Abstract
Background
Donors after brain death develop a systemic proinflammatory state that may predispose the kidneys to injury after transplantation. Because it is not known whether this inflammatory environment similarly affects the kidneys from expanded criteria donor (ECD) and standard criteria donors (SCD), we sought to evaluate differences in the gene expression of inflammatory cytokines in preimplantation biopsies (PIBx) from ECD and SCD kidneys.
Methods
Cytokines gene expression was measured in 80 PIBx (SCD, 52; ECD, 28) and associated with donor variables.
Results
Normal histology and chronic histological lesions were not different between both types of kidneys. ECD kidneys showed significant increase in the transcripts of MCP-1, RANTES, TGF-β1, and IL-10 when compared with SCD. Kidneys presenting normal histology had similar inflammatory profile except by a higher expression of RANTES observed in ECD (P = 0.04). Interstitial fibrosis and tubular atrophy (interstitial fibrosis and tubular atrophy ≥ 1) were associated with higher expression of TGF-β1, RANTES, and IL-10 in ECD compared with SCD kidneys. Cold ischemia time of 24 hours or longer was significantly associated with upregulation of FOXP3, MCP-1, RANTES, and IL10, whereas longer duration of donor hospitalization significantly increased gene expression of all markers. High FOXP3 expression was also associated with lower level of serum creatinine at 1 year. Donor age was not associated with any of the transcripts studied.
Conclusions
PIBx of ECD exhibit a higher gene expression of inflammatory cytokines when compared with SCD kidneys. This molecular profile may be a specific ECD kidney response to brain death and may help to predict the posttransplant outcomes of ECD recipients.
A high demand and limited supply of organs for kidney transplantation (Tx) has resulted in a growing waiting list for the procedure, leading transplant centers to accept “unideal” kidneys recovered from expanded criteria donors (ECD).1,2 ECD outcomes seem to be inferior to those from standard criteria donors (SCD) and the tools available to assess organ quality have low predictive power to be used in clinical practice. For this reason, an elevated discard rate of recovered ECD kidneys has been observed among transplant teams, which has a negative impact on the waiting list for renal transplantation.3-5
It is known from animal models and clinical studies that donor after brain death (DBD) generates a proinflammatory state that can cause damage to the kidney tissue influencing graft outcomes. The nature of the inflammatory process seems to be different among the various types of donors because distinct patterns of cytokines gene expression were reported in kidneys recovered from DBD compared with kidneys from cardiac death or living donors.6-9 So far, it has not been reported whether the DBD-induced inflammatory response equally affects ECD and SCD kidneys.
In the present study, we hypothesized that ECD kidneys could have a higher inflammatory burden than SCD. Thus, we aimed to evaluate differences in the cytokines gene expression in preimplantation kidney biopsies (PIBx) from ECD and SCD.
MATERIALS AND METHODS
Biopsies and Clinical Data Definitions
In this prospective, single-center preliminary study, 80 PIBx from DBD (SCD, 52; ECD, 28) were analyzed. Wedge biopsies were performed immediately before implantation in kidneys from DBD after obtaining prior permission from the transplant recipients. The study was approved by the Research Ethics Committee of the Medical School of São José do Rio Preto (2011-7), in accordance with current standards for research involving human beings. One half of the preimplantation biopsy was used for histological analysis, and the rest was immediately immersed in RNAlater (Ambion, Applied Biosystems) for molecular evaluation.
The histopathological diagnosis was assessed by a pathologist blinded to the genomic analysis, and the histological chronic lesions were classified using the Banff 2007 criteria and according to the degree of the glomerulosclerosis.10,11 ECD were defined as older than 60 years, or between 50 and 59 years of age, with at least 2 of the following underlying risks: hypertension, death caused by cerebrovascular accident, or serum creatinine level higher than 133 μmol/L (1.5 mg/dL).12
Delayed graft function (DGF) was defined as the need for dialysis within the first week after Tx.13 Acute rejection (AR) was diagnosed in the clinical setting by graft dysfunction and histology (biopsy-proven AR), according to Banff's 2007 histopathological criteria.10 Graft failure was defined by the date of return to chronic dialysis.
Quantification of Intragraft Gene Expression Through Real-Time Polymerase Chain Reaction
Graft preimplantation biopsy fragments were macerated and processed for the RNA extraction using TRIzol reagent (Ambion; Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. Total RNA (2.0 μg) was reverse transcribed with the high-capacity cDNA Reverse Transcription Kit, according to the manufacturer's instructions, in a final volume of 20 μL (Applied Biosystems).
For the evaluation of gene expression levels, real-time polymerase chain reaction quantitative was performed according to the TaqMan protocols (Applied Biosystems). TaqMan Assay reagents enable the detection and quantification of FOXP3, IL-10, TGF-β1, CCL5/RANTES, and CCL2/MCP-1 genes. All samples were run in triplicate on the StepOnePlus Real-Time PCR System (Applied Biosystems) and normalized to the expression levels of glyceraldehyde 3-phosphate dehydrogenase and β-actin. Relative quantification was performed by the 2−ΔΔCt method (threshold cycle).
Statistical Analysis
The data are presented as absolute numbers, means ± SD, medians, or percentages. Gene quantifications are displayed as box-plot graphs with logarithmic transformation of the data. For continuous variables, statistical significance was assessed by Student t test or, if the variable deviated from a normal distribution, by nonparametric test (Mann-Whitney). Qualitative variables were analyzed by the χ2 test or Fisher exact test. The Kaplan-Meier log-rank test was used to compare the survival rates. All analyses were performed using Stats Direct version 2.5.7 (Stats Direct Ltd.). Throughout the study, a P value less than 0.05 was considered significant. To prevent false-positive due to multiple comparisons, when appropriate a Bonferroni correction was applied.
RESULTS
Donor and Recipient Demographics
The variables analyzed for SCD and ECD donors are summarized in Table 1. A comparison between both donor groups showed that the ECD were significantly older (mean age of 57 ± 6 vs 37 ± 13 years; P = 0.0001) and had a higher death rate due to cerebrovascular accident (P = 0.02) and arterial hypertension (P = 0.001). Cold ischemia time (CIT) and length of hospital stay were similar in both groups.
TABLE 1.
Donor and recipient demographic characteristics and transplant data
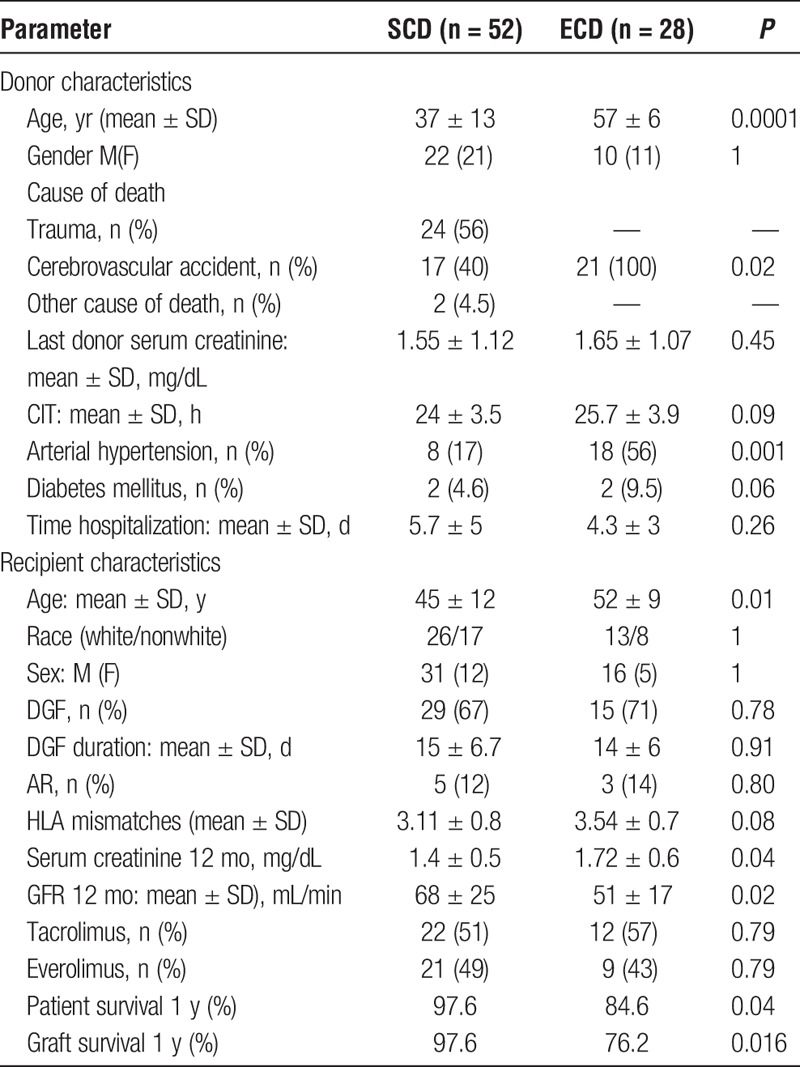
The demographics of the recipients, the type of kidney received and the clinical outcomes are shown in Table 1. Rates of AR for SCD and ECD were 12% versus 14%, respectively (P = 0.8). DGF occurrence (67% vs 71%; P = 0.78) and duration (15 ± 6.7 days vs 14 ± 6 days; P = 0.91) were similar for both groups. Serum creatinine level (SCD, 1.4 ± 0.55 mg/dL vs ECD, 1.72 ± 0.65 mg/dL; P = 0.04) and the glomerular filtration rate (GFR) (SCD, 68 ± 25 mL/min vs ECD, 51 ± 17 mL/min; P = 0.02) at 1-year post-Tx were worst in the ECD group. One-year patient and graft survival rates were significantly lower for ECD (P = 0.04) than for SCD (P = 0.016) recipients (Table 1).
Histological Findings
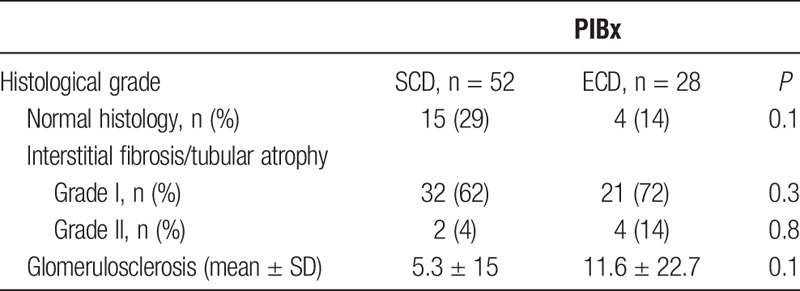
Normal histology was observed in 29% and 14% of SCD and ECD, respectively, and the percentage of glomerulosclerosis and IFTA was also similar in both types of kidneys, although IFTA II and glomerulosclerosis were numerically higher in the ECD group (Table 2).
TABLE 2.
Histological Banff scores and degree of glomerulosclerosis of kidney biopsies
Molecular Profile of PIBx
Gene expression of MCP-1 (P = 0.01), RANTES (P = 0.008), TGF-β1 (P = 0.04), and IL-10 (P = 0.0005) was significantly higher in ECD than in SCD kidneys. FOXP3 gene expression was similarly upregulated in both types of kidneys (Figure 1).
FIGURE 1.
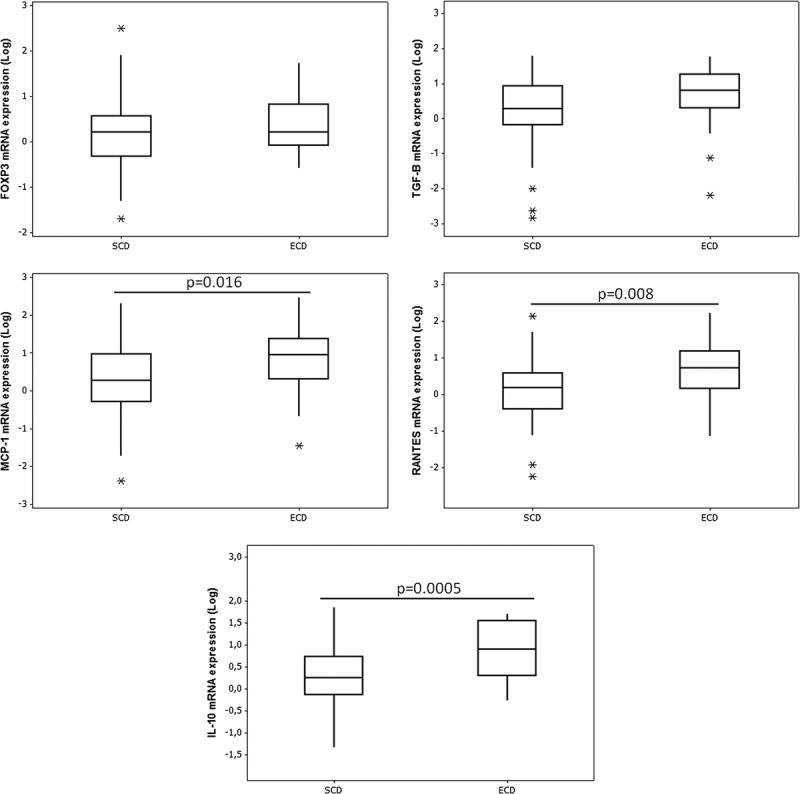
Relative mRNA expression of FOXP3, TGF-B, MCP-1, RANTES, and IL-10 genes in PIBx. Box-plot representation of median and quartile values. The whiskers are indicating range and asterisks represent outliers.
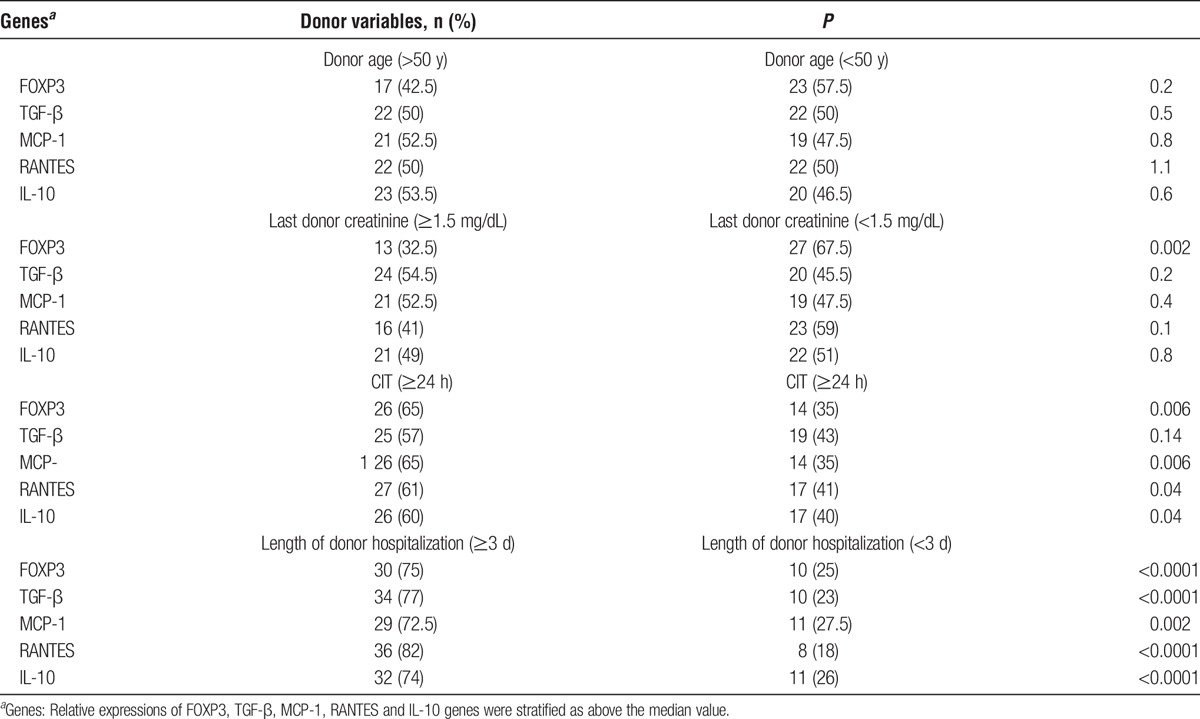
Analysis of donor variables and genetic expression showed that upregulation of FOXP3 (P = 0.006), MCP-1 (P = 0.006), RANTES (P = 0.04), and IL-10 (P = 0.04) were associated with CIT of 24 hours or longer. Duration of donor hospitalization longer than 3 days was strongly associated with upregulation of all markers, whereas high FOXP3 expression (P = 0.002) was only associates with lower level of serum creatinine. Donor age was not associated with any of the transcripts studied (Table 3).
TABLE 3.
Association analysis of genes expression with donor variables in preimplantation biopsies
Molecular Profile and Histology
Kidneys presenting normal histology had similar expression of molecular markers, except by higher RANTES expression in ECD (P = 0.04; Figure 2). However, it is noteworthy that despite presenting normal histology, ECD kidneys showed a tendency to have higher gene expression molecules than SCD kidneys (FOXP3, P = 0.06; MCP-1, P = 0.08; IL-10, P = 0.1). IFTA ≥ 1 were associated with increased gene expression and were higher in the kidneys ECD (TGF-β1, P = 0.045; RANTES, P = 0.009; IL-10, P = 0.005; Figure 2).
FIGURE 2.
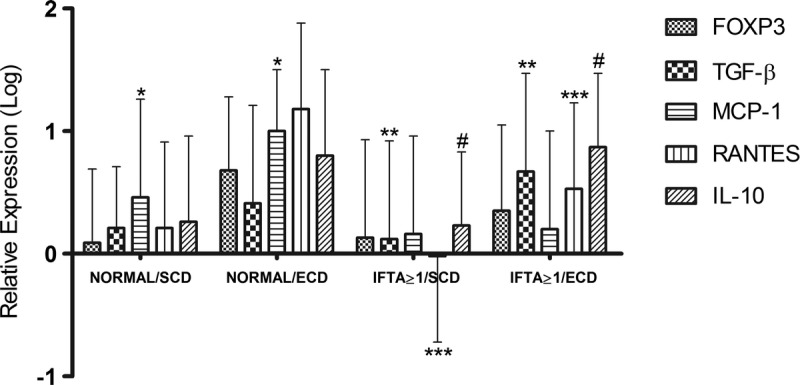
Relative mRNA expression of FOXP3, TGF-B, MCP-1, RANTES and IL-10 genes in preimplantation biopsies according to donor type and histological findings. *P < 0.05 vs normal/ECD; **P < 0.05 vs IFTA ≥ 1/ECD; ***P < 0.01 vs IFTA ≥ 1/ECD; #P < 0.001 vs. IFTA ≥ 1/ECD.
DISCUSSION
Our data corroborate reports showing that ECD recipients had inferior renal function and lower graft and patient survivals compared to SCD recipients.14,15 Additionally, we lend support to previous studies showing that kidneys from DBD present a proinflammatory state.8,16-18 The present findings add new information by showing that kidneys from SCD and ECD respond differently to the systemic inflammation induced by brain death, as the proinflammatory cytokines MCP-1, RANTES and the anti-inflammatory molecules IL-10 and TGF-β1 were significantly over expressed in ECD, in comparison with SCD kidneys (Figure 1).
The massive release of inflammatory cytokines has been reported in the plasma and in PIBx of living donors, DBD and in cardiac dead donors. However, differences in the cytokines gene expression profiles of SCD and so far, ECD kidneys have not been investigated.8,17
We found that upregulation of all markers was associated with longer duration of donor hospitalization (Table 3), corroborating previous report suggesting that an evolving inflammatory process started before organ retrieval.18 The higher expression of FOXP3, MCP-1, RANTES and IL-10 were significantly associated with elevated CIT may be due to prolonged ischemia activations a of complex sequence of events that sustain kidney damage, through the, release of proinflammatory cytokines.19,20 On the other hand, association of increased FOXP3 and lower serum creatinine suggest a nephroprotective effect of Tregs in response to the proinflammatory environment.21
Histological findings based on the Banff consensus could not be correlated with the molecular markers studied because, in spite of normal histology both types of kidneys showed increased expression of the molecular marker. Additionally, ECD kidneys without histological lesions presented massive RANTES expression and all others markers marginally more expressed than in SCD kidneys. Similarly, both types of kidney donors with same degree of IFTA had increased transcripts of TGF-β1, RANTES, and IL-10 in ECD compared with SCD kidneys. Some transcriptomic studies analyses have provided mechanistic insights about the value of molecular markers over the histological findings.22
Although the proinflammatory profile found in the PIBx can be explained as a response to the insult occurring during recovering of organs from DBD, it does not explain why the molecular profile of ECD kidneys presents a much more vigorous inflammatory response than SCD (Figure 1). We suggest that this difference could be related to ischemia-driven major histocompatibility complex-independent activation of innate immunity, because it is accepted that dying cells, under conditions of cellular stress, can release various damage-associated molecular pattern molecules into the extracellular environment, triggering a sterile inflammation.23-25
Another nonmutually exclusive hypothetical factor involving damage-associated molecular patterns is the degree of hypoxia occurring before organ retrieval. In this context, ECD kidneys are likely to release higher amounts of cellular debris and endogenous signaling molecules due to a longer hospital stay and more tissue damage, resulting in more potent stimuli for the sterile inflammation and higher production of cytokines.23,25,26 Moreover, changes in the number of infiltrating neutrophils, resident dendritic cells and CD4, CD8+, and γδ T cells into the ECD kidney may also influence the intensity of the sterile inflammation associated with the T cell–mediated adaptive immune response.27,28
Although the panel of cytokine genes that we selected for the study does not provide a detailed mechanistic explanation for the findings, it was chosen to represent certain molecules that are essential for directing, positioning or suppressing cells involved in the inflammatory process.29,30 Our study has several limitations. This was a pilot study with small sample size, and therefore our results must be validated independently using larger cohorts. However, the primary goal of this project was focused on determining whether ECD kidneys may have tissue-specific characteristics resulting in a distinct molecular profile from SCD kidneys. Other limitations of this study include some potential bias in the histological interpretation of PIBx and in the transcriptome analysis, as well as the cost and feasibility of additional molecular tests in a clinical setting.
In conclusion, to the best of our knowledge, this is the first demonstration that brain death causes a heavier inflammatory response in ECD kidneys than in SCD kidneys. These results strongly suggest that ECD kidneys may have tissue-specific characteristics resulting in a distinct molecular profile from SCD kidneys. Larger studies using molecular markers may provide a better predictive power analysis and therefore should be conducted to validate our results. If confirmed, the present data may also help to clarify the “organ quality” and to reduce discard rates. Alternatively, it may give an impetus to revisit donor pretreatment strategies targeting to reduce the inflammatory molecular profile of ECD kidneys and thereby to improve the clinical outcomes of ECD recipients. However, our findings must be interpreted cautiously due to the fact that the patient sample was relatively small and we have performed a large number of statistical comparisons. This may increase the probability of false positives due to the number of statistical tests performed. Furthermore, the design of the present study is not suitable to show causality between gene expression and the type of graft, only that there seems to be an association.
ACKNOWLEDGMENTS
This academic study has been partially supported by Novartis. The opinions expressed in this manuscript are those of the authors, and Novartis Pharmaceuticals Corporation had no influence on the contents.
Footnotes
Published online 23 June, 2017.
C.M.M.-F. and H.C.C. contributed equally to this article.
M.A.F., I.M.M.F.C., and M.A.S.F.B. received grant funding for medical research. The remaining authors declare that they have no competing interests.
The authors declare no conflicts of interest.
C.Z.D and C.M.M.F. participated in the collection and assembly of data. I.M.M.F.C. participated in the collection and assembly of data, data analysis, and interpretation. M.A.S.F.B. participated in the data analysis histological and interpretation. H.C.C. participated in the data analysis and interpretation, administrative support. M.A.F. participated in the conception and design, collection and assembly of data, data analysis and interpretation, article writing, final approval of the article.
REFERENCES
- 1.Assis-Borba L, Cristelli MP, Paula MI, et al. Expanding the use of expanded criteria donors in kidney transplantation. Int Urol Nephrol. 2014;46:1663–1671. [DOI] [PubMed] [Google Scholar]
- 2.García-Rubio JH, García JR, Hernández PC, et al. Correlation between dual kidney biopsy in expanded-criteria donors and transplant survival. Transplant Proc. 2013;45:3606–3608. [DOI] [PubMed] [Google Scholar]
- 3.Grifasi C, D'Alessandro V, D'Armiento M, et al. Can only histological evaluation determine the allocation of ECD kidneys? BMC Nephrol. 2014;15:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanriover B, Mohan S, Cohen DJ, et al. Kidneys at higher risk of discard: expanding the role of dual kidney transplantation. Am J Transplant. 2014;14:404–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sung RS, Christensen LL, Leichtman AB, et al. Determinants of discard of expanded criteria donor kidneys: impact of biopsy and machine perfusion. Am J Transplant. 2008;8:783–792. [DOI] [PubMed] [Google Scholar]
- 6.Snoeijs MG, Van Bijnen A, Swennen E, et al. Tubular epithelial injury and inflammation after ischemia and reperfusion in human kidney transplantation. Ann Surg. 2011;253:598–604. [DOI] [PubMed] [Google Scholar]
- 7.Floerchinger B, Oberhuber R, Tullius SG. Effects of brain death on organ quality and transplant outcome. Transplant Rev (Orlando). 2012;26:54–59. [DOI] [PubMed] [Google Scholar]
- 8.de Vries DK, Lindeman JH, Ringers J, et al. Donor brain death predisposes human kidney grafts to a proinflammatory reaction after transplantation. Am J Transplant. 2011;11:1064–1070. [DOI] [PubMed] [Google Scholar]
- 9.Caramori ML, Basgen JM, Mauer M. Glomerular structure in the normal human kidney: differences between living and cadaver donors. J Am Soc Nephrol. 2013;14:1901–1903. [DOI] [PubMed] [Google Scholar]
- 10.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–760. [DOI] [PubMed] [Google Scholar]
- 11.Gaber LW, Moore LW, Alloway RR, et al. Glomerulosclerosis as a determinant of posttransplant function of older donor renal allografts. Transplantation. 1995;60:334–339. [DOI] [PubMed] [Google Scholar]
- 12.Metzger RA, Delmonico FL, Feng S, et al. Expanded criteria donors for kidney transplantation. Am J Transplant. 2003;4:114–125. [DOI] [PubMed] [Google Scholar]
- 13.Ojo AO, Wolfe RA, Held PJ, et al. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63:968. [DOI] [PubMed] [Google Scholar]
- 14.Merion RM, Ashby VB, Wolfe RA, et al. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA. 2005;294:2726–2733. [DOI] [PubMed] [Google Scholar]
- 15.Mezrich JD, Pirsch JD, Fernandez LA, et al. Differential outcomes of expanded-criteria donor renal allografts according to recipient age. Clin J Am Soc Nephrol. 2012;7:1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nijboer WN, Schuurs TA, van der Hoeven JA, et al. Effect of brain death on gene expression and tissue activation in human donor kidneys. Transplantation. 2004;15:978–986. [DOI] [PubMed] [Google Scholar]
- 17.Saat TC, Susa D, Roest HP, et al. A comparison of inflammatory, cytoprotective and injury gene expression profiles in kidneys from brain death and cardiac death donors. Transplantation. 2014;98:15–21. [DOI] [PubMed] [Google Scholar]
- 18.Westendorp WH, Leuvenink HG, Ploeg RJ. Brain death induced renal injury. Curr Opin Organ Transplant. 2011;16:151–156. [DOI] [PubMed] [Google Scholar]
- 19.Debout A, Foucher Y, Trébern-Launay K, et al. Each additional hour of cold ischemia time significantly increases the risk of graft failure and mortality following renal transplantation. Kidney Int. 2015;87:343–349. [DOI] [PubMed] [Google Scholar]
- 20.Kosieradzki M, Rowiński W. Ischemia/reperfusion injury in kidney transplantation: mechanisms and prevention. Transplant Proc. 2008;40:3279–3288. [DOI] [PubMed] [Google Scholar]
- 21.Baan CC, Peeters AM, Demmers MW, et al. FoxP3 T cells and the pathophysiologic effects of brain death and warm ischemia in donor kidneys. Clin J Am Soc Nephrol. 2012;7:1481–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naesens M. Zero-time renal transplant biopsies: a comprehensive review. Transplantation. 2016;100:1425–1439. [DOI] [PubMed] [Google Scholar]
- 23.Shen H, Kreisel D, Goldstein DR. Processes of sterile inflammation. J Immunol. 2013;191:2857–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otterbein LE, Fan Z, Koulmanda M, et al. Innate immunity for better or worse govern the allograft response. Curr Opin Organ Transplant. 2015;20:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iyer SS, Pulskens WP, Sadler JJ, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009;106:20388–20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann SC, Kampen RL, Amur S, et al. Molecular and immunohistochemical characterization of the onset and resolution of human renal allograft ischemia-reperfusion injury. Transplantation. 2002;74:916–923. [DOI] [PubMed] [Google Scholar]
- 28.Schwacha MG, Rani M, Zhang Q, et al. Mitochondrial damage-associated molecular patterns activate γδ T-cells. Innate Immun. 2014;20:261–268. [DOI] [PubMed] [Google Scholar]
- 29.Gyoneva SM, Ransohoff RM. Inflammatory reaction after traumatic brain injury: therapeutic potential of targeting cell-cell communication by chemokines. Trends Pharmacol Sci. 2015;36:471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chu HX, Arumugam TV, Gelderblom M, et al. Role of CCR2 in inflammatory conditions of the central nervous system. J Cereb Blood Flow Metab. 2014;34:1425–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]


