Abstract
Background
Both prolonged cold ischemia time (CIT) and donor history of diabetes mellitus (DM) are associated with reduced graft survival after liver transplantation. However, it is unknown whether the adverse effect of prolonged CIT on posttransplant graft survival is more pronounced after transplant with DM versus non-DM donor grafts.
Methods
The study sample included 58 226 liver transplant recipients (2002-2015) from the Scientific Registry of Transplant Recipients. Multivariable Cox survival regression with interaction analysis was used to quantify the extent to which history of donor DM (n = 6478) potentiates the adverse effect of prolonged (≥8 hours) CIT (n = 18 287) on graft survival.
Results
Donor DM and CIT 8 hours or longer were each associated with increased risk of graft failure (GF) (adjusted hazard ratio [aHR], 1.19; 95% confidence interval [CI], 1.06-1.35 and aHR, 1.42; 95% CI, 1.32-1.53, respectively) compared with transplanted grafts without either risk factor. However, the combination of DM and CIT 8 hours or longer was associated with a higher risk of GF than either factor alone (aHR, 1.79; 95% CI, 1.55-2.06) and had a synergy index of 1.30. The interaction was significant on a multiplicative scale in the later postoperative period, days 31 to 365 (P = 0.047).
Conclusions
These results suggest that liver grafts from DM donors are more susceptible to the adverse effects of prolonged CIT than livers from non-DM donors. We need to be cognizant that they are more susceptible to ischemic injury, and this may be considered during the allocation process.
Liver transplantation (LT) provides an effective treatment for patients with end-stage liver disease. Due to persistent organ shortages, approximately 2000 patients die while on the liver waiting list every year.1 To overcome this gap, there has been an increase in the use of organs from deceased donors that have multiple comorbidities and other factors that are associated with increased risk of graft failure (GF).
The prevalence of diabetes mellitus (DM) among US adults in 2011 was 23.7 million and this number is expected to increase to 29.6 million in 2030.2 Only few studies regarding the effect of donor DM on the risk of GF have been carried out.3,4 It has been suggested that DM in donor livers may be associated with adverse outcomes posttransplant.3 DM often results in systemic vascular damage (diabetic microangiopathy)5 and is a risk factor for chronic hepatic injury due to nonalcoholic fatty liver disease and subsequent progression to more advanced liver diseases.6
Prolonged cold ischemia time (CIT) is a well-established risk factor for GF. Ischemic injury damages the liver graft at the cellular level and may lead to primary nonfunction, delayed graft function, and ischemic cholangiopathy.7 Ischemia-reperfusion injury (IRI) is associated with the release of reactive oxygen species and proinflammatory mediators, which causes damage of the hepatic sinusoidal epithelium and severe hepatic microcirculatory impairment.8
We hypothesized that prolonged cold ischemia aggravates preexisting microvascular changes that are seen in diabetic donors grafts, leading to inferior graft survival. We explored this question using over a decade of comprehensive US Transplant Registry data and quantified the extent to which the effects of donor DM on risk of GF 1 year post-LT are modified by prolonged CIT.
MATERIALS AND METHODS
Study Design
This retrospective cohort study includes subjects that underwent first LT between April 1, 2002, and April 1, 2015, using Scientific Registry of Transplant Recipients (SRTR) data (Figure 1). Exclusion criteria included: recipient age, younger than 18 years (n = 6562), multiorgan (n = 4801) or living donor (n = 2823) transplants, and patients with missing or extreme values (>18 hours for CIT) on key predictor variables, donor DM (n = 306) and CIT (n = 3391). Retransplants were also excluded from analysis.
FIGURE 1.
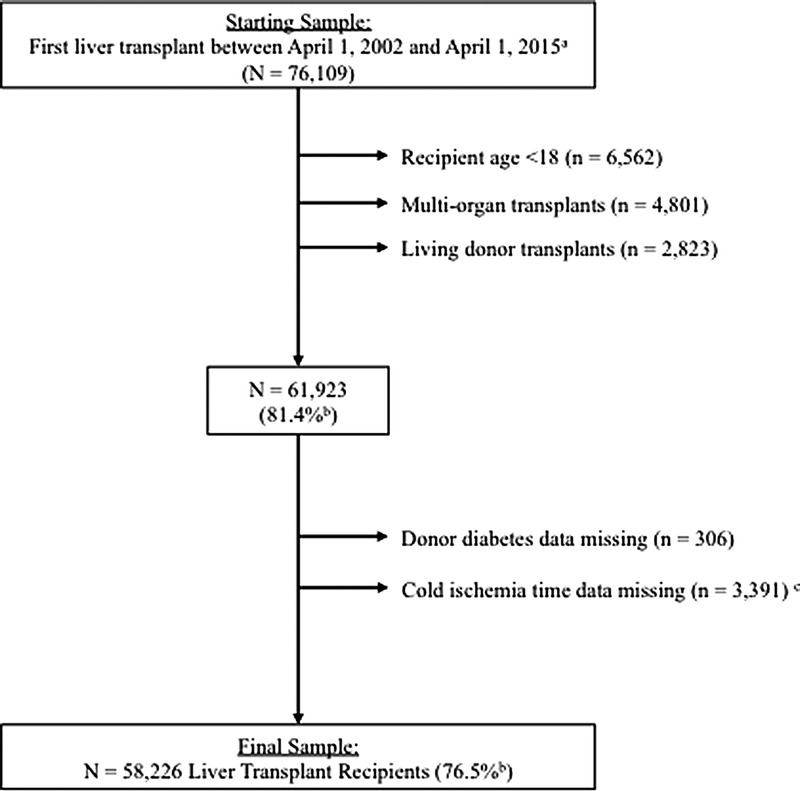
Study Inclusion/exclusion criteria flowchart. Scientific Registry of Transplant Recipients. a Retransplants were not included in analyses. b Percent = N remaining / N starting sample. c Extreme values (>18 hours) change to missing.
The primary study endpoint was 1-year GF, defined as time to all-cause GF or retransplant. 1-month and 3-year GF were included as secondary outcomes to investigate potential early, and long-term consequences of IRI. Separate survival models were fit for 1-year and 3-year results with appropriate censoring as each person either died or was lost to follow up. The primary exposure variables were prolonged CIT (≥8 hours) and history of donor DM (DM+) versus no DM (DM−). CIT was evaluated as a potential effect measure modifier of donor DM on graft survival rate. CIT threshold was dichotomized into CIT 8 hours or longer versus CIT less than 8 hours based on previous publications.3 Sensitivity analyses were done for CIT of 6, 8, and 10 hours as well as using up to 5 categories of CIT. The results were not presented in the final analyses, because they did not meaningfully change the results.
Potential confounders included both donor and recipient factors and were identified based on established clinical evidence and literature review.3,4,9 Recipient characteristics were collected perioperatively pretransplant and included age, sex, race/ethnicity, body mass index (BMI), history of diabetes, last laboratory Model for End-Stage Liver Disease (MELD) score, United Network for Organ Sharing (UNOS) Status 1, primary liver diagnosis, hepatitis C status, Child-Pugh score (and individual components albumin, bilirubin, encephalopathy, and ascites), medical condition (intensive care unit, hospitalized, or home) and whether patients were receiving ventilatory support. Donor characteristics included Donor Risk Index and its components (age, race/ethnicity, cause of death, donation after cardiac death, partial/split liver, height, allocation type), sex, BMI, and history of hypertension.
Statistical Analyses
Variables were assessed for missingness and extreme values; variables with more than 5% missing values were not included in tables or regression analyses. A full list of variables in the SRTR database can be found on the associated website.10 Complete-case analyses were conducted, whereas only subjects with complete data on all variables included in the statistical equation were included in the models. We evaluated the relationships between potential confounders and the primary exposure variable using t tests for continuous variables and χ2 tests for categorical variables. Results are expressed as mean ± SD unless otherwise indicated.
Graft survival rates were estimated and tested using the Kaplan-Meier method and log-rank tests, respectively. Cox proportional hazards survival regression was used to examine the relationships between the primary predictors of interest and graft survival. Multivariable models were built for each predictor (donor DM, CIT) separately and then in combination using a forward (manual) approach, sequentially adding conceptually meaningful groups of variables while assessing model fit. Only variables that were statistically significant were retained in the final model. Model goodness-of-fit and proportional hazards assumptions were assessed graphically and confirmed with the Grønnesby and Borgan test using martingale residuals.11
Effect measure modification was assessed by evaluating departures from additivity using dummy variables for each possible combination of DM and CIT (short CIT, DM- (referent group) / short CIT, DM+ / prolonged CIT, DM- / prolonged CIT, DM+) and multiplicative effects were assessed using a product term (interaction) for DM x CIT.
Results are expressed as hazard ratios (HR) (95% confidence intervals [CIs]); P value of 0.05 or less was considered significant. All analyses were conducted using Stata software version 13 (StataCorp LP).
RESULTS
Sample Characteristics
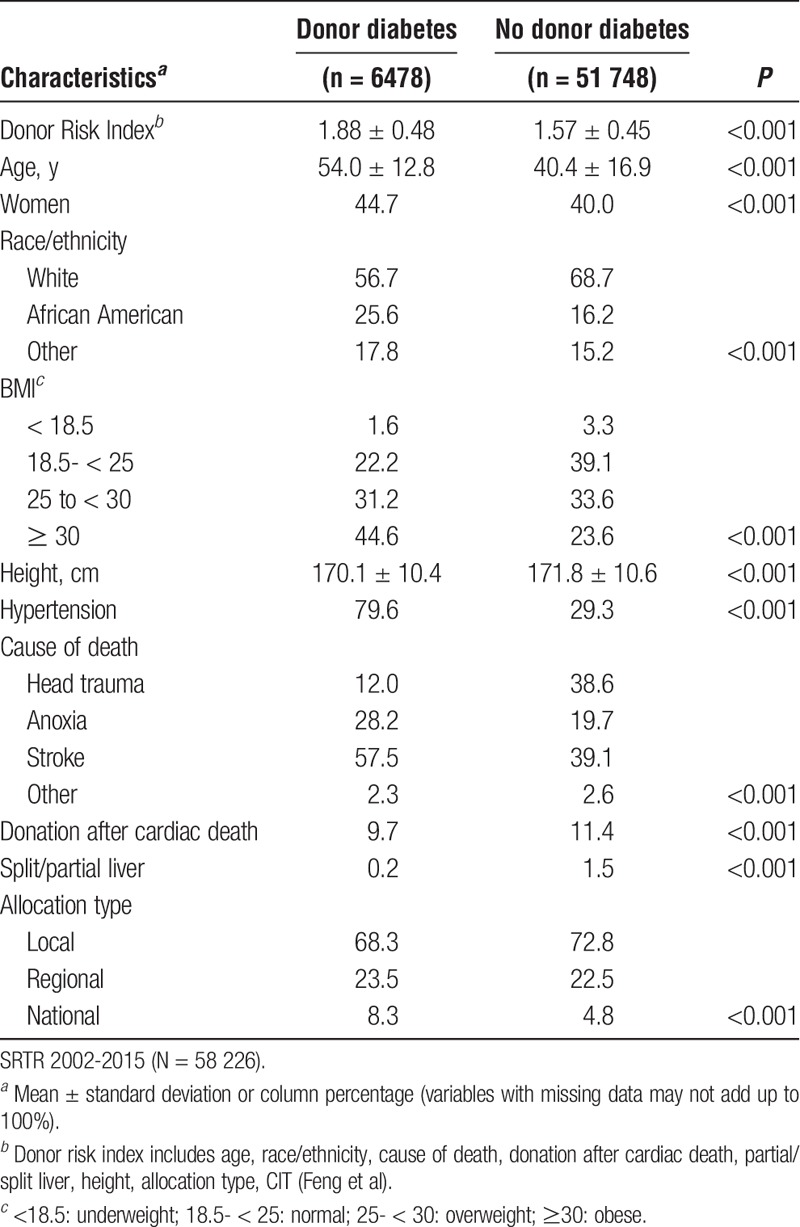
After excluding 3697 patients with missing data on key variables (only considering variables with <5% missingness) the final sample used in complete cases analyses included 58 226 subjects. The average age was 54.1 (±10.0) years and 1/3 (32.4%) were women. About one-quarter (72.2%) of the sample was White, compared to 12.8% Hispanic and 9.3% African American. The average MELD score was 20.9 ± 9.7. Eleven percent (n = 6478) of donors had a history of DM and 88.9% (n = 51 748) did not.
Table 1 shows recipient pretransplant characteristics by donor DM status. Recipient characteristics were comparable among those that received a diabetic versus nondiabetic donor graft.
TABLE 1.
Recipient pretransplant characteristics by donor DM status
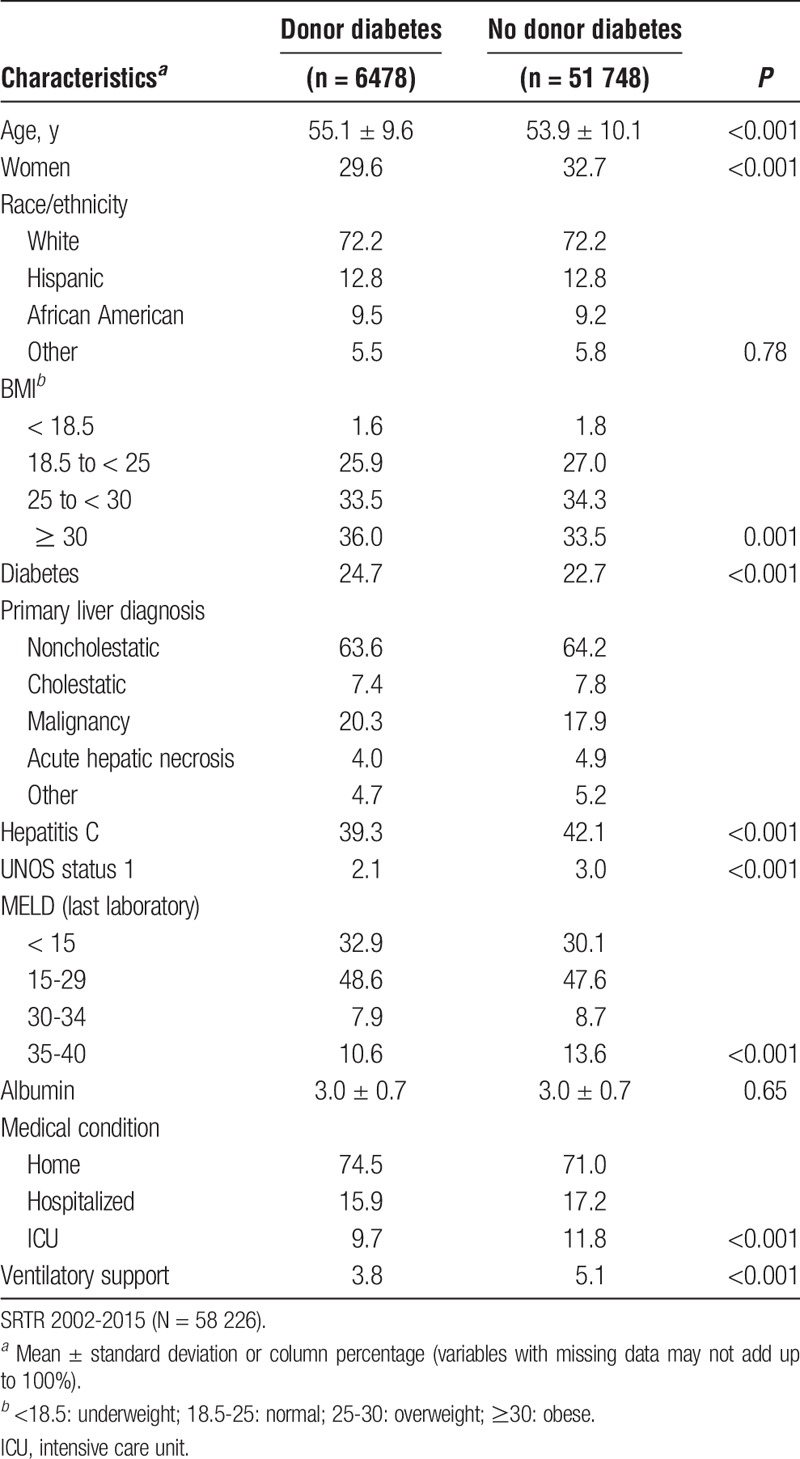
Table 2 shows donor characteristics by donor DM status. DM donors were older and more likely to be African American or Hispanic. A greater proportion of DM donors was obese and/or suffered from hypertension. DM donors more often died from anoxia or stroke than trauma.
TABLE 2.
Donor characteristics by donor DM status
GF
The median time at risk was 38 months and 11.7% (6797 subjects) experienced GF at any point during the study period. Graft survival at 30 days and 1 year posttransplant was 97.5% (1456 events) and 93.0% (3755 events), respectively.
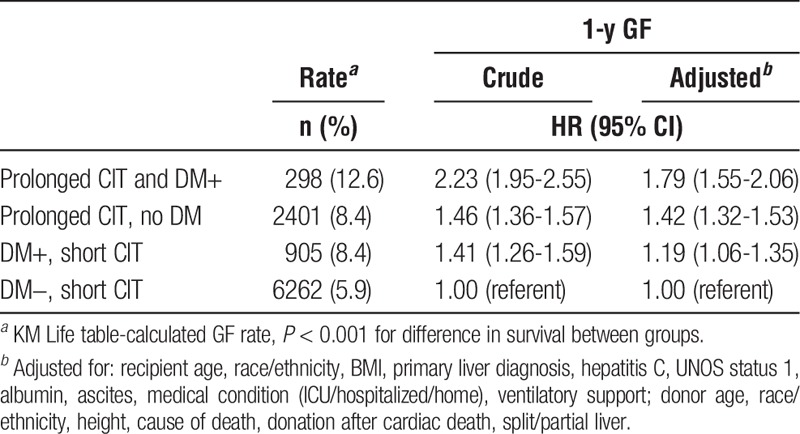
Table 3 shows HRs for GF within 1 year posttransplant for DM and CIT. The GF rate was higher for diabetic donor grafts and for prolonged CIT. Adjustment did not meaningfully alter (>10% change in estimate) the HR for risk associated with CIT. The only risk factor that meaningfully altered the HR for DM (and remained statistically significant) in the final models was donor age.
TABLE 3.
HRs (95% CIs) for 1-year GF by donor DM status and CIT
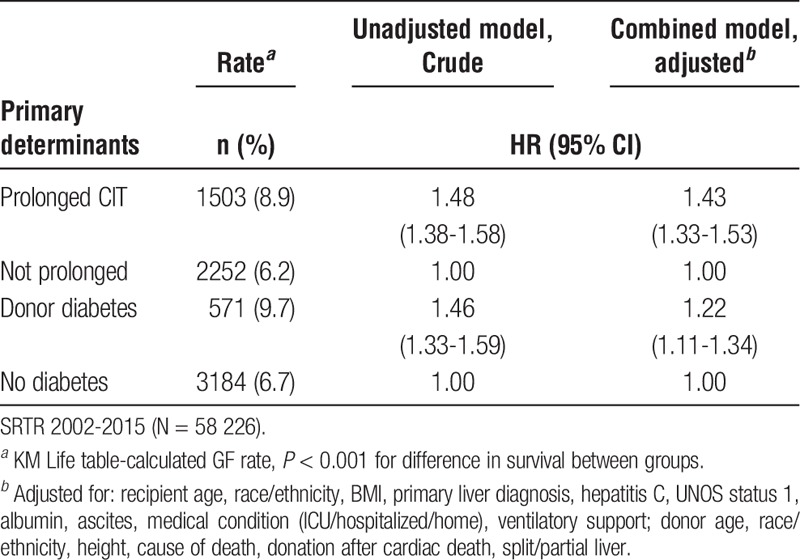
Figure 2 illustrates the (unadjusted) Kaplan-Meier curves by DM and CIT strata. GF rate was highest in the immediate postoperative period (days 0-30) for all groups. Graft survival was lowest for patients that received a diabetic donor graft with prolonged CIT for up to 3 years posttransplant and survival functions were significantly different across strata on log-rank test (P < 0.001).
FIGURE 2.
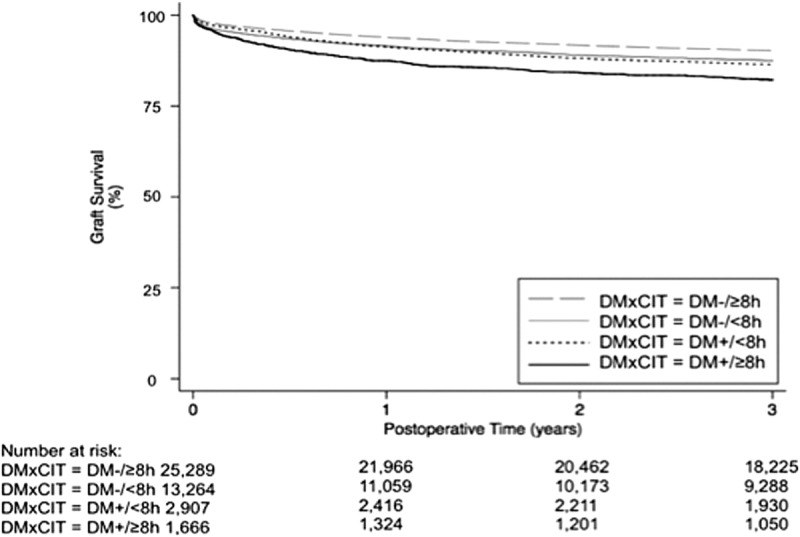
Kaplan-Meier curves: Liver graft survival up to 3 years posttransplant, stratified by donor DM status and short (<8 hours) versus long (≥8 hours) CIT. SRTR 2002-2010 (N = 33 980).
Table 4 shows the HRs for patients with either or both risk factors relative to neither. On adjusted analyses, the probability of GF within 1 year of transplant was highest for recipients with a combination of donor DM and prolonged CIT compared to for recipients of grafts with short CIT from donors without any history of DM (adjusted HR, 1.79; 95% CI, 1.55-2.06). The unadjusted HRs for subjects with either factor alone versus neither factor were comparable, but the HR for donor DM (relative to subjects with neither factor) declined by about 16% after adjustment.
TABLE 4.
HRs (95% CI) for 1-year GF stratified by donor DM status and CIT relative to neither risk factor
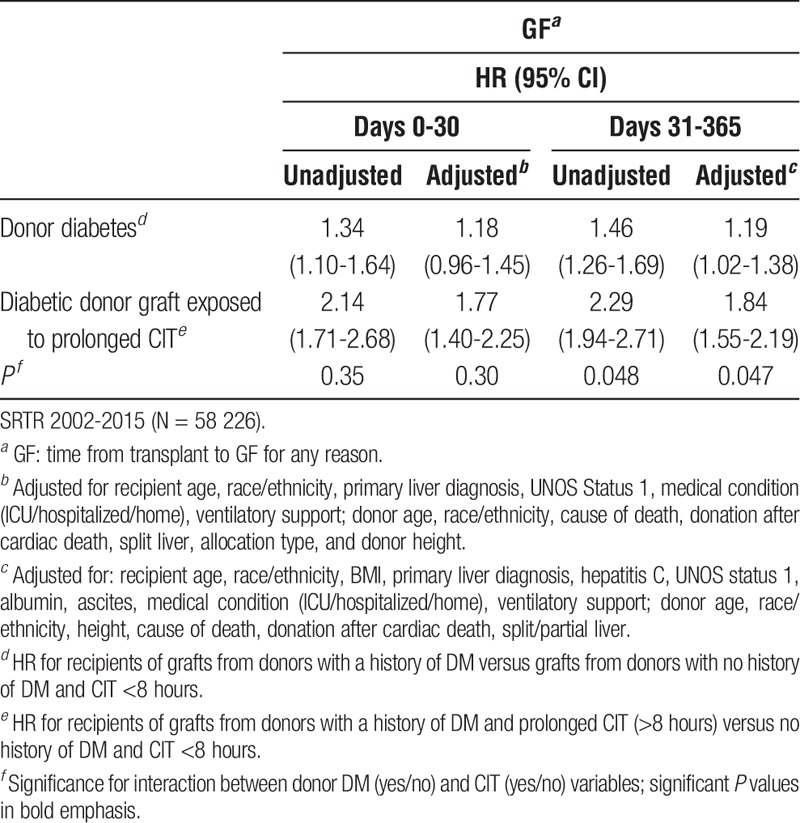
After multivariable adjustment, 10% of the risk for grafts with both prolonged CIT and donor DM history was attributable to interactive effect between CIT and DM, suggestive of effect measure modification, with a synergy index of 1.30. In other words, there are likely grafts that would fail in the presence of both factors that would not otherwise fail without the added injury from prolonged CIT in a given range of time. Thus, although the product term (multiplicative interaction) for CIT and DM was not significant for overall 1-year GF, we further subdivided postoperative periods into 0 to 30 days and 31 to 365 days and found that there was a significant synergistic interaction between DM and CIT in the latter postoperative period (Table 5).
TABLE 5.
HRs (95% CIs) for GF for recipients of diabetic donor grafts and the effect of prolonged CIT
DISCUSSION
We used the SRTR database to analyze the extent to which donor DM modifies the effect of prolonged CIT on the risk of GF after LT. Our results show that the combination of prolonged CIT in a diabetic liver graft has a synergistic effect on the risk of GF.
Our results are supported by prior literature on GF risk associated with donor DM and further advance the field by providing evidence on the interaction between DM and CIT. A recent study describing 26 645 liver transplant recipients demonstrated that recipients of diabetic donor grafts have an increased risk of mortality after LT (HR, 1.11; 95% CI, 1.02-1.19).3 In this study, 34.8% of recipients of grafts from DM donors experience GF compared with 27.8% of recipients that received a non-DM donor graft (P < 0.001). Another study evaluated 27 033 transplant cases and showed that donor diabetes was a strong independent risk factor for GF (HR, 1.20; P = 0.006) in hepatitis C virus positive transplant recipients.4 Segev et al showed no effect modification by donor DM on the effects of prolonged CIT. Our results did not show a significant interaction between CIT and donor DM on overall 1-year GF either. The synergistic interaction between DM and CIT was significant in the latter postoperative period only.9
Type 2 DM is associated with hepatic steatosis, a form of nonalcoholic fatty liver disease, which can progress to nonalcoholic steatohepatitis.12,13 Hepatic fat accumulation can result in liver inflammation through the release of various cytokines and ultimately cause liver fibrosis.5,14,15 Several studies have shown that steatotic livers are more likely to experience IRI, leading to worse clinical outcomes after LT.16,17
Furthermore, the microvascular changes in diabetic donor grafts, like damage to the sinusoidal lining cells and disruption of the microvasculature, impair the hepatic microcirculation. Prolonged CIT can aggravate these microvascular changes and consequently increase susceptibility to IRI.18-21 Surprisingly, the impact of donor DM status on graft survival was more pronounced 30 days after LT. This seems to be counterintuitive, but can be explained by the fact that donor DM status can increase the incidence of acute rejection episodes and ischemic cholangiopathy. To support this hypothesis, a recent study on 88 primary LTs demonstrated a significant association between donor DM and ischemic-type biliary lesions with an HR of 9.5 (P = 0.009), suggesting that DM promotes chronic changes in the biliary vessels, thereby increasing susceptibility to IRI.22
There were several limitations to our study. First, it should be noted that the statistical significance was only marginal. Second, this is a retrospective analysis and it is impossible to eliminate allocation biases. As is common in analyses of large administrative databases, missing data and reliability of the entered data must be thoroughly evaluated and appropriately handled in analyses, and in our study, we did not use any variables missing more than 5% of values. In addition, we were also unable to evaluate the direct effect of steatosis in the donor graft, because biopsy results are not captured in this database. To address this limitation, we evaluated BMI as a surrogate of graft steatosis but found it was not associated with GF after multivariable adjustment and was not retained in any of our final models. Lastly, we were not able to assess the impact of the duration of DM, since the SRTR does not have a variable that quantifies duration of DM. Future suggestions would be to investigate whether increasing GF rates occur with increasing CIT and duration of DM in a study with a smaller sample size.
Over the last decade more marginal donor organs are being accepted to increase the shrinking donor organ pool. This study demonstrates for the first time that liver grafts from a DM donor are more susceptible to prolonged CIT compared to nondiabetic donor grafts. The risk of GF within 1 year after LT is significantly higher in liver grafts from DM donors with CIT 8 hours or longer compared with diabetic liver grafts with CIT less than 8 hours. Although the outcomes of diabetic liver grafts are acceptable, we need to be cognizant that they are more susceptible to ischemic injury in transit and IRI intraoperatively.
Expediting allocation of diabetic donor grafts will help to reduce CIT and therefore decrease graft injury. In conclusion, this study confirmed that donor DM status is an independent risk factor and contributes synergistically with prolonged CIT to reduce graft survival.
Footnotes
Published online 12 June, 2017.
Funding of this study was supported by a research grant of the Maag, Lever, Darm Stichting (MLDS), the Netherlands.
The authors declare no conflicts of interest.
A.B. and P.N.A.M. equally contributed.
I.M.A.B. and N.H.D. equally contributed.
I.M.A. participated in research design, drafting the work, and data analysis. No conflict of interest to declare. N.H.D. participated in research design, drafting the work, and data analysis. R.J.P. participated in research design, critical revising of the work, and data analysis. A.B. participated in research design, critical revising of the work, and data analysis. P.N.M. participated in research design, critical revising of the work, and data analysis.
REFERENCES
- 1.Thuluvath PJ, Guidinger MK, Fung JJ, et al. Liver transplantation in the United States, 1999-2008. Am J Transplant. 2010;10(4 Pt 2):1003–1019. [DOI] [PubMed] [Google Scholar]
- 2.Whiting DR, Guariguata L, Weil C, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. [DOI] [PubMed] [Google Scholar]
- 3.Zheng J, Xiang J, Zhou J, et al. Liver grafts for transplantation from donors with diabetes: an analysis of the Scientific Registry of Transplant Recipients database. PLoS One. 2014;9:e98104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y, Ahmed A, Kamal A. Donor diabetes mellitus is an independent risk factor for graft loss in HCV positive but not HCV negative liver transplant recipients. Dig Dis Sci. 2013;58:574–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotronen A, Juurinen L, Tiikkainen M, et al. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology. 2008;135:122–13. [DOI] [PubMed] [Google Scholar]
- 6.Lv WS, Sun RX, Gao YY, et al. Nonalcoholic fatty liver disease and microvascular complications in type 2 diabetes. World J Gastroenterol. 2013;19:3134–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. [DOI] [PubMed] [Google Scholar]
- 8.Gracia-Sancho J, Casillas-Ramírez A, Peralta C. Molecular pathways in protecting the liver from ischaemia/reperfusion injury: a 2015 update. Clin Sci (Lond). 2015;129:345–362. [DOI] [PubMed] [Google Scholar]
- 9.Segev DL, Kucirka LM, Nguyen GC, et al. Effect modification in liver allografts with prolonged cold ischemic time. Am J Transplant. 2008;8:658–666. [DOI] [PubMed] [Google Scholar]
- 10.Scientific Registry of Transplant Recipients. SAF Data Dictionary. https://www.srtr.org/requesting-srtr-data/saf-data-dictionary/. Updated 2017. Accessed February 6, 2017.
- 11.Grønnesby JK, Borgan O. A method for checking regression models in survival analysis based on the risk score. Lifetime Data Anal. 1996;2:315–328. [DOI] [PubMed] [Google Scholar]
- 12.Leite NC, Villela-Nogueira CA, Cardoso CR, et al. Non-alcoholic fatty liver disease and diabetes: from physiopathological interplay to diagnosis and treatment. World J Gastroenterol. 2014;20:8377–8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leite NC, Villela-Nogueira CA, Pannain VL, et al. Histopathological stages of nonalcoholic fatty liver disease in type 2 diabetes: prevalences and correlated factors. Liver Int. 2011;31:700–706. [DOI] [PubMed] [Google Scholar]
- 14.Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467–1476. [DOI] [PubMed] [Google Scholar]
- 15.Paradis V, Perlemuter G, Bonvoust F, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34(4 Pt 1):738–744. [DOI] [PubMed] [Google Scholar]
- 16.Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105–113. [DOI] [PubMed] [Google Scholar]
- 17.Gaffey MJ, Boyd JC, Traweek ST, et al. Predictive value of intraoperative biopsies and liver function tests for preservation injury in orthotopic liver transplantation. Hepatology. 1997;25:184–189. [DOI] [PubMed] [Google Scholar]
- 18.Cursio R, Gugenheim J. Ischemia-reperfusion injury and ischemic-type biliary lesions following liver transplantation. J Transplant. 2012;2012:164329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schön MR, Kollmar O, Akkoc N, et al. Cold ischemia affects sinusoidal endothelial cells while warm ischemia affects hepatocytes in liver transplantation. Transplant Proc. 1998;30:2318–2320. [DOI] [PubMed] [Google Scholar]
- 20.Seifalian AM, Chidambaram V, Rolles K, et al. In vivo demonstration of impaired microcirculation in steatotic human liver grafts. Liver Transpl Surg. 1998;4:71–77. [DOI] [PubMed] [Google Scholar]
- 21.Teramoto K, Bowers JL, Kruskal JB, et al. Hepatic microcirculatory changes after reperfusion in fatty and normal liver transplantation in the rat. Transplantation. 1993;56:1076–1082. [DOI] [PubMed] [Google Scholar]
- 22.Ghinolfi D, De Simone P, Lai Q, et al. Risk analysis of ischemic-type biliary lesions after liver transplant using octogenarian donors. Liver Transpl. 2016;22:588–598. [DOI] [PubMed] [Google Scholar]


