Abstract
Background
Deceased-donor kidneys are exposed to ischemic events from donor instability during the process of donation after circulatory death (DCD). Clinicians may be reluctant to transplant DCD kidneys with prolonged cold ischemia time (CIT) for fear of an additional deleterious effect.
Methods
We performed a retrospective cohort study examining US registry data between 1998 and 2013 of adult first-time kidney-only recipients of paired kidneys (derived from the same donor transplanted into different recipients) from DCD donors.
Results
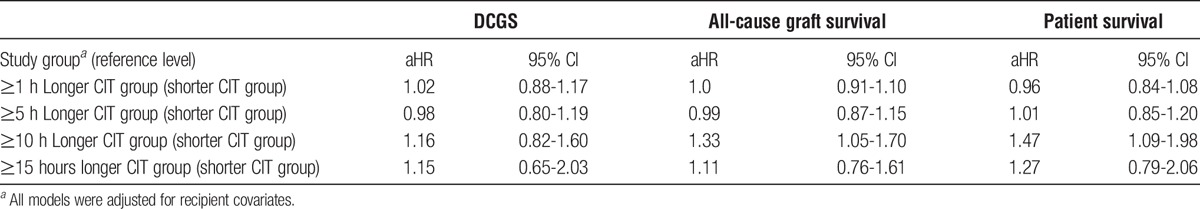
On multivariable analysis, death-censored graft survival (DCGS) was comparable between recipients of kidneys with higher CIT relative to paired donor recipients with lower CIT when the CIT difference was 1 hour or longer (adjusted hazard ratio, [aHR], 1.02; 95% confidence interval [CI], 0.88-1.17; n = 6276), 5 hours or longer (aHR, 0.98; 95% CI, 0.80-1.19; n = 3130), 10 hours or longer (aHR, 1.15; 95% CI, 0.82-1.60; n = 1124) or 15 hours (aHR, 1.15; 95% CI, 0.66-1.99; n = 498). There was a higher rate of primary non function in the long CIT groups for delta 1 hour or longer (0.89% vs 1.63%; P = 0.006), 5 hours (1.09% vs 1.67%, P = 0.13); 10 hours (0.53% vs 1.78%; P = 0.03), and 15 hours (0.40% vs 1.61%; P = 0.18), respectively. Between each of the 4 delta CIT levels of shorter and longer CIT, there was a significantly and incrementally higher rate of delayed graft function in the long CIT groups for delta 1 hour or longer (37.3% vs 41.7%; P < 0.001), 5 hours (35.9% vs 42.7%; P < 0.001), 10 hours (29.4% vs 44.2%, P < 0.001), and 15 hours (29.6% vs 46.1%, P < 0.001), respectively. Overall patient survival was comparable with delta CITs of 1 hour or longer (aHR, 0.96; 95% CI, 0.84-1.08), 5 hours (aHR, 1.01; 95% CI, 0.85-1.20), and 15 hours (aHR, 1.27; 95% CI, 0.79-2.06) but not 10 hours (aHR, 1.47; 95% CI, 1.09-1.98).
Conclusions
These results suggest that in the setting of a prior ischemic donor event, prolonged CIT has limited bearing on long-term outcomes.
As the disparity between kidney need and availability continues to widen, efforts to reduce kidney discard are critically important. This has led many clinicians to consider donation after circulatory death (DCD) donors; however, 23% of recovered DCD kidneys in the United States are discarded.1 Placement of deceased-donor kidneys to centers outside of the local donor service area offers the potential to reduce discard; however, kidney placement is often delayed because of time required to find an accepting center.2 This leads to prolonged cold ischemia time (CIT) which may contribute to the perception of the graft as being suboptimal since DCD kidneys may be considered less tolerant of CIT.3 In fact, previous reports recommend restriction of CIT to 12 to 18 hours4,5 when transplanting DCD kidneys and a recent UK registry analysis identified increased risks of DCD graft failure with CIT longer than 12 hours.3
Organ donation through the DCD pathway is characterized by variable lengths of warm ischemia. The time between withdrawal of life support and administration of organ preservation solution, referred to as the warm ischemia time (WIT), exposes the kidney to the insults of hypoxia and hypoperfusion. Placement of a kidney that has sustained warm ischemic injury into a cold ischemic environment slows the rate of ischemic injury but does not reverse it. During cold storage, cellular energy stores are exhausted ultimately resulting in activation of apoptotic and necrotic cell death upon reperfusion.6 After transplantation, manifestations of this reperfusion injury depend on the extent of ischemic insult and include delayed graft function (DGF), primary nonfunction, and possibly indolent yet chronically progressive damage leading to higher rates of chronic graft loss.7,8
To analyze the risks associated with transplantation of DCD kidneys with prolonged CIT, we examined national registry data for outcomes of adult transplant recipients of DCD kidneys in which both kidneys from the same donor were transplanted into separate recipients with different CITs. An analysis of mate kidneys from the same donor is optimal to control for the predominant effects of donor quality while illustrating the effects of CIT.
MATERIALS AND METHODS
Sources of Data
We used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, waitlisted candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network. The Health Resources and Services Administration, U.S. Department of Health and Human Services provides oversight to the activities of the Organ Procurement and Transplantation Network and SRTR contractors. This study was approved by the Institutional Review Board of the Albert Einstein College of Medicine. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Study Population
SRTR data of all adult, first-time, deceased-donor kidney-only recipients between January 1998 and October 2013 with a common donor that met criteria for donation after circulatory death (DCD) were examined. Donors meeting DCD criteria are those who are near death but do not meet formal brain death criteria and where cessation of cardiac function occurred before the organs were procured. Recipient outcomes were compared between paired donors stratified by CIT Group in which the kidney with the shorter CIT was placed in the short-CIT Group and its mate with the longer CIT in the long-CIT Group per CIT differences (delta CIT) of at least 1 hour, 5 hours or longer, 10 hours or longer and 15 hours or longer. Exclusions were previous organ transplant, hepatitis C or human immunodeficiency virus positivity, research study participation, missing information on CIT, and missing donor DCD status (Figure 1). Paired donors were categorized using a donor identifier available in the registry.
FIGURE 1.
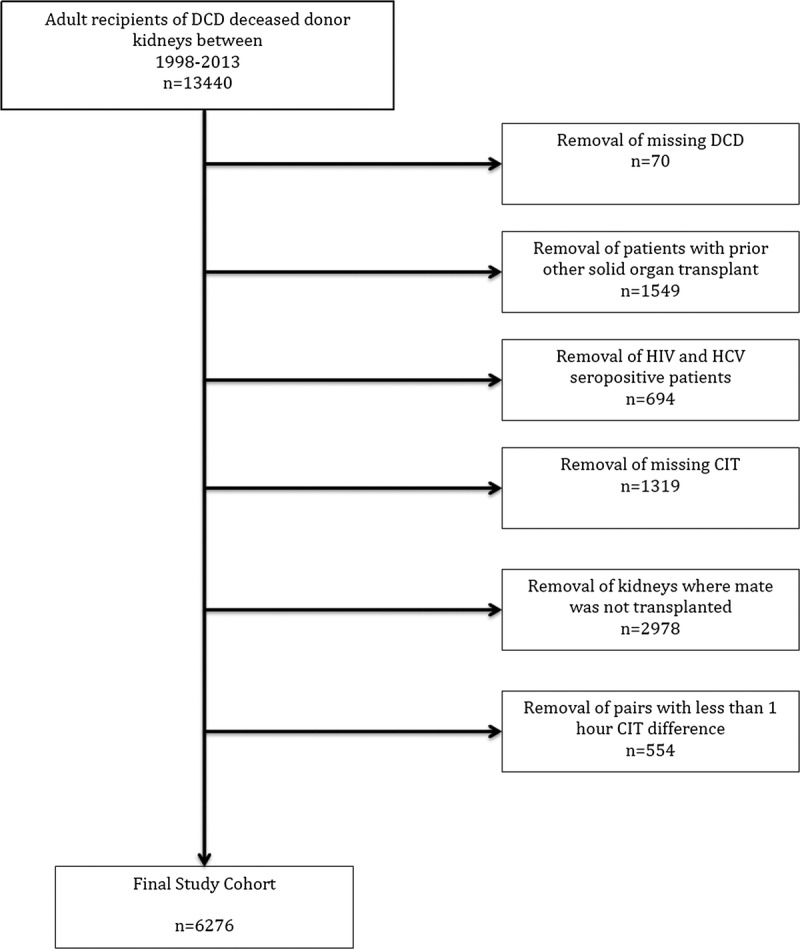
Flow diagram of cohort creation.
Outcomes
The primary outcomes were time to death-censored graft survival (DCGS) (defined as return to chronic dialysis, allograft nephrectomy, or retransplantation). Secondary outcomes were (a) all-cause graft survival (defined as return to chronic dialysis, allograft nephrectomy, retransplantation, or death), (b) patient survival; (c) DGF (defined as dialysis within 7 days posttransplantation), (d) 1-year acute rejection (defined as presence of rejection regardless of treatment as coded in follow up forms at 3, 6, or 12 months), and (e) primary nonfunction (defined as a graft that never achieved sufficient function to allow discontinuation of dialysis).
Covariates
Recipient covariates included in each of the multivariable models were age (continuous), sex, race (black, other), diabetes mellitus (as primary diagnosis of end-stage renal disease or presence of diabetes), polycystic kidney disease (PCKD), duration of pretransplant maintenance dialysis (none,<3, ≥3 years, missing), number of HLA-A, -B, and -DR mismatches (HLAMM; ≤3, >3), panel-reactive antibody (PRA) level (>30%, ≤30%, missing), body mass index (BMI) (< 18.5, 18.5-30, 31-40, > 40 kg/m2, missing), nonprivate insurance, comorbidity (chronic obstructive pulmonary disease, peptic ulcer disease, angina, cerebrovascular disease, peripheral vascular disease, and/or malignancy), transplant year, machine perfusion, and donor/recipient weight ratio. BMI was calculated as weight (kg)/height (m2). BMI outliers (<10 and >60) were coded as missing. The appropriate functional form of model covariates was determined by exploratory data analysis in unadjusted models and perceived impact on clinical meaningfulness.
Statistical Analysis
Discrete variables were expressed as percentages and continuous variables, whose distributions approximated normality, were expressed as means and standard deviations. Survival distributions were depicted with Kaplan-Meier curves and compared using the log-rank test. Cox proportional hazards models were fit to estimate hazard ratios (HR) and 95% confidence intervals (95% CI) for exposure groups after accounting for potential confounders for time to outcome data with the dependence of observations derived from the kidneys from the same donor accounted for with adjustment of the standard error of the HR (sandwich estimator). Exposure groups were examined for adherence to the proportional hazard assumption. Proportional hazards assumptions were confirmed by visual inspection of complementary long-log plots. No important departures from proportionality were observed. Ties in the failure time were handled using the Breslow method. Time to outcome was defined as time from the transplant date until date of outcome (death or graft failure), censored for loss to follow-up, and end of study period (October 31, 2013). For variables that had missing data greater than 1%, a missing category was created to conduct main multivariable analyses; complete case analysis was also conducted in sensitivity analyses. Other sensitivity analyses for the primary endpoint included (a) conversion of pretransplant dialysis, BMI, and PRA from categorical to continuous variables in the model; (b) cohort restriction to the most recent decade (2003-2013); (c) cohort restriction to inclusion of centers with DCD volume at or above the 75th percentile; and (d) validation of our findings by testing whether there exists a threshold level of absolute CIT at which graft outcomes diminish by comparing paired kidney transplants performed with a CIT 0 to 18 hours relative to mate kidneys transplanted with CITs of 19 to 30 and longer than 30 hours. The odds of DGF was assessed between paired patients where both had at least 7 days graft survival based on their status as being at risk for this event and neither had primary nonfunction. One-year acute rejection was assessed between paired patients where both had at least 1 year of graft survival.
All data were analyzed using SAS software, version 9.4. Two-sided P values less than 0.05 were considered statistically significant.
RESULTS
Recipient Characteristics
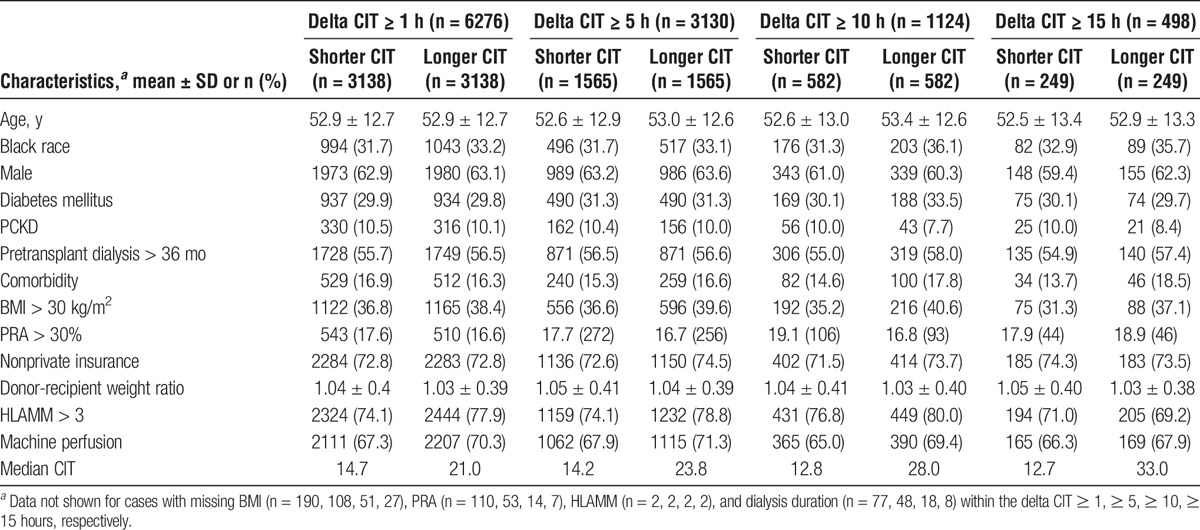
The study populations for the delta CIT 1 hour or longer, 5 hours or longer, 10 hours or longer, and 15 hours or longer groups consisted of 6276, 3130, 1124, and 498 kidney transplant recipients, respectively. The median duration of increased CIT in the pair with longer time was 6.3, 9.6, 15.2, and 20.3 hours, respectively. Although differences were small, the longer CIT recipients were more likely to be black, have diabetes, long pretransplant dialysis duration, nonprivate insurance, comorbidity, BMI greater than 30, HLAMM greater than 3, machine perfused kidney, and were less likely to have PCKD (Table 1). These differences (except for machine perfusion) generally incrementally increased as the delta CIT widened.
TABLE 1.
Recipient and transplant characteristics by delta cold ischemia time
Graft and Patient Survival
Unadjusted DCGS between patients with delta CITs of 1 hour or longer, 5 hours or longer, 10 hours or longer, and ≥15 hours or longer, were not significantly different between recipients with higher CIT relative to the paired donor recipients with lower CIT (Figures 2A-D). In risk-adjusted analysis, no significant differences in DCGS between short and long CIT groups were noted regardless of the extent of CIT difference (Table 2).
FIGURE 2.
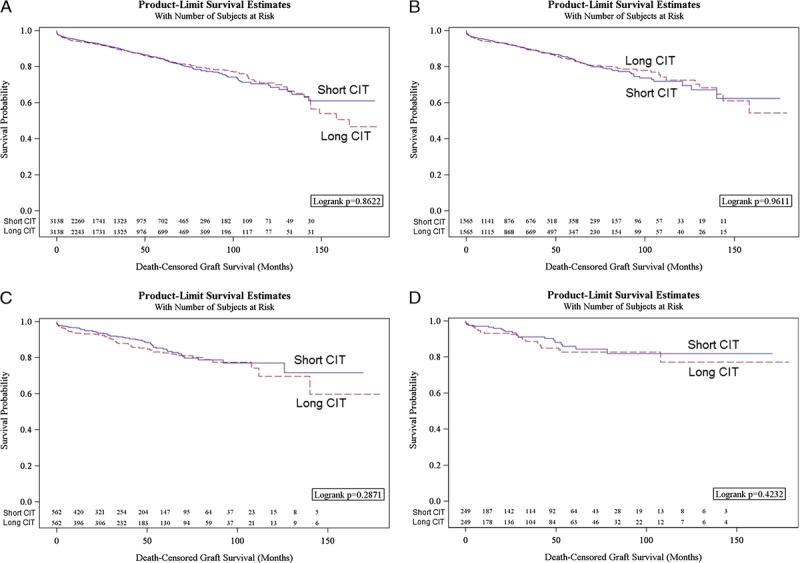
A, Kaplan-Meier plots of DCGS, all-cause graft survival, and patient survival in pairs with shorter and longer cold ischemia time with 1 hour or longer difference. B, Kaplan-Meier plots of DCGS, all-cause graft survival, and patient survival in DCD donor pairs with shorter and longer cold ischemia time with 5 hours or longer difference. C, Kaplan-Meier plots of DCGS, all-cause graft survival, and patient survival in pairs with shorter and longer cold ischemia time with 10 hours or longer difference. D, Kaplan-Meier plots of DCGS, all-cause graft survival, and patient survival in pairs with shorter and longer cold ischemia time with 15 hours or longer difference.
TABLE 2.
Multivariable models for DCGS, all-cause graft survival, and patient survival, by delta CIT groups
Unadjusted all-cause graft survival between recipients with higher CIT relative to the paired donor recipients with lower CIT were not significantly different within most of the delta CITs groups (Figures 2A-D). On multivariable analysis, the absence of significant difference remained with delta CIT 1 hour or longer, 5 hours or longer, and 15 hours or longer; in the delta CIT 10 hours or longer group, the significant decrement in all-cause graft survival in the longer relative to the shorter CIT group remained (Table 2).
Unadjusted overall patient survival between recipients with higher CIT relative to the paired donor recipients with lower CIT were not significantly different within most of the delta CITs groups (Figures 2A-D). On multivariable analysis, the absence of significant difference remained with delta CIT 1 hours or longer, 5 hours or longer, and 15 hours or longer,; and, in the delta CIT 10 hours or longer group, the significant decrement in overall patient survival in the longer relative to the shorter CIT group remained (Table 2).
DGF
The incidence of DGF by CIT group is displayed in Figure 3. Between each of the 4 delta CIT levels of shorter and longer CIT, there was a significantly and incrementally higher rate of DGF in the long CIT groups for delta 1 hour or longer (37.3% vs 41.7% P < 0.001), 5 hours or longer (35.9% vs 42.7%, P < 0.001), 10 hours or longer (29.4% vs 44.2%, P < 0.001), and 15 hours or longer (29.6% vs 46.1%, P < 0.001).
FIGURE 3.
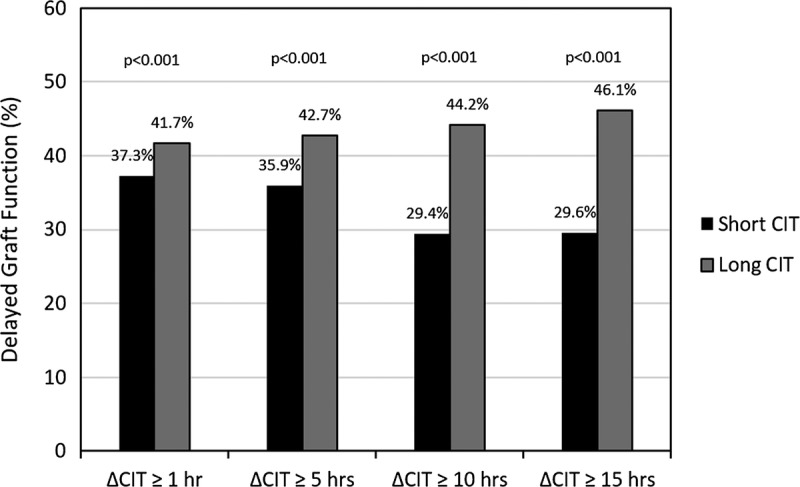
Proportion of patients with DGF by delta CIT.
Acute Rejection
The cumulative incidence of acute rejection at 1 year by CIT Group is displayed in Figure 4. Between each of the 4 delta CIT levels of shorter and longer CIT, there were no statistically significant differences in the proportion of acute rejection at delta 1 hour or longer (8.2% vs 7.7% P = 0.5), 5 hours or longer (6.9% vs 7.0%, P = 0.9), 10 hours or longer (8.4% vs 7.6%, P = 0.7), and 15 hours or longer (8.9% vs 8.9%, P = 1.0).
FIGURE 4.
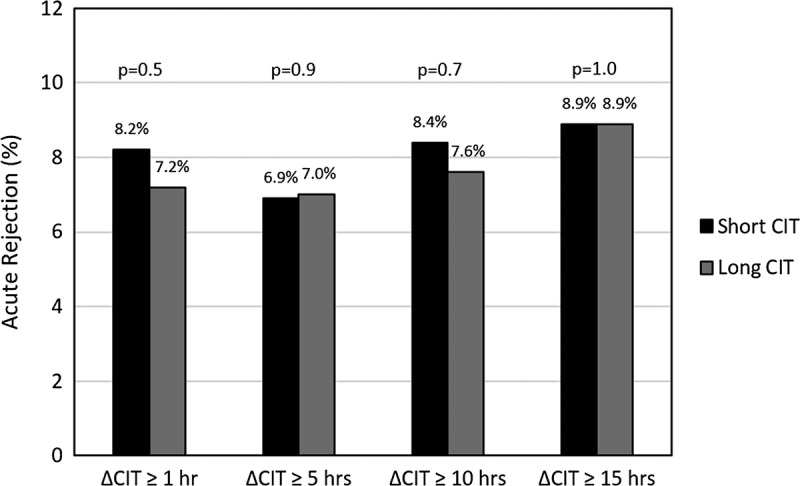
Proportion of patients with acute rejection at 1 year by delta CIT.
Primary Nonfunction
The incidence of primary non function (PNF) by CIT group is displayed in Figure 5. Between each of the 4 delta CIT levels of shorter and longer CIT, there was a higher rate of PNF in the long CIT groups for delta 1 hour or longer (0.89% vs 1.63% P = 0.006), 5 hours or longer (1.09% vs 1.67%, P = 0.13), 10 hours or longer (0.53% vs 1.78%, P = 0.03), and 15 hours or longer (0.40% vs 1.61%, P = 0.18).
FIGURE 5.
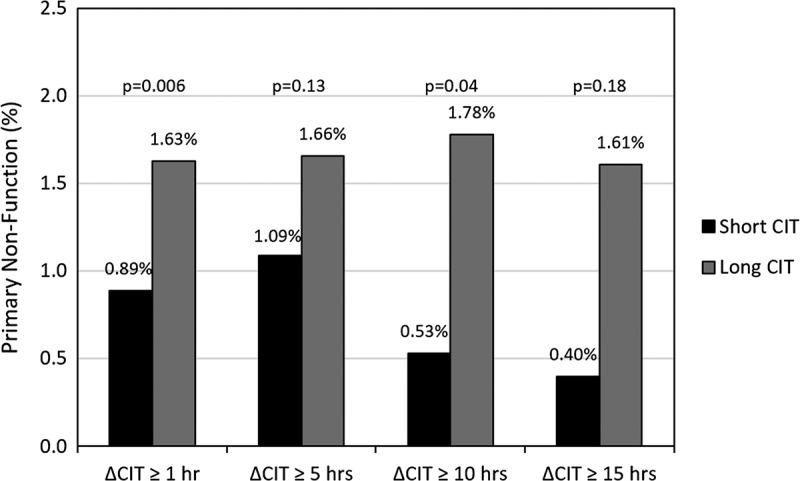
Proportion of patients with primary nonfunction by delta CIT.
Sensitivity Analyses
Additional analyses were conducted to evaluate the robustness of the primary results. There was little impact on the magnitude of the HR or the significance of the findings of DCGS after exclusion of cases with missing data or conversion of categorical values to continuous where applicable, after restriction of the cohort to the most recent decade, or after cohort restriction to include centers within the top 75th percentile of transplant centers by DCD volume (ie, of 54 centers, all of which performed at least 39 kidney transplants from DCD donors [3642 patients]), the DCGS findings were recapitulated.
Additional analyses were performed to validate our findings and further test whether there exists a threshold level of absolute CIT at which graft outcomes diminish by comparing paired kidney transplants was performed with a CIT of 0 to 18 hours relative to mate kidneys transplanted with CITs of 19 to 30 hours and longer than 30 hours. Compared with the 0 to 18 hours group, DCGS was similar (adjusted HR [aHR], 1.04; 95% CI, 0.80-1.35; n = 1878) to mate kidneys transplanted with an absolute CIT of 19 to 30 hours. Compared with the 0 to 18 hours group, DCGS was similar (aHR, 1.07; 95% CI, 0.41-2.79; n = 172) to mate kidneys transplanted with an absolute CIT longer than 30 hours.
DISCUSSION
Uncertainties exist regarding the extent and reversibility of renal injury that occurs during donation after circulatory death. These uncertainties are often compounded when kidneys are also subjected to prolonged CITs incurred while attempting to place kidneys often resulting in reduced acceptance rates. Using SRTR data of paired kidney transplants between 1998 and 2013, we found similar DCGS regardless of differences in CIT between the paired kidneys from DCD donors. Our results suggest important evidence that despite the occurrence of other ischemic events because of DCD type kidney recovery, kidneys with prolonged CIT offer acceptable outcomes to recipients and are a potential source to expand the donor pool. In addition to the lack of dose dependent effect with CIT differences up to 15 hours, we also found that the extent of absolute CIT that is tolerable without impacting DCGS of DCD kidneys is at least up to 30 hours. Longer CITs may also be tolerable but were not robustly analyzable due to the small sample size of cases with CIT beyond 30 hours. These findings have important implications for transplant centers considering utilization of DCD kidneys with anticipated prolonged CITs. Kidneys currently discarded due to CIT are likely to provide a significant benefit to patients. The perception that these kidneys are too high risk, which may not be fully supported by empirical evidence, may have led to discard, whereas results of this study could potentially highlight the utility of these organs.
Donor kidneys subjected to moderate degrees of hypoxia/hypoperfusion due to obligatory warm ischemic time, in the case of DCD, are generally thought to have reversible insults.9-11 Most evidence shows that of kidneys chosen for transplantation long-term graft survival is comparable in recipients of kidneys from DCD donors compared with brain dead donor kidneys.11,12 Despite these optimistic data, kidneys from DCD donors are generally underused with 23% of recovered DCD kidneys in the United States ultimately discarded.1 Whereas DCD kidneys may be discarded for many reasons, particularly if the initial ischemic event is perceived as being irreversible, another likely reason for refusal is prolonged CIT.13 Our finding of a lack of an adverse effect of CIT on the long-term graft survival of kidneys with a prior ischemic event is in keeping with many other investigations on the impact of CIT on graft outcomes which also suggest an absence of an effect of CIT on graft survival, at least to the extent that CIT thresholds are practiced.3,14-17 In contrast, a recent UK registry analysis found an increased risk of graft loss from circulatory death (but not brain-dead) donors with prolonged CIT3; however, a major limitation of this study is the likely inability of multivariate models to accurately adjust for important donor quality confounders, which may not always be codified in standard registries, a problem that is ameliorated with the use of a paired kidney analysis. Two recent studies out of the United Kingdom examined DCD transplants from 2002 to 2009. Although there were increased rates of DGF, long-term outcomes were similar between the 2 groups.18,19 Our data suggest that given the availability of a DCD with an extent of WIT that is considered acceptable, the additional “insult” of cold ischemia does not alter the long-term function of these organs.
We found that despite a correlation of CIT with DGF, DCGS was similar regardless of CIT differences of 15 hours or more over the CIT of the first transplanted mate kidney. DGF has clearly been associated with longer hospitalization and increased resource utilization, but usually recovers without long-term sequela. DGF has been associated with excess graft failure in some studies, but much of this is likely due to donor quality not completely accounted for in multivariable models. A previous mate kidney analysis by our group noted an absence of an effect of CIT-induced DGF on graft survival.15 Another study by Kayler et al20 demonstrated that using SRTR data, although DGF rates were higher with increasing CIT, overall graft survival with and without DGF was similar. This analysis was like that in the current study, and when looking within the cohort at DCD standard criteria donors, again, although there was more DGF, this did not affect graft survival. Similarly, despite the terminal WIT inherent in the DCD organ recovery process, resulting in DGF rates of over 50%,17,21 it has been shown that graft survival rates are similar between brain death and DCD standard-criteria donors, suggesting that ischemic injury is usually a reversible lesion.11,12 Furthermore, the common belief that DGF “causes” progressive late deterioration is not supported by phenotype data: biopsy studies of troubled transplants show that most late graft losses are attributable to definable entities, such as rejection, nonadherence, and recurrent disease, and no phenotype of late unexplained deterioration associated with DGF has been identified.22,23
In terms of patient survival, our analysis demonstrated an adverse association of longer CIT with patient mortality within 1 delta CIT group (≥10) but not the other 3 groups. The absence of a clear trend across the CIT differences suggests an absence of relationship of CIT and mortality. We also noted an adverse association of longer CIT with all-cause graft survival and the same delta CIT Group (≥10); based on the shape of the Kaplan-Meier curves, this appeared primarily to be due to the influence of mortality. These discordant findings may also be related to selection bias in the types of recipients that receive DCD kidneys with long CIT. Although our analyses were adjusted for known recipient confounders, there may be other unmeasured risk factors or combinations of donor and recipient characteristics that influence outcomes but are not captured in our multivariable models. Alternatively, the higher mortality may be due to poorer function due to receipt of a longer CIT-DCD graft; however, this is not ascertainable due to the absence of long-term data on the quality of the graft.
Acute rejection was not associated with CIT among recipients of DCD kidneys in our study. Whereas our results compare to a recent registry analysis that did not find an association of CIT with acute rejection,21 others have found positive associations including a 20% increase in adjusted rejection risk with CIT longer than 36 hours,8 a 4% increased risk of acute rejection for every hour of CIT,24 and higher unadjusted acute rejection rates for the second of transplanted mate kidneys (28.1% vs 22.3%, respectively, P < 0.01),25 supporting the hypothesis that prolonged cold storage results in increased allograft immunogenicity. Reports examining a relationship with DCD and acute rejection are mixed.3,26-28 Our results might suggest, considering the conflicting results in the literature, that our understanding of the relationship between ischemic injury and acute rejection is unclear.
Primary nonfunction was significantly more likely among long CIT (1.63%) relative to short CIT (0.89%) paired kidneys. Although the clinical meaningfulness of this finding is likely minor because overall event rates were low, the between-group difference was less than 1%, and long-term outcomes were comparable, this finding suggests that PNF among DCD kidneys may be related to either the effects of ischemia-reperfusion injury concurrent with prolonged CIT and/or is related to other contributing factors associated with long CIT not included in the analysis (not available in SRTR registry). PNF has been reported to be associated with CIT in both DCD and brain dead donors.30-32 A Dutch Organ Transplant Registry Study of 6322 kidney transplant patients in the Netherlands found a significant association with increasing hourly CIT and PNF (adjusted odds ratio, 1.05; 95% CI, 1.02-1.1) after adjustment for first and second WIT, donor type, DCD Maastricht classification, donor and recipient age and sex, and number of retransplants.29 Matsuno and colleagues30 reported that total ischemic time (warm plus cold time) of 720 minutes or longer (n = 69) correlated with 19.3% PNF compared with 5.8% among DCD transplants with shorter total ischemic times (n = 57). The effect of CIT was unclear due to lack of adjustment for WIT which was longer in the group with worse outcomes (21 vs 7 minutes). Roodnat and coworkers31 found that among 1124 living and brain-dead deceased donor kidney recipients, the adjusted relative risk of DCGS increases with increasing CIT with the largest risk of CIT being in the first week and disappearing after a year. The authors suggested that the highest risk of CIT was in the postoperative phase, but there was no risk beyond a year.
Our results are subject to the limitations inherent in observational data. Because recipients are often not randomly selected to receive kidneys, it is possible that they were in some unmeasured way systemically healthier such that a decrease in risk could have prevented an increase in graft failure or death despite an increase in CIT. There is the possibility for residual confounding because of recipient- or center-related factors not captured in registry data. Our analyses included many but not all the factors that may confer risks at or after transplantation, such as implantation technique, anastomosis time, machine perfusion, immunosuppression type and dosing, recipient anatomic abnormalities, and anastomosis time. The paired kidney analysis allows for the adjustment for most donor factors, but it is not possible to capture anatomical abnormalities in 1 of the kidneys that could lead to technical difficulties, independent of recipient factors. The analysis was unable to assess choice of kidney and timing of surgery made based on machine perfusion parameters. Evaluation of characteristics of DCD donors that would portend a poor prognosis when CIT is prolonged is not an option within a mated analysis. There may be a CIT threshold at which graft outcomes begins to deteriorate that would not be detected in the analysis due to the paucity of cases with extremely long CITs. This study included adult recipients undergoing their first kidney transplant, and therefore the results cannot be generalized to all kidney recipients. Potential issues relating to the determination of acute rejection include missing or incomplete data, reporting bias, sampling and technique errors, measures of quantification, and subjective interpretation.
There has been extensive focus in the field of transplantation on recovery and placement of all possible donor organs. This study suggests that prolonged CIT (at least up to 30 hours) does not negatively impact long-term graft survival outcomes of kidneys from DCD donors. An association of prolonged CIT with primary nonfunction was identified and, although event rates and differences between groups were low, warrants further study. These data may suggest important opportunities to increase transplantation of previously discarded organs.
Footnotes
Published online 23 June, 2017.
The authors declare no funding or conflicts of interest.
The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government.
L.K. participated in research design, the writing of the article, the performance of the research and data analysis. X.Y. participated in writing of the article and performance of the research. C.C. participated in writing of the article and performance of the research. M.L. participated in writing of the article and performance of the research. P.F. participated in writing of the article and data analysis.
REFERENCES
- 1.Shapiro R, Halloran PF, Delmonico FL, et al. The “two, one, zero” decision: what to do with suboptimal deceased donor kidneys. Am J Transplant. 2010;10:1959–1960. [DOI] [PubMed] [Google Scholar]
- 2.Massie AB, Desai NM, Montgomery RA, et al. Improving distribution efficiency of hard-to-place deceased donor kidneys: predicting probability of discard or delay. Am J Transplant. 2010;10:1613–1620. [DOI] [PubMed] [Google Scholar]
- 3.Summers DM, Johnson RJ, Hudson A, et al. Effect of donor age and cold storage time on outcome in recipients of kidneys donated after circulatory death in the UK: a cohort study. Lancet. 2013;381:727–734. [DOI] [PubMed] [Google Scholar]
- 4.Locke JE, Segev DL, Warren DS, et al. Outcomes of kidneys from donors after cardiac death: implications for allocation and preservation. Am J Transplant. 2007;7:1797–1807. [DOI] [PubMed] [Google Scholar]
- 5.Opelz G, Döhler B. Multicenter analysis of kidney preservation. Transplantation. 2007;83:247–253. [DOI] [PubMed] [Google Scholar]
- 6.McCully JD, Wakiyama H, Hsieh YJ, et al. Differential contribution of necrosis and apoptosis in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2004;286:H1923–H1935. [DOI] [PubMed] [Google Scholar]
- 7.Land W. Possible role of postischemic reperfusion injury as initiator of allorecognition/alloactivation. Transplant Proc. 1998;30:4269. [DOI] [PubMed] [Google Scholar]
- 8.Halloran PF, Homik J, Goes N, et al. The “injury response”: a concept linking nonspecific injury, acute rejection, and long-term transplant outcomes. Transplant Proc. 1997;29:79–81. [DOI] [PubMed] [Google Scholar]
- 9.Lewers DT, Mathew TH, Maher JF, et al. Long-term follow-up of renal function and histology after acute tubular necrosis. Ann Intern Med. 1970;73:523–529. [DOI] [PubMed] [Google Scholar]
- 10.Wood KE, Becker BN, McCartney JG, et al. Care of the potential organ donor. N Engl J Med. 2004;351:2730–2739. [DOI] [PubMed] [Google Scholar]
- 11.Sudhindran S, Pettigrew GJ, Drain A, et al. Outcome of transplantation using kidneys from controlled (Maastricht category 3) non–heart-beating donors. Clin Transplant. 2003;17:93–100. [DOI] [PubMed] [Google Scholar]
- 12.Singh SK, Kim SJ. Does expanded criteria donor status modify the outcomes of kidney transplantation from donors after cardiac death? Am J Transplant. 2013;13:329–336. [DOI] [PubMed] [Google Scholar]
- 13.Garonzik-Wang JM, James NT, Weatherspoon KC, et al. The aggressive phenotype: center-level patterns in the utilization of suboptimal kidneys. Am J Transplant. 2012;12:400–408. [DOI] [PubMed] [Google Scholar]
- 14.Lim WH, McDonald SP, Russ GR. Effect on graft and patient survival between shipped and locally transplanted well-matched cadaveric renal allografts in Australia over a 10-year period. Nephrology (Carlton). 2006;11:73–77. [DOI] [PubMed] [Google Scholar]
- 15.Kayler LK, Sokolich J, Magliocca J, et al. Import kidney transplants from nonmandatory share deceased donors: characteristics, distribution and outcomes. Am J Transplant. 2011;11:77–85. [DOI] [PubMed] [Google Scholar]
- 16.Stegall MD, Dean PG, McBride MA, et al. Survival of mandatorily shared cadaveric kidneys and their paybacks in the zero mismatch era. Transplantation. 2002;74:670–675. [DOI] [PubMed] [Google Scholar]
- 17.Watson CJ, Wells AC, Roberts RJ, et al. Cold machine perfusion versus static cold storage of kidneys donated after cardiac death: a UK multicenter randomized controlled trial. Am J Transplant. 2010;10:1991–1999. [DOI] [PubMed] [Google Scholar]
- 18.Pine JK, Goldsmith PJ, Ridgway DM, et al. Impact of cold ischemia on renal transplant outcomes following donation after cardiac death. Transplant Proc. 2010;42:3951–3953. [DOI] [PubMed] [Google Scholar]
- 19.Goldsmith PJ, Ridgway DM, Pine JK, et al. Sequential transplant of paired kidneys following donation after cardiac death: impact of longer cold ischemia time on the second kidney on graft and patient outcome. Transplant Proc. 2010;42:3960–3962. [DOI] [PubMed] [Google Scholar]
- 20.Kayler LK, Srinivas TR, Schold JD. Influence of CIT-induced DGF on kidney transplant outcomes. Am J Transplant. 2011;11:2657–2664. [DOI] [PubMed] [Google Scholar]
- 21.Jochmans I, Moers C, Smits JM, et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: a multicenter, randomized, controlled trial. Ann Surg. 2010;252:756–764. [DOI] [PubMed] [Google Scholar]
- 22.Kreepala C, Famulski KS, Chang J, et al. Comparing molecular assessment of implantation biopsies with histologic and demographic risk assessment. Am J Transplant. 2013;13:415–426. [DOI] [PubMed] [Google Scholar]
- 23.Singh RP, Farney AC, Rogers J, et al. Kidney transplantation from donation after cardiac death donors: lack of impact of delayed graft function on post-transplant outcomes. Clin Transplant. 2011;25:255–264. [DOI] [PubMed] [Google Scholar]
- 24.Mikhalski D, Wissing KM, Ghisdal L, et al. Cold ischemia is a major determinant of acute rejection and renal graft survival in the modern era of immunosuppression. Transplantation. 2008;85(7 Suppl):S3–S9. [DOI] [PubMed] [Google Scholar]
- 25.Giblin L, O’Kelly P, Little D, et al. A comparison of long-term graft survival rates between the first and second donor kidney transplanted—the effect of a longer cold ischaemic time for the second kidney. Am J Transplant. 2005;5:1071–1075. [DOI] [PubMed] [Google Scholar]
- 26.Cooper JT, Chin LT, Krieger NR, et al. Donation after cardiac death: the University of Wisconsin experience with renal transplantation. Am J Transplant. 2004;4:1490–1494. [DOI] [PubMed] [Google Scholar]
- 27.Farney AC, Hines MH, al-Geizawi S, et al. Lessons learned from a single center’s experience with 134 donation after cardiac death donor kidney transplants. J Am Coll Surg. 2011;212:440–451. [DOI] [PubMed] [Google Scholar]
- 28.Smits JM, van Houwelingen HC, De Meester J, et al. Permanent detrimental effect of nonimmunological factors on long-term renal graft survival: a parsimonious model of time-dependency. Transplantation. 2000;70:317–323. [DOI] [PubMed] [Google Scholar]
- 29.Van der Vliet JA, Warlé MC, Cheung CL, et al. Influence of prolonged cold ischemia in renal transplantation. Clin Transplant. 2011;25:E612–E616. [DOI] [PubMed] [Google Scholar]
- 30.Matsuno N, Tashiro J, Uchiyama M, et al. Early graft function in kidney transplantation from non–heart-beating donors. Ann Transplant. 2004;9:21–22. [PubMed] [Google Scholar]
- 31.Roodnat JI, Mulder PG, van Riemsdijk IC, et al. Ischemia times and donor serum creatinine in relation to renal graft failure. Transplantation. 2003;75:799–804. [DOI] [PubMed] [Google Scholar]
- 32.Kayler LK, Magliocca J, Zendejas I, et al. Impact of cold ischemia time on graft survival among ECD transplant recipients: a paired kidney analysis. Am J Transplant. 2011;11:2647–2656. [DOI] [PubMed] [Google Scholar]


