Abstract
Cell division associated 1 (CDCA1) was screened as an oncogene that is overexpressed on several cancers, including prostate cancer. A highly immunogenic HLA‐A*2402‐restricted epitope peptide corresponding to part of the CDCA1 protein was also identified. A phase I clinical trial was conducted for patients with castration resistant prostate cancer (CRPC) using a CDCA1 peptide vaccination. Twelve patients having HLA‐A*2402 with CRPC after failure of docetaxel chemotherapy were enrolled. They received subcutaneous administration of the CDCA1 peptide as an emulsion with Montanide ISA51VG once a week in a dose‐escalation manner (doses of 1.0 or 3.0 mg/body, six patients received each dose). The primary endpoint was safety, and the secondary endpoints were the immunological and clinical responses. Vaccination with CDCA1 peptide was well tolerated without any serious adverse events. Peptide‐specific cytotoxic T lymphocyte (CTL) responses using ELISPOT assay and dextramer assay were observed in three patients receiving the 1.0 mg dose and five patients receiving the 3.0 mg dose. The median overall survival time was 11.0 months and specific CTL reacting to CDCA1 peptide were recognized in long‐surviving patients. CDCA1‐derived peptide vaccine treatment was tolerable and might effectively induce peptide‐specific CTLs for CRPC patients. This novel peptide vaccine therapy for CRPC appears promising. (ClinicalTrials.gov number, NCT01225471).
Keywords: Cancer peptide vaccine, castration resistant prostate cancer, cell division associated 1, oncogene, peptide specific CTL
Prostate cancer is the second most frequently diagnosed cancer worldwide and is the second leading cause of cancer deaths in men.1 Approximately 5–10% of patients present with metastatic disease at initial diagnosis, and another 25% with localized or locally advanced prostate cancer will develop metastases during the course of their disease. A standard initial therapy for metastatic prostate cancer consists of androgen deprivation therapy. However, the disease becomes castration‐resistant and can progress even at undetectable levels of testosterone. Therefore, this stage of the disease is termed castration‐resistant prostate cancer (CRPC). Docetaxel has been approved as a first‐line chemotherapy for metastatic prostate cancer because two independent phase III trials showed an increased survival benefit for patients treated with docetaxel compared with mitoxantrone.2, 3 Since then, docetaxel has become the standard treatment for patients with metastatic CRPC. Recently, several new agents, abiraterone,4 cabazitaxel5 and enzalutamide,6 have become available for the treatment of CRPC. However, these treatments caused severe adverse events, such as febrile neutropenia. Therefore, new treatments that provide durable disease control are still needed.
A greater understanding of basic immunologic principles and advances in immunologic and molecular techniques led to the development of therapeutic cancer vaccines for prostate cancer. Sipuleucel‐T (Provenge) was the first cancer vaccine approved by the FDA for the treatment of CRPC in 2010.7 Recently there has been remarkable progress in cancer immunotherapy using anti‐CTLA4 antibody, anti‐programmed death‐1 (PD‐1) antibody, and anti‐programmed death‐ligand 1 (PD‐L1) antibody for several cancers including prostate cancer. However, a phase III trial using anti‐CTLA4 antibody (ipilimumab) for CRPC patients showed no significant difference between the ipilimumab group and the placebo group in terms of overall survival in the primary analysis.8
We previously performed a genome‐wide expression profile analysis by cDNA microarray and identified cell division associated 1 (CDCA1),9 which was also overexpressed in various cancers including prostate cancer.10 Because the expression of CDCA1 was hardly detectable in normal organs, we considered CDCA1 a good candidate for the development of a cancer specific antigen. We screened and identified an HLA‐A*2402‐restricted epitope peptide, CDCA1‐A2456‐64, that has a high antigenic activity to induce cytotoxic T lymphocytes (CTL).11 We also identified several peptide epitopes derived from cancer‐testis antigens or onco‐fetal proteins, and these were investigated in translational studies targeting several types of human cancer.12, 13, 14, 15 Here, we report the results of a Phase I clinical trial using the CDCA1‐A2456‐64 peptide vaccination for patients with CRPC.
Materials and Methods
Peptide
The CDCA1‐A2456‐64 peptide (VYGIRLEHF) previously reported11 was manufactured to Good Manufacturing Practice (GMP) grade for the clinical trial by the American Peptide Company Inc. (Sunnyvale, CA, USA). Analysis showed a purity of >95%. An HLA‐A*2402‐restricted HIV‐derived epitope peptide (RYLRDQQLL) was used as a negative control.16
Study design
This clinical trial was an open label phase I clinical trial with a dose‐escalation of the CDCA1 peptide for patients with CRPC. This study was approved by the Ethics Committee of Iwate Medical University and Oita University. The primary endpoint was the safety of peptide vaccination, and the secondary endpoints were immunological responses and clinical outcome. Immunological monitoring was performed at the central laboratory by enzyme‐linked immunospot (ELISPOT) assay and dextramer assay using in vitro cultured lymphocytes derived from peripheral blood lymphocytes at pre‐ and post‐vaccination periods. Clinical outcomes were assessed by changes in serum prostate specific antigen (PSA), and computed tomography (CT) or magnetic resonance imaging (MRI) if measurable lesions were present at baseline. This trial was registered with ClinicalTrials.gov (no. NCT01225471).
Patient eligibility
CRPC was defined as a serum testosterone level of less than 50 ng per deciliter (17 nmol/L) despite surgical or medical castration. Patients with CRPC were enrolled in this study from July, 2009 to November, 2011. The eligibility criteria were: (i) diagnosis of prostate cancer on the basis of histological examinations; (ii) HLA‐A*2402 genotype as determined using commercially‐available genomic DNA typing tests (SRL, Tokyo, Japan); (iii) age between 20 and 85 years, and an Eastern Cooperative Oncology Group performance status (PS) of 0–2; (iv) no expectation of response to other therapies such as radiation; (v) no prior therapy within 4 weeks; (vi) adequate hepatic, renal, and bone marrow function (white blood cell counts ≥2000/L, platelets ≥70 000/L, aspartate aminotransferase ≤100 IU/L, alanine aminotransferase ≤100 IU/L, total bilirubin ≤1.5 g/dL, and serum creatinine ≤1.5 mg/dL). The exclusion criteria were: (i) active infection, other active malignancy; (ii) pregnancy or lactation; and (iii) treatment with immunosuppressive agents (e.g., steroids). All patients were informed of the investigational nature of the study and signed informed consent in accordance with each institutional review board.
Treatment protocol
A skin test was performed before the first vaccination by intradermal injection of 10 μg of the peptide to avoid the risk of acute hypersensitivity. A positive skin reaction was defined as >30 mm diameter of erythema and induration compared with the negative control using saline. CDCA1‐A2456‐64 peptide was administered in liquid form, emulsified in 1 mL incomplete Freund's adjuvant (IFA; Montanide ISA51VG, Seppic, Paris, France), and injected at the axilla or inguinal regions. The vaccination was given subcutaneously once a week, and at 4 weeks as one cycle. After a 1‐week interval, the next cycle was performed and continued until the judgment of progressive disease (PD) or doctor's assessment. We planned the administration of two incremental doses of peptide (1.0 and 3.0 mg/body) and enrolled six patients for each dose.
Clinical monitoring and toxicity assessment
Baseline studies gained data by physical examination, blood examination including PSA and image analysis. Decreased PSA levels of ≥50% were defined as partial responses (PR) and confirmed by two separate measurements ≥4 weeks apart. Decreases of less than 50% or increases of less than 25% from the baseline were interpreted as stable disease (SD).17 For measurable lesions, Response Evaluation Criteria in Solid Tumors (RECIST) was used. Image analysis was performed every one to three cycles, and once every 3 months after three cycles. PD was defined as radiological progression, or if defined using PSA level alone, three consecutive increases in PSA level and 125% of the baseline PSA value. Overall survival (OS) was estimated from the date of the initial vaccination to the date of death. All patients were followed up until cancer death, intolerance, or patient withdrawal of consent. Toxicity assessments were performed at least once a week using National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (NCI‐CTCAE v3.0). Dose‐limiting toxicity was defined as a hematological toxicity of grade 4 or greater, and non‐hematologic toxicity of grade 3 or greater. Reaction at the injection sites (RAI) was defined by erythema and/or induration at the injection site of the vaccine. Clinical and laboratory assessments were checked at each visit.
Analysis of immunological responses
IFN‐γ ELISPOT kit and AEC substrate set (BD Pharmingen, San Diego, CA, USA) were used to measure CTL responses throughout the clinical study as previously reported. Peripheral blood mononuclear cells (PBMCs) were obtained from patients and immediately frozen before vaccination and at the end of each course. Frozen PBMCs were thawed and used for in vitro sensitization. In brief, PBMCs were cultured in 1 mL of complete media (prepared with a mix of AIM‐V and RPMI media, 50:50) containing 10% fetal bovine serum in a 48‐well plate with 10 μg/mL of CDCA1‐A2456‐64 peptide and 120 IU/mL of IL‐2 at 37°C under conditions of 5% CO2 for 2 weeks. At day 7, half of the medium was removed from each well and 500 μL of fresh medium containing the epitope peptide was added for sensitization. After a 2‐week incubation, CD4‐positive cells were removed using the Dynal CD4 positive isolation kit (Invitrogen, Carlsbad, CA, USA), and harvested cells were co‐cultured with peptide‐pulsed TISI cells (2 × 104 cells per well) at 37°C for 20 h. ELISPOT assay was performed in triplicate. CDCA1‐A2456‐64‐specific CTL responses were defined according to the evaluation tree algorithm.18 In vitro cultured T cells were subjected to the dextramer assay to confirm peptide‐specificity. HLA‐A24/CDCA1‐peptide dextramer (Immudex) staining in combination with anti‐CD8, ‐CD4 and ‐CD3 mAbs were performed and analyzed by flow cytometry. HLA‐A24/HIV‐peptide (RYLRDQQLL) dextramer (Immudex) was used as a negative control. CTL and dextramer assays were performed using PBMCs which were collected before the vaccination and after each single course of the vaccination.
Statistical analysis
Overall survival was analyzed by the Kaplan–Meier method, and the statistical differences were analyzed by the log‐rank method. All the statistical analyses were performed using JMP Version 10.0.0 software (SAS Institute Inc., Cary, NC, USA). Statistical significance was defined by a value of P < 0.05.
Results
Patient characteristics
Table 1 shows the patient characteristics. Six patients were administered each dose (1.0 or 3.0 mg/body) and 12 patients were enrolled in this trial. The median age was 75.5 years (range: 51–84 years). The performance status was 0 in 3 patients, 1 in 3 patients and 2 in 6 patients. The median PSA was 52.2 ng/mL (range: 8.2–5260 ng/mL) and the median Gleason score was 8. Most patients had multiple bone metastases and lymph node metastases. All patients had received docetaxel that was repeated every 3–4 weeks in combination with oral prednisone. The median cycle of docetaxel was 6 (range: 4–12) and showed resistance to treatment.
Table 1.
Patient characteristics
| No. | Dose of peptides (mg) | Age (years) | ECOG PS | PSA pre vaccination (ng/mL) | Gleason Score | Site of metastasis | Cycle of docetaxel |
|---|---|---|---|---|---|---|---|
| 1 | 1.0 | 74 | 2 | 66.4 | 8 | LN and bone | 6 |
| 2 | 1.0 | 67 | 1 | 11.1 | 7 | LN, bone | 12 |
| 3 | 1.0 | 79 | 2 | 37.6 | 7 | bone | 6 |
| 4 | 1.0 | 75 | 2 | 179 | 8 | LN and bone | 6 |
| 5 | 1.0 | 76 | 2 | 579 | 10 | bone and liver | 5 |
| 6 | 1.0 | 84 | 1 | 74.1 | 7 | bone | 4 |
| 7 | 3.0 | 68 | 2 | 5260 | 8 | LN and bone | 4 |
| 8 | 3.0 | 62 | 0 | 18.5 | 7 | LN and bone | 10 |
| 9 | 3.0 | 77 | 0 | 8.2 | 6 | LN | 8 |
| 10 | 3.0 | 51 | 0 | 29 | 8 | bone | 10 |
| 11 | 3.0 | 76 | 2 | 38 | 9 | LN and bone | 5 |
| 12 | 3.0 | 81 | 1 | 145 | 8 | bone | 12 |
LN, lymph node.
Toxicity
The numbers of vaccinations for each patient were 6 to 68. There were no grade 4 toxicities and no treatment‐related deaths. The overall toxicities are shown in Table 2. The most frequent adverse events (AEs) were dermatological reactions at injection sites (n = 8). Two of 6 patients (33.3%) developed erythema and/or induration at a dose of 1.0 mg, and 5 of 6 patients (83.3%) developed a skin reaction at a dose of 3.0 mg. Dose‐limiting toxicity and dose‐specific AEs were not seen. Severe AEs assessed as grade 3 were anemia and hypoalbuminemia. The patient (No. 5) who was occurred severe AEs had multiple organ metastases and rapidly progressing disease during vaccination. According to the independent safety evaluation committee in this trial, these severe AEs were not directly associated with the vaccinations, but rather with cancer progression. These results suggest that CDCA1 peptide vaccine therapy was well tolerated for CRPC patients.
Table 2.
Adverse Events
| G1 | G2 | G3 | G4 | Total | |
|---|---|---|---|---|---|
| 1.0 mg dose group | |||||
| Injection site reaction | 1 | 3 | 0 | 0 | 4 |
| Anemia | 2 | 2 | 0 | 0 | 4 |
| AST increased | 1 | 1 | 0 | 0 | 2 |
| ALT increased | 1 | 0 | 0 | 0 | 2 |
| Hypoalbuminemia | 1 | 2 | 0 | 0 | 3 |
| Bone pain | 2 | 1 | 0 | 0 | 3 |
| Fatigue | 3 | 1 | 0 | 0 | 4 |
| Appetite loss | 0 | 3 | 0 | 0 | 3 |
| Edema peripheral | 0 | 2 | 0 | 0 | 2 |
| Eruption | 0 | 1 | 0 | 0 | 1 |
| 3.0 mg dose group | |||||
| Injection site reaction | 0 | 4 | 0 | 0 | 4 |
| Anemia | 0 | 2 | 1 | 0 | 3 |
| AST increased | 1 | 0 | 0 | 0 | 1 |
| ALT increased | 1 | 0 | 0 | 0 | 1 |
| Hypoalbuminemia | 1 | 1 | 1 | 0 | 3 |
| Bone pain | 1 | 1 | 0 | 0 | 2 |
| Fatigue | 0 | 3 | 0 | 0 | 3 |
| Appetite loss | 0 | 1 | 0 | 0 | 1 |
| Edema peripheral | 0 | 1 | 0 | 0 | 1 |
Immunological responses
In vitro cultured T cells were subjected to ELISPOT and dextramer assays. Representative ELISPOT and dextramer assays specific to the CDCA1 peptide are shown in Figure 1. The ELISPOT assay indicated a substantial number of T cell responses were specific to the CDCA1 peptide compared with the irrelevant peptide. This CDCA1‐specific T cell response was further confirmed by the CDCA1‐dextramer assay with the value of dextramer (+) CD8(+) cells among CD3(+) CD4(‐) cells. CDCA1 peptide‐specific strong CTL responses were seen in 3 (50.0%) of 6 patients receiving the 1.0 mg dose, and 5 (83.3%) of 6 patients receiving the 3 mg dose after two or three courses of vaccination (Table 3). Strong CTL responses were observed in 8 (66.7%) of 12 patients, and these patients also had peptide‐specific CTLs by dextramer assay.
Figure 1.
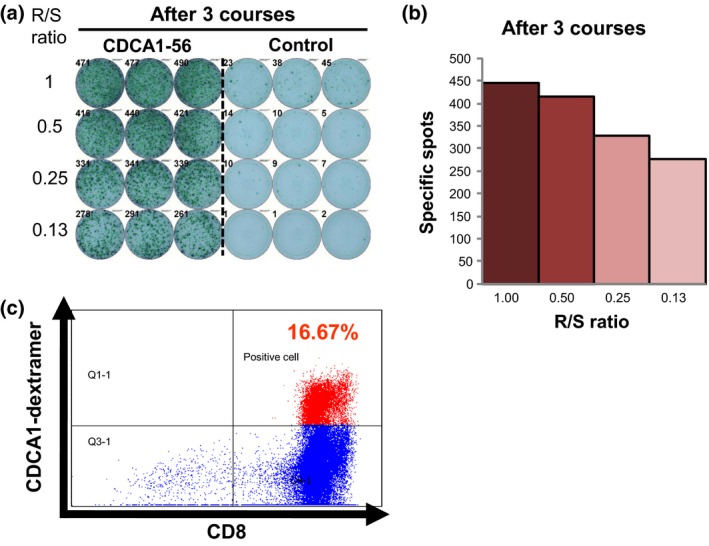
Representative CDCA1 peptide‐specific CTL responses. peripheral blood lymphocytes obtained from patient case 8 pre‐vaccination and after the 3rd vaccination were cultured in rIL‐2 for 14 days with 2 CDCA1‐peptide stimulations. (a) The cultured lymphocytes were subjected to ELISPOT assay after depletion of CD4‐positive cells by magnetic beads. TISI cells were incubated with responder cells in the presence of CDCA1 peptide or HIV peptide as an irrelevant control, and the spot counts were quantified (b). (c) The cultured lymphocytes were analyzed with HLA‐A2402/HIV‐dextramer pre vaccination (left) or HLA‐A2402/CDCA1‐dextramer (right) combined with anti‐CD8 and ‐CD3 mAbs by flow cytometry. The value of dextramer (+)/CD8(+) cells among CD3(+) cells is shown. R/S, responder/stimulator.
Table 3.
Immunological response and clinical outcome
| No. | Number of vaccination | CTL responsea | CDCA1 dextramer + / CD3+CD4‐CD8+ (%) | PSA response | Tumor response | OS (months) | Injection site reaction | ||
|---|---|---|---|---|---|---|---|---|---|
| Pre | After | Pre | After | ||||||
| 1 | 10 | − | − | − | − | SD | PD | 5.2 | − |
| 2 | 26 | − | +++ | 0.03 | 13.0 | SD | SD | >20.9 | + |
| 3 | 29 | − | +++ | 0.04 | 0.11 | PD | – | 9.7 | + |
| 4 | 19 | − | − | − | − | PD | SD | 6.2 | − |
| 5 | 6 | − | − | − | − | SD | PD | 1.7 | − |
| 6 | 20 | − | +++ | 0.12 | 0.32 | SD | – | >20.1 | + |
| 7 | 6 | − | − | − | − | SD | PD | 3.9 | − |
| 8 | 12 | − | +++ | 0.03 | 16.7 | SD | SD | >22.5 | + |
| 9 | 24 | − | +++ | 0.01 | 3.03 | SD | SD | 14.1 | + |
| 10 | 52 | − | +++ | 0.02 | 2.87 | PR | – | >25.7 | + |
| 11 | 8 | − | +++ | 0.01 | 16.54 | SD | PD | 3.0 | + |
| 12 | 68 | − | +++ | 0.01 | 0.28 | SD | – | >31.8 | + |
The positivity of the CTL response was classified into four grades (−, +, ++, and +++) depending on previous evaluations.18
Clinical responses
All patients received four or more vaccinations (at least one cycle). Only one patient (8.3%) showed a PSA decrease greater than 50%. This patient (No. 10) had slight bone metastasis with a PS of 0. His PSA value gradually decreased and he showed PR after four cycles. Of the patients with measurable lesions, four patients (33.3%) achieved SD after three courses of vaccination and four patients (33.3%) revealed PD after less than three cycles according to RECIST criteria (Table 3). No objective responses were observed in this study. All 12 patients were analyzed for OS with a median follow‐up of 10.9 months. At the time of analysis, seven deaths had occurred. The Kaplan–Meier curve for OS is shown in Figure 2. The median OS for all patients was 11.0 months. When the OS was compared between the CTL strong response and negative/weak response groups, patients with a strong CTL response exhibited a significantly longer OS than the negative/weak CTL response group (OS; not reached versus 4.6 months, P = 0.0040). These observations indicate that the strong immunological response induced by the CDCA1 peptide vaccination contributed to an improved prognosis of CRPC patients.
Figure 2.
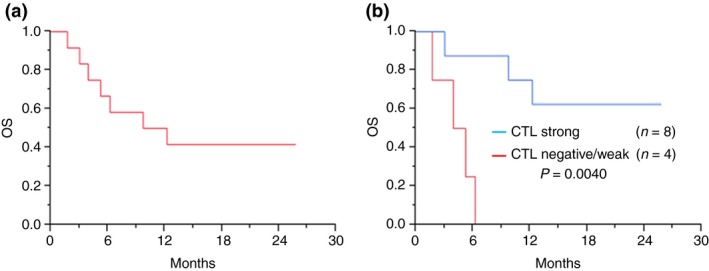
Overall survival of CDCA1 peptide vaccine therapy patients. Total median overall survival (OS) was 25.8 months (a). According to peptide‐specific CTL responses, the CTL strong response group showed a significantly longer OS than the CTL negative/weak response group (OS; not reached versus 4.6 months, P = 0.0040) (b).
Discussion
In general, the ideal target molecules for the development of cancer vaccines have high immunogenicity, are specifically expressed in cancer cells with a high frequency among patients, and are essential molecules for cell survival (to avoid escape from immune cells by loss of antigen expression).1, 19, 20 In this regard, the CDCA1 molecule used in the present trial is considered appropriate because it is expressed in the majority of prostate cancers and specifically in cancer cells and testis (cancer‐testis antigens), is essential for the survival of cancer cells, and most importantly showed very strong immunogenicity.
We did not observe severe hematological or non‐hematological adverse events related to the CDCA1‐A2456‐64 peptide vaccine treatment with dose escalation in this trial. Although grade 3 AE with anemia and hypoalbuminemia were shown, these AEs were considered associated with cancer progression. Specific adverse events caused by this vaccine treatment were only RAI, but this event is tolerable during vaccination. Because no dose‐limiting toxicities were observed, this protocol is considered very safe and well tolerated for advanced CRPC patients.
The strong specific CTL responses against CDCA1 peptide by ELISPOT assay were observed in 3 (50.0%) of 6 patients receiving the 1.0 mg dose and 5 (83.3%) of 6 patients receiving the 3.0 mg. Therefore, we assume the optimal dose of the peptide is 3.0 mg for further clinical trials. Patients who showed a strong peptide‐specific CTL response had long survival, but those with no or weak immune responses developed rapid disease progression. Although treatment with cancer vaccines increased circulating tumor antigen‐specific T cells,21 we demonstrated direct evidence of a positive correlation between the extent of peptide‐specific CTL responses and a better OS. Therefore, our present study supports the concept that vaccination‐induced immune responses contribute to the improvement of prognosis of CRPC patients. However, the log‐rank test is generally not suitable for subgroup parameters of time‐related variable values, such as immune response to the vaccination. Cox hazard analysis is more suitable, but the number of patients in this study was too small. A detailed statistical analysis using many cases is necessary in a future study. The skin reactions following vaccination were suggested to be associated with clinical outcome in some vaccine therapies.12, 22 We observed RAI correlated with strong CTL responses in this study. This suggests that RAI might be a surrogate marker to predict CTL responses for this protocol, although the biological significance and specificity for RAI are unclear. Further studies are required because the number of subjects in our study was too small to confirm an association between the development of RAI and clinical efficacy. We also performed immunohistochemical analysis as immune regulatory molecules using cancer tissues of some patients who were enrolled in this study (Table S1 and Fig. [Link], [Link], [Link]). However, the association between expression of immune molecules and effect of peptide vaccine therapy was unclear. The comprehensive expression analysis using many immunocompetent molecules as cancer specific antigen, HLA‐class I, innate or acquired immune molecules, and immune regulatory molecules would be required for a biomarker research of the cancer vaccine therapy.
Recently, several new treatments have been approved for metastatic CRPC after progression with docetaxel chemotherapy, each of which extended overall survival.5, 6, 23, 24 However, new treatments that provide durable disease control are still needed. In this study, the median OS time was 11.0 months although a reduction effect on the tumor was not seen. Noguchi et al. reported a median OS time of 14.8 months using a personalized peptide vaccine for docetaxel‐based chemotherapy‐resistant CRPC patients.25 In our study, many patients had worse general conditions with a PS of 2 and a high PSA value. Recently, it was reported that patients with early‐stage CRPC that were PS 0 or 1 and PSA < 10 ng/mL may receive more preferable clinical benefits using peptide vaccine treatment.26 Cancer immunotherapies elicit antitumor effects by inducing or enhancing a patient's immune responses. These effects can be delayed and may manifest as a gradual reduction in tumor growth, resulting in prolonged OS, which is not often accompanied with objective short‐term tumor responses. We considered that a further trial with early‐stage CRPC patients based on OS as the primary endpoint of efficacy should be initiated in the future. The PSA response following cancer vaccine therapy for CRPC was generally low. Only a few studies have reported a decrease in PSA ≥50% in a small number of patients. The lack of PSA response with vaccine‐based therapies makes quantifying the clinical benefit of cancer vaccines especially difficult. In a phase 3 study with Sipuleucel‐T using a dendritic cell‐based cancer vaccine therapy for metastatic CRPC, the PSA response rate was only 2.6%.7 In our study, only one patient showed a gradual decline in PSA. We consider that survival advantage is the true effect of the vaccine treatment for CRPC patients even if PSA decline and tumor cell destruction are not observed.
More recently, immune checkpoint blockade by anti‐PD‐1/PD‐L1 or anti‐CTLA‐4 antibodies is considered one of the most promising methods to treat advanced cancer patients. For this type of treatment, the presence of CTLs recognizing a cancer‐specific antigen with an HLA class I molecule on cancer cells is now suggested as a critical predictor to better clinical outcome. Particularly, CTLs recognizing neoantigens generated by somatic mutations in cancer cells are considered strong inducers for CTLs in cancer tissues.27, 28 CTLs specifically recognizing an oncoantigen‐derived peptide such as CDCA1, which are broadly expressed on cancer cells but not in normal cells, may also contribute to the clinical outcome of immune checkpoint blockade therapies. Currently, although neoantigens are certainly more cancer‐specific than tumor antigen‐derived peptides, we still do not know which induces a higher level of anti‐tumor immune responses in cancer patients. In addition, it is also certain that HLA‐restricted cancer peptide vaccines derived from oncoantigens can be more widely applicable to a larger subset of cancer patients than individualized neoantigens. We suspect that a combination of immune checkpoint blockade with CTL‐inducing active immunotherapy with neoantigens or oncoantigens could enhance the clinical benefit.
In conclusion, CDCA1‐peptide vaccine therapy was well tolerated without severe adverse events, and it effectively induced peptide‐specific CTLs. This vaccine therapy might also provide clinical benefit by extending survival and maintaining the QOL of CRPC patients. In future, a randomized, controlled clinical trial will be essential to demonstrate the clinical benefits.
Disclosure Statement
The authors have no conflict of interest.
Abbreviations
- AE
Adverse Event
- CDCA1
Cell division associated 1
- CRPC
castration resistant prostate cancer
- CT
computed tomography
- ELISPOT
Enzyme‐Linked ImmunoSpot
- GMP
Good Manufacturing Practice
- MRI
magnetic resonance imaging
- NCI‐CTC
National Cancer Institute Common Terminology Criteria
- PBMCs
Peripheral blood mononuclear cells
- PD
progressive disease
- PSA
prostate specific antigen
- RECIST
Response Evaluation Criteria in Solid Tumors
Supporting information
Fig. S1. Representative results of immunohistochemical analysis using cancer tissues of prostate biopsy obtained from case 4. (×200). (a) Expression of HLA class I was strongly positive, expression of CDCA1 was positive in tumor cells. (b) No expression of CD4 or CD56 was shown, and expression of CD3, CD8, and CD16 were weakly positive in tumor‐infiltrating lymphocytes. Staining of FOXP3 was strongly positive.(c) No expression of PD‐1 was shown, and expression of PD‐L1 and PD‐L2 was strongly positive in tumor cells. The following Abs were employed; anti‐HLA class I‐A,B,C (mouse monoclonal, EMR8‐56, HoKudo, Sapporo Japan), anti‐Nuf2 (synonymous to CDCA1) antibody ((mouse monoclonal, 29‐37, Abcam, Cambridge, UK), anti‐CD3 antibody (rabbit polyclonal; DAKO Glostrup Denmark), anti‐CD4 antibody (mouse monoclonal, 4B12, DAKO), anti‐CD8 antibody (mouse monoclonal, C8/144B, DAKO), anti‐CD56 antibody (mouse monoclonal, 123C3, DAKO), anti‐FOXP3 antibody (mouse monoclonal, 22510, Abcam), anti‐PD‐1 antibody (rabbit monoclonal, SP269, spring bioscience, California USA), anti‐PD‐L1 antibody (rabbit monoclonal, 28‐8, Abcam), anti‐PD‐L2 antibody (rabbit polyclonal, Abcam).
Table S1. Immunohistochemical analysis using multiple immune molecules
Acknowledgments
We would like to thank Koji Yoshida and Takuya Tsunoda for useful advice, and Reiko Shinagawa for technical assistance at Iwate Medical University.
Cancer Sci 108 (2017) 1452–1457
References
- 1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60: 277–300. [DOI] [PubMed] [Google Scholar]
- 2. Petrylak DP, Tangen CM, Hussain MH et al Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004; 351: 1513–20. [DOI] [PubMed] [Google Scholar]
- 3. Tannock IF, de Wit R, Berry WR et al Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–12. [DOI] [PubMed] [Google Scholar]
- 4. de Bono JS, Logothetis CJ, Molina A et al Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364: 1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Bono JS, Oudard S, Ozguroglu M et al Prednisone plus cabazitaxel or mitoxantrone for metastatic castration‐resistant prostate cancer progressing after docetaxel treatment: a randomised open‐label trial. Lancet 2010; 376: 1147–54. [DOI] [PubMed] [Google Scholar]
- 6. Scher HI, Fizazi K, Saad F et al Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367: 1187–97. [DOI] [PubMed] [Google Scholar]
- 7. Kantoff PW, Higano CS, Shore ND et al Sipuleucel‐T immunotherapy for castration‐resistant prostate cancer. N Engl J Med 2010; 363: 411–22. [DOI] [PubMed] [Google Scholar]
- 8. Kwon ED, Drake CG, Scher HI et al Ipilimumab versus placebo after radiotherapy in patients with metastatic castration‐resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184‐043): a multicentre, randomised, double‐blind, phase 3 trial. Lancet Oncol 2014; 15: 700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hayama S, Daigo Y, Kato T et al Activation of CDCA1‐KNTC2, members of centromere protein complex, involved in pulmonary carcinogenesis. Cancer Res 2006; 66: 10339–48. [DOI] [PubMed] [Google Scholar]
- 10. Harao M, Hirata S, Irie A et al HLA‐A2‐restricted CTL epitopes of a novel lung cancer‐associated cancer testis antigen, cell division cycle associated 1, can induce tumor‐reactive CTL. Int J Cancer 2008; 123: 2616–25. [DOI] [PubMed] [Google Scholar]
- 11. Tomita Y, Yuno A, Tsukamoto H et al Identification of CDCA1‐derived long peptides bearing both CD4+ and CD8+ T‐cell epitopes: CDCA1‐specific CD4+ T‐cell immunity in cancer patients. Int J Cancer 2014; 134: 352–66. [DOI] [PubMed] [Google Scholar]
- 12. Miyazawa M, Ohsawa R, Tsunoda T et al Phase I clinical trial using peptide vaccine for human vascular endothelial growth factor receptor 2 in combination with gemcitabine for patients with advanced pancreatic cancer. Cancer Sci 2010; 101: 433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoshitake Y, Fukuma D, Yuno A et al Phase II clinical trial of multiple peptide vaccination for advanced head and neck cancer patients revealed induction of immune responses and improved OS. Clin Cancer Res 2015; 21: 312–21. [DOI] [PubMed] [Google Scholar]
- 14. Obara W, Karashima T, Takeda K et al Effective induction of cytotoxic T cells recognizing an epitope peptide derived from hypoxia‐inducible protein 2 (HIG2) in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother 2017; 66: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suzuki N, Hazama S, Iguchi H et al A phase IotaI clinical trial of peptide cocktail therapy for patients with advanced pancreatic cancer: VENUS‐PC study. Cancer Sci 2017; 108: 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tomita Y, Imai K, Senju S et al A novel tumor‐associated antigen, cell division cycle 45‐like can induce cytotoxic T‐lymphocytes reactive to tumor cells. Cancer Sci 2011; 102: 697–705. [DOI] [PubMed] [Google Scholar]
- 17. Scher HI, Halabi S, Tannock I et al Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008; 26: 1148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kono K, Iinuma H, Akutsu Y et al Multicenter, phase II clinical trial of cancer vaccination for advanced esophageal cancer with three peptides derived from novel cancer‐testis antigens. J Transl Med 2012; 10: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cecco S, Muraro E, Giacomin E et al Cancer vaccines in phase II/III clinical trials: state of the art and future perspectives. Curr Cancer Drug Targets 2011; 11(1): 85–102. [DOI] [PubMed] [Google Scholar]
- 20. Lesterhuis WJ, Haanen JB, Punt CJ. Cancer immunotherapy–revisited. Nat Rev Drug Discov 2011; 10: 591–600. [DOI] [PubMed] [Google Scholar]
- 21. Germeau C, Ma W, Schiavetti F et al High frequency of antitumor T cells in the blood of melanoma patients before and after vaccination with tumor antigens. J Exp Med 2005; 201: 241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Vries IJ, Bernsen MR, Lesterhuis WJ et al Immunomonitoring tumor‐specific T cells in delayed‐type hypersensitivity skin biopsies after dendritic cell vaccination correlates with clinical outcome. J Clin Oncol 2005; 23: 5779–87. [DOI] [PubMed] [Google Scholar]
- 23. Fizazi K, Scher HI, Molina A et al Abiraterone acetate for treatment of metastatic castration‐resistant prostate cancer: final overall survival analysis of the COU‐AA‐301 randomised, double‐blind, placebo‐controlled phase 3 study. Lancet Oncol 2012; 13: 983–92. [DOI] [PubMed] [Google Scholar]
- 24. Parker C, Nilsson S, Heinrich D et al Alpha emitter radium‐223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369: 213–23. [DOI] [PubMed] [Google Scholar]
- 25. Noguchi M, Moriya F, Suekane S et al Phase II study of personalized peptide vaccination for castration‐resistant prostate cancer patients who failed in docetaxel‐based chemotherapy. Prostate 2012; 72: 834–45. [DOI] [PubMed] [Google Scholar]
- 26. Yoshimura K, Minami T, Nozawa M et al A Phase 2 randomized controlled trial of personalized peptide vaccine immunotherapy with low‐dose dexamethasone versus dexamethasone alone in chemotherapy‐naive castration‐resistant prostate cancer. Eur Urol 2016; 70(1): 35–41. [DOI] [PubMed] [Google Scholar]
- 27. Snyder A, Makarov V, Merghoub T et al Genetic basis for clinical response to CTLA‐4 blockade in melanoma. N Engl J Med 2014; 371: 2189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Le DT, Uram JN, Wang H et al PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med 2015; 372: 2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Representative results of immunohistochemical analysis using cancer tissues of prostate biopsy obtained from case 4. (×200). (a) Expression of HLA class I was strongly positive, expression of CDCA1 was positive in tumor cells. (b) No expression of CD4 or CD56 was shown, and expression of CD3, CD8, and CD16 were weakly positive in tumor‐infiltrating lymphocytes. Staining of FOXP3 was strongly positive.(c) No expression of PD‐1 was shown, and expression of PD‐L1 and PD‐L2 was strongly positive in tumor cells. The following Abs were employed; anti‐HLA class I‐A,B,C (mouse monoclonal, EMR8‐56, HoKudo, Sapporo Japan), anti‐Nuf2 (synonymous to CDCA1) antibody ((mouse monoclonal, 29‐37, Abcam, Cambridge, UK), anti‐CD3 antibody (rabbit polyclonal; DAKO Glostrup Denmark), anti‐CD4 antibody (mouse monoclonal, 4B12, DAKO), anti‐CD8 antibody (mouse monoclonal, C8/144B, DAKO), anti‐CD56 antibody (mouse monoclonal, 123C3, DAKO), anti‐FOXP3 antibody (mouse monoclonal, 22510, Abcam), anti‐PD‐1 antibody (rabbit monoclonal, SP269, spring bioscience, California USA), anti‐PD‐L1 antibody (rabbit monoclonal, 28‐8, Abcam), anti‐PD‐L2 antibody (rabbit polyclonal, Abcam).
Table S1. Immunohistochemical analysis using multiple immune molecules


