an evolving event, and verification of immediate communication of those adjacent units with the Emergency Operations Center. The updated plan was of value in a February 2016 brief (but dramatic) internal flood affecting the floor immediately above this same clinical laboratory.
Discussion
The concept of service lines was introduced to the health-care industry in the 1970s as a form of “product line management” brought over from industry.17 Although service lines had an initial fad status through the 1980s, they did not achieve universal appeal until the turn of the new century, when skyrocketing health costs demanded new approaches to the delivery of health care.18 In recent years, clinical service lines have become a major feature of the health-care landscape. While improved financial performance is part of the appeal,19 the more important reason for implementing service lines is improved clinical care.20 Service lines can help knit together the dispersed components a health-care system needs for care of patients with specific disorders,21 can help drive integration of clinical care during institutional mergers,22 and can serve the three missions of an academic enterprise.23
Despite the underpinning of almost all health care by Pathology and Laboratory Medicine diagnostic services, only one institution has written of successful implementation of a laboratory service line.24 The reported benefits of the service line include working with clinical colleagues to optimize testing protocols, reducing unnecessary testing, guiding treatment by helping to personalize therapy, designing laboratory information technology solutions to promote accurate and complete data mining, and administering cost-effective laboratories.
In this report, we describe the founding principles of our laboratory service line and its implementation across a 19-hospital health system. We then highlight a further benefit of a pan-system service line which is to help ensure continued support of patient care during threatened interruptions in service. While not part of the founding principles, the ability to deal with emergencies is a stunning example of how a laboratory service line can benefit a health system. The preexisting strengths of the Northwell Health Laboratory Service Line were essential for our successful responses to these emergencies.
Threats to laboratory services are dramatic, demand response at the highest levels of the organization, and must be addressed on an immediate basis. In some instances, mitigating the threat evolves over hours or even days, in other instances the response time is measured in seconds to minutes. An important lesson from our experience is that health systems face threatened interruptions in service on a regular basis. For our laboratories and the geographic region we support, on average once a year the operations of a clinical laboratory were at risk of cessation. For 5 of those threats (2 fires, 1 flood, 1 threatened floor subsidence, and 1 threat to regional hospital), our laboratory service line was able to keep the threatened laboratory in operation.
In 2 instances (abrupt closure of an unrelated regional laboratory and Hurricane Sandy), laboratories closed. Our Core Laboratory immediately took on the requisite case load. In the first instance, we were able to handle the regional surge but were glad to give the surge back to another laboratory after 5 days. In the second instance, following 2 weeks of supporting the emergent relocation of SIUH Microbiology to the Core Laboratory following Hurricane Sandy, we were forced to conclude that we had exceeded our capacity for assistance and had to decant SIUH Microbiology to an outside laboratory for almost 2 years. Only after a rigorous build of infrastructure, and just-in-time hiring of staff, were we able to bring SIUH Microbiology back into the system. To the extent that this is a “lesson learned,” it is that a laboratory service line does not have infinite capacity to provide assistance.
Based on our experience, the strengths of the laboratory service line in support of such threats are given in Table 6. Perhaps the most important is the last strength: the 2000-plus employees of the Northwell Health Laboratories perform their work with the daily knowledge that they are part of a highly integrated laboratory network. We have a culture of supporting one another across the different practice sites. The working relationships nurtured through an integrated service line ensure that our laboratory management and leadership at the many sites interact with one another on essentially a daily basis. When an unexpected event happens, our response can be immediate because we already know each other so very well. Moreover, “mini-disasters” happen every day, and a laboratory service line can both take these events in-stride and ensure that there is a continuous cycle of process improvement across the entire health system.
Table 6.
Strengths of Laboratory Service Line in Response to Threatened Interruptions in Service.
| Standardized equipment and technologies |
| Standardized procedures |
| Standardized quality and performance measures |
| Integrated management |
| Integrated physician leadership |
| Integrated system logistics and client support |
| Available workforce for emergency reassignment |
| Testing capacity at alternate sites |
| Breadth of expertise |
| Financial resources for emergency response |
| Experience in dealing with emergencies |
| Preexisting working relationships |
Conclusion
A laboratory service line can be a major asset to an integrated health system. In this report, we show that from the perspective of a large health system, emergency events that threaten the continuous operation of one or another clinical laboratory occur on a seemingly regular basis. A pan-system laboratory service line enables rapid and effective response to these threats, helping to ensure continuous support of patient care despite the potential severity of the laboratory event. The laboratory service line also constitutes a valuable regional resource when other health-care providers need emergent assistance in support of their clinical laboratory operations. These emergency responses are a valuable case study, for they highlight the intrinsic benefits of a laboratory service line to the daily operations of a health system.
Supplementary Material
Acknowledgment
The authors express their deepest gratitude to the many employees of the Northwell Health system who work in, or provide support to, the clinical laboratories. While the authors provided leadership in managing the events described herein, it is the entirety of the workforce that actually provided patient care. We also thank civic authorities, particularly the New York State Department of Health, for their close work with the Northwell Health system, in general, and the Northwell Health Laboratories, in particular, prior to, during, and following the extraordinary events that have been described in this report.
Note
From 1996 to December 2015, the health system was known as “North Shore-LIJ Health System (NSLIJ).” The system name changed to “Northwell Health” on January 1, 2016. When chronologically appropriate, reference is to the former name of the health system.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: The online [appendices/data supplements/etc] are available at http://apc.sagepub.com/supplemental.
References
- 1. Rauch CA, Nichols JH. Laboratory accreditation and inspection. Clin Lab Med. 2007;27(4):845–858, vii. [DOI] [PubMed] [Google Scholar]
- 2. Wadsworth Center Clinical Laboratory Evaluation Program: Guide to Program Requirements and Services. Web site http://www.wadsworth.org/regulatory/clep. Updated October 2005, Accessed March 1, 2016.
- 3. 2014 North Shore-LIJ Annual Report. Web site https://www.northwell.edu/sites/default/files/NSLIJAR2014.pdf. Accessed March 1, 2016.
- 4. Groppi DE, Alexis CE, Sugrue CF, Bevis CC, Bhuiya TA, Crawford JM. Consolidation of the North Shore- LIJ Health System anatomic pathology services: the challenge of subspecialization, operations, quality management, staffing and education. Am J Clin Pathol. 2013;140(1):20–30. [DOI] [PubMed] [Google Scholar]
- 5. Merriam-Webster. An Encyclopedia Britannica Company. Springfield, MA: Merriam-Webster; Web site http://www.merriam-webster.com/dictionary/crisis. Accessed August 9, 2014. [Google Scholar]
- 6. Crawford JM, Stallone R, Zhang F, et al. Laboratory Surge Response to Pandemic (H1N1) 2009 Outbreak, New York City Metropolitan Area, USA. Emerg Infect Dis. 2010;16(1):8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ginocchio CC, Zhang F, Manji R, et al. Evaluation of multiple test methods for the detection of the novel 2009 influenza A (H1N1) during the New York City outbreak. J Clin Virol. 2009;45(3):191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ginocchio CC, St George K. Likelihood that an unsubtypeable influenza A virus result obtained with the Luminex xTAG respiratory virus panel is indicative of infection with novel A/H1N1 (swine-like) influenza virus. J Clin Microbiol. 2009;47(7):2347–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. NSLIJ. News Room. 2010 President’s Award. August 2, 2010. Great Neck, NY: NSLIJ; Web site http://www.northshorelij.com/hospitals/news/2010-president-award. Accessed August 12, 2014. [Google Scholar]
- 10. U.S. Department of the Interior report. “New York and Long Island Water Tables More Than Two Feet Higher since April 2009”. 2010. Web site http://www.usgs.gov/newsroom/article.asp?ID=2503#.VsHcPED5cjY. Updated June 9, 2010. Accessed February 15, 2016.
- 11. NYS Department of Health. St. John’s Episcopal Hospital Laboratory Permit Suspended for 30 Days. Albany, NY: NYS Department of Health; 2011. Web site http://www.health.ny.gov/press/releases/2011/2011-12-16_laboratory_permit_suspended.htm. Accessed August 30, 2014. [Google Scholar]
- 12. New York Times. “Down to One Hospital, Rockaway Braces for Summer Crowds”. New York, NY: New York Times; 2016. Web site http://www.nytimes.com/2012/05/21/nyregion/closing-of-peninsula-hospital-in-rockaway-raises-fears.html?r=0. Accessed February 15, 2016. [Google Scholar]
- 13. Hurricane Sandy: Covering the Storm. NY Times. NY/Region; 2012. Web site http://www.nytimes.com/interactive/2012/10/28/nyregion/hurricane-sandy.html?_r=0. Updated November 6, 2012, Accessed August 17, 2014. [Google Scholar]
- 14. Baum S. MedCity News; 2012. Web site http://medcitynews.com/2012/10/hurricane-sandy-underscores-new-yorks-health-information-exchange-and-data-storage-logistics/ . Updated October 30, 2012, Accessed August 17, 2014.
- 15. Vassilakos T. Staten Island, Together and Strong. Braving the Storm: SIUH’s Response to Hurricane Sandy. North Shore-LIJ Health System; 2013. Web site http://www.siuh.edu/documents/publications/HealthFocus_Q1_2013.pdf. Accessed August 17, 2014.
- 16. Southern TR, VanSchooneveld TC, Bannister DL, et al. Implementation and performance of the BioFire FilmArray® Blood Culture Identification panel with antimicrobial treatment recommendations for bloodstream infections at a Midwestern academic tertiary hospital. Diagn Microbiol Infect Dis. 2015;81(2):96–101. [DOI] [PubMed] [Google Scholar]
- 17. Fligstein N. The spread of the multidivisional form among large firms, 1919-1979. Am Sociol Rev. 1985;50(3):377–391. [Google Scholar]
- 18. Jain AK, Thompson JM, Kelley SM, Schwartz RW. Fundamentals of service lines and the necessity of physician leaders. Surg Innov. 2006;13(2):136–144. [DOI] [PubMed] [Google Scholar]
- 19. Longshore GF. Service-line management/bottom-line management. J Health Care Finance. 1998;24(4):72–79. [PubMed] [Google Scholar]
- 20. Parker VA, Charns MP, Young J. Clinical service lines in integrated delivery systems: an initial framework and exploration. J Healthc Manag. 2001;46(4):261–275. [PubMed] [Google Scholar]
- 21. Turnipseed WD, Lund DP, Sollenberger D. Product line development: a strategy for clinical success in academic centers. Ann Surg. 2007;246(4):585–590;discussion 590-592. [DOI] [PubMed] [Google Scholar]
- 22. Corwin SJ, Cooper MR, Leiman JM, Stein DE, Pardes H, Berman MA. Model for a merger: New York-Presbyterian’s use of service lines to bring two academic medical centers together. Acad Med. 2003;78(11):1114–1120. [DOI] [PubMed] [Google Scholar]
- 23. Wenzel RP, Kontos HA. Clinical service-line structures can better carry out the missions of traditional clinical departments. Acad Med. 1999;74(10):1055–1057. [DOI] [PubMed] [Google Scholar]
- 24. Sussman I, Prystowsky MB. Pathology service line: a model for accountable care organizations at an academic medical center. Hum Pathol. 2012;43(5):629–631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.