Abstract
Objective
To determine trends in management and factors associated with men receiving either chemotherapy or radiation therapy post-orchiectomy for Clinical Stage I (CSI) seminoma in a contemporary setting.
Methods and Materials
The National Cancer Data Base was queried for all patients with CSI seminoma from 1998 to 2012. Adjuvant treatment after orchiectomy was classified into three groups: surveillance, radiotherapy (XRT), and chemotherapy (chemo). Yearly trends in management are described. Sub-group analysis for the years 2010–2012 was completed using logistic regression to determine predictors of receiving treatment.
Results
Of 80,385 patients with testicular cancer, 16,931 had CSI seminoma. There was a progressive decline in the use of post-orchiectomy treatment from 1998–2012. In the years 2010–2012 (n=5,816), 59.9% of patients chose surveillance compared to 25.1% receiving XRT and 15.0% chemo. Regression modeling demonstrated that men aged 18–30 were less likely [OR 0.83, 95% CI 0.69–1.00, p=0.048] to receive treatment than those aged 31–37. Increasing pathologic stage was associated with a greater likelihood of treatment [OR 1.77, 95% CI 1.52–2.06] while patients treated at academic hospitals were less likely to receive adjuvant therapy [OR 0.77, 95% CI 0.62–0.94].
Conclusion
Despite a trend towards increased use of post-orchiectomy surveillance for CSI seminoma patients, a significant portion are still receiving treatment. Pathologic stage and treating hospital type have the strongest association with management decisions. Improved guideline adherence may reduce the potential for adverse effects after chemotherapy or radiation for CSI seminoma.
Keywords: chemotherapy, radiotherapy, seminoma, surveillance, testicular cancer
INTRODUCTION
Testis cancer is the most common malignancy among young men1. Fortunately, long term survival rates are excellent, approaching 99% for clinically localized disease 2. For clinical stage I (CSI) seminoma, post-orchiectomy management options include surveillance, radiation therapy (XRT), and platinum-based chemotherapy3, 4. The rate of post-orchiectomy recurrence of CSI seminoma managed with surveillance is between 15–20% at 5 years.5, 6 Treatment (chemotherapy or XRT) reduces the recurrence rate to 5% at 5 years but inherently involves overtreatment of a significant portion of patients who would otherwise not recur.7
The long-term adverse effects of chemotherapy8 and XRT9 in testis cancer survivors include an increased risk of cardiovascular disease, secondary malignancy, and other toxicities. Though this literature is largely based on a variety of chemotherapy and radiation protocols, when these adverse-effects occur, they are not innocuous. Studies have demonstrated a 41% 5-year survival rate after diagnosis of a secondary malignancy and 64% 5-year survival rate after diagnosis of cardiovascular disease among patients treated for testicular cancer10. As an alternative to adjuvant therapy, salvage therapies for seminoma recurrence still result in cure rates of nearly 99%11, 12. As such, national treatment guidelines are now recommending surveillance as the preferred post-orchiectomy management decision for CSI seminoma4.
We hypothesized that despite data demonstrating the risks of adjuvant treatment for CSI seminoma and management guidelines indicating a preference for surveillance, a significant number of patients are still receiving treatment after orchiectomy. Therefore, the objectives of this study were to determine trends of adjuvant management of CSI seminoma and to better understand how patients are currently managed in a contemporary national cohort. Understanding these factors may help reduce treatment related adverse effects and further improve recognition of the benefits of surveillance for CSI seminoma.
PATIENTS AND METHODS
Data Source
The National Cancer Data Base (NCDB) is an oncology dataset that captures approximately 70% of newly diagnosed cancers in the United States.13 It is a joint project of the American Cancer Society and the Commission on Cancer (CoC) of the American College of Surgeons and receives data from approximately 1,450 CoC-accredited cancer programs. Institutional Review Board review was waived by the host institution secondary to de-identified data. Data was abstracted from the most recent available years of the NCDB participant use file, from 1998 to 2012.
Patients
All patients with Clinical Stage I (CSI) seminoma of the testis from January 1, 1998 to December 31, 2012 were identified. Staging was determined using classification from the AJCC Cancer Staging Manual, 7th Edition. Cases with T-classification of T0/Tis (n=14, 0.05%), T4 (n=35, 0.11%), and Tx (n=406, 1.3%) were excluded from analysis. Men with stage 1S (elevated tumor markers) were not included for analysis. Seminoma histology was determined using International Classification of Diseases for Oncology, Third Edition (ICD-O-3) codes 9061 and 9062 for seminoma and anaplastic seminoma, respectively.
Patients were categorized into age groups in evenly distributed quartiles: 18–30, 31–37, 38–44, ≥45 years old. Race and ethnicity were categorized as non-Hispanic white, non-Hispanic black, Hispanic/Spanish, Asian, and other/unknown. Insurance status was grouped into three categories: uninsured/unknown, government insurance including Medicare and Medicaid, and private insurance. Further demographic data were grouped by reporting hospital location and by median income quartiles derived from patient’s home zip code from the 2012 American Community Survey data. Distance to treating hospital was categorized as less than 10 miles, between 10 and 50 miles, and greater than 50 miles “crowfly” distance from hospital to patients’ home zip code. Reporting hospital type was determined by CoC accreditation status (based on hospital volume, presence of postgraduate medical education trainees, and clinical research among other factors) and was dichotomized into either academic/research programs or community cancer programs.
Adjuvant management after orchiectomy was classified into four mutually exclusive groups: surveillance, XRT, chemotherapy, and retroperitoneal lymph node dissection (RPLND). Since RPLND is not indicated as primary treatment for CSI seminoma and there is no information regarding the timing of RPLND collected in the database, we were unable to determine if these were adjuvant or salvage RPLNDs. Therefore, patients who received RPLND were excluded (265 patients overall, 0.8%) from analysis. To be considered to have had adjuvant XRT or chemotherapy, a patient must have had treatment within 60 days of diagnosis and after orchiectomy. Any chemotherapy regimen (either single or multi-agent chemotherapy) was considered as chemotherapy if it was given. Surveillance was designated only if all three other adjuvant options (XRT, chemo, RPLND) were negatively coded. Roughly 16% (4858) of cases were unable to be definitively classified according to this system and were not included.
Statistical Analyses
Basic descriptive statistics identified demographic information in our cohort. Using our designations of treatment, we explored rates over time for each adjuvant management option. To minimize contamination from hospitals that dropped in/out of the NCDB due to accreditation status and to abstract a proper population based sample, only hospitals that reported at least one case during all fifteen years were included (16,931 cases total). Trends were assessed over periods of three years in order to smooth for yearly variation.
Factors associated with receiving treatment in a contemporary setting were assessed. To do this, we isolated all 5,816 CSI seminoma cases during the years 2010 to 2012 rather than the entire dataset (1998–2012). No hospital-level exclusion criteria were applied for this cohort. Since our clinical hypothesis involved the binary decision of either surveillance or adjuvant treatment, chemotherapy and XRT were combined to dichotomize the variable. Overall rates of surveillance or adjuvant treatment were then compared for all possible covariates for an unadjusted rate and compared via chi-squared test. Next, a mixed-effects logistic regression analysis was conducted using age, race, insurance status, “crowfly” travel distance, income, hospital type, hospital location, and stage as fixed effects and individual hospital identifier as a random effect. Primary tumor size and rete testis involvement were not included in the model as they were not contributory.
The threshold for statistical significance was set at p=0.05 based on two-sided tests. All data analysis was performed using STATA 13.1 (College Station, TX, USA).
Results
Rates of Surveillance Over Time
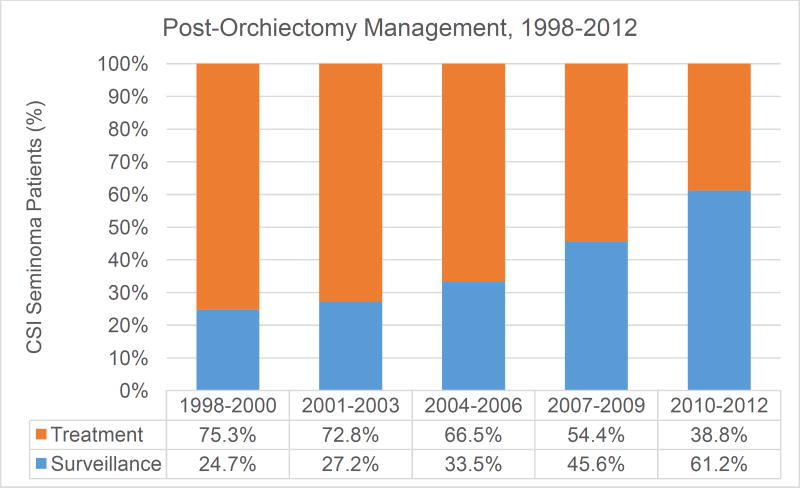
The initial cohort included 16,931 patients from hospitals with continuous reporting through all 15 study years. Figure 1 displays increasing use of surveillance among patients with CSI seminoma for all year groups from 1998 to 2012. The largest rate of increase was seen between years 2007–2009 and 2004–2006 (36.3% and 34.2%, respectively). Surveillance rates among the most recently treated patients (2010–2012), more than doubled compared to years 1998–2000 (61.2% from 24.8%).
Figure 1.
Rates of management for clinical stage I seminoma of the testis for the years 1998–2012 from hospitals with consistent reporting throughout all study years. Treatment is defined as receiving either chemotherapy or radiation in the adjuvant post-orchiectomy period.
Contemporary Cohort Evaluation
Our contemporary cohort (Table 1) contained 5,816 patients; complete treatment information was available for 5,045 patients (86.7%). The median age was 37 [IQR 30–46] and the majority (76.2%, n=4,434) of patients had Stage IA disease. Treatment of the primary tumor was accomplished via orchiectomy in 99.5% of patients while the remaining 0.5% had a partial orchiectomy. The majority of patients (67.1%, n=3,902) were treated at community cancer programs and lived within 10 miles of their treating hospital (54.2%, n=3,154). Overall, during 2010–2012, 59.9% (n=3,021) of patients chose surveillance with 25.1% (n=1,267) electing for radiation therapy and 15.0% (n=757) receiving chemotherapy- 79.5% (604) of which received single agent treatment.
Table 1.
Demographic data for CSI testicular seminoma patients for the years 2010–2012
| n | % | ||
|---|---|---|---|
| Age (median, IQR) | 37 | [30–46] | |
| Stage | |||
| 1A | 4,434 | 76.2 | |
| 1B | 1,382 | 23.8 | |
| Race | |||
| White | 4,614 | 79.3 | |
| Black | 171 | 2.9 | |
| Hispanic/Spanish | 496 | 8.5 | |
| Asian | 117 | 2.0 | |
| Other/Unknown | 418 | 7.2 | |
| Reporting hospital location | |||
| Northeast | 1,323 | 22.8 | |
| Mid-West | 1,574 | 27.1 | |
| Mountain/Pacific | 1,130 | 19.4 | |
| South | 1,789 | 30.8 | |
| Distance to Treating Hospital | |||
| <10 miles | 3,154 | 54.2 | |
| 10–50 miles | 2,258 | 38.8 | |
| >50 miles | 404 | 7.0 | |
| Insurance | |||
| Uninsured/Unknown | 724 | 12.5 | |
| Gov't Insurance | 748 | 12.9 | |
| Private Insurance | 4,344 | 74.7 | |
| Median Income (Quartiles) | |||
| <$38,000 | 740 | 12.9 | |
| $38000–$47999 | 1,210 | 21.0 | |
| $48000–$62999 | 1,551 | 27.0 | |
| $63000+ | 2,252 | 39.1 | |
| Cases (by year) | |||
| 2010 | 1,993 | 34.3 | |
| 2011 | 1,926 | 33.1 | |
| 2012 | 1,897 | 32.6 | |
| Reporting hospital type | |||
| Community | 3,902 | 67.1 | |
| Academic | 1,914 | 32.9 | |
| Treatment | |||
| Surveillance | 3,021 | 59.9 | |
| Chemo | 757 | 15.0 | |
| Radiation | 1,267 | 25.1 | |
Unadjusted rates of treatment type are shown in Table 2. Significant findings include higher rates of treatment among older patients, patients who lived less than 50 miles from their treating hospital, and in patients outside of the Northeast region. Patients with IB staging were managed with surveillance 50.4% of the time compared to 62.8% for stage IA (p<0.001). Those treated in the years 2011 (61.2%) and 2012 (65.9%) had higher rates of surveillance compared to those in 2010 (52.7%). Patients treated at academic hospitals also had higher rates of surveillance (65.6% vs 57.1%, p<0.001).
Table 2.
Unadjusted rates of treatment and surveillance for CSI testicular seminoma patients for the years 2010–2012.
| Surveillance | Treatment | |||||
|---|---|---|---|---|---|---|
| n | % | n | % | p-value | ||
| Age (quartiles) | <0.001 | |||||
| 18–30 | 823 | 64.3% | 456 | 35.7% | ||
| 31–37 | 777 | 60.2% | 514 | 39.8% | ||
| 38–44 | 585 | 56.1% | 458 | 43.9% | ||
| 45+ | 836 | 58.4% | 596 | 41.6% | ||
| Stage | <0.001 | |||||
| 1A | 2429 | 62.8% | 1441 | 37.2% | ||
| 1B | 592 | 50.4% | 583 | 49.6% | ||
| Race | 0.001 | |||||
| White | 2367 | 58.6% | 1669 | 41.4% | ||
| Black | 93 | 63.7% | 53 | 36.3% | ||
| Hispanic/Spanish | 271 | 68.1% | 127 | 31.9% | ||
| Asian | 56 | 56.0% | 44 | 44.0% | ||
| Other/Unknown | 234 | 64.1% | 131 | 35.9% | ||
| Reporting hospital location | <0.001 | |||||
| Northeast | 747 | 66.8% | 371 | 33.2% | ||
| Mid-West | 762 | 54.5% | 637 | 45.5% | ||
| Mountain/Pacific | 574 | 59.5% | 391 | 40.5% | ||
| South | 938 | 60.0% | 625 | 40.0% | ||
| Distance to Treating Hospital | <0.001 | |||||
| <10 miles | 1650 | 60.6% | 1074 | 39.4% | ||
| 10–50 miles | 1126 | 57.4% | 835 | 42.6% | ||
| >50 miles | 245 | 68.1% | 115 | 31.9% | ||
| Insurance | 0.215 | |||||
| Uninsured/Unknown | 372 | 62.4% | 224 | 37.6% | ||
| Gov't Insurance | 357 | 57.5% | 264 | 42.5% | ||
| Private Insurance | 2292 | 59.9% | 1536 | 40.1% | ||
| Median Income (Quartiles) | <0.001 | |||||
| <$38,000 | 366 | 58.0% | 265 | 42.0% | ||
| $38000–$47999 | 596 | 56.4% | 461 | 43.6% | ||
| $48000–$62999 | 778 | 57.9% | 566 | 42.1% | ||
| $63000+ | 1242 | 63.5% | 715 | 36.5% | ||
| Cases (by year) | <0.001 | |||||
| 2010 | 895 | 52.7% | 803 | 47.3% | ||
| 2011 | 1023 | 61.2% | 649 | 38.8% | ||
| 2012 | 1103 | 65.9% | 572 | 34.1% | ||
| Reporting hospital type | <0.001 | |||||
| Community | 1943 | 57.1% | 1459 | 42.9% | ||
| Academic | 1078 | 65.6% | 565 | 34.4% | ||
In mixed-effects regression modeling (Table 3), younger patients (18–30) were found to be less likely (OR 0.83, 95% CI 0.69–1.00) to receive treatment than the referent group (ages 31–37). Patients treated at academic hospitals (OR 0.77 [95% CI 0.62–0.94]) and those who lived farther than 50 miles from their treating hospital (OR 0.59 [95% CI 0.43, 0.80], were significantly less likely to receive post-orchiectomy treatment. Increased clinical stage (1B compared to 1A) was associated with a greater likelihood of treatment (OR 1.77 [95% CI 1.52–2.06]). Compared to 2010, each increasing year was associated with a decreased likelihood of receiving treatment with all other covariates held constant.
Table 3.
Mixed-effects logistic regression analysis predicting treatment over surveillance for all covariates in CS1 testicular seminoma patients for the years 2010–2012.
| Odds Ratio [95% Confidence Interval] | p-value | ||
|---|---|---|---|
| Age (quartiles) | |||
| 18–30 | 0.83 [0.69, 1.00] | 0.048 | |
| 31–37 | REF | ||
| 38–44 | 1.18 [0.97, 1.43] | 0.09 | |
| 45+ | 1.00 [0.83, 1.19] | 0.96 | |
| AJCC TNM Stage | |||
| 1A | REF | ||
| 1B | 1.77 [1.52, 2.06] | <0.001 | |
| Race | |||
| White | REF | ||
| Black | 0.71 [0.48, 1.07] | 0.1 | |
| Hispanic/Spanish | 0.78 [0.59, 1.03] | 0.8 | |
| Asian | 1.47 [0.92, 2.35] | 0.11 | |
| Other/Unknown | 0.80 [0.60, 1.06] | 0.12 | |
| Location (region) | |||
| Northeast | REF | ||
| Mid-West | 1.69 [1.31, 2.20] | <0.001 | |
| Mountain/Pacific | 1.41 [1.05, 1.88] | 0.02 | |
| South | 1.29 [1.00, 1.67] | 0.049 | |
| Distance to Treating Hospital | |||
| <10 miles | 0.88 [0.76, 1.01] | 0.68 | |
| 10–50 miles | REF | ||
| >50 miles | 0.59 [0.43, 0.80] | 0.001 | |
| Insurance Payor | |||
| Uninsured/Unknown | 0.79 [0.60, 1.04] | 0.09 | |
| Medicare/Medicaid | REF | ||
| Private Insurance | 0.87 [0.71, 1.07] | 0.19 | |
| Income (Median) | |||
| <38,000 | REF | ||
| $38000–$47999 | 1.06 [0.83, 1.34] | 0.65 | |
| $48000–$62999 | 0.98 [0.77, 1.24] | 0.85 | |
| $63000+ | 0.83 [0.65, 1.04] | 0.11 | |
| Year | |||
| 2010 | REF | ||
| 2011 | 0.67 [0.58, 0.79] | <0.001 | |
| 2012 | 0.52 [0.45, 0.62] | <0.001 | |
| Reporting Hospital Type | |||
| Community | REF | ||
| Academic | 0.77 [0.62, 0.94] | 0.01 | |
Discussion
In CSI seminoma, long-term survival is expected and remote toxicities from treatment may affect the duration or quality of patients’ lives long after their testicular cancer is cured. An improved understanding of the morbidity associated with adjuvant chemotherapy14 and radiation15 for testicular cancer has altered the treatment approach in recent years. As with any new strategy in medicine, widespread dissemination is a gradual process. Therefore, in this study we sought to 1) gain a current perspective on treatment trends for patients with CSI seminoma and 2) to determine factors associated with receiving treatment instead of surveillance in a contemporary cohort. We confirmed our hypothesis that despite greater use of surveillance (Figure 1) over the study years, many patients with CSI seminoma are still receiving adjuvant treatment post-orchiectomy. We also identified several factors associated with receiving treatment.
Two prior studies described the population level trend towards greater use of surveillance in seminoma patients16, 17. These studies provided a longitudinal overview of the change in clinical management but did not specifically examine the most contemporary years as a separate, isolated entity. We expected and found an even more substantial shift toward using surveillance in recent years (2010–2012). Use of surveillance increased from 52.7% in 2010 to 65.9% in 2012 (Table 1). This 25% relative increase can be compared to a 9.9% relative increase from year groups 1998–2000 to 2001–2003 (24.7% vs. 27.2%). Further, year of diagnosis was significantly associated (2011 OR 0.67, 2012 OR 0.52 compared to 2010, p<0.001) with reduced odds of treatment.
Several other findings from our study should be highlighted. First, younger patients (18–30) had lower odds of treatment compared to the referent group (31–37), which contains the median age (37) at time of diagnosis. Information about reasons a specific treatment option was selected is not found in the database. However, one possibility is that the increasing interest in fertility preservation as an important element of cancer survivorship may be contributing to this treatment option18. From a health disparities perspective, neither race, income, nor insurance status were associated with risk-adjusted treatment odds among patients treated during 2010–2012 in our cohort. This is an encouraging development as race and socioeconomic status were found to be negatively associated with outcomes in a SEER study of testicular cancer during 1998–200619.
Another important finding of this study is that hospital type was significantly associated with a patient receiving surveillance, even after controlling for distance from hospital. Patients treated at an academic hospital were 23% less likely to receive treatment compared to community cancer hospitals, with all factors held constant. Academic hospitals are generally higher volume centers involved in training residents and fellows. This may lead to an increased familiarity with cancer guideline recommendations or greater comfort in managing patients with surveillance. There are data to support this hypothesis as Yu et al discovered a disparity in surveillance protocols between referral centers and the community in CSI testis cancer20. Patient non-adherence has also been demonstrated to hinder the success of testicular cancer surveillance programs. One study reported non-adherence rates as high as 22% for clinic visits and 35.7% for imaging studies21. As such, larger academic centers with greater resources to track patients may be more likely to recommend surveillance than community centers. Additionally, external factors such as financial incentives and physician availability may also influence management choices.
Regional variation was also seen in our data as patients in the Northeast were less likely than those in all other regions to receive treatment. Regional variation, particularly in the use of surgery, has been well demonstrated22. This can be extrapolated to our study as similar environmental factors such as health care spending, specialist access, and the social landscape likely influence general treatment decisions (including chemo/XRT) as well. The significant concentration of large academic hospitals in the Northeast may also be influential despite the random-effect modeling used to reduce hospital cluster influence in our regression analysis. Hospital distance greater than 50 miles from patients’ home was associated with a lesser likelihood of receiving treatment. This is somewhat surprising given that treatment typically reduces the need for multiple follow up visits and imaging tests. In centers where compliance is a concern21, this could lead to worse outcomes for patients. It is also possible that men were either referred to, or simply went to high-volume centers (that employ greater use of surveillance) farther from their homes due to lack of a local community hospital.
Arguably the most important finding of this study is that patients with stage IB seminoma had a significantly greater association (OR 1.77, 95% CI 1.52–2.06) with receiving treatment than patients with stage 1A cancer, controlling for all other covariates. At this time, no evidence exists showing a difference in outcomes between IA and IB seminoma, including those upstaged due to LVI.23 On the contrary, for non-seminomatous germ cell tumors (NSGCT), lymphovascular invasion, and therefore a stage increase from IA to IB, is the strongest independent predictor of relapse24. Confusion regarding the difference in treatment strategy between the two histologic classifications or lack of awareness of this small but important detail could contribute to seminomas being treated similarly to NSGCT. This is a potential target for quality improvement on a large scale with educational programing.
There are several limitations to this study, primarily due to the retrospective nature of the data. Individual patient factors related to treatment decisions are not available and may significantly bias trends on the patient-level. An individualized, shared-decision making discussion should always be employed during treatment.25 Further, one of the most important aspects of a successful surveillance protocol is patient acceptance and regular follow up (with appropriate imaging and tumor markers), which is not captured by this dataset.26 The NCDB also lacks data on cancer recurrence. To best separate adjuvant treatment from treatment for recurrence, we isolated the 60 days post orchiectomy as the time frame to be considered adjuvant. The NCCN guidelines recommend starting radiotherapy within 7 weeks of orchiectomy4, so 60 days is a conservative time frame that may slightly over-report radiation rates. The NCDB also lacks data on secondary malignancy rates and cardiovascular comorbidity to link long-term treatment complications to this patient population.
Conclusion
Despite increased use of post-orchiectomy surveillance for patients with CSI seminoma, a significant proportion of patients still receiving either XRT or chemotherapy in this contemporary cohort. Age, stage and treating hospital type had the most significant associations with management decisions. Improved dissemination of guidelines, especially highlighting the nuanced differences in recurrence risk, as well as a greater understanding of the morbidity associated with chemotherapy and radiation may help to increase rates of surveillance, standardize management, and decrease post-treatment morbidity.
Acknowledgments
The authors wish to thank Dr George J. Bosl for his expertise and invaluable review of our manuscript.
The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presentation: Study accepted for podium presentation at AUA National Conference in May 2016
Disclaimers/Disclosures: none
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Hanna N, Einhorn LH. Testicular cancer: a reflection on 50 years of discovery. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:3085–3092. doi: 10.1200/JCO.2014.56.0896. [DOI] [PubMed] [Google Scholar]
- 3.Albers P, Albrecht W, Algaba F, et al. Guidelines on Testicular Cancer: 2015 Update. European urology. 2015;68:1054–1068. doi: 10.1016/j.eururo.2015.07.044. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Jonasch E, Agarwal N, et al. Testicular Cancer, Version 2.2015. Journal of the National Comprehensive Cancer Network : JNCCN. 2015;13:772–799. doi: 10.6004/jnccn.2015.0092. [DOI] [PubMed] [Google Scholar]
- 5.Warde P, Gospodarowicz MK, Banerjee D, et al. Prognostic factors for relapse in stage I testicular seminoma treated with surveillance. The Journal of urology. 1997;157:1705–1709. discussion 1709–1710. [PubMed] [Google Scholar]
- 6.Aparicio J, Garcia del Muro X, Maroto P, et al. Multicenter study evaluating a dual policy of postorchiectomy surveillance and selective adjuvant single-agent carboplatin for patients with clinical stage I seminoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2003;14:867–872. doi: 10.1093/annonc/mdg241. [DOI] [PubMed] [Google Scholar]
- 7.Oliver RT, Mason MD, Mead GM, et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet. 2005;366:293–300. doi: 10.1016/S0140-6736(05)66984-X. [DOI] [PubMed] [Google Scholar]
- 8.Fung C, Fossa SD, Milano MT, Sahasrabudhe DM, Peterson DR, Travis LB. Cardiovascular Disease Mortality After Chemotherapy or Surgery for Testicular Nonseminoma: A Population-Based Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:3105–3115. doi: 10.1200/JCO.2014.60.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Travis LB, Curtis RE, Storm H, et al. Risk of second malignant neoplasms among long-term survivors of testicular cancer. Journal of the National Cancer Institute. 1997;89:1429–1439. doi: 10.1093/jnci/89.19.1429. [DOI] [PubMed] [Google Scholar]
- 10.van den Belt-Dusebout AW, de Wit R, Gietema JA, et al. Treatment-specific risks of second malignancies and cardiovascular disease in 5-year survivors of testicular cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:4370–4378. doi: 10.1200/JCO.2006.10.5296. [DOI] [PubMed] [Google Scholar]
- 11.Kollmannsberger C, Tandstad T, Bedard PL, et al. Patterns of relapse in patients with clinical stage I testicular cancer managed with active surveillance. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:51–57. doi: 10.1200/JCO.2014.56.2116. [DOI] [PubMed] [Google Scholar]
- 12.Cohn-Cedermark G, Stahl O, Tandstad T, Swenoteca Surveillance vs. adjuvant therapy of clinical stage I testicular tumors - a review and the SWENOTECA experience. Andrology. 2015;3:102–110. doi: 10.1111/andr.280. [DOI] [PubMed] [Google Scholar]
- 13.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Annals of surgical oncology. 2008;15:683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meinardi MT, Gietema JA, van der Graaf WT, et al. Cardiovascular morbidity in long-term survivors of metastatic testicular cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18:1725–1732. doi: 10.1200/JCO.2000.18.8.1725. [DOI] [PubMed] [Google Scholar]
- 15.Travis LB, Fossa SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. Journal of the National Cancer Institute. 2005;97:1354–1365. doi: 10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- 16.Gray PJ, Lin CC, Sineshaw H, Paly JJ, Jemal A, Efstathiou JA. Management trends in stage I testicular seminoma: Impact of race, insurance status, and treatment facility. Cancer. 2015;121:681–687. doi: 10.1002/cncr.29094. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman KE, Chen MH, Punglia RS, Beard CJ, D'Amico AV. Influence of year of diagnosis, patient age, and sociodemographic status on recommending adjuvant radiation treatment for stage I testicular seminoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:3937–3942. doi: 10.1200/JCO.2008.16.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kort JD, Eisenberg ML, Millheiser LS, Westphal LM. Fertility issues in cancer survivorship. CA: a cancer journal for clinicians. 2014;64:118–134. doi: 10.3322/caac.21205. [DOI] [PubMed] [Google Scholar]
- 19.Sun M, Abdollah F, Liberman D, et al. Racial disparities and socioeconomic status in men diagnosed with testicular germ cell tumors: a survival analysis. Cancer. 2011;117:4277–4285. doi: 10.1002/cncr.25969. [DOI] [PubMed] [Google Scholar]
- 20.Yu HY, Madison RA, Setodji CM, Saigal CS. Quality of surveillance for stage I testis cancer in the community. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:4327–4332. doi: 10.1200/JCO.2008.19.9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ernst DS, Brasher P, Venner PM, et al. Compliance and outcome of patients with stage 1 non-seminomatous germ cell tumors (NSGCT) managed with surveillance programs in seven Canadian centres. The Canadian journal of urology. 2005;12:2575–2580. [PubMed] [Google Scholar]
- 22.Birkmeyer JD, Reames BN, McCulloch P, Carr AJ, Campbell WB, Wennberg JE. Understanding of regional variation in the use of surgery. Lancet. 2013;382:1121–1129. doi: 10.1016/S0140-6736(13)61215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warde P, Specht L, Horwich A, et al. Prognostic factors for relapse in stage I seminoma managed by surveillance: a pooled analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:4448–4452. doi: 10.1200/JCO.2002.01.038. [DOI] [PubMed] [Google Scholar]
- 24.Tandstad T, Dahl O, Cohn-Cedermark G, et al. Risk-adapted treatment in clinical stage I nonseminomatous germ cell testicular cancer: the SWENOTECA management program. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:2122–2128. doi: 10.1200/JCO.2008.18.8953. [DOI] [PubMed] [Google Scholar]
- 25.Oldenburg J, Aparicio J, Beyer J, et al. Personalizing, not patronizing: the case for patient autonomy by unbiased presentation of management options in stage I testicular cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2015;26:833–838. doi: 10.1093/annonc/mdu514. [DOI] [PubMed] [Google Scholar]
- 26.de Wit R, Fizazi K. Controversies in the management of clinical stage I testis cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:5482–5492. doi: 10.1200/JCO.2006.07.9434. [DOI] [PubMed] [Google Scholar]