Abstract
Background
Lack of dietary fiber has been suggested to increase the risk of developing various chronic inflammatory diseases, while supplementation of diets with fiber might offer an array of health promoting benefits. Consistent with this theme, we recently reported that, in mice, compositionally defined diets (CDD) that are made with purified ingredients and lack fermentable fiber promote low-grade inflammation and metabolic syndrome, both of which could be ameliorated by supplementation of such diets with the fermentable fiber inulin.
Methods
Herein, we examined if, relative to a grain-based mouse diet (chow), CDD consumption would impact development of intestinal inflammation induced by dextran sodium sulfate (DSS) and, moreover, whether DSS-induced colitis might also be attenuated by diets supplemented with inulin.
Results
Analogous to their promotion of low-grade inflammation, CDD of high- and low-fat content with cellulose increased the severity of DSS-induced colitis relative to chow. However, in contrast to the case of low-grade inflammation, addition of inulin, but not the insoluble fiber cellulose, further exacerbated the severity of colitis and its associated clinical manifestations (weight loss and bleeding) in both low and high fat diets.
Conclusions
While inulin, and perhaps other fermentable fibers, can ameliorate low-grade inflammation and associated metabolic disease, it also has the potential to exacerbate disease severity in response to inducers of acute colitis.
Keywords: Inulin, intestinal inflammation, microbiota
INTRODUCTION
The vast majority of rodents used in biomedical research are fed grain-based “chow” diets, which are conglomerates of partially processed and relatively unrefined plant and animal products. In addition, most of these chows are ‘closed’ formulas, meaning their formula composition is kept secret and not available to the public. Because of the variability in such products and their poorly-characterized nature, they are not capable of being used in rodent-based studies that seek to define how diet composition influences health and disease. In contrast, purified-ingredient compositionally defined diets (CDD, also known as purified diets) are ‘open’ formulas, made with highly refined ingredients which allows for nutrient manipulations and minimizes variability among batches (1). Therefore, CDDs are perfectly suited to understand the influence of diet composition and can be modified to ‘push’ a particular disease phenotype or to limit disease development. Although it is possible to formulate a CDD to be similar to chow for macronutrient levels, producing a low-fat control CDD that would closely mimic chow’s phenotypic effect on the host has remained an elusive goal. For example, we recently reported that, relative to chow-fed mice, mice fed a commonly-used low fat control CDD with an insoluble fiber (cellulose), that is virtually unfermentable, had lower intestinal mass that was associated with low-grade inflammation and indices of metabolic syndrome (2). Such loss of gut mass correlated with reduced levels of colonic short-chain fatty acids (SCFA), suggesting that the CDD’s lack of fermentable fiber contributed to the difference in phenotype between chow-fed and CDD-fed mice. Moreover, supplementation of this CDD with fermentable fiber inulin was sufficient to restore colonic SCFA and gut mass, as well as reduce adiposity and eliminate evidence of low-grade inflammation (2). Together, these results indicated that a dearth of fermentable fiber promotes low-grade intestinal inflammation and associated obesity, and that diet supplementation with inulin, and perhaps other fermentable fibers, might be an effective approach to remediate this disease state.
The goal of the present study was to examine the extent to which fiber content would have an analogous impact on robust (i.e. classic) acute colitis, which is frequently modeled in mice by the administration of a chemical colitogen, dextran sulfate sodium (DSS) (3). We observed that, analogous to its promotion of low-grade inflammation, feeding mice a CDD lacking fermentable fiber worsened disease severity following challenge with DSS, irrespective of dietary fat content. However, in stark contrast to the case of low-grade inflammation, supplementation of CDD with inulin did not protect against, but rather, dramatically exacerbated disease severity induced by DSS, thus demonstrating the impact of fiber content on gut inflammation is context-dependent.
MATERIALS AND METHODS
Mice
Wild-type C57BL/6 mice were purchased from Jackson Laboratories. IL22KO animals were obtained from Genentech (4). All mice were then housed at Georgia State University, Atlanta, Georgia, USA under institutionally-approved protocols (Institutional Animal Care and Use Committee # A14033). Mice were fed Purina rodent chow (cat# 5001) from LabDiet (normal chow diet, NCD), unless another diet is specified.
Diets
Diets were produced by Research Diets, Inc, New Brunswick, NJ. The composition of all diets used in this study was detailed in tables 1 and 2. Fiber was added on a similar per kcal basis in low and high fat diet formulas, and inulin was considered as 1.0 kcal/g replacing an equal amount of kcals from corn starch (or maltodextrin-10 in formulas with 60 kcal% fat). We considered cellulose to provide no calories.
Table 1.
Purified diets used in the study.
| Product # | D12450B | D12492 | D12450J-1.5V | D13081108-1.5V | D13081107 | D13081106 | |
|---|---|---|---|---|---|---|---|
| Protein source | Casein | Casein | Casein | Casein | Casein | Casein | |
| Fiber source | Cellulose | Cellulose | Cellulose | Inulin | Cellulose | Inulin | |
| Irradiated | No | No | Yes | Yes | No | No | |
| kcal% fat | 10 | 60 | 10 | 10 | 60 | 60 | |
| Protein (kcal%) | 20 | 20 | 20 | 20 | 20 | 20 | |
| Carbohydrate (kcal%) | 70 | 20 | 70 | 65 | 20 | 20 | |
| Fat (kcal%) | 10 | 60 | 10 | 10 | 60 | 60 | |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | |
| kcal/gm | 3.85 | 5.24 | 3.8 | 3.5 | 4.4 | 4.6 | |
| Ingredient (g) | kcal/gm | ||||||
| Casein | 4 | 200 | 200 | 200 | 200 | 200 | 200 |
| L-Cystine | 4 | 3 | 3 | 3 | 3 | 3 | 3 |
| Corn Starch | 4 | 315 | 0 | 506.2 | 456.2 | 0 | 0 |
| Maltodextrin 10 | 4 | 35 | 125 | 125 | 125 | 125 | 75 |
| Sucrose | 4 | 350 | 68.8 | 63.8 | 63.8 | 68.8 | 68.8 |
| Cellulose | 0 | 50 | 50 | 50 | 0 | 200 | 0 |
| Inulin | 1 | 0 | 0 | 0 | 200 | 0 | 200 |
| Soybean Oil | 9 | 25 | 25 | 25 | 25 | 25 | 25 |
| Lard | 9 | 20 | 245 | 20 | 20 | 245 | 245 |
| Mineral Mix, S10026 | 0 | 10 | 10 | 10 | 10 | 10 | 10 |
| DiCalcium Phosphate | 0 | 13 | 13 | 13 | 13 | 13 | 13 |
| Calcium Carbonate | 0 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 |
| Potassium Citrate, 1 H2O | 0 | 16.5 | 16.5 | 16.5 | 16.5 | 16.5 | 16.5 |
| Vitamin Mix, V10001 | 4 | 10 | 10 | 15 | 15 | 10 | 10 |
| Choline Bitartrate | 0 | 2 | 2 | 2 | 2 | 2 | 2 |
| FD&C Red Dye #40 | 0 | 0.05 | 0 | 0.05 | 0.025 | 0.025 | 0.05 |
| FD&C Blue Dye #1 | 0 | 0 | 0.05 | 0 | 0 | 0.025 | 0 |
| FD&C Yellow Dye #5 | 0 | 0 | 0 | 0 | 0.025 | 0 | 0 |
| Total gm | 1055.05 | 773.85 | 1055.05 | 1155.05 | 923.85 | 873.85 | |
| Total kcals | 4057 | 4057 | 4057 | 4057 | 4057 | 4057 | |
| Used in figure: | 1 | 1 | 2, 3, S1 | 2, 3, S1 | 4, 5 | 4, 5 | |
Table 2.
Chow-based diets used in the study.
| Product # | C11000 | C13062904 |
|---|---|---|
| Protein source | Multiple | Multiple |
| Fiber source | Multiple | Multiple+ Added Inulin |
| Ingredient (g) | ||
| Purina 5001 | 1000 | 800 |
| Casein | 0 | 0 |
| Soy Protein | 0 | 0 |
| Cellulose | 0 | 0 |
| Inulin | 0 | 200 |
| FD&C Red Dye #40 | 0 | 0 |
| FD&C Blue Dye #1 | 0 | 0.075 |
| Total | 1000 | 1000.075 |
| Used in figure: | 1, 2, 3, 4, 5, 6, S1 | 6 |
Mice treatment
Mice were fed with NCD or specified compositionally defined diets (CDD) for a total period of 14 days. After 7 days of diet-treatment, animals were further treated with 2.5% Dextran Sulfate Sodium (DSS) in water for 5–7 additional days (3). We previously reported that effects of purified diet on intestinal homeostasis are rapid, with altered gut morphology (colon weight, colon length and ceacum weight) and increase in peri-epididymal fat pad weight occurring as soon as following 2 and 4 days of diet treatment, respectively (2).
Body weights were measured every day and are expressed as a percentage gain compared to body weight post diet/pre DSS, defined as day 0 and as 100% (the initial body weight of each individual mice was defined as 100%, and subsequent body weight were expressed as relative to this initial body weight, making changes in body weight easily appreciable). At the end of diet/DSS treatment, mice were fasted for 5 h. Mice were then euthanized and colon length, colon weight (wet weight, without luminal content), caecum weight and spleen weight were measured. Organs were collected for downstream analysis.
Quantification of fecal lipocalin-2 (Lcn-2) by ELISA
For quantification of fecal Lcn-2 by ELISA, frozen fecal samples were reconstituted in PBS containing 0.1% Tween 20 to a final concentration of 100 mg/mL and vortexed for 20 min to get a homogenous fecal suspension (5). These samples were then centrifuged for 10 min at 14 000 g and 4°C. Clear supernatants were collected and stored at −20°C until analysis. Lcn-2 levels were estimated in the supernatants (following appropriate dilution) using Duoset murine Lcn-2 ELISA kit (R&D Systems, Minneapolis, Minnesota, USA) using the colorimetric peroxidase substrate tetramethylbenzidine, and optical density (OD) was read at 450 nm (Versamax microplate reader).
Haematoxylin and eosin staining of colonic tissue and histopathologic analysis
Following euthanasia, mouse colons were fixed in 10% buffered formalin for 24 h at room temperature and then embedded in paraffin. Tissues were sectioned at 5 μm thickness and stained with haematoxylin and eosin (H&E) using standard protocols. H&E stained slides were scored in a blinded manner. Each colon was assigned four scores based on the degree of epithelial damage (0 = normal; 1 = hyperproliferation, irregular crypts; 2 = mild to moderate crypt loss; 3 = severe crypt loss; 4 = complete crypt loss, surface epithelium intact; 5 = small- to medium-sized ulcer; 6 = large ulcer), inflammatory infiltrate in the mucosa (0 = normal, 1 = mild, 2 = modest, 3 = severe), submucosa (0 = normal, 1 = mild, 2 = modest) and muscularis/serosa (0 = normal, 1 = mild,), as previously described (6). Each of the four scores was multiplied by 1 if the change was focal, 2 if it was patchy and 3 if it was diffuse, as previously described (5). The 4 individual scores per colon were added, resulting in a total scoring range of 0–36 per mouse.
Water consumption
Groups of mice were placed in a clean cage with a known amount of water. Forty-eight hours later, the amount of remaining water was measured with the difference viewed as water consumption.
Statistical analysis
Significance was determined using one-way ANOVA with Bonferroni’s multiple comparisons test (GraphPad Prism software, version 6.01). Differences were noted as significant at *P≤0.05.
RESULTS
Severe disease in DSS-treated mice fed a compositionally-defined diet (CDD)
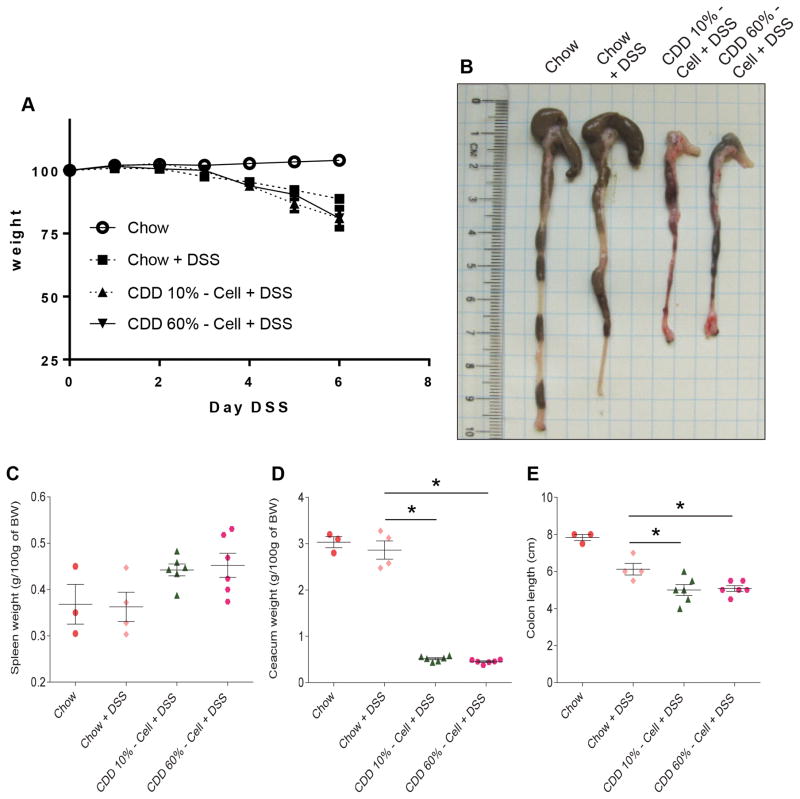
Switching the diet of chow-fed mice to a commonly-used control-CDD containing 10% fat (CDD-10%), which was designed to be similar in the macronutrient and calorie content to some commonly used chow diets, results in loss of gut mass that begins within 2-days of administration of the CDD, reaches maximal reduction within 10 days, and stays at stable but markedly reduced levels thereafter while on this diet (2). Such reduction in gut mass is irrespective of CDD fat content and associates with low-grade inflammation, which promotes metabolic syndrome (7). Here, we sought to examine how the CDD-induced phenotype might impact the response to an inducer of robust gut inflammation, namely dextran sulfate sodium (DSS) (3). Six-week-old mice that had been maintained on chow were switched to control CDD-10% or high-fat CDD-60% 1 week before administering drinking water containing 2.5% DSS and monitored for disease. The primary clinical-type readout to monitor ongoing DSS-induced colitis is weight loss. While all mice administered DSS exhibited weight loss, the extent of weight loss was, relative to chow-fed mice, modestly but significantly greater in mice fed CDD-10% and CDD-60% (Figure 1A). Such increased disease severity was not a consequence of diet-mediated increased water consumption, as reported figure S1. Mice were euthanized on day 6 of DSS treatment, a time at which about half of the CDD-fed mice but not the chow-fed mice had reached end points (weight loss or moribund appearance) that required euthanasia. In accord with the notion that both CDD-10% and CDD-60% fed mice had worse colitis following DSS treatment, the colons and caeca appeared grossly necrotic and bloody (Figure 1B). One relatively straightforward means to quantitate gut inflammation is to measure gross organ parameters. Specifically, acute gut inflammation induced by DSS (or other agents) is often marked by shortening of the colon, shrinkage of the caecum and, splenomegaly. Analysis of these parameters also indicated exacerbated inflammatory disease in CDD-fed DSS-treated mice compared to chow fed-mice (Figure 1C–E). Thus, although CDD can, by itself, affect all of the parameters used to assay disease following DSS treatment (see (2) and below), collectively, these results indicate that consumption of CDD-10% or CDD-60% eventuates in worse gut inflammatory disease following DSS treatment.
Figure 1. Compositionally-defined diet results in severe disease in DSS-treated mice.
C57Bl/6 male mice were maintained on the specified diet for 7 days and subsequently treated with DSS 2.5% for 6 days. A. Body weight over time. B. Caecum/colon gross morphology. C. Spleen weight. D. Caecum weight. E. Colon length. Data are represented as means ± SEM of N=3–6 mice per group. Significance was determined by Student t test. *P < 0.05.
Inulin does not protect against DSS challenge
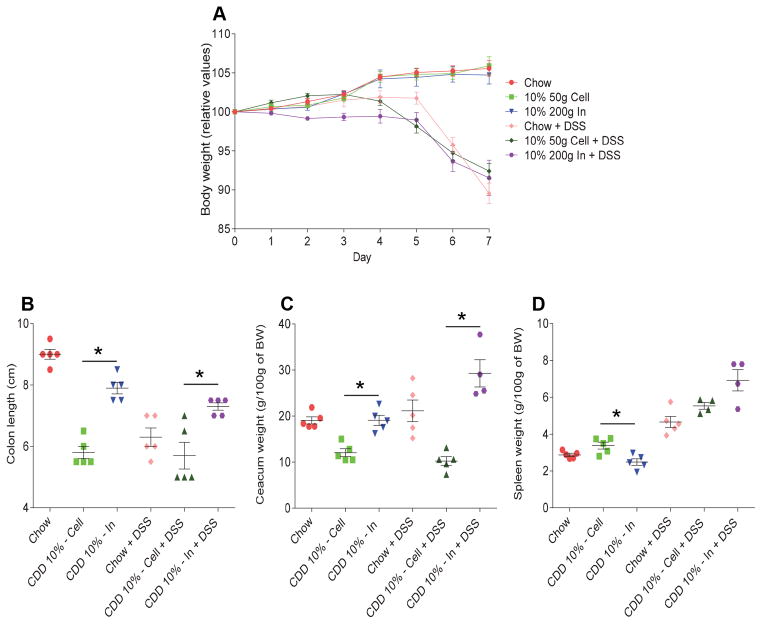
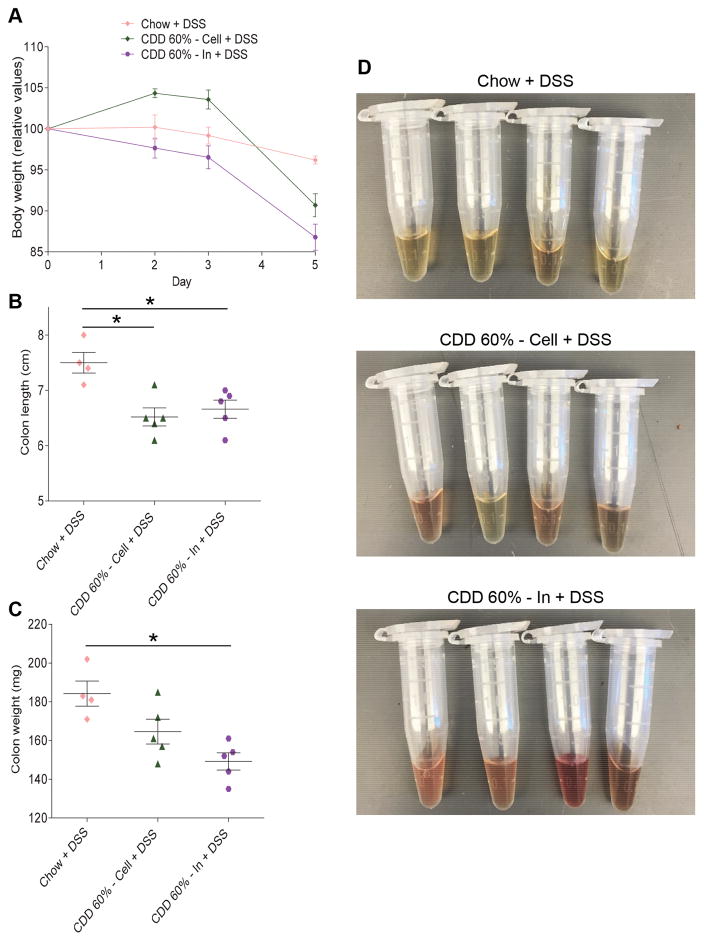
The commonly-used CDD-10% and CDD-60% utilized herein, and in our recently published work (2), lacks fermentable fiber and rather contains only a modest amount of insoluble fiber, namely cellulose. Incorporating a fermentable fiber, namely inulin, into such CDD largely prevents loss of intestinal mass and its associated low-grade inflammation (2). Hence, we hypothesized that supplementation of CDD with inulin, but not cellulose, would ameliorate the exacerbated colitis observed in CDD-fed mice. Relative to DSS-treated mice consuming CDD-10% supplemented with cellulose, addition of inulin to the CDD-10% did not significantly impact body weight loss (Figure 2A and S2) and eventuated in a longer colon and greater caecum weight following DSS (Figure 2B–C). Moreover, mice consuming inulin-enriched diet exhibited greater splenomegaly (Figure 2D), which often indicates severe systemic inflammatory disease. In accordance, fecal Lcn-2, which can be used to track gut inflammation (5), was slightly elevated in CDD-10% + inulin fed mice on day 3 and then lower in those mice on day 7 (Figure 3A–C). More strikingly, all mice consuming diets enriched in inulin, but not cellulose, exhibited obvious gross rectal bleeding (Figure 3D) and had a generally ill appearance that dictated need for euthanasia. In accord with these observations, histopathologic analysis indicated severe gut pathology in mice consuming diet enriched in inulin (Figure 3E), namely near complete destruction of some sections of the colon, which may explain the relative lack of fecal Lcn-2 (fecal Lcn-2 is made in major part by gut epithelia, (8)). Collectively, these data indicate that, despite inulin’s ability to restore gut mass, it did not reduce, and in fact exacerbated, gut pathology and associated disease in response to DSS.
Figure 2. Fermentable fiber inulin does not protect against DSS challenge in purified diet-fed mice.
C57Bl/6 male mice were maintained on the specified diet for 7 days and subsequently treated with DSS 2.5% for 7 days. A. Body weight over time. B. Colon length. C. Caecum weight. D. Spleen weight. Data are represented as means ± SEM of N=5 mice per group. Significance was determined by Student t test. *P < 0.05.
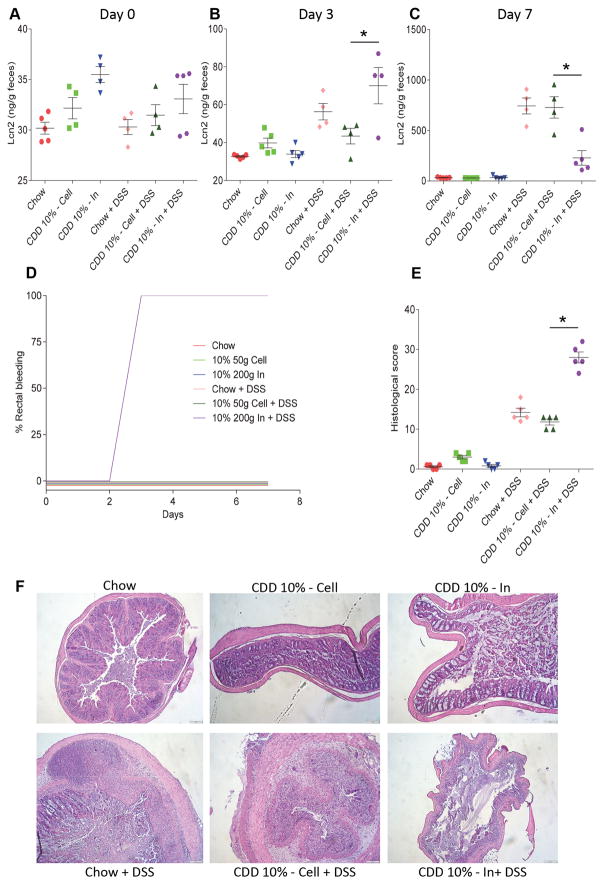
Figure 3. Fermentable fiber inulin worsens intestinal inflammation in DSS-treated mice under purified diet.
C57Bl/6 male mice were maintained on the specified diet for 7 days and subsequently treated with DSS 2.5% for 7 days. A–C. Fecal lipocalin-2 levels at day 0 (A), day 3 (B) and day 7 (C). D. Development of rectal bleeding over time. E. Colonic histopathological scoring. F. H&E staining of colonic tissues. Data are represented as means ± SEM of N=5 mice per group. Significance was determined by Student t test. *P < 0.05.
Neither fat content nor absence of IL-22 impact inulin’s exacerbation of DSS-induced colitis
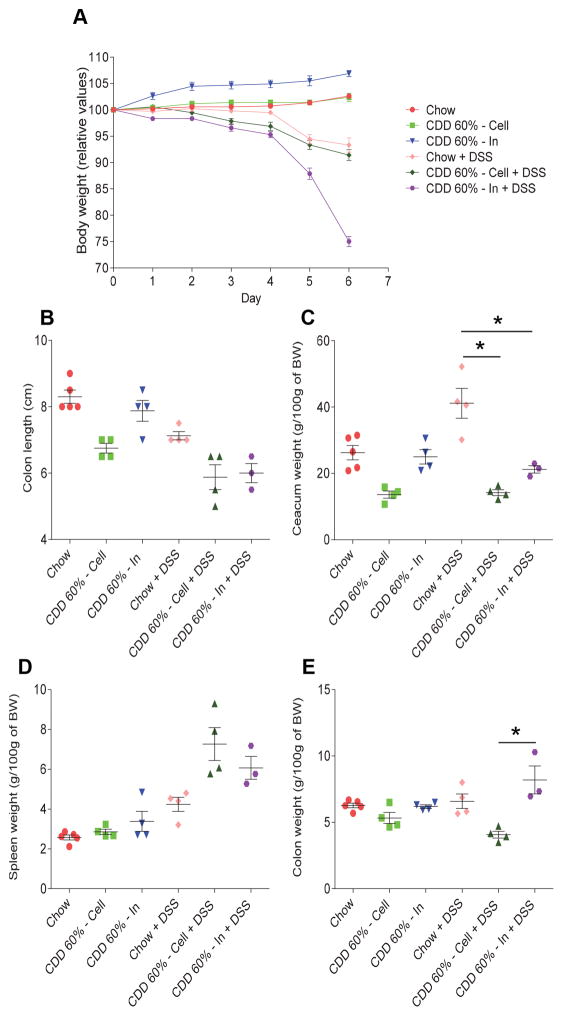
While CDD’s previously observed reduction of gut mass was irrespective of fat content, the extent to which CDD induced metabolic syndrome, which was associated with low-grade inflammation, was detrimentally impacted by fat content (2). Thus, we next examined if supplementation of CDD-60% fat with inulin would impact DSS-induced colitis. Analogous to the results obtained with control CDD-10%, addition of inulin but not cellulose to CDD-60% resulted in very severe disease in response to DSS. Briefly, all of the DSS-treated mice consuming inulin-enriched diets, and none of the chow-fed or cellulose-supplemented mice, exhibited gross rectal bleeding within 4 days of DSS treatment (data not shown). Moreover, DSS-treated mice consuming the inulin-enriched diet exhibited severe weight loss and required euthanasia on day 6 (Figure 4A). Such severe disease associated with splenomegaly, and occurred despite inulin resulting in an increased gut mass (Figure 4B–E). Thus, irrespective of fat content, addition of inulin to CDD results in severe disease in response to DSS. In considering why inulin would promote more severe disease, especially gross bleeding, which was its most striking feature, we noted that, although some studies indicate that IL22 protects against gut inflammation, including DSS colitis (9), our findings seemed reminiscent of recently-described IL-23 transgenic mice that exhibit IL-22-dependent ulcerations that causes severe bleeding in the intestinal tract (10). Hence, we utilized IL-22 deficient mice to test the hypothesis that fermentation of inulin into SCFA might be promoting IL-22 production that drove colonic ulcers in DSS-treated animals. However, inulin-fed mice still exhibited greater weight loss (Figure 5A) and, moreover, uniform gross bleeding (Figure 5D), thus arguing against the notion that increased induction of IL-22 drove inulin’s exacerbation of DSS colitis (Figure 5).
Figure 4. Fermentable fiber inulin does not protect against DSS challenge in high-fat diet-fed mice.
C57Bl/6 male mice were maintained on the specified diet for 7 days and subsequently treated with DSS 2.5% for 6 days. A. Body weight over time. B. Colon length. C. Caecum weight. D. Spleen weight. E. Colon weight. Data are represented as means ± SEM of N=3–5 mice per group. Significance was determined by Student t test. *P < 0.05.
Figure 5. Absence of IL-22 does not impact inulin’s exacerbation of DSS colitis.
IL-22KO male mice were maintained on the specified diet for 7 days and subsequently treated with DSS 2.5% for 5 days. A. Body weight over time. B. Colon length. C. Colon weight. D. Feces supernatant gross picture. Data are represented as means ± SEM of N=4–5 mice per group. Significance was determined by Student t test. *P < 0.05.
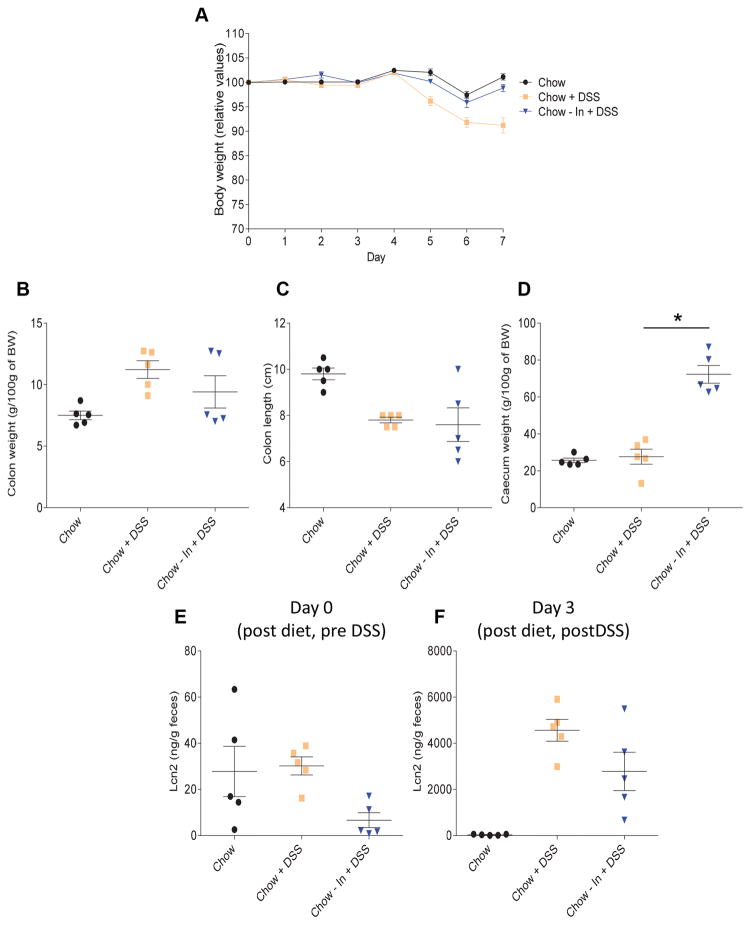
Supplementation of chow with inulin does not exacerbate DSS colitis
Lastly, we investigated whether addition of inulin to NCD would also enhance DSS-induced colitis, or whether inulin only did so in the context of a CDD. We observed that addition of 20% inulin to chow did not exacerbate colitis in response to DSS treatment and, in fact, moderately reduced disease severity, as indicated by weight loss, gross organ parameters and inflammatory marker fecal Lcn-2 (Figure 6). Thus, supplementation of a diet with inulin does not inherently worsen inflammatory disease but rather impacts DSS-induced colitis in a manner that is very much dependent on other aspects of diet composition.
Figure 6. Fermentable fiber inulin dampened basal intestinal inflammation but does not protect against DSS challenge in chow-fed mice.
C57Bl/6 male mice were maintained on the specified diet for 7 days and subsequently treated with DSS 2.5% for 7 days. A. Body weight over time. B. Colon weight. C. Colon length. D. Caecum weight. E–F. Fecal lipocalin-2 levels at day 0 (E, post diet/pre DSS) and day 3 (F, post diet/post DSS). Data are represented as means ± SEM of N=5 mice per group. Significance was determined by Student t test. *P < 0.05.
DISCUSSION
Human epidemiological studies suggest that consuming diets high in fiber promotes health, and consequently protects against an array of chronic diseases, especially those associated with inflammation (7). In order to gain a more mechanistic understanding of how fiber influences health, many researchers, ourselves included, have sought to model these effects in mice, whose diets and genetics can be controlled and manipulated. Indeed, numerous studies in mice indicate that supplementation of diet with fiber ameliorates a range of chronic inflammatory diseases, including mouse models of colitis, cancer and obesity (11). One mechanism thought to underlie some of fiber’s beneficial effects, particularly fermentable fibers, is that they promote growth of beneficial bacteria that metabolize the fiber to short-chain fatty acids (SCFA), especially butyrate, that have an array of anti-inflammatory and other beneficial effects on the host (12–15). Hence, we presumed that feeding mice diets low in fiber would promote inflammatory diseases while supplementing such diets with inulin, which is known to be the most effective fiber at raising levels of colonic butyrate (16), would ameliorate inflammation and thus promote health. This was indeed the case in our studies relating to low-grade inflammation, in that replacing the cellulose (non-fermentable) in compositionally-defined diets (CDD) with inulin ameliorated indices of low-grade inflammation and metabolic syndrome in both control and high-fat CDD (2). Moreover, analogous to recent findings by Macia and collaborators, we observed that CDD lacking fiber results in severe colitis upon DSS challenge (17). However, in the current study, we observed that supplementing such CDD with inulin further exacerbates colitis, resulting in very severe disease characterized by destruction of the colonic mucosa and gross bleeding. Similarly to our findings, Goto et al., previously reported that consumption of fructooligosaccharide, a shorter polymer chain length than inulin, exacerbated diarrhea and weight loss in mice fed a purified diet, while it reduced bleeding in mice fed a non-purified diet (18).
The notion that consumption of high amounts of fiber is safe and, moreover, has the potential to protect against robust inflammation, including DSS colitis, was previously demonstrated in the context of a CDD containing 40% fiber by Macia and collaborators (17). While total amount of fiber in this diet was around twice what we used herein, the amount of fermentable fiber was similar to ours (around 200 gm/kg), although their study used guar gum while we used inulin. Such differences suggest that, in the context of CDD and DSS, inulin, but not guar gum, may promote severe disease. We would thus suggest that careful comparison of the impact of these (and other) fibers on colitis is warranted but do not, at present, have a good potential mechanistic explanation for any differences that might be observed. In any case, we do not question that, in many contexts, sometimes including acute colitis, supplementation of diet with fiber would protect against disease. Indeed, we observed that supplementing chow with inulin offered some, albeit modest, protection against DSS-induced disease. Moreover, we observed a trend that inulin supplementation of chow and CDD ameliorated colitis in IL-10 deficient mice, although no statistically significant results were obtained (data not shown). Given the great interest in how diet impacts health, it will be important for future research to decipher how inulin intersects with other CDD’s components to exacerbate intestinal inflammation while seemingly not dramatically impacting animals whose base diet was rodent chow. A role for intestinal microbiota, which is altered by CDD as well as by inulin, seems likely to be involved.
In conclusion, viewing our findings from the perspective of trying to create a defined, tractable rodent diet that would allow rigorous rational testing of how diet composition influences health, we conclude that while inulin-enriched CDD results in a basal phenotype that more closely resembles that of chow-fed mice, this approximation comes apart upon challenge with DSS. Thus, further efforts to develop a CDD that would fully phenocopy chow diet are warranted. However, there are many commercially available chows, each of which vary in composition, making difficult to recapitulate or predict the phenotype of all chows. When viewed from the perspective of trying to predict how fiber consumption might impact human health, our findings caution against the notion that health of persons consuming a low-fiber diet can be broadly and safely promoted by consumption of foods containing fermentable fiber. Rather, it argues that recommendations to supplement foods with presumably beneficial ingredients should be given with a high degree of caution. Lastly, our results underscore the need for more studies to better define the impact of various fibers on phenotype and, moreover, decipher the underlying mechanisms that mediate fibers’ effects.
Supplementary Material
Figure S1: Effect of the various diets on water consumption. C57Bl/6 male mice were maintained on the specified diet and water consumption was determined over a period of 48h. Data are represented as means ± SEM of N=5 per group.
Figure S2: Fermentable fiber inulin does not protect against DSS challenge in purified diet-fed mice. C57Bl/6 male mice were maintained on the specified diet for 7 days and subsequently treated with DSS 2.5% for 7 days. Body weight over time is represented. Lines represent the mean of each group, with individual mouse value represented with symbols. N=5 mice per group.
Acknowledgments
FUNDING
This work was supported by NIH grant DK099071 and DK083890. BC is a recipient of the Career Development Award from the Crohn’s and Colitis Foundation of America (CCFA).
Abbreviations
- DIO
diet-induced obesity
- CDD
compositionally-defined diet
- NCD
normal chow diet
- HFD
high-fat diet
- TLR
Toll-like receptor
- SCFA
short-chain fatty acid
- LPS
lipopolysaccharide
Footnotes
COMPETING INTEREST
None.
References
- 1.Pellizzon M, Ricci M. Nutriphenomics in rodent models: Impact of dietary choices on toxicological biomarkers. In: Gupta Ramesh., editor. Biomarkers in Toxicology. Chapter: 36. Elsivier, Inc; 2015. pp. 629–643. [Google Scholar]
- 2.Chassaing B, Miles-Brown J, Pellizzon M, et al. Lack of soluble fiber drives diet-induced adiposity in mice. Am J Physiol Gastrointest Liver Physiol. 2015;309:G528–541. doi: 10.1152/ajpgi.00172.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chassaing B, Aitken JD, Malleshappa M, et al. Dextran sulfate sodium (DSS)-induced colitis in mice. Current protocols in immunology/edited by John E Coligan [et al] 2014;104(Unit 15):25. doi: 10.1002/0471142735.im1525s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng Y, Valdez PA, Danilenko DM, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 5.Chassaing B, Srinivasan G, Delgado MA, et al. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One. 2012;7:e44328. doi: 10.1371/journal.pone.0044328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katakura K, Lee J, Rachmilewitz D, et al. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest. 2005;115:695–702. doi: 10.1172/JCI22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5:1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh V, Yeoh BS, Chassaing B, et al. Microbiota-inducible Innate Immune, Siderophore Binding Protein Lipocalin 2 is Critical for Intestinal Homeostasis. Cell Mol Gastroenterol Hepatol. 2016;2:482–498. e486. doi: 10.1016/j.jcmgh.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zenewicz LA, Yancopoulos GD, Valenzuela DM, et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L, He Z, Slinger E, et al. IL-23 activates innate lymphoid cells to promote neonatal intestinal pathology. Mucosal Immunol. 2015;8:390–402. doi: 10.1038/mi.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andoh A, Bamba T, Sasaki M. Physiological and anti-inflammatory roles of dietary fiber and butyrate in intestinal functions. JPEN J Parenter Enteral Nutr. 1999;23:S70–73. doi: 10.1177/014860719902300518. [DOI] [PubMed] [Google Scholar]
- 12.Kim CH, Park J, Kim M. Gut microbiota-derived short-chain Fatty acids, T cells, and inflammation. Immune network. 2014;14:277–288. doi: 10.4110/in.2014.14.6.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaiko GE, Ryu SH, Koues OI, et al. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell. 2016;167:1137. doi: 10.1016/j.cell.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 16.Scott KP, Martin JC, Duncan SH, et al. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol Ecol. 2014;87:30–40. doi: 10.1111/1574-6941.12186. [DOI] [PubMed] [Google Scholar]
- 17.Macia L, Tan J, Vieira AT, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nature communications. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 18.Goto H, Takemura N, Ogasawara T, et al. Effects of fructo-oligosaccharide on DSS-induced colitis differ in mice fed nonpurified and purified diets. J Nutr. 2010;140:2121–2127. doi: 10.3945/jn.110.125948. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Effect of the various diets on water consumption. C57Bl/6 male mice were maintained on the specified diet and water consumption was determined over a period of 48h. Data are represented as means ± SEM of N=5 per group.
Figure S2: Fermentable fiber inulin does not protect against DSS challenge in purified diet-fed mice. C57Bl/6 male mice were maintained on the specified diet for 7 days and subsequently treated with DSS 2.5% for 7 days. Body weight over time is represented. Lines represent the mean of each group, with individual mouse value represented with symbols. N=5 mice per group.