Abstract
It is well known that comedo necrosis is closely associated with an aggressive phenotype of ductal carcinoma in situ (DCIS) of human breast, but its molecular mechanisms remain largely unclear. Therefore, in this study, we first examined the gene expression profile of comedo DCIS based on microarray data and identified CYC1 as a gene associated with comedo necrosis. Cytochrome c1 (CYC1) is a subunit of complex III in the mitochondrial oxidative phosphorylation that is involved in energy production. However, the significance of CYC1 has not yet been examined in DCIS. We therefore immunolocalized CYC1 in 47 DCIS cases. CYC1 immunoreactivity was detected in 40% of DCIS cases, and the immunohistochemical CYC1 status was significantly associated with tumor size, nuclear grade, comedo necrosis, van Nuys classification, and Ki‐67 labeling index. Subsequent in vitro studies indicated that CYC1 was significantly associated with mitochondrial membrane potential in MCF10DCIS.com DCIS cells. Moreover, CYC1 significantly promoted proliferation activity of MCF10DCIS.com cells and the cells transfected with CYC1 siRNA decreased pro‐apoptotic caspase 3 activity under hypoxic or anoxic conditions. Considering that the center of DCIS is poorly oxygenated, these results indicate that CYC1 plays important roles in cell proliferation and comedo necrosis through the elevated oxidative phosphorylation activity in human DCIS.
Keywords: Comedo necrosis, CYC1, DCIS, immunohistochemistry, proliferation
Breast cancer is the most common malignant neoplasm in women worldwide. Among these, the incidence of DCIS has been markedly increasing during the past two decades, and now accounts for >20% of all breast cancer diagnoses.1 Ductal carcinoma in situ is generally considered a precursor lesion of invasive ductal carcinoma;2 it is very important to examine the biological features of DCIS to improve clinical outcomes for breast cancer patients.
Ductal carcinoma in situ encompasses a wide spectrum of diseases ranging from low‐grade lesions that are not life threatening to high‐grade lesions. It is typically classified according to architectural pattern (e.g., solid, cribriform, papillary), grade of nuclear atypia, and presence or absence of comedo necrosis that exists in the center of DCIS lesion with calcification.3 Comedo necrosis is reportedly associated with higher proliferation activity4 and clinically aggressive behavior,5 and it is considered an important factor predicting local recurrence after surgical treatment in DCIS patients.6, 7 Comedo necrosis can be easily recognized in clinical practice, but the occurring mechanism remains largely unknown. Therefore, in this study, we studied the gene expression profile of comedo DCIS based on microarray data and identified CYC1 as a gene closely associated with comedo necrosis.
It is well known that mitochondria are indispensable for energy metabolism. Approximately 90% of cellular ATP is generated in mitochondria through the OXPHOS pathway,8 and CYC1 (also referred to cytochrome c1) is a heme‐containing subunit of complex III. Overexpression of CYC1 mRNA has been reported in human osteosarcoma and nasopharyngeal carcinoma,9, 10 and very recently Han et al.11 reported that CYC1 immunoreactivity was increased in breast carcinoma compared to the benign mass. However, the significance of CYC1 has not been known in DCIS to the best of our knowledge. Therefore, we examined CYC1 in DCIS using immunohistochemistry and in vitro studies to explore its clinical and biological significance.
Materials and Methods
Patients and tissues
Two sets of tissue specimens were evaluated in this study. The first set consisted of four specimens of ER‐positive pure DCIS (two comedo DCIS and two non‐comedo DCIS cases) obtained from Japanese female patients (age range, 51–77 years) who underwent surgical treatment between 2003 and 2008 in the Department of Surgery, Tohoku University Hospital (Sendai, Japan). These specimens were stored at −80°C for subsequent microarray analysis.
As a second set, 47 specimens of pure DCIS were obtained from Japanese female patients (age range, 39–80 years) who underwent surgical treatment between 1998 and 2005 in the Department of Surgery, Tohoku University Hospital. All specimens had been fixed in 10% formalin and embedded in paraffin wax. The tumor size was histologically evaluated in 39 cases. Nuclear grade12 and van Nuys classification6 were determined according to previous reports. The mean follow‐up time after surgery was 46 months (range, 8–104 months) in this study, and no patients had recurrent disease or died within the duration.
The research protocol was approved by the Ethics Committee at Tohoku University School of Medicine.
Laser capture microdissection/microarray analysis
Gene expression profiles of ER‐positive pure DCIS were examined using microarray analysis, and these were assembled previously.13 Briefly, DCIS cells were dissected under the light microscope and laser transferred from these frozen sections. Whole Human Genome Oligo Microarray (G4112F; ID: 012391; Agilent Technologies, Waldbronn, Germany), containing 41 000 unique probes, was used in this study, and sample preparation and processing were carried out according to the manufacturer's protocol. The relative levels of gene expression were calculated by global normalization, and scatter plot and GO analyses of the microarray data were carried out using GeneSpring 12.6.1 (Agilent Technologies).
Immunohistochemistry
Rabbit polyclonal antibody for CYC1 (PA5‐25257) and mouse mAb for Ki‐67 (MIB1) were purchased from Thermo Fisher Scientific (Waltham, MA, USA) and Dako (Carpinteria, CA, USA), respectively. The antigen–antibody complex was visualized with DAB solution and counterstained with hematoxylin. We used human tissue of the stomach as a positive control based on a data sheet of CYC1 antibody by Novus Biologicals (http://www.novusbio.com/CYC1-Antibody_NBP1-86872.html#dsTab0) and normal rabbit IgG instead of the primary antibody as a negative control for CYC1 immunostaining in this study.
Immunohistochemistry for ER (CONFIRM anti‐ER [SP1]; Roche Diagnostics Japan, Tokyo, Japan) and PR (CONFIRM anti‐PR [1E2]; Roche Diagnostics Japan) was carried out with Ventana Benchmark XT (Roche Diagnostics Japan), and that for HER2 was carried out using HercepTest (Dako).
Scoring of immunoreactivity
CYC1 was immunolocalized in the cytoplasm of carcinoma cells, and weakly present in the epithelium of non‐neoplastic mammary glands. We evaluated CYC1‐positive carcinoma cells if the CYC1 immunoreactivity was stronger compared to the non‐neoplastic mammary glands. Cases that had more than 10% positive carcinoma cells were considered positive for CYC1 status, as the 10% cut‐off value is frequently used as a reproducible evaluation of various cytoplasmic immunostaining.14, 15 Immunoreactivity for ER, PR, and Ki‐67 was detected in the nucleus, and the percentage of immunoreactivity (labeling index; LI) was determined. Cases with ER LI >1% were considered ER‐positive breast carcinoma according to a previous report.16 HER2 immunoreactivity was evaluated according to the grading system proposed in HercepTest (Dako).
Cell line
The MCF10DCIS.com DCIS cell line was purchased from Asterand (Detroit, MI, USA), and cultured in RPMI‐1640 (Sigma‐Aldrich, St.Louis, MO, USA) containing 10% FBS (Thermo Fisher Scientific). MCF10DCIS.com cells generated basal‐type lesions and formed comedo DCIS in a mouse transplantation model.17, 18
Small interfering RNA transfection
Two siRNA oligonucleotides for siCYC1 used in this study were designed as follows: siCYC1‐1 (sense, 5′‐rGrCUrGUUrCrGrArCUrAUUUrCrCrCrATT‐3′; antisense, 5′‐UrGrGrGrArArAUrArGUrCrGrArArCrArGrCTT‐3′) and siCYC1‐2 (sense, 5′‐rGUrCrArCUrGrCrGrGrGrArArGrGUrCUrCTT‐3′; antisense, 5′‐rGrArGrArCrCUUrCrCrCrGrCrArGUrGrArCTT‐3′). These were purchased from Sigma‐Aldrich. MISSION siRNA Universal Negative Control (Sigma‐Aldrich) was used as siCTRL. The siRNAs were transfected using the Lipofectamine 3000 Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol.
Plasmid transfection
The CYC1 expression plasmid was constructed by inserting a full‐length ORF of human CYC1 mRNA (NM_001916) into pBApo‐CMV Pur vector (Takara Bio, Otsu, Japan) using the restriction enzymes BamHI and HindIII (CYC1 plasmid). The plasmid was transfected into MCF10DCIS.com cells using Lipofectamine 3000 Reagent (Invitrogen). As a control, empty vector pcDNA3.1(−) was transfected in this study.
Real‐time PCR
Total RNA was extracted using TRI Reagent (Molecular Research Center, Cincinnati, OH, USA), and cDNA was synthesized using a ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo, Osaka, Japan). Real‐Time PCR was carried out using the THUNDERBIRD SYBR qPCR Mix (Toyobo). The primer sequences of CYC1 and RPL13A were: CYC1 (NM_001916), 5′‐GAGGTGGAGGTTCAAGACGG‐3′ (forward) and 5′‐TAGCTCGCACGATGTAGCTG‐3′ (reverse)19; and RPL13A (NM_012423), 5′‐CCTGGAGGAGAAGAGGAAAGAGA‐3′ (forward) and 5′‐TTGAGGACCTCTGTGTATTTGTCAA‐3′ (reverse). The CYC1 mRNA level was calculated as the ratio of the RPL13A mRNA level in this study.
Immunoblotting
The mitochondrial protein from MCF10DCIS.com cells was extracted according to a previous report20 and M‐PER (Thermo Fisher Scientific Pierce Biotechnology, Rockford, IL, USA). The lysate proteins (2 μg) were subjected to SDS–PAGE (10% acrylamide gel) and transferred onto Hybond PVDF membranes (GE Healthcare, Little Chalfont, UK). The primary anti‐CYC1 antibody used was the same as that in the immunohistochemistry. Antimitochondrial voltage‐dependent anion channel 1 antibody (sc‐390996; Santa Cruz Biotechnology, Dallas, TX, USA) was also used as an internal control for mitochondrial protein.21
Measurement of mitochondrial membrane potential
Mitochondrial potential was analyzed using a JC‐1 Mitochondrial Membrane Potential Assay Kit (Cayman Chemical, Ann Arbor, MI, USA). After staining, flow cytometric analysis was undertaken acquiring FL1 (green; JC‐1 monomers) and FL2 (red; JC‐1 aggregates) signals using a fluorescence microscope (FSX100; Olympus, Tokyo, Japan).
Cell proliferation assay and caspase 3 activity assay under hypoxic conditions
The cell proliferation status was measured by the WST‐8 method using Cell Counting Kit‐8 (Dojindo Molecular Technologies, Kumamoto, Japan) 4 days after the transfection in these cells. Hypoxic (10% 02) and anoxic (0% 02) conditions were generated using an Anaeropack (Mitsubishi Gas Chemicals, Tokyo, Japan). Pro‐apoptotic caspase 3 activity was evaluated using a CaspGLOW Fluorescein Active Caspase‐3 Staining Kit (BioVision, Milpitas, CA, USA). The luminescence of each sample in a plate‐reading luminometer was measured as directed by the luminometer manufacturer (PHERAstar FS; BMG LABTECH JAPAN, Saitama, Japan).
Statistical analysis
Immunohistochemical CYC1 status and clinicopathologic factors were evaluated by Student's t‐test or a cross‐table using the χ2‐test. In the in vitro experiments, the statistical analyses were carried out using Student's t‐test and Fisher's PLSD test. The statistical analyses were undertaken using StatView 5.0J software (SAS Institute, Cary, NC, USA), and P < 0.05 was considered significant in this study.
Results
Gene expression profile in comedo DCIS
We first examined gene expression profiles of DCIS cells according to the status of comedo necrosis by microarray analysis. In this analysis, when the expression ratio of a gene in the comedo DCIS group compared to that in the non‐comedo group was >2.0 or <0.5, we determined that the gene was predominantly expressed in the comedo or non‐comedo group respectively, according to the cut‐off value previously used.22 As shown in Figure 1(a), a scatter plot revealed that 2871 genes (7%) were predominantly expressed in the comedo type (group A; comedo DCIS/non‐comedo DCIS ratio >2.0), 1750 genes (4%) were mainly expressed in the non‐comedo type (group B; ratio <0.5), and 36 472 genes (89%) had a similar expression level in both groups (group C; ratio 0.5–2.0).
Figure 1.
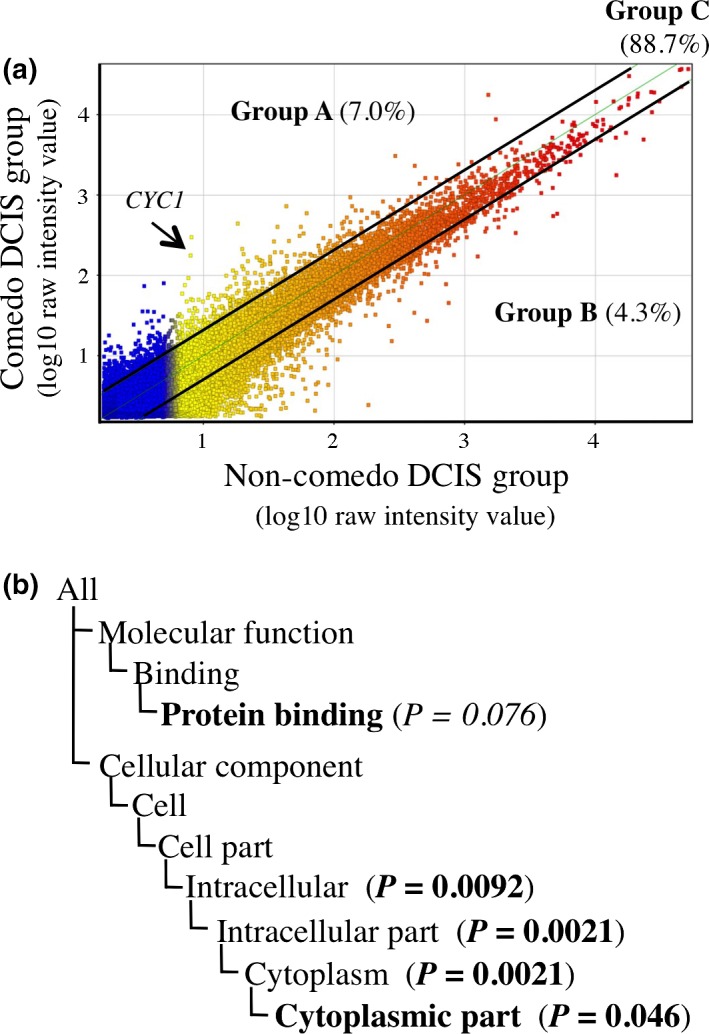
Gene expression profile in comedo ductal carcinoma in situ (DCIS). (a) Scatter plot analysis of microarray data of DCIS according to the status of comedo necrosis (n = 2 each group), in which 41 093 genes were plotted on the logarithmic graph. Those genes that were more than 2.0‐fold higher in the comedo DCIS group or non‐comedo DCIS group were located outside of the diagonal line, and classified as group A or group B, respectively. Genes with ≤2.0‐fold change were classified as group C. Arrow, location of cytochrome c1 (CYC1). (b) Gene ontology (GO) terms enriched in the comedo DCIS group compared to all the DCIS cases in group A genes by GO analysis. P‐value <0.05 and 0.05 ≤ P‐value < 0.10 were considered significant and marginally significant, respectively. GO terms are summarized. Bold, GO terms whose gene lists are summarized in Table 1. P‐values are summarized following the Gene Ontology Consortium (http://geneontology.org/page/go-enrichment-analysis). P‐values indicate the probability or chance of seeing at least x number of genes out of the total n genes in the list annotated to a particular GO term, given the proportion of genes in the whole genome that were annotated to that GO term. The closer the P‐value is to zero, the more significant the particular GO term associated with the group of genes (i.e., the less likely the observed annotation of the particular GO term to a group of genes occurred by chance).
When we undertook GO analysis in group A genes, “intracellular” (P = 0.0092), “intracellular part” (P = 0.0021), “cytoplasm” (P = 0.0021) and “cytoplasmic part” (P = 0.046) were identified as significantly enriched GO terms in comedo DCIS compared to all the DCIS cases, and “protein binding” (P = 0.075) was marginally significant (Fig. 1b). The definitions of these GO terms are summarized in Table S1. As shown in Table 1, CYC1 showed the highest increase (31‐fold) among the genes in the “cytoplasmic part”, indicating its possible involvement in comedo necrosis. S100A8 showed the highest increase (34‐fold) among the genes in “protein binding,” which was a marginally enriched GO term in comedo DCIS in this study. The known functions of these genes are briefly described in Table S2.
Table 1.
Top 10 genes in “cytoplasmic part” (P = 0.046) and “protein binding” (P = 0.075) in gene ontology analysis of comedo and non‐comedo ductal carcinoma in situ (DCIS) tissue
| Cytoplasmic part | Protein binding | ||
|---|---|---|---|
| Fold | Gene symbol | Fold | Gene symbol |
| 31.2 | CYC1 | 33.7 | S100A8 |
| 15.2 | S100A7 | 15.2 | S100A7 |
| 13.4 | KCNA5 | 14.6 | WISP2 |
| 12.2 | KCNK1 | 14.6 | GSTM3 |
| 11.7 | PLEK | 13.5 | CD24 |
| 9.55 | HSD17B1 | 13.4 | KCNA5 |
| 9.19 | CX3CL1 | 11.8 | MET |
| 8.73 | PTN | 11.7 | PLEK |
| 8.31 | CYB5R2 | 9.34 | IGFBP7 |
| 7.98 | CXCR4 | 9.22 | S100A9 |
Bold text indicates a gene identified by immunohistochemistry. Italic text shows genes described in the Discussion section. Fold, expression ratio in comedo DCIS group to that in non‐comedo DCIS group.
CYC1 immunolocalization in human DCIS
Immunoreactivity of CYC1 was detected in the cytoplasm of carcinoma cells in DCIS tissues (Fig. 2a–c). CYC1 was also weakly present in the cytoplasm of epithelial cells in non‐neoplastic glands, but it was negative in the stroma (Fig. 2d). In the positive control, CYC1 was immunolocalized in the epithelium of the fundic glands in the stomach (Fig. 2e,f).
Figure 2.
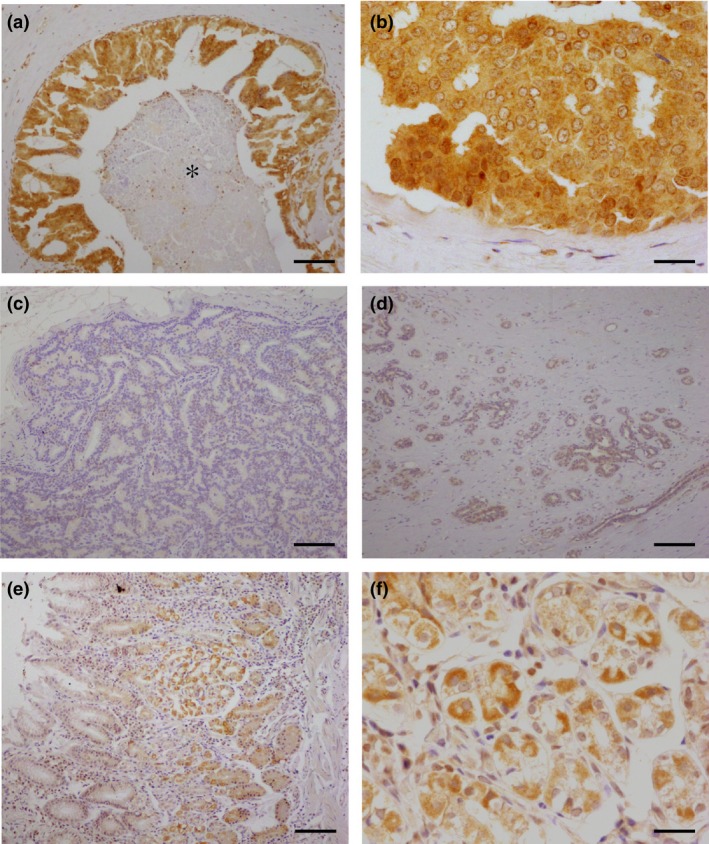
Immunohistochemistry for cytochrome c1 (CYC1) in ductal carcinoma in situ (DCIS). (a,b) Higher magnification, CYC1 was immunolocalized in the cytoplasm of DCIS cells. *Comedo necrosis. (c) CYC1‐negative DCIS case. (d) CYC1 immoreactivity was weakly observed in the morphologically normal mammary gland. (e,f) Higher magnification, in a positive control section, CYC1 immunoreactivity was detected in the fundic glands of the stomach. Bar = 100 μm (a,c–f) or 400 μm (b,f).
Associations between immunohistochemical CYC1 status and clinicopathologic parameters in DCIS are shown in Table 2. When DCIS cases showed stronger CYC1 immunointensity compared with the non‐neoplastic mammary gland, it was considered positive for CYC1 status. The number of CYC1‐positive DCIS was 19 of 47 (40%) cases examined. The CYC1 status was significantly associated with tumor size (P = 0.048), nuclear grade (P = 0.032), comedo necrosis (P = 0.0065), van Nuys classification (P = 0.0021), and Ki‐67 LI (P = 0.011). No significant association was detected between CYC1 status and patient age, menopausal status, ER status, PR LI, or HER2 status in this study.
Table 2.
Association between immunohistochemical cytochrome c1 (CYC1) status and clinicopathological parameters in 47 cases of ductal carcinoma in situ
| CYC1 status | P‐value | ||
|---|---|---|---|
| + (n = 19) | − (n = 28) | ||
| Age, years† | 60.3 ± 2.5 | 58.9 ± 1.7 | 0.650 |
| Menopausal status | |||
| Premenopausal | 3 | 6 | |
| Postmenopausal | 16 | 22 | 0.630 |
| Tumor size, cm† | 4.0 ± 0.6 | 2.7 ± 0.3 | 0.048 |
| Nuclear grade | |||
| 1 + 2 | 12 | 25 | |
| 3 | 7 | 3 | 0.032 |
| Comedo necrosis | |||
| Present | 17 | 15 | |
| Absent | 2 | 13 | 0.007 |
| van Nuys classification | |||
| 1 | 0 | 12 | |
| 2 | 12 | 13 | |
| 3 | 7 | 3 | 0.002 |
| ER status | |||
| Positive | 18 | 26 | |
| Negative | 1 | 2 | 0.800 |
| PR LI, %†, ‡ | 49.8 ± 7.4 | 44.0 ± 6.1 | 0.550 |
| HER2 status | |||
| Positive | 10 | 9 | |
| Negative | 9 | 19 | 0.160 |
| Ki‐67 LI, %† | 6.5 ± 0.8 | 4.1 ± 0.6 | 0.011 |
†Data are presented as mean ± SEM. All other values represent the number of cases. ‡Three estrogen receptor (ER)‐negative cases were excluded. Statistical analysis was evaluated by Student's t‐test or a cross‐table using the χ2‐test. P < 0.05 were considered significant (shown in bold). HER2, human epidermal growth factor receptor 2; LI, labeling index; PR, progesterone receptor.
We could not examine the association between CYC1 status and prognosis of DCIS patients in this study, because no patients had recurrent disease or died within the follow‐up duration after surgery.
Association between CYC1 expression and mitochondrial membrane potential in DCIS cells
In this study, microarray data identified CYC1 as a gene associated with comedo necrosis in ER‐positive DCIS, and the following immunohistochemical analysis showed CYC1 immunoreactivity was detected in DCIS tissues regardless of the ER status. To further evaluate the biological functions of CYC1 in human breast DCIS cells, we next transfected specific siRNA for CYC1 in ER‐negative MCF10DCIS.com cells, because no ER‐positive DCIS cells were available to the best of our knowledge. The mRNA expression level of CYC1 was significantly decreased in cells transfected with siCYC1‐1 (Fig. 3a, upper panel) and siCYC1‐2 (Fig. 3b, upper panel) in a dose‐dependent manner at 3 days after transfection compared with those in cells transfected with siCTRL (10 nM). When the cells were transfected with 10 nM siRNA, the ratio of CYC1 mRNA level to the control level and its P‐value were 30% and P < 0.001 (siCYC1‐1), and 39% and P < 0.001 (siCYC1‐2). The mitochondrial protein levels of CYC1 were also markedly decreased in cells transfected with CYC1 siRNA compared with those in the control cells under the same conditions (Fig. 3a,b, lower panels).
Figure 3.
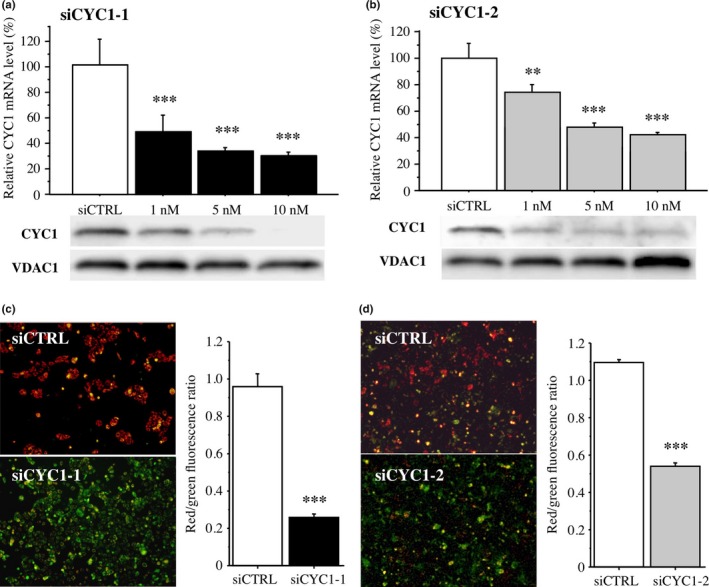
Effects of cytochrome c1 (CYC1) on mitochondrial membrane potential in MCF10DCIS.com ductal carcinoma in situ cells. (a,b) Upper panel shows expression of CYC1 mRNA in MCF10DCIS.com cells transfected with CYC1‐specific siRNA (siCYC1‐1 [a, closed bar] and siCYC1‐2 [b, gray bar]) or negative control siRNA (siCTRL, open bar) by real‐time PCR. Lower panel shows the corresponding CYC1 immunoreactivity by immunoblotting. Voltage‐dependent anion channel 1 (VDAC1) immunoreactivity is shown as an internal control of mitochondrial protein. (c,d) JC‐1 assays in MCF10DCIS.com cells transfected with siCYC1‐1 (c; 10 nM, closed bar) and siCYC1‐2 (d; 10 nM, gray bar) or siCTRL (open bar). Left panels show representative merged images. Green fluorescence shows JC‐1 monomer (low mitochondrial membrane potential) and red fluorescence represents JC‐1 aggregate (high mitochondrial membrane potential); cells show yellow if both fluorescence are similar levels. Right panels show their red/green fluorescence ratio. Data are presented as mean ± SD (n = 3). Statistical analyses were carried out using Student's t‐test and Fisher's PLSD test. **P < 0.01; ***P < 0.001 versus control cells transfected with siCTRL (left open bar).
JC‐1 produces two fluorescence emission peaks. The JC‐1 monomer, which is the predominant form at low mitochondrial membrane potential, emits green fluorescence, and the JC‐1 aggregates, the predominant species at high mitochondrial membrane potential, emit red fluorescence. When we carried out the JC‐1 assay, green fluorescence was predominantly detected in MCF10DCIS.com cells transfected with siCYC1‐1 (Fig. 3c) and siCYC1‐2 (Fig. 3d), whereas red fluorescence was mainly detected in MCF10DCIS.com cells transfected with siCTRL, in the merged image. The red/green ratio, which reflects mitochondrial membrane potential,23 was significantly lower in cells transfected with siCYC1‐1 (27%, P < 0.001) and siCYC1‐2 (49%, P < 0.001). These results suggest that CYC1 is associated with mitochondrial membrane potential in MCF10DCIS.com cells.
Effects of CYC1 on cell proliferation and caspase 3 activity under hypoxia in DCIS cells
As CYC1 status was significantly associated with Ki‐67 LI in DCIS tissues in this study, we next examined the effects of CYC1 expression on cell proliferation in MCF10DCIS.com cells. As shown in Figure 4(a,b), the number of cells transfected with CYC1 siRNA was dose‐dependently decreased compared to that of the control cells transfected with siCTRL cells 4 days after transfection (10 nM; 63% and P < 0.001 for siCYC1‐1 and 59% and P < 0.001 for siCYC1‐2).
Figure 4.
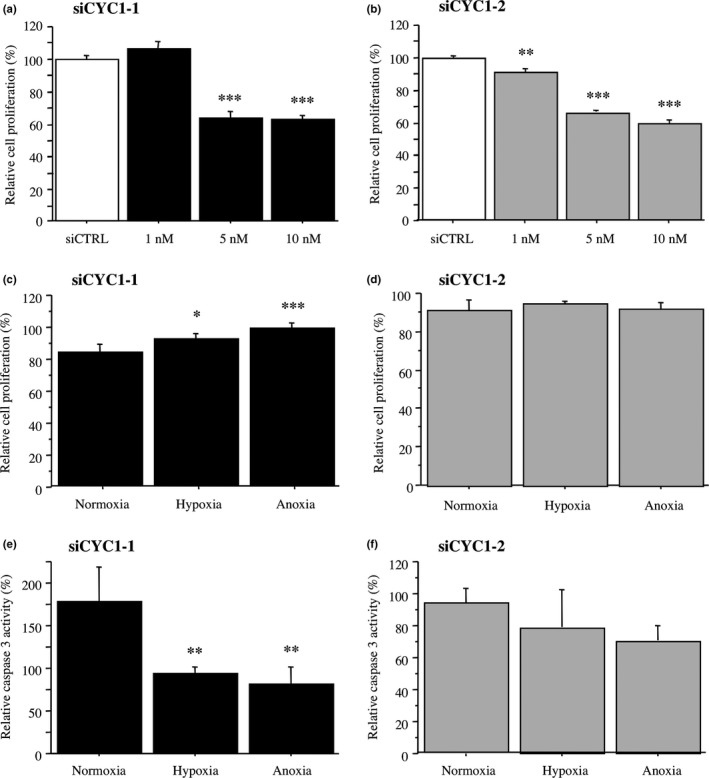
Effects of cytochrome c1 (CYC1) knockdown on cell proliferation and caspase 3 activity under hypoxia in MCF10DCIS.com ductal carcinoma in situ cells. (a,b) WST‐8 assay in MCF10DCIS.com cells transfected with siCYC1‐1 (a, closed bar), siCYC1‐2 (b, gray bar), or negative control siRNA (siCTRL; open bar). Relative proliferative activity of these cells was summarized as a ratio (%) compared to the control cells transfected with siCTRL at 4 days after treatment (left open bar). (c,d) WST‐8 assay in MCF10DCIS.com cells transfected with siCYC1‐1 (10 nM) (c) or siCYC1‐2 (10 nM) (d) under normoxia, hypoxia, or anoxia for 2 days. Relative cell proliferation activity was evaluated as a ratio (%) compared to the mean value of control cells transfected with siCTRL under the same conditions. (e,f) Caspase 3 activity assays in MCF10DCIS.com cells transfected with siCYC1‐1 (10 nM) (e) or siCYC1‐2 (10 nM) (f). Cells were incubated under the indicated conditions for 5 h. Relative caspase 3 activity was evaluated as a ratio (%) compared to the mean value of control cells transfected with siCTRL under the same condition. Data were presented as the mean ± SD (n = 3). Statistical analyses were carried out using Fisher's PLSD test. *P < 0.05; **P < 0.01; ***P < 0.001 versus left bar.
Our immunohistochemical analysis also indicated that CYC1 status was significantly associated with comedo necrosis, suggesting that CYC1 is involved in apoptosis under hypoxic conditions because the center of DCIS is poorly oxygenated.24 Proliferation activity was significantly increased in MCF10DCIS.com cells transfected with siCYC1‐1 cells under hypoxia or anoxia for 2 days (1.1‐fold and P < 0.05 under hypoxia; 1.2‐fold and P < 0.001 under anoxia), whereas it was not significant in MCF10DCIS.com cells transfected with siCYC1‐2 cells under the same conditions (Fig. 4d,e). On the contrary, as shown in Figure 4(e), caspase 3 activity was significantly decreased in MCF10DCIS.com cells transfected with siCYC1‐1 cells when these cells were incubated under hypoxic and anoxic conditions (53% and P < 0.01 under hypoxia; 46% and P < 0.01 under anoxia). Similar tendency was also detected in the cells transfected with siCYC1‐2, although P‐values did not reach significance (Fig. 4f).
We next transfected CYC1 plasmid in MCF10DCIS.com cells. As shown in Figure 5, CYC1 expression level was markedly increased both at mRNA (22‐fold and P < 0.01) (Fig. 5a, upper panels) and protein (Fig. 5a, lower panels) levels; however, the mitochondrial membrane potential (1.03‐fold and P = 0.44) (Fig. 5b) and cell proliferation activity (1.1‐fold and P = 0.26) (Fig. 5c) were not significantly changed. Cell proliferation activity was similar level in MCF10DCIS.com cells transfected with CYC1 plasmid regardless of the oxygen level (Fig. 5d), while the caspase 3 activity was decreased (82% and P < 0.05 under hypoxia; 77% and P < 0.05 under anoxia) (Fig. 5e).
Figure 5.
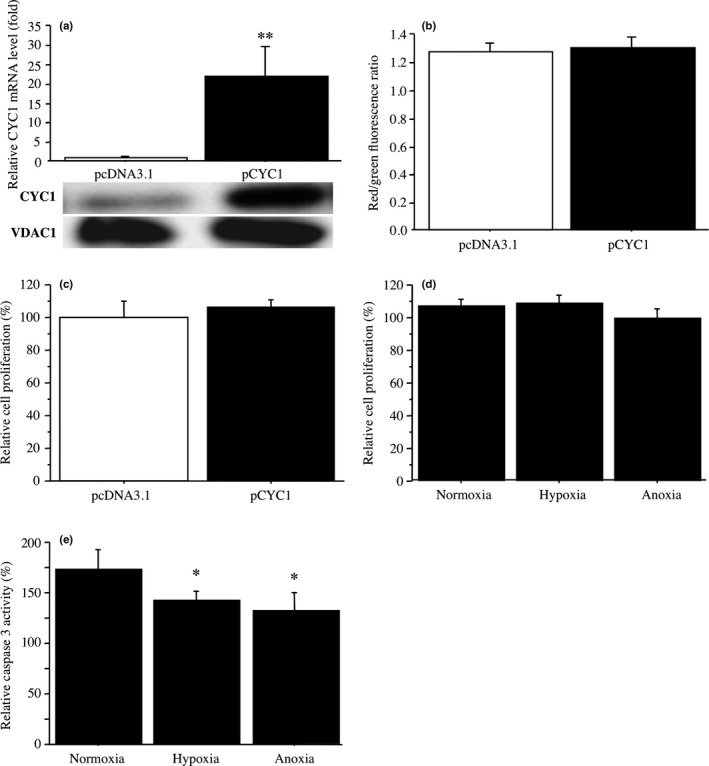
Effects of cytochrome c1 (CYC1) overexpression on mitochondrial membrane potential, cell proliferation, and caspase 3 activity under hypoxia in MCF10DCIS.com ductal carcinoma in situ cells. (a) Upper panel, expression of CYC1 mRNA in MCF10DCIS.com cells transfected with CYC1 plasmid (pCYC1; closed bar) or control plasmid (pcDNA3.1; open bar) by real‐time PCR. Lower panel, corresponding CYC1 immunoreactivity by immunoblotting. (b) JC‐1 assays in MCF10DCIS.com cells transfected with CYC1 plasmid (closed bar) or control plasmid (open bar). Mitochondrial membrane potential was evaluated by red/green fluorescence ratio. (c) WST‐8 assay in MCF10DCIS.com cells transfected with CYC1 plasmid (closed bar). Relative proliferative activity of these cells was summarized as a ratio (%) compared to control cells transfected with control plasmid (closed bar) at 2 days after treatment. (d) WST‐8 assay in MCF10DCIS.com cells transfected with CYC1 plasmid under normoxia, hypoxia, or anoxia for 2 days. Relative cell proliferation activity was evaluated as a ratio (%) compared to the mean value of control cells transfected with control plasmid under the same condition. (e) Caspase 3 activity assays in MCF10DCIS.com cells transfected with CYC1 plasmid under normoxia, hypoxia, or anoxia for 5 h. Relative caspase 3 activity was evaluated as a ratio (%) compared to the mean value of control cells transfected with control plasmid under the same conditions. Data are presented as mean ± SD (n = 3). Statistical analyses were carried out using Fisher's PLSD test. *P < 0.05; **P < 0.01 versus left bar.
Discussion
Gene expression profiling is a very important method to survey molecular features of a selective group of breast carcinoma13, 25 in addition to conventional clinical and histopathologic examinations. This is the first study to show gene expression profiles of comedo DCIS. Results of our microarray analysis did reveal that “cytoplasmic part” and “protein binding” were enriched GO terms in comedo DCIS. Among these genes (Table 1), S100A7 (psoriasin), which is a member of the S100 family of calcium‐binding proteins, and CD24 were linked and related to the differentiation of mammary epithelial cells.26 S100A7 was expressed in DCIS at the highest levels and it is involved in the transition to invasive breast carcinoma.27 S100A8 (calgranulin A) and S100A9 (calgranulin B), also members of the S100 family, have been shown to be highly expressed in DCIS,28 and play important roles in growth, invasion, and metastasis in breast cancer cells.29 Muggerud et al.25 identified a subgroup of high‐grade DCIS that show a distinctive gene expression profile compared to other DCIS and an invasive‐like phenotype by microarray analysis. S100A8 was highly expressed in this subgroup. Taken together with these previous reports and our present results, comedo DCIS is suggested to show aggressive molecular features. CYC1 showed the highest expression ratio in genes of “cytoplasmic part,” a significantly enriched GO term in comedo DCIS (Table 1), but its significance has not yet been established in DCIS to the best of our knowledge.
This is the first study to reveal the clinical significance of CYC1 in DCIS. In this study, CYC1 immunoreactivity was detected in 40% of DCIS cases, whereas it was very weak in the non‐neoplastic epithelium. The CYC1 status was significantly associated with nuclear atypia, comedo necrosis, and van Nuys classification in the DCIS cases. The van Nuys classification was reported to be significantly associated with local recurrence of DCIS, and it has been established as a potent prognostic classification for DCIS patients.6 Previously, Fang et al.9 found that CYC1 expression was upregulated in nasopharyngeal carcinomas compared to non‐cancerous nasopharyngeal tissues by microarray analysis, and Li et al.10 reported that CYC1 was overexpressed in human osteosarcoma tissues and cell lines. Moreover, Han et al.11 very recently reported that CYC1 immunoreactivity was significantly increased in breast carcinoma compared to benign breast tumors. Our present results are consistent with these previous reports, and it is suggested that CYC1 expression is increased in an aggressive phenotype of DCIS compared to normal breast tissue.
Expression of CYC1 is induced by oxygen and repressed by glucose.30 Ivanova et al.31 reported that CYC1 expression was stimulated by estrogen through nuclear respiratory factor 1 in MCF‐7 breast carcinoma cells. However, considering that CYC1 immunoreactivity was relatively widely detected in DCIS tissues, CYC1 expression may be induced in the process of breast carcinogenesis by other factors such as oncogenes. Limited information is available about the regulatory mechanisms of CYC1 expression, and further examination is required.
In our present study, CYC1 immunoreactivity was significantly associated with Ki‐67 LI in DCIS tissues. Ki‐67 antibody recognizes cells in all phases of the cell cycle except G0 (resting) phase, and Ki‐67 LI is well correlated with proliferation activity of breast carcinoma.32 In addition, results of in vitro studies showed that MCF10DCIS.com cells transfected with siRNA against CYC1 significantly decreased the cell proliferation, although ER‐positive DCIS cells were not available for our in vitro experiments. Previously, Li et al.10 reported that CYC1 silencing by shRNA transfection inhibited proliferation activity of osteosarcoma cells in vitro and suppressed tumor growth in a mouse xenograft model in vivo. Han et al.11 also showed that silencing CYC1 suppressed proliferation of invasive breast carcinoma cells (MDA‐MB‐231), consistent with our present results. Our in vitro experiments revealed that CYC1 expression levels reflected mitochondrial membrane potential in MCF10DCIS.com cells, and Owens et al.33 reported that immunoreactivity of UQCRFS1/RISP, which is another subunit of complex III in mitochondrial OXPHOS, was increased in DCIS compared to benign breast tissue. Therefore, it is suggested that CYC1 plays an important role in the cell proliferation of DCIS, possibly through elevated OXPHOS activity.
Although CYC1 is an important subunit of mitochondria complex III,11 MCF10DCIS.com cells transfected with CYC1 plasmid did not significantly increase mitochondrial membrane potential or cell proliferation activity (Fig. 5b,c) in this study. Considering that CYC1 was abundantly expressed in MCF10DCIS.com cells, as shown in Figure 3(a,b), forcibly expressed CYC1 might not cause further increment of OXPHOS activity in MCF10DCIS.com cells.
It is known that proliferating carcinoma cells preferentially use anaerobic glycolysis rather than OXPHOS for energy production, and the metabolic alteration is commonly referred to as the Warburg effect.34 Although efficiency of ATP synthesis in aerobic glycolysis is much lower compared to OXPHOS, the glycolytic pathway can provide precursors for biomolecules necessary for proliferation.8, 35 Indeed, Sato‐Tadano et al.15 showed that immunoreactivity of hexokinase II, which is involved in the rate‐limiting step of glycolysis, was detected in 44% of invasive breast carcinomas and it was a potent worse prognostic factor. However, both OXPHOS and glycolysis are active inside tumor cells at various rates; for instance, the contribution of OXPHOS to the total cellular energy was 80% in MCF‐7 breast carcinoma cells.36 It is also true that the sensitivity of glucose‐based PET is much lower for detecting DCIS than invasive breast cancer.37
In our present in vitro study, MCF10DCIS.com cells transfected with siRNA against CYC1 significantly decreased pro‐apoptotic caspase 3 activity under anaerobic conditions compared to normoxia. Mitochondrial OXPHOS also regulates apoptosis and enhanced OXPHOS function increased intracellular oxidative stress and apoptosis.35, 38, 39 Ducts in DCIS are frequently enlarged beyond 200 μm in diameter, although diffusion of oxygen from periductal vessels into ducts is limited to 100 μm, suggesting that the center of DCIS becomes poorly oxygenated.24 Taken together with these previous findings and our present results, mitochondrial OXPHOS is suggested to play an important role in energy production of DCIS, at the early stages of breast carcinoma, and elevated OXPHOS activity through CYC1 overexpression might cause both increased proliferation activity and central comedo necrosis in DCIS. We could not examine the effects of CYC1 on formation of comedo necrosis in DCIS cells using a mouse transplantation model in this study, and further investigations are required.
In summary, we examined gene expression profiles of comedo DCIS using microarray analysis and showed that CYC1 is associated with comedo necrosis. CYC1 immunoreactivity was detected in 40% of DCIS cases, and it was associated with tumor size, nuclear grade, comedo necrosis, van Nuys classification, and Ki‐67 LI. In vitro studies indicated that CYC1 was associated with mitochondrial membrane potential, increased proliferation activity, and increased pro‐apoptotic caspase 3 activity under hypoxia. These results suggest that CYC1 plays an important role in cell proliferation and comedo necrosis by increasing OXPHOS activity in human DCIS.
Disclosure Statement
The authors have no conflict of interest.
Abbreviations
- CYC1
cytochrome c1
- DCIS
ductal carcinoma in situ
- ER
estrogen receptor
- GO
gene ontology
- HER2
human epidermal growth factor receptor 2
- LI
labeling index
- OXPHOS
oxidative phosphorylation
- PR
progesterone receptor
- RPL13A
ribosomal protein L13A
- siCTRL
negative control siRNA
Supporting information
Table S1. Definition of enriched gene ontology terms in comedo ductal carcinoma in situ cases.
Table S2. Brief functions of genes listed in Table 1.
Cancer Sci 108 (2017) 1510–1519
Funding Information
None declared.
References
- 1. American Cancer Society . Cancer Facts & Figures 2015. Atlanta: American Cancer Society; 2015. Available from URL: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. [Google Scholar]
- 2. Erbas B, Provenzano E, Armes J, Gertig D. The natural history of ductal carcinoma in situ of the breast: a review. Breast Cancer Res Treat 2006; 97: 135–44. [DOI] [PubMed] [Google Scholar]
- 3. Virnig BA, Wang SY, Shamilyan T, Kane RL, Tuttle TM. Ductal carcinoma in situ: risk factors and impact of screening. J Natl Cancer Inst Monogr 2010; 2010: 113–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meyer JS. Cell kinetics of histologic variants of in situ breast carcinoma. Breast Cancer Res Treat 1986; 7: 171–80. [DOI] [PubMed] [Google Scholar]
- 5. Lagios MD, Margolin FR, Westdahl PR, Rose MR. Mammographically detected duct carcinoma in situ. Frequency of local recurrence following tylectomy and prognostic effect of nuclear grade on local recurrence. Cancer 1989; 63: 618–24. [DOI] [PubMed] [Google Scholar]
- 6. Silverstein MJ, Poller DN, Waisman JR et al Prognostic classification of breast ductal carcinoma‐in‐situ. Lancet 1995; 345: 1154–7. [DOI] [PubMed] [Google Scholar]
- 7. Pinder SE, Duggan C, Ellis IO, George WD; UK Coordinating Committee on Cancer Research (UKCCCR) . Ductal Carcinoma In Situ (DCIS) Working Party. A new pathological system for grading DCIS with improved prediction of local recurrence: results from the UKCCCR/ANZ DCIS trial. Br J Cancer 2010; 103: 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang Y, Karakhanova S, Hartwig W et al Mitochondria and mitochondrial ROS in cancer: novel targets for anticancer therapy. J Cell Physiol 2016; 231: 2570–81. [DOI] [PubMed] [Google Scholar]
- 9. Fang W, Li X, Jiang Q et al Transcriptional patterns, biomarkers and pathways characterizing nasopharyngeal carcinoma of Southern China. J Transl Med 2008; 6: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li G, Fu D, Liang W et al CYC1 silencing sensitizes osteosarcoma cells to TRAIL‐induced apoptosis. Cell Physiol Biochem 2014; 34: 2070–80. [DOI] [PubMed] [Google Scholar]
- 11. Han Y, Sun S, Zhao M et al CYC1 predicts poor prognosis in patients with breast cancer. Dis Markers 2016; 3528064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The Consensus Conference Committee . Consensus conference on the classification of ductal carcinoma in situ. Cancer 1997; 80: 1798–802. [DOI] [PubMed] [Google Scholar]
- 13. Ebata A, Suzuki T, Takagi K et al Oestrogen‐induced genes in ductal carcinoma in situ: their comparison with invasive ductal carcinoma. Endocr Relat Cancer 2012; 19: 485–96. [DOI] [PubMed] [Google Scholar]
- 14. Oka K, Suzuki T, Onodera Y et al Nudix‐type motif 2 in human breast carcinoma: a potent prognostic factor associated with cell proliferation. Int J Cancer 2011; 128: 1770–82. [DOI] [PubMed] [Google Scholar]
- 15. Sato‐Tadano A, Suzuki T, Amari M et al 2013 Hexokinase II in breast carcinoma: a potent prognostic factor associated with hypoxia‐inducible factor‐1α and Ki‐67. Cancer Sci 2013; 104: 1380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hammond ME, Hayes DF, Dowsett M et al American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med 2010; 134: e48–72. [DOI] [PubMed] [Google Scholar]
- 17. Miller FR, Santner SJ, Tait L, Dawson PJ. MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ. J Natl Cancer Inst 2000; 92: 1185–6. [DOI] [PubMed] [Google Scholar]
- 18. Behbod F, Kittrell FS, LaMarca H et al An intraductal human‐in‐mouse transplantation model mimics the subtypes of ductal carcinoma in situ. Breast Cancer Res 2009; 11: R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grube S, Göttig T, Freitag D, Ewald C, Kalff R, Walter J. Selection of suitable reference genes for expression analysis in human glioma using RT‐qPCR. J Neurooncol 2015; 123: 35–42. [DOI] [PubMed] [Google Scholar]
- 20. Clayton DA, Shadel GS. Isolation of mitochondria from tissue culture cells. Cold Spring Harb Protoc 2014; 10: 1109–111. pdb.prot080002. [DOI] [PubMed] [Google Scholar]
- 21. Zhu Y, Li M, Wang X et al Caspase cleavage of cytochrome c1 disrupts mitochondrial function and enhances cytochrome c release. Cell Res 2012; 22: 127–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suzuki S, Takagi K, Miki Y et al Nucleobindin 2 in human breast carcinoma as a potent prognostic factor. Cancer Sci 2012; 103: 136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kong M, Ba M, Liang H, Shao P, Yu T, Wang Y. Regulation of adenosine triphosphate‐sensitive potassium channels suppresses the toxic effects of amyloid‐beta peptide (25–35). Neural Regen Res 2013; 8: 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bussolati G, Bongiovanni M, Cassoni P, Sapino A. Assessment of necrosis and hypoxia in ductal carcinoma in situ of the breast: basis for a new classification. Virchows Arch 2000; 437: 360–4. [DOI] [PubMed] [Google Scholar]
- 25. Muggerud AA, Hallett M, Johnsen H et al Molecular diversity in ductal carcinoma in situ (DCIS) and early invasive breast cancer. Mol Oncol 2010; 4: 357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vegfors J, Petersson S, Kovács A, Polyak K, Enerbäck C. The expression of Psoriasin (S100A7) and CD24 is linked and related to the differentiation of mammary epithelial cells. PLoS One 2012; 7: e53119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Emberley ED, Murphy LC, Watson PH. S100A7 and the progression of breast cancer. Breast Cancer Res 2004; 6: 153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petersson S, Bylander A, Yhr M, Enerbäck C. S100A7 (Psoriasin), highly expressed in ductal carcinoma in situ (DCIS), is regulated by IFN‐gamma in mammary epithelial cells. BMC Cancer 2007; 7: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yin C, Li H, Zhang B et al RAGE‐binding S100A8/A9 promotes the migration and invasion of human breast cancer cells through actin polymerization and epithelial‐mesenchymal transition. Breast Cancer Res Treat 2013; 14: 297–309. [DOI] [PubMed] [Google Scholar]
- 30. Martens C, Krett B, Laybourn PJ. 2001 RNA polymerase II and TBP occupy the repressed CYC1 promoter. Mol Microbiol 2001; 40: 1009–19. [DOI] [PubMed] [Google Scholar]
- 31. Ivanova MM, Luken KH, Zimmer AS et al Tamoxifen increases nuclear respiratory factor 1 transcription by activating estrogen receptor beta and AP‐1 recruitment to adjacent promoter binding sites. FASEB J 2011; 25: 1402–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Azambuja E, Cardoso F, de Castro G et al Ki‐67 as prognostic marker in early breast cancer: a meta‐analysis of published studies involving 12,155 patients. Br J Cancer 2007; 96: 1504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Owens KM, Kulawiec M, Desouki MM, Vanniarajan A, Singh KK. Impaired OXPHOS complex III in breast cancer. PLoS One 2011; 6: e23846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Warburg O. On the origin of cancer cells. Science 1956; 123: 309–14. [DOI] [PubMed] [Google Scholar]
- 35. Księżakowska‐Łakoma K, Żyła M, Wilczyński JR. Mitochondrial dysfunction in cancer. Prz Menopauzalny 2014; 13: 136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guppy M, Leedman P, Zu X, Russell V. Contribution by different fuels and metabolic pathways to the total ATP turnover of proliferating MCF‐7 breast cancer cells. Biochem J 2002; 364: 309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Azuma A, Tozaki M, Ito K, Fukuma E, Tanaka T, O'uchi T. Ductal carcinoma in situ: correlation between FDG‐PET/CT and histopathology. Radiat Med 2008; 26: 488–93. [DOI] [PubMed] [Google Scholar]
- 38. Dey R, Moraes CT. Lack of oxidative phosphorylation and low mitochondrial membrane potential decrease susceptibility to apoptosis and do not modulate the protective effect of Bcl‐x(L) in osteosarcoma cells. J Biol Chem 2000; 275: 7087–94. [DOI] [PubMed] [Google Scholar]
- 39. Yadav N, Kumar S, Marlowe T et al Oxidative phosphorylation‐dependent regulation of cancer cell apoptosis in response to anticancer agents. Cell Death Dis 2015; 6: e1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Definition of enriched gene ontology terms in comedo ductal carcinoma in situ cases.
Table S2. Brief functions of genes listed in Table 1.


