Abstract
Background
ABO and HLA antibody incompatible (HLAi) renal transplants (AIT) now comprise around 10% of living donor kidney transplants. However, the relationship between pretransplant factors and medium-term outcomes are not fully understood, especially in relation to factors that may vary between centers.
Methods
The comprehensive national registry of AIT in the United Kingdom was investigated to describe the donor, recipient and transplant characteristics of AIT. Kaplan-Meier analysis was used to compare survival of AIT to all other compatible kidney transplants performed in the United Kingdom. Cox proportional hazards regression modeling was used to determine which pretransplant factors were associated with transplant survival in HLAi and ABOi separately. The primary outcome was transplant survival, taking account of death and graft failure.
Results
For 522 HLAi and 357 ABO incompatible (ABOi) transplants, 5-year transplant survival rates were 71% (95% confidence interval [CI], 66-75%) for HLAi and 83% (95% CI, 78-87%) for ABOi, compared with 88% (95% CI, 87-89%) for 7290 standard living donor transplants, and 78% (95% CI, 77-79%) for 15 322 standard deceased donor transplants (P < 0.0001). Increased chance of transplant loss in HLAi was associated with increasing number of donor specific HLA antibodies, center performing the transplant, antibody level at the time of transplant, and an interaction between donor age and dialysis status. In ABOi, transplant loss was associated with no use of IVIg, cytomegalovirus seronegative recipient, 000 HLA donor-recipient mismatch; and increasing recipient age.
Conclusions
Results of AIT were acceptable, certainly in the context of a choice between living donor AIT and an antibody compatible deceased donor transplant. Several factors were associated with increased chance of transplant loss, and these can lead to testable hypotheses for further improving therapy.
After cases of hyperacute rejection in the 1960s, transplantation across ABO incompatibility (ABOi) and across preformed donor specific HLA antibodies causing a positive crossmatch was vetoed for many years.1-3 In the 21st century, it has become possible to transplant across antibody barriers and such transplants are performed in large numbers around the world. There are several national guidelines and consensus documents indicating the current understanding of best practice, and such transplants may be performed outside research programme as part of routine care.4-7
However, the medium-term results of antibody incompatible transplantation (AIT) are not fully clear and practice is not fully informed by an evidence base.8 Analysis of the UK AIT Registry enabled a more comprehensive answer to questions about outcomes than previously possible. In ABOi renal transplantation, excellent results are reported but also some larger series suggest an early increase in graft loss, or an increase in posttransplant mortality.9-15 In HLA antibody incompatible (HLAi) renal transplantation many reports continue to be guarded about the outcomes, especially in transplants with high levels of donor specific HLA antibodies (DSA) and in the longer term in all transplants.16-23 A consensus article published in 2013 suggested that transplantation across a positive complement-dependent cytotoxic (CDC) crossmatch (performed using antihuman globulin enhancement) should not be performed because of poor results though some units in the United Kingdom do perform transplantation at these antibody levels.7 A survival benefit for transplantation from an antibody incompatible donor is reported, compared with either remaining on the transplant list or receiving a deceased donor transplant (DDT).24,25
Assessment of the results of larger multicenter series of such transplants is also complicated by having appropriate comparison groups. For example, it is desirable for the results of AIT to be set in the context of all “standard” transplants. This especially includes kidney sharing through paired/pooled transplantation; the targeting of nondirected altruistic donor kidneys to highly sensitized patients; and the results of transplanting deceased donor kidneys into highly sensitized recipients with no donor specific antibody barrier. Our comprehensive registry avoids having any comparison group that is biased by the inclusion of AIT within the “standard” group.
To understand better the outcomes of AIT the regulatory body for organ transplantation in the United Kingdom, NHS Blood and Transplant (NHSBT), established the 1st comprehensive national registry of AIT. Analysis of this Registry allows for complete inclusion of AIT cases and their analysis against appropriate and complete comparison groups in a comprehensive manner. This study has concentrated on those factors that clinicians have control over before the transplant, namely patient selection and risk stratification, and pretransplant therapies.
MATERIALS AND METHODS
Cohort
NHSBT is the national body overseeing transplantation in the United Kingdom and maintains records of all transplants performed in the United Kingdom including follow-up data for the duration of function of the transplant. In 2008, an additional data set was established for all patients who had been transplanted across preformed DSA or ABO incompatibility. This included transplants where HLA antibodies were detectable by solid phase assay (SPA) in the absence of a positive cellular crossmatch, so long as the center felt that a potentially clinically relevant preformed antibody was present. Transplants performed since 2000 were eligible for inclusion. Centers completed a detailed data set for each case, which included pretransplant therapies and, for HLAi, some data on the specificities and strength of pretransplant DSA.
Data were extracted from the UK Transplant Registry, held by NHS Blood and Transplant, on all kidney only antibody incompatible transplants performed in the United Kingdom between January 1, 2001, and December 31, 2012. Seventeen HLAi transplants were excluded from the analysis where data specifically relating to antibody incompatibility was not reported. Six HLAi transplants were excluded due to a lack of follow up information. There were only 3 deceased donor ABOi transplants, and these were also excluded. The cohort was split by incompatibility type: 521 HLAi and 357 ABOi (55 combined HLAi and ABOi transplants were included within the HLAi cohort) and were analyzed separately. Two centers performed FC but not CDC crossmatches, an additional category of flow positive (FC) and CDC not tested (NT) was used in analysis.
Analysis
Transplant survival was chosen as the outcome of interest and was determined as the time from transplant until the earlier of graft failure or patient death. Kaplan-Meier analysis was used to compare AIT with all other compatible living and DDTs in the United Kingdom. Recipients were defined as highly sensitized if the calculated reaction frequency of their HLA antibodies in blood group compatible donors within the most recent 10 000 UK donor pool was greater than or equal to 85%. Because incompatible transplants made up only a small minority of all kidney only transplants between 2001 and 2004 (0.2%, n = 16/6849, 11 HLAi and 5 ABOi), transplant survival estimates were compared with all kidney transplants performed between January 1, 2005, and December 31, 2012.
Cox proportional hazards regression models were fitted to assess the combined effect of risk factors on the 5-year transplant survival for AIT transplants. A standard stepwise approach with a 10% significance level was used to judge whether a factor should be added to or eliminated from the model. All factors in Tables 1 and 2 were considered as risk factors for the HLAi and ABOi models, respectively. Some variables, most notably recipient waiting time to transplant, donor and recipient cytomegalovirus (CMV), cold ischemic time, antibody removal method and dialysis status at registration, contained missing observations. Multiple imputation requires suitable predictors of the variables where the missing data appear. In this instance, it was felt that there were not enough suitable predictor variables accurately to impute the values which were missing. Therefore, missing observations in categorical variables were grouped together, whereas for continuous variables the complete case was considered. The final model did not contain any continuous variables which had missing observations, therefore the complete cohort was used. In the ABOi model, the analysis presented includes 40 patients for whom additional data on the incompatibility was not returned to the Registry. For these 40 patients, values were coded as missing for the 7 variables obtained from the incompatibility data set for this analysis.
TABLE 1.
Demographics for antibody incompatible transplants in the United Kingdom between January 1, 2001, and December 31, 2012
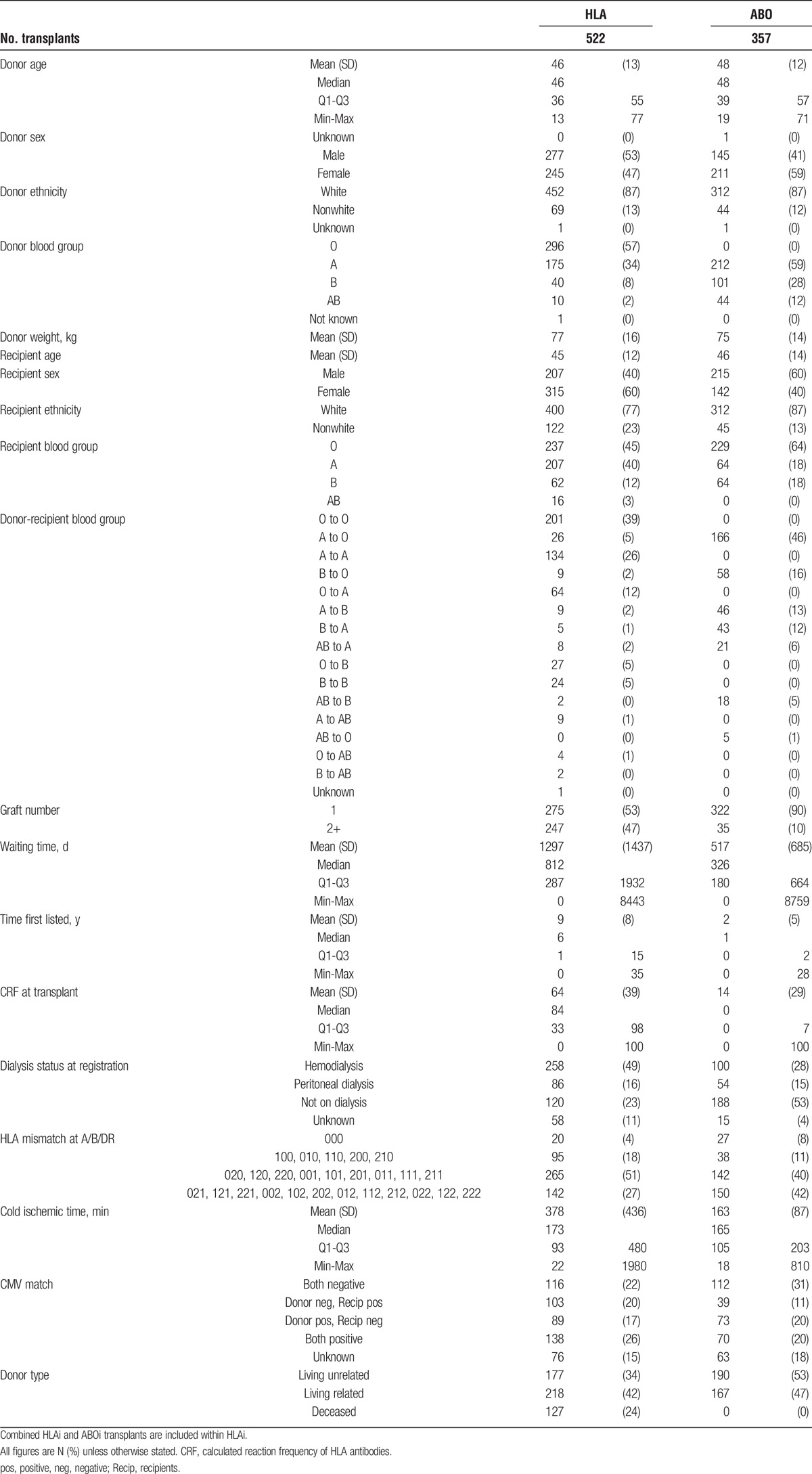
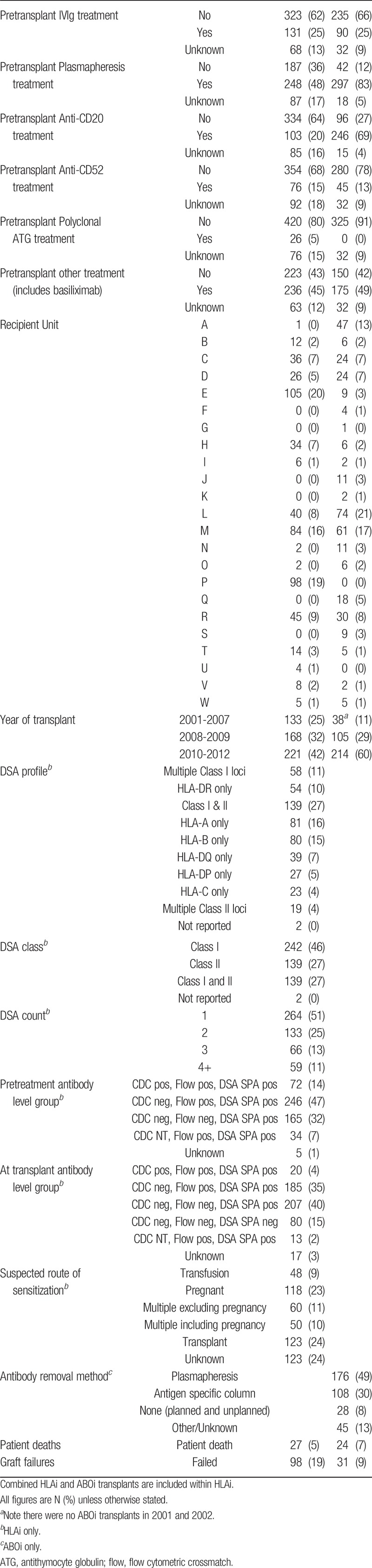
TABLE 2.
Treatment for antibody incompatible transplants in the United Kingdom between January 1, 2001, and December 31, 2012
RESULTS
The characteristics of 522 HLAi transplants and 357 ABOi transplants are presented in Tables 1 and 2. The overall outcomes (Figure 1) show that the 5 year transplant survival for ABOi and HLAi transplants (83% and 71%, respectively) were not as good as for “standard” living donor transplantation in highly sensitized or other individuals (87% and 88% respectively), but were closer to the outcomes of standard DDT in highly sensitized and other individuals (73% and 78%).
FIGURE 1.
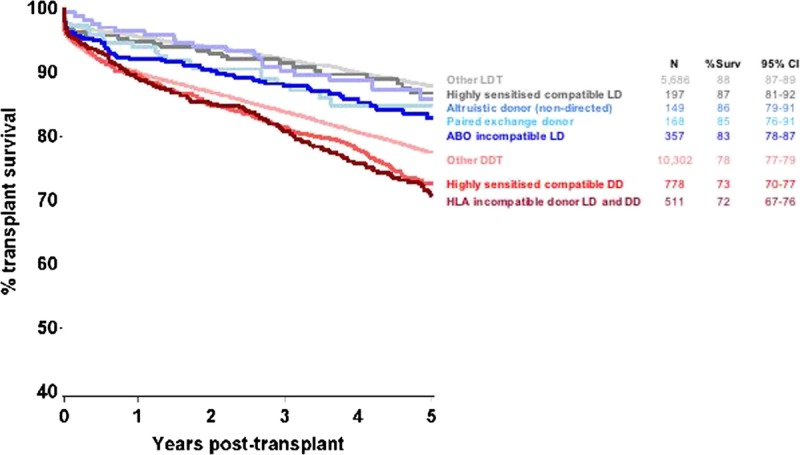
Five-year transplant survival for all kidney only transplants in the United Kingdom between January 1, 2005, and December 31, 2012.
The causes of graft failure in the AIT group, as recorded on the NHS BT Registry, were hyperacute rejection (3); nonviable kidney (5); rejection while taking immunosuppression (50); vascular or ureteric problems, including vascular thrombosis (11); infection of graft (13); recurrent primary disease (9); other (30). ‘Infection of graft’ as a cause of graft failure in the NHSBT database does not distinguish between bacterial and viral (for example CMV or BK virus) infections, but there were only 13 graft failures in this category, 10% of the total failures. The causes of death in the AIT group were vascular and cardiac including pulmonary embolus (8), infection (18), lymphoid malignancy (2), other and unidentified (23). The numbers of events were not high enough to include in multivariate analysis.
Figure 2 shows the 5-year transplant survival for the HLAi cohort divided per the pretreatment HLA antibody reactivities. This shows reduced transplant survival in all levels of HLAi, including those who were CDC and FC crossmatch negative but positive by SPA analysis using microbeads. At 3 and 5 years posttransplant, 82% and 54% of patients, respectively, had complete follow-up data (when counting those who had failed or died before the time point as having complete follow up data).
FIGURE 2.
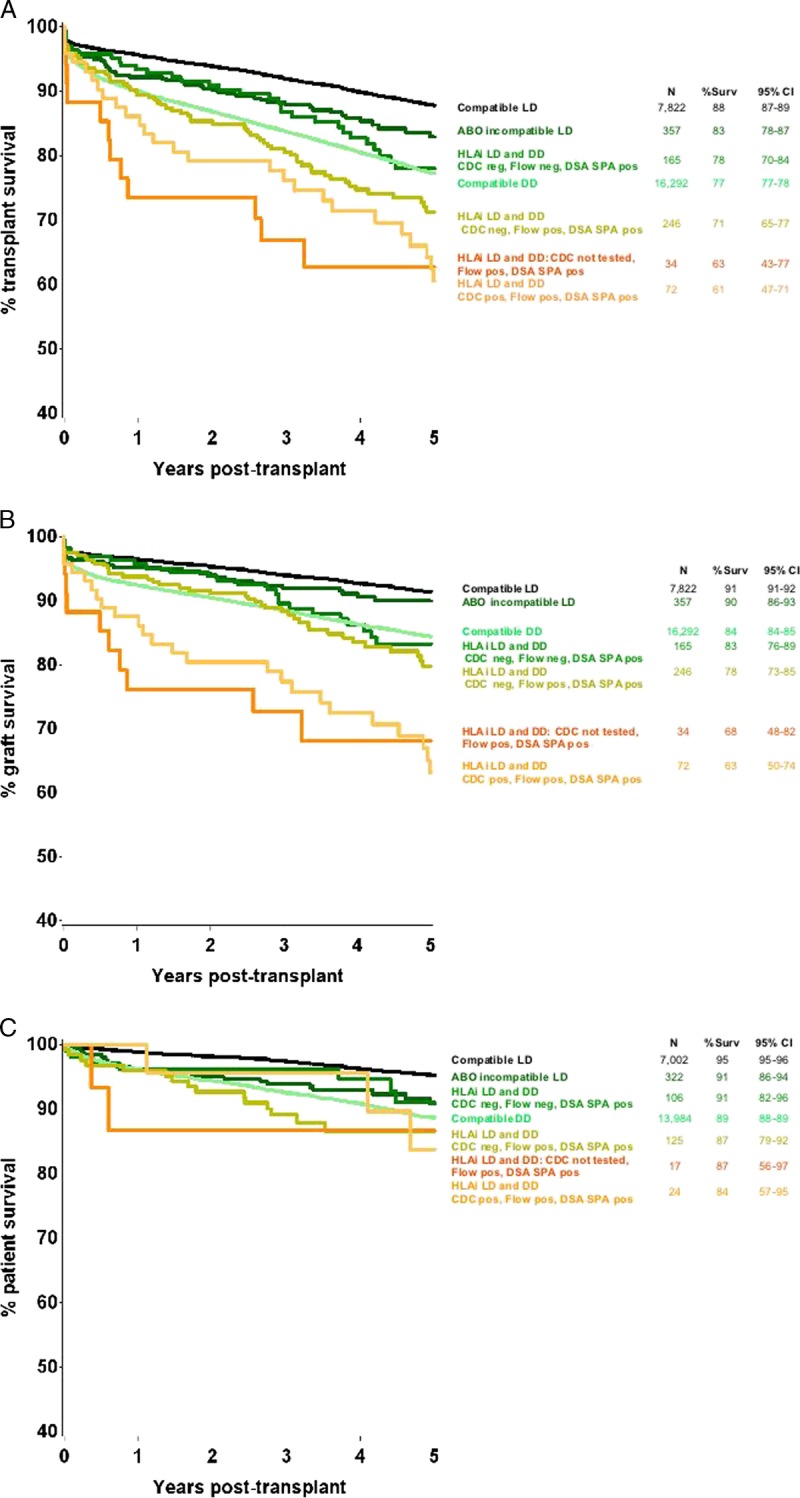
Five-year transplant survival for all kidney only transplants in the United Kingdom between January 1, 2005, and December 31, 2012. A, all transplant survival; B, graft survival; C, patient survival. Levels of antibody reported pretreatment. flow, flow cytometric crossmatch; NT, not tested. Five HLAi transplants which had an unknown pretreatment antibody level group have been excluded from this figure.
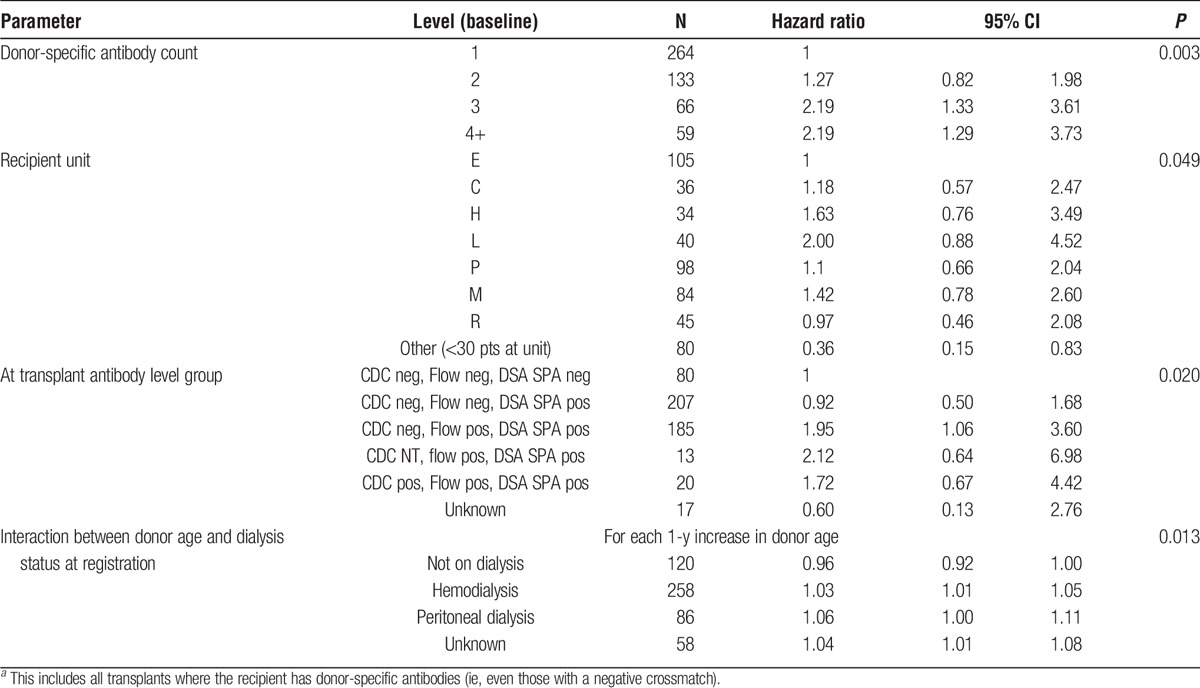
Three factors and 1 interaction were found to be significant in the model assessing 5-year transplant outcomes within the HLAi cohort (Table 3). The model found that an increased chance of transplant failure was associated with the presence of 3 or more DSA. A decreased chance of transplant failure was found for those where the transplant was performed in units which had performed less than 30 HLAi transplants during the period. Donor age and recipient dialysis status at registration were also found to be statistically significant (P = 0.013) in the model. If the recipient was not on dialysis, then an increasing donor age showed a protective effect with a reduced chance of transplant failure. On the other hand, if the patient was on dialysis an increased donor age was associated with increased chances of transplant failure. This effect was greatest for peritoneal dialysis. In this analysis, pretreatment crossmatch status ceased to be significant, as there was a stronger association with pretransplant crossmatch status, in other words the final sample tested before the transplant surgery, after any conditioning therapy. Additional ABO incompatibility was not significantly associated with outcome.
TABLE 3.
Cox regression model showing the factors that affect 5 year HLAia kidney transplant outcomes, between January 1, 2001, and December 31, 2012
A range of combinations of pretransplant therapies was reported, showing wide intercenter and intracenter variation. There was perhaps a tendency in many centers to start with lower levels of immunosuppression and escalate posttransplant (either as a matter of routine or if rejection occurred), for example in 239 HLAi transplants (46% of total), no pretransplant use of antibody therapy (antithymocyte globulin, CD20, CD52, or “other therapy”) was reported.
Donor source was not associated with outcome. Although transplant survival was lower with DDTs at 2 years posttransplant, by 5 years, there was little difference. The 5 year transplant survivals were 69.1% (95% confidence interval [CI] 59.8-76.7%) for deceased donors (n = 127), 70.4% (95% CI, 63.3-70.4%) for living related donors (n = 218), 72.5% (95% CI, 63.9-79.4%) for living unrelated donors (n = 177).
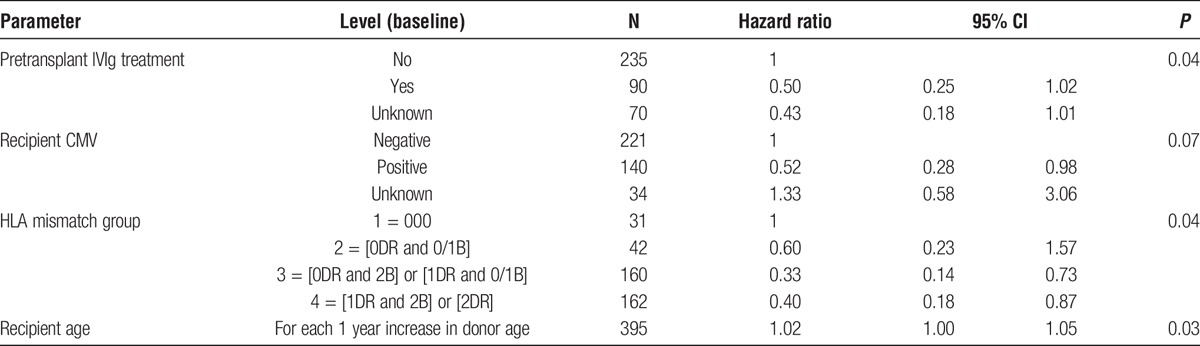
In the model assessing 5 year transplant outcome in the ABOi cohort, 4 factors were found to be significant: recipient age, pretransplant IVIg treatment, CMV status, and HLA mismatch group (Table 4). No pretransplant IVIg treatment, recipient negative for CMV antibodies pretreatment, a transplant with 000 donor-recipient HLA mismatches and an increased recipient age were all associated with an increased chance of transplant failure. In the ABOi cohort, there were 357 transplants, with 31 grafts failures and 24 deaths. In the 000 subgroups, there were 27 transplants, with 6 graft failures and 3 deaths. Donor and recipient blood groups were not significantly associated with outcome, including donor blood groups A1 and A2. Of the various treatments used, many combinations were reported. The pretransplant use of CD52 and CD 20 was segregated, though, with 4 patients receiving both pretransplant, compared with compared with 242 who received CD20 but not CD52, and 41 who received CD52 but not CD20.
TABLE 4.
Cox regression model showing the factors that affect 5 year ABOi* kidney transplant outcomes, January 1, 2001, and December 31, 2012
DISCUSSION
AIT is now an important part of the landscape of kidney transplantation. During the study period over 10% of living donor kidney transplants were HLAi or ABOi or both, making an important contribution to transplant numbers. All transplant units in the United Kingdom reported some AIT activity, though some units had performed only a few such transplants and others concentrated on 1 type of transplant (for example, 1 unit reported 98 HLAi transplants and 0 ABOi, and another reported 1 HLAi and 47 AB0i).
This comprehensive registry, which separates all AIT transplants from standard transplants, is unique internationally and is important in defining risk accurately. Although all registries can determine which transplants were ABO-incompatible HLA antibody incompatible transplants are not universally defined except in the United Kingdom. It is important that a reference group of “standard” transplants does not contain a significant number of HLAi transplants or the outcomes of AIT cannot be accurately defined and may be overestimated.
The aim of this study, the first large report from the registry, was to examine the 5-year outcomes of antibody incompatible transplants considering pretransplant immunological risk and pretransplant therapies.
All Transplants
The overall outcomes (Figure 1) show that the 5-year transplant survivals for ABOi and HLAi transplants (83% and 71%, respectively) were inferior when compared with “standard” living donor transplantation in highly sensitized or other individuals (87% and 88%, respectively), but were closer to the outcomes of standard DDT in highly sensitized and other individuals (73% and 78%). This is important information for the patient who may have a very real choice between going ahead with an incompatible living donor transplant (LDT) or waiting for an antibody compatible DDT. The outcome in ABOi transplantation was less good than that for standard transplantation, and can also be compared with ABOi outcomes in some previous studies. Graft survival at 5 years in our study was 90%, which is comparable with some single centers (for example, 90% at 4 years26) and cohort reports (for example, 82.6%10), though not as high as reported from Japan (around 95%9). Patient survival at 5 years in our study was 91%, lower than that in some studies (for example, 98% in a single center study26) but comparable with US data (88%).10
The data confirm the continuing success of the UK kidney sharing scheme27 with 5-year transplant survival in recipients of transplants from nondirected altruistic donors and exchange transplants being close to other standard LDTs. Potential recipients with a living donor who is antibody incompatible should therefore consider entry into the national sharing scheme as a preferred choice.
HLAi Multivariate Analysis
Multivariate analysis for the outcomes of HLAi transplantation showed significant associations for loss with the number of DSA, the center performing the transplant, the antibody crossmatch status at the time of transplantation (ie, after conditioning therapy), and in an interaction between donor age and dialysis status.
The risk of increasing number of DSA specificities was more important in this analysis than the crossmatch status (though the 2 factors are associated). The class of HLA specificity was not significant, in other words, combinations of DSA including those to HLA DP and DQ as well as to HLA DR were associated with increased risk, especially in combination with HLA class I DSA, as previously suggested.28
NHS BT performs continuous comparison of the outcomes of all transplant centers in the United Kingdom, and there is borderline significant difference in outcomes for “standard” transplants. There are also known differences in risk appetite between UK centers, for example, the proportions of living and DDTs vary between centers, and the NHS BT donor risk index for DDTs varies between centers.29 There is also variation in the proportion of patients within the HLAi cohort at each center who were CDC positive, ranging from 4% to 16%, though the center effect in our multivariate analysis was not due to CDC status because this was included in the analysis. The center effect for HLAi requires further investigation as it is potentially paradoxical, with the centers performing fewer AIT having lower risk at RR 0.36 (95% CI, 0.15-0.83) compared with the reference center, which was the largest reporter to the Registry. The highest relative risk for loss was 2.0 (95% CI, 0.88-4.52), in a center reporting 40 HLAi transplants. It is possible that the smaller centers were relatively risk averse and selected cases with less comorbidity who were better able to receive more immunosuppression.
The risk of loss was associated with the crossmatch status of DSA reported at the time of the transplant, rather than the pretreatment level. This could indicate that the cut off levels for positivity of the FC crossmatch and CDC do not reflect the risks of transplant loss, or that the extent of reduction in DSA reactivity during pretransplant antibody removal is important, whatever the starting level.
The results in relation to transplants where pretransplant DSA were detectable by SPA using microbeads but not by cellular crossmatching are interesting, especially because this is an area of continuing uncertainty and controversy in clinical practice. Figure 2 shows that the outcomes in transplants with negative crossmatching but with DSA detectable by SPA were lower than for standard transplants, in line with previous reports.16,17,20,21 However, multivariate analysis indicated no increased risk of transplant loss when DSA is only detectable by SPA, additionally including cases with a positive cellular crossmatch rendered negative by pretransplant conditioning (Table 2). This suggests that subjects with a single DSA and low reactivity (even if FC crossmatch positive) are at low risk, whereas subjects with multiple DSA are at increased risk, even if the antibody reactivity is positive only by SPA.
Donor age and dialysis status were also associated with outcomes after HLAi transplantation, with an interaction such that older age was associated with increased risk for those on dialysis, and reduced risk for those transplanted predialysis. This finding requires examination in the light of the rates of antibody-mediated rejection posttransplant.
ABOi Multivariate Analysis
Multivariate analysis for factors associated with losses after ABOi transplantation showed associations with IVIg therapy, CMV status, HLA mismatching and recipient age.
IVIg therapy is used (though not universally) because it replaces immunoglobulins removed by plasmapheresis, and because it may reduce the risk of antibody mediated rejection regardless of therapeutic antibody removal.8,14,15,21,30 It is not currently possible to determine from our registry data whether the association with IVIg therapy was related to reductions in graft loss or in death (or both) owing to the small numbers of events in each category. Transplant survival was not associated with other differences in pretransplant therapies, such as Rituximab, method of antibody removal, or the center performing the transplant. One large center in the analysis used IVIg in 74% of their transplants, other units used it infrequently, with 6 smaller centers not reporting its use pretransplant at all. The possible beneficial effect of IVIg should be investigated further.
CMV status indicated an increased risk of loss if the recipient was CMV antibody-negative pretreatment. The Registry cannot at this stage determine whether the increased losses associated with CMV seronegative status pretransplant were directly consequent on CMV infection, or whether there was a difference in rejection rates, or whether transplant loss was associated with different clinical management of CMV prophylaxis or disease.
An increased risk of graft loss in ABOi transplants where there was a 000 donor-recipient HLA mismatch at loci A, B, and DR was an unexpected finding, and requires further examination. Possible explanations include transplantation at higher ABO antibody levels or reduced routine immunosuppression because clinicians perceived a lower risk of HLA driven rejection. More detailed study of the losses in this group is required.
Although this is one of the largest reports of the outcomes of AIT and the only 1 from a comprehensive national registry, there are some limitations to this analysis. One of these is variation in the details of measurement of antibody levels between units. Although there is little major variation in the method for measuring CDC crossmatch (no UK units routinely uses antihuman globulin enhancement), there are variations between laboratories in the determination of the FC crossmatch and the level at which HLA antibodies are determined to be clinically significant when an SPA method is used. The finding in this study that the number of DSA was more strongly associated with outcome than their levels suggests that the methods used to measure DSA levels gave poor risk stratification. After the final date for inclusion in this analysis, there have been changes in laboratory practice to reduce prozone effect,31 and work suggesting DSA characteristics such as complement fixation32 or IgG subclass profile33 might provide better risk stratification. Likewise, there is variation in the measurement of ABO antibodies. This is known to be considerable,34 and ABO antibody titres were not included in the initial registry data set.
Transplant survival, the chosen outcome in this study, is a composite of graft loss and death. These are likely to be correlated, because increasing administration of immunosuppression for prevention or treatment of antibody-mediated rejection would be expected to make death more likely. A further study would give deeper understanding of the interactions by studying death and graft loss as separate outcome measures, and including the administration of posttransplant immunosuppression and occurrence of rejection. At present, the numbers of events are not enough for conventional multivariate analysis to produce robust conclusions, but the database will accumulate rapidly, and other techniques for analysis such as data-driven machine learning may give additional insights.
In summary, this study indicates that AIT is widely performed in the United Kingdom and that the outcomes are good, at least for a patient facing a choice between an incompatible living donor and waiting for an antibody compatible DDT. The advantage of the kidney sharing scheme is confirmed. The results of multivariate analysis assist with risk stratification of current transplants and indicate important areas for future work. The value of IVIg therapy in ABOi transplantation should be investigated further. The association of HLAi outcomes with DSA number and the crossmatch status at transplant, and not pretreatment, is intriguing and suggests that the response of antibody level to therapy might be important, as well as its initial level. The finding that levels of DSA that did not cause a positive FC crossmatch might only be significant if there were multiple DSA might also be important, allowing more accurate identification of low-risk transplants that do not need augmented monitoring and therapy.
Footnotes
Published online 26 June, 2017.
The authors declare no funding or conflicts of interest.
L.P. participated in the study design; statistical analysis, writing and reviewing the article. A.H. participated in the study design; statistical analysis; writing and reviewing article. L.M. participated in the study design; statistical analysis; writing and reviewing the article. M.W. participated in the data collection; writing and reviewing the article. J.G. participated in the data collection; writing and reviewing the article. O.S. participated in the data collection; writing and reviewing the article. R.T. participated in the data collection; writing and reviewing the article. C.P. participated in the data collection; writing and reviewing article. D.T. participated in the data collection; writing and reviewing the article. S.G. participated in the data collection; writing and reviewing article. N.T. participated in the data collection; writing and reviewing the article. S.B. participated in the data collection; writing and reviewing the article. B.C. participated in the data collection; writing and reviewing the article. D.B. participated in the data collection; writing and reviewing the article. S.V.F. participated in the study design; data collection and analysis; writing and reviewing the article. R.M.H. participated in the study design; data collection; writing and reviewing the article.
REFERENCES
- 1.Palmer A, Taube D, Welsh K, et al. Removal of anti-HLA antibodies by extracorporeal immunoadsorption to enable renal transplantation. Lancet. 1989;1:10–12. [DOI] [PubMed] [Google Scholar]
- 2.Higgins RM, Bevan DJ, Carey BS, et al. Prevention of hyperacute rejection by removal of antibodies to HLA immediately before renal transplantation. Lancet. 1996;348:1208–1211. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery RA, Zachary AA, Racusen LC, et al. Plasmapheresis and intravenous immune globulin provides effective rescue therapy for refractory humoral rejection and allows kidneys to be successfully transplanted into cross-match-positive recipients. Transplantation. 2000;70:887–895. [DOI] [PubMed] [Google Scholar]
- 4.Montgomery RA, Hardy MA, Jordan SC, et al. Consensus opinion from the antibody working group on the diagnosis, reporting and risk assessment for antibody-mediated rejection and desensitisation protocols. Transplantation. 2004;78:181–185. [DOI] [PubMed] [Google Scholar]
- 5.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–155. [DOI] [PubMed] [Google Scholar]
- 6.British Society for Histcompatibility and Immunogenetics, British Transplantation Society. The detection and characterisation of clinically relevant antibodies in allotransplantation. https://bts.org.uk/wp-content/uploads/2016/09/06_BTS_BSHI_Antibodies-1.pdf. Updated December 2016. [DOI] [PubMed]
- 7.Tait BD, Süsal C, Gebel HM, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013;95:19–47. [DOI] [PubMed] [Google Scholar]
- 8.Lo P, Sharma A, Craig JC, et al. Preconditioning therapy in ABO-incompatible living kidney transplantation: a systematic review and meta-analysis transplantation. Transplantation. 2016;100:933–942. [DOI] [PubMed] [Google Scholar]
- 9.Tanabe K, Ishida H, Inui M, et al. ABO-incompatible kidney transplantation: long-term outcomes. Clin Transpl. 2013;307–12. [PubMed] [Google Scholar]
- 10.Montgomery JR, Berger JC, Warren DS, et al. Outcome of ABO-incompatible kidney transplantation in the United States. Transplantation. 2012;93:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Opelz G, Morath C, Süsal C, et al. Three-year outcomes following 1420 ABO-incompatible living-donor kidney transplants performed after ABO antibody reduction: results from 101 centers. Transplantation. 2015;99:400–404. [DOI] [PubMed] [Google Scholar]
- 12.Becker LE, Siebert D, Süsal C, et al. Outcomes following ABO-incompatible kidney transplantation performed after desensitization by nonantigen-specific immunoadsorption. Transplantation. 2015;99:2364–2371. [DOI] [PubMed] [Google Scholar]
- 13.Aikawa A, Saito K, Takahashi K. Trends in ABO-incompatible kidney transplantation. Exp Clin Transplant. 2015;13(Suppl 1):18–22. [PubMed] [Google Scholar]
- 14.Couzi L, Manook M, Perera R, et al. Difference in outcomes after antibody-mediated rejection between ABO-incompatible and positive cross-match transplantations. Transpl Int. 2015;28:1205–1215. [DOI] [PubMed] [Google Scholar]
- 15.Lentine KL, Axelrod D, Klein C, et al. Early clinical complications after ABO-incompatible live-donor kidney transplantation: a national study of Medicare-insured recipients. Transplantation. 2014;98:54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins R, Lowe D, Hathaway M, et al. HLA antibody incompatible renal transplantation: excellent medium term outcomes with negative cytotoxic crossmatch. Transplantation. 2011;92:900–906. [DOI] [PubMed] [Google Scholar]
- 17.Willicombe M, Brookes P, Santos-Nunez E, et al. Outcome of patients with preformed donor-specific antibodies following alemtuzumab induction and tacrolimus monotherapy. Am J Transplant. 2011;11:470–477. [DOI] [PubMed] [Google Scholar]
- 18.Bentall A, Cornell LD, Gloor JM, et al. Five-year outcomes in living donor kidney transplants with a positive crossmatch. Am J Transplant. 2013;13:76–85. [DOI] [PubMed] [Google Scholar]
- 19.Lefaucheur C, Loupy A, Hill GS, et al. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. 2010;21:1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh N, Djamali A, Lorentzen D, et al. Pretransplant donor-specific antibodies detected by single-antigen bead flow cytometry are associated with inferior kidney transplant outcomes. Transplantation. 2010;90:1079–1084. [DOI] [PubMed] [Google Scholar]
- 21.Orandi BJ, Garonzik-Wang JM, Massie AB, et al. Quantifying the risk of incompatible kidney transplantation: a multicenter study. Am J Transplant. 2014;14:1573–1580. [DOI] [PubMed] [Google Scholar]
- 22.Vo AA, Sinha A, Haas M, et al. Factors predicting risk for antibody-mediated rejection and graft loss in highly human leukocyte antigen sensitized patients transplanted after desensitization. Transplantation. 2015;99:1423–1430. [DOI] [PubMed] [Google Scholar]
- 23.Garonzik Wang JM, Montgomery RA, Kucirka LM, et al. Incompatible live-donor kidney transplantation in the United States: results of a national survey. Clin J Am Soc Nephrol. 2011;6:2041–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montgomery RA, Lonze BE, King KE, et al. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011;365:318–326. [DOI] [PubMed] [Google Scholar]
- 25.Orandi BJ, Luo X, Massie AB, et al. Survival benefit with kidney transplants from HLA-incompatible live donors. N Engl J Med. 2016;374:940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chow KV, Flint SM, Shen A, et al. Histological and extended clinical outcomes following ABO-incompatible renal transplantation without splenectomy or rituximab. Transplantation. 2017;6:1433–1440. [DOI] [PubMed] [Google Scholar]
- 27.Johnson RJ, Allen JE, Fuggle SV, et al. Early experience of paired living kidney donation in the United Kingdom. Transplantation. 2008;86:1672–1677. [DOI] [PubMed] [Google Scholar]
- 28.Higgins R, Lowe D, Hathaway M, et al. Rises and falls in donor-specific and third-party HLA antibody levels after antibody incompatible transplantation. Transplantation. 2009;87:882–888. [DOI] [PubMed] [Google Scholar]
- 29.NHS Blood and Transplant. Annual report on kidney transplantation. http://www.odt.nhs.uk/uk-transplant-registry/organ-specific-reports/. Published July 2016.
- 30.Galliford J, Charif R, Chan KK, et al. ABO incompatible living renal transplantation with a steroid sparing protocol. Transplantation. 2008;86:901–906. [DOI] [PubMed] [Google Scholar]
- 31.Schnaidt M, Weinstock C, Jurisic M, et al. HLA antibody specification using single-antigen beads—a technical solution for the prozone effect. Transplantation. 2011;92:510–515. [DOI] [PubMed] [Google Scholar]
- 32.Loupy A, Lefaucheur C, Vernerey D, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 2013;369:1215–1226. [DOI] [PubMed] [Google Scholar]
- 33.Khovanova N, Daga S, Shaikhina T, et al. Subclass analysis of donor HLA specific IgG in antibody incompatible renal transplantation reveals a significant association of IgG4 with rejection and graft failure. Transpl Int. 2015;28:1405–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bentall A, Barnett AN, Braitch M, et al. Clinical outcomes with ABO antibody titer variability in a multicenter study of ABO-incompatible kidney transplantation in the United Kingdom. Transfusion. 2016;56:2668–2679. [DOI] [PubMed] [Google Scholar]


