Abstract
Background
Posttransplant lymphoproliferative disease (PTLD) is an important cause of morbidity and mortality in solid organ transplants. Epstein Barr virus (EBV) plays a major role in PTLD development. Guidelines recommend EBV viral load (VL) monitoring in high-risk populations in the first year.
Methods
Retrospective observational study in all adult patients who had at least 1 EBV-VL performed in the postkidney transplant (KT) period from January 2005 to December 2014 at the Policlinico Modena Hospital. We compared patients with negative EBV-DNA to patients with positive EBV-DNA and we described PTLD developed in the study period.
Results
One hundred ninety (36.3%) KT patients of 523 were screened for EBV-DNA with 796 samples. One hundred twenty-eight (67.4%) of 190 tested patients presented at least 1 positive sample for EBV. Older age, the use of sirolimus, everolimus, and steroids were associated with EBV-DNA positivity in the univariate analysis. Nine (1.7%) of 523 patients had PTLD. Incidence rate of PTLD in the KT cohort was 0.19/100 person year follow-up (95% confidence interval, 0.09-0.37). One of 9 patients developed early PTLD and was a high-risk patient. Only this PTLD case was positive for EBV. No PTLD case had an EBV-VL superior to 4000 copies/mL.
Conclusions
Our results suggest that the keystone of PTLD diagnosis is the clinical suspicion. Our study suggests that, in line with guidelines, EBV-VL assays may be avoided in low-risk patients in the absence of a strong clinical PTLD suspicion without increasing patients' risk of developing PTLD. This represents a safe and cost-saving clinical strategy for our center.
Posttransplant lymphoproliferative disease (PTLD) is increasingly recognized as an important cause of morbidity and mortality in solid organ transplants.1 Kidney transplant recipients are at relatively low risk (1-3%).2
Epstein-Barr virus (EBV) is ubiquitous, and about 90% of the world adult population have anti-EBV antibodies.3 In Italy, the seroprevalence in the adult population is 88.4%,4 and the primary infection usually occurs early in life. Effectively, 72.7% of Italian children aged 10 years already show EBV immunity.5 Most EBV infections in immunocompetent hosts are asymptomatic in children, whereas primary infections in adults frequently result in infectious mononucleosis.6,7 Over 50% of patients with infectious mononucleosis manifest fever, lymphadenopathy, and pharyngitis. EBV also plays a role in the development of nasopharyngeal carcinoma, Burkitt lymphoma,6 and PTLD.2 In kidney transplant recipients, PTLD has shown bimodal patterns of incidence, with peaks in the first year and then in the later posttransplantation period.8-10 Patient survival after PTLD diagnosis is 64% at 1 year, 48% at 5 years, and 37% at 10 years.11 The EBV genome is found in more than 90% of B cell PTLD occurring during the first year after transplantation, while up to 45% of late onset PTLD are EBV negative.1,12 The pathogenesis of these disorders is complex and related to EBV ability to transform and immortalize B lymphocytes, combined with secondary genetic or epigenetic events that occur during uncontrolled proliferation.2 Although the role of EBV in EBV-negative PTLD is uncertain, recent data support the hypothesis that over time, immune escape occurs in initially EBV-driven lymphoproliferation, with cellular mutations replacing the functions of EBV oncogenes.2,13
Due to the impaired immunity after transplant, kidney transplant recipients are at risk for viral reactivation.14 Indeed, immunosuppression is associated with EBV, cytomegalovirus (CMV), β-herpesviruses,15 and polyoma BK reactivation. Potential microbial interactions between viruses have been suggested and can modify the clinical presentation of infections.14
Kidney Disease: Improving Global Outcomes guidelines16 suggest monitoring high-risk kidney transplants (defined as donor EBV seropositive and recipient EBV seronegative) for EBV by nucleic acid testing after transplantation once in the first week, monthly for the first 3 to 6 months, then every 3 months until the end of the first posttransplant year, and additionally after treatment for acute rejection. Kidney Disease: Improving Global Outcomes guidelines recommend reducing immunosuppressive medication in EBV-seronegative patients with an increasing EBV viral load (VL) and in patients with EBV disease, including PTLD. The more recent American Society of Transplantation guidelines2 state that there are data17 to support quantitative EBV-VL monitoring for PTLD prevention only in high-risk populations in the first year. Data to support monitoring in the population at low-risk for PTLD are lacking.2,16
In contrast with the guidelines, a recent survey published by the European Study Group of Infections in Compromised Hosts18 showed that EBV-VL measurements are frequently used in Europe to guide both the diagnostic workup and the reduction of immunosuppression in solid organ transplants. EBV monitoring is routinely used in 86% of the transplant programs; in particular, 38% of renal transplant centers perform EBV-VL surveillance in all recipients, independently from the EBV risk evaluation. Furthermore, 77% perform preemptive treatments for patients with high-risk EBV DNAemia levels such as the reduction of immunosuppression (50.9%), and the conversion to mammalian target of rapamaycin inhibitors (mTORi) (30.9%). Up to 14.5% had used rituximab for this indication and 7.3% reported the use of immune- adoptive T cell therapy.
EBV DNAemia levels considered significant can vary between centers.19,20 In our study, the value of 4000 copies/mL has been chosen based on literature data evaluating such a threshold as a risk factor for PTLD onset.21
In view of the difference between guidelines and clinical practice in Europe, the aims of our study were to assess the clinical utility of EBV-VL monitoring and risk factors for PTLD in the kidney transplant cohort from Azienda Ospedaliero-Universitaria (AOU) Policlinico in Modena, Italy.
MATERIALS AND METHODS
Study Design
A retrospective, observational, single-center study was performed in patients in follow-up after kidney transplantation (KT) from January 1987 to December 2014 in the Clinic of Nephrology at AOU Policlinico Modena Hospital. All adult (≥18 years) patients who had at least one quantitative EBV-DNA plasma assay performed after KT from January 2005 to December 2014 were included.
Data were extracted from the clinical database and from the computerized microbiological database. Patients with negative plasma EBV-VL were compared with patients with positive EBV-VL (any titer). In addition, patients with positive EBV-VL greater than 4000 copies/mL were compared to patients with EBV-VL less than 4000 copies/mL. Diagnosis of PTLD was made according to World Health Organization criteria.22 Furthermore, PTLD characteristics in cases identified in the study period were described.
Microbiological Samples
All microbiological samples were analyzed in the Microbiology and Virology Laboratory of the AOU Policlinico Modena Hospital, as routine clinical practice. EBV-DNA was detected and quantified by EBV ELITe MGB real-time polymerase chain reaction of ELITech Group with a threshold of sensitivity for detection of 225 copies/mL and a linearity range from 225 copies/mL to 22 500 000 copies/mL. CMV-DNA was detected and quantified by the ABBOTT RealTime CMV assay of ABBOTT group, with a threshold of sensitivity for detection of 62 UI/mL and a linearity range from 62 to 156 000 000 UI/mL. Polyoma BK DNA was detected and quantified by BKV ELITe MGB real-time polymerase chain reaction of ELITech group, with a threshold of sensitivity for detection of 140 copies/mL and a linearity range from 140 to 140 000 000 copies/mL. The tests provide an internal control of extraction and amplification for each sample; furthermore, a positive and a negative control are added for each session. The laboratory regularly participates in the external quality control QCMD for: CMV, EBV, and Polyoma-virus BK.
Data Collection
To describe patient characteristics, the following parameters were recorded at the time of kidney transplantation: recipient age, gender, transplant indications, copathologies, graft and transplantation type, EBV serological status of the recipient and of the donor, and retransplantation. Postoperative data included duration of hospital stay, acute rejection, graft survival (in months), induction and maintenance immunosuppressive regimen, cause of death, date of death, diagnosis of PTLD, PTLD type, and PTLD date of diagnosis. In addition, the following data at the time of EBV-VL sampling were recorded: date of EBV-VL sample, presence of symptoms usually correlated with PTLD at the time of EBV-VL sample or at PTLD diagnosis, CMV or BK positivity, ongoing immunosuppressive therapy, and creatinine (mg/dL). Potential factors associated with positive EBV-VL (any titer) or EBV-VL greater than 4000 copies/mL or PTLD were age, sex, creatinine value (mg/dL), EBV serostatus, the use of basiliximab versus thymoglobulin, and the use of all different immunosuppressive drugs.
Statistical Methods
Continuous variables were presented with mean (SD) or median (interquartile range [IQR]), respectively, if with normal or not normal distribution. Categorical variables were presented with frequencies (percentages). Comparisons between patients with and patients without EBV-VL positivity were analyzed with t test, Wilcoxon (Mann-Whitney) U test, χ2 test or Fisher exact test when appropriate. The analysis of incidence was conducted by the method of Poisson. Univariate Poisson regression analyses were carried out to assess the risk factors associated with positive EBV-VL (any titer), EBV-VL greater than 4000 copies/mL, and PTLD, respectively. The level of statistical significance was fixed for an α error of 0.5. The analysis has been conducted using the software package STATA 13.1 for Mac (StataCorp ltd., College Station, TX).
Ethical Approval
An approval from the Modena ethical committee was obtained (Approval Number 131/15, 10/06/2015). Due to the retrospective nature of this study the need for informed consent was waived.
KT Patient Cohort Follow-Up
After transplantation, the patients were evaluated as outpatients 2 times a week for the first month, once a week until the third month, once every 2 weeks up to 6 months and once a month from 6 months up to the end of the year. After 1 year, patients were evaluated every 2 months, with monthly blood exams. In our center, EBV-VL assay was performed in high-risk patients in the first year after transplant, in case of signs and symptoms suggesting EBV-related disease or primary infection (fever, cytopenia, night sweats, and lymphadenopathy), or in patients considered at major risk of viral reactivation by the clinicians (eg, increased immunosuppression, human immunodeficiency virus, and so on). Samples of patients with EBV-VL persistently superior to 4000 copies/mL despite the reduction of immunosuppressive medication were sent to the Laboratory of Transplant Immunology and Pediatric Hematology/Oncology, Fondazione IRCCS Policlinico S. Matteo, University of Pavia, Italy, to evaluate T cell-specific response using ELISpot method.23 If T cell-specific response was absent,23 EBV-specific cytotoxic T lymphocytes (CTL)24 were prepared and given to the patient.
RESULTS
Five hundred twenty-three patients, corresponding to 4175.3 person year follow-up (PYFU), received a kidney transplant between 1987 and 2014 and were followed in the Clinic of Nephrology at AOU Policlinico Modena Hospital after transplantation. The median follow-up post-KT was 94.6 months (IQR, 55-148). All recipients were adults with a median age of 46 years (IQR, 35-57). Of these, 340 (65.0%) patients were men. Forty-four (8.4%) patients had a living-donor transplant, 21 patients (4.0%) a combined kidney-liver transplant and 22 patients (4.2%) a combined pancreas-kidney transplant. Two hundred sixty-five (50.7%) transplants were performed in Modena. The other transplants were performed in other Italian centers.
During the entire 10-year follow-up period, 796 samples were tested for EBV viremia. Total numbers of patients screened for EBV-VL were 190 (36.3%) of 523, corresponding to 1768.8 PYFU. Sixty-four (33.7%) of 190 patients had EBV-VL performed in the first year after transplant, 71 (37.4%) of 190 patients had EBV-VL performed because they had at least 1 symptom suggestive for EBV reactivation or primary infection.
We could collect EBV serology data in 105 (55.3%) of 190 patients. Among patients for whom EBV serology data were not available, 31 (36.5%) patients were transplanted before 2000. Only 8 (7.6%) of 105 patients had a negative pre-KT EBV serology. The median age of EBV-seronegative patients was 42 years (range, 21-51). One hundred twenty-eight (67.4%) of 190 tested patients presented at least one positive sample for EBV. The median time between KT and the first sample tested for EBV-VL was 1548 days (IQR, 253-4204). Figure 1 shows the flowchart of the study.
FIGURE 1.
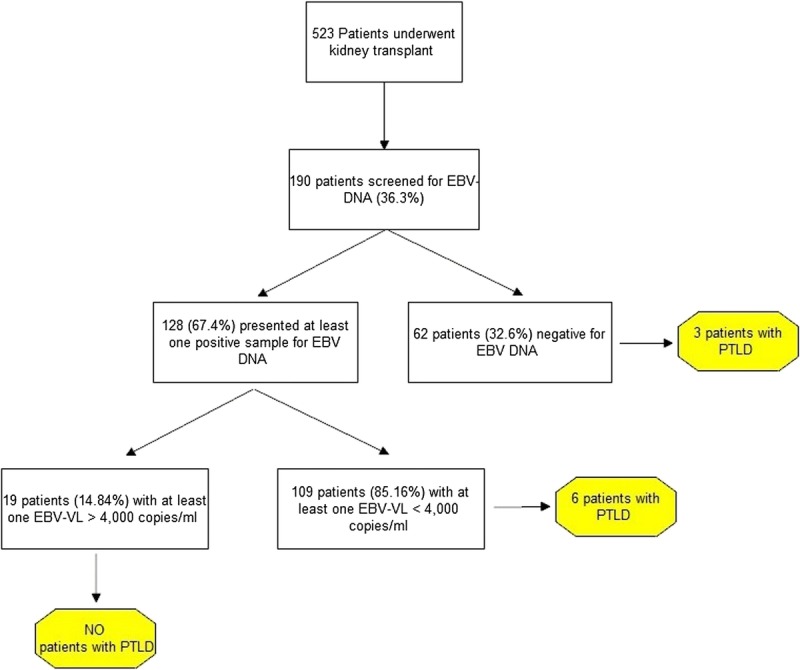
Study flowchart.
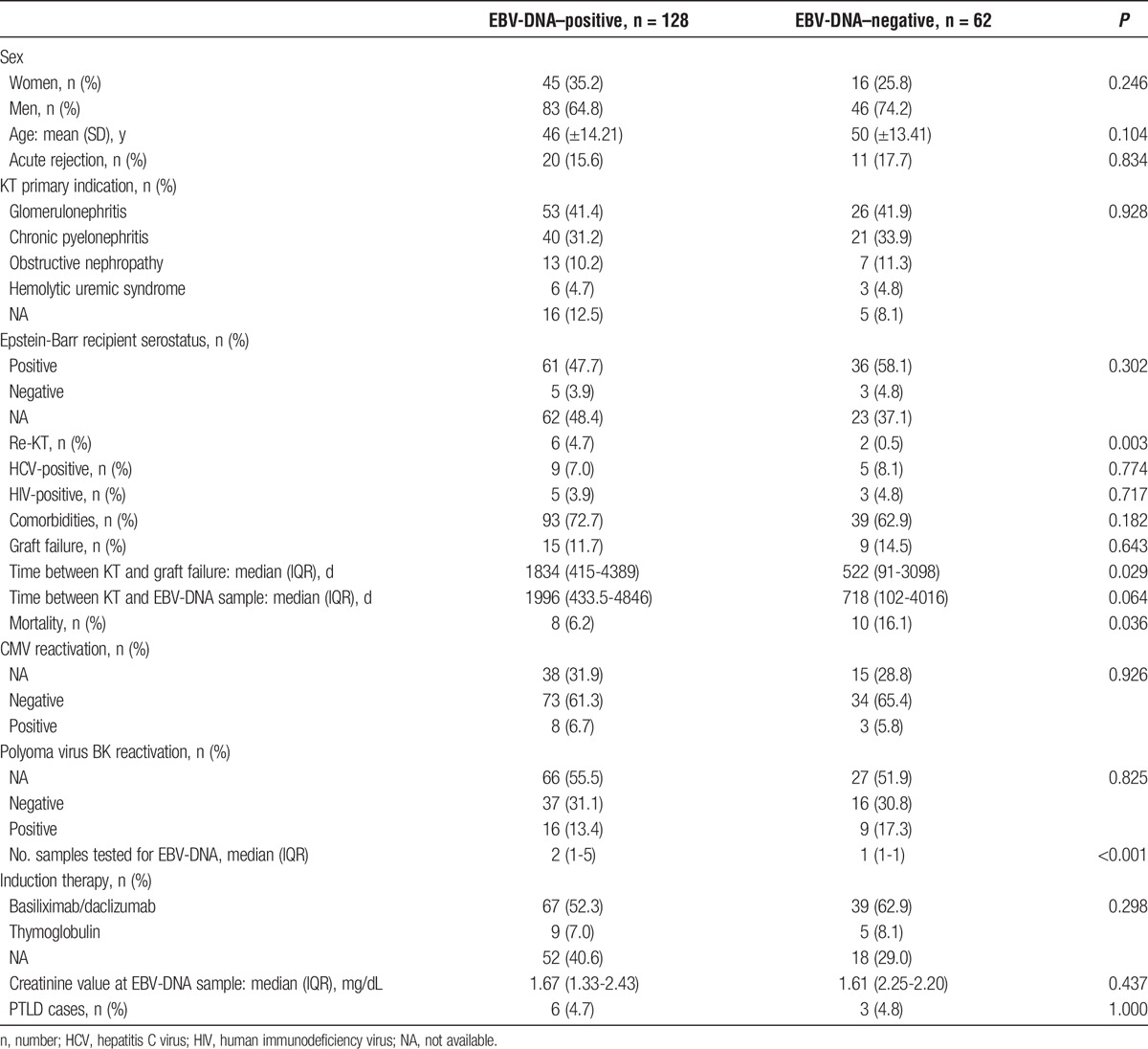
Table 1 describes patient characteristics in EBV-DNA positive and EBV-DNA negative patients.
TABLE 1.
Characteristics of EBV-DNA positive and EBV-DNA negative kidney transplant patients
In EBV-DNA positive patients, the median EBV-VL at the first available evaluation was 319.5 copies/mL (IQR, 114-1070).
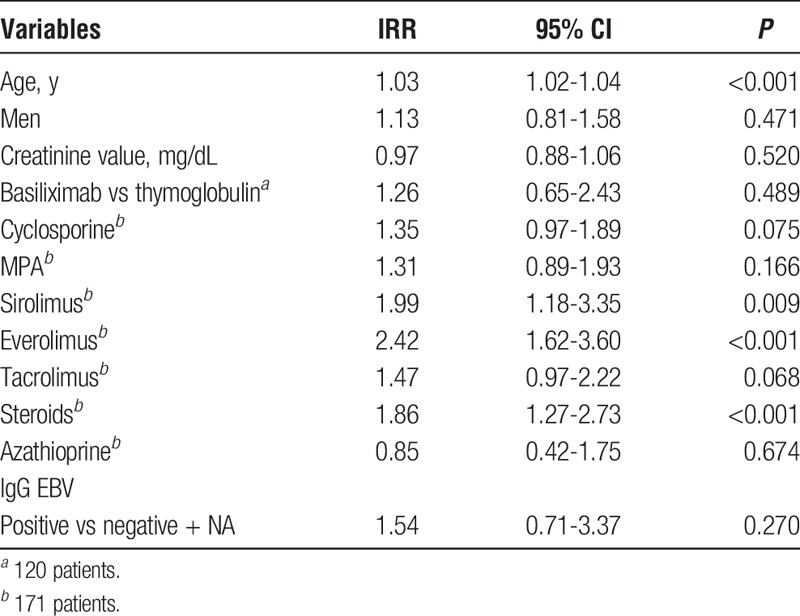
We found statistically significant differences between EBV-positive and EBV-negative patients for the percentage of retransplant (4.69 vs 0.50, P = 0.003), time between KT and graft failure (1834 vs 522, p 0.029), mortality (6.25 vs 16.12%, P = 0.036), and number of samples tested for EBV-VL (2 vs 1, P < 0.001). Table 2 shows the results of univariate Poisson regression analysis for factors associated with EBV-DNA positivity. Older age, the use of sirolimus, everolimus, and steroids were factors associated with EBV-DNA positivity in the univariate analysis.
TABLE 2.
Univariate Poisson regression analysis for factors associated with EBV-DNA positivity (n = 190 patients)
Fifty-one patients presented persistent viremia with at least 2 EBV-positive sample (median, 4; range, 2-66). The median age of patients with persistent viremia was 44 years (IQR, 33-51). Two (3.9%) of 51 patients experienced PTLD. Two patients (3.9%) had an induction therapy with thymoglobulin, 27 (52.9%) with basiliximab or daclizumab, and 22 (43.1%) had missing data about induction therapy. One patient (2.0%) died.
Nineteen (14.8%) of 128 patients had at least one EBV-VL superior to 4000 copies/mL. Incidence rate of EBV greater than 4000 copies/mL was 1.07/100 PYFU (95% confidence interval [CI], 0.65-1.68). The characteristics of patients with EBV-VL greater than 4000 copies/mL and EBV-VL less than 4000 copies/mL were compared (Table 3). There were no statistically significant differences.
TABLE 3.
Characteristics of patients with EBV-VL >4000 and <4000 copies/mL
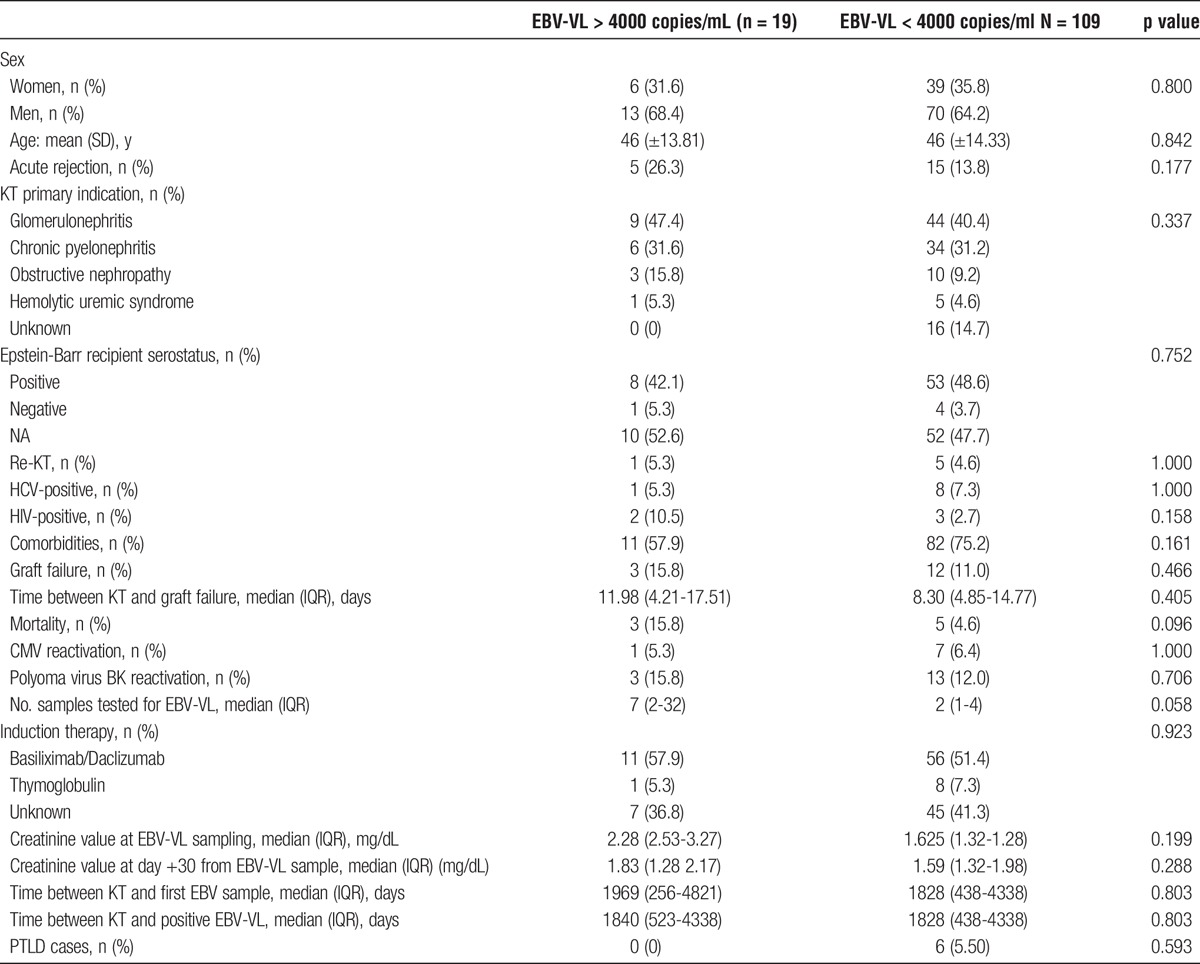
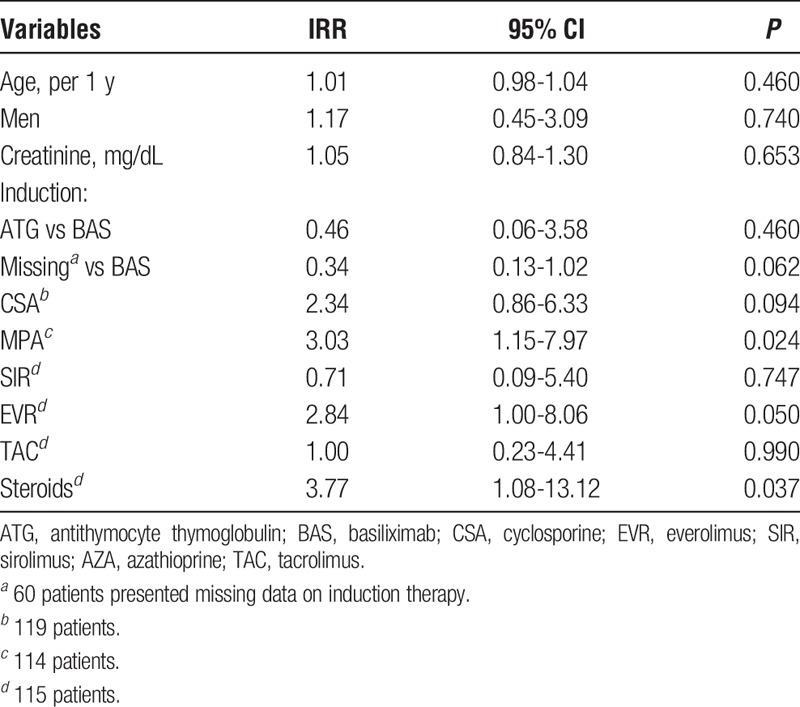
Table 4 shows the results of univariate Poisson regression analysis for factors associated with EBV-VL greater than 4000 copies/mL. The use of micofenolic acid (MPA) and steroids were associated with EBV-VL superior to 4000 copies/mL in the univariate analysis.
TABLE 4.
Univariate Poisson regression analysis for factors associated with EBV-VL >4000 copies/mL (n = 128 patients)
To avoid the development of PTLD in patients with significant EBV-VL, different preemptive strategies were used: 13 (68.4%) of 19 patients had an EBV-VL follow-up in two weeks, five patients (26.3%) had a reduction of the immunosuppressive regimen, six patients (31.6%) had more tests performed to exclude a PTLD, and in five patients (26.3%) T cell-specific response was evaluated. None of them developed a PTLD. T cell-specific response was also evaluated in a patient with early PTLD. The patient with early PTLD had EBV-specific autologous CTLs infusions whereas the T cell specific response of the other 5 patients was considered sufficient.
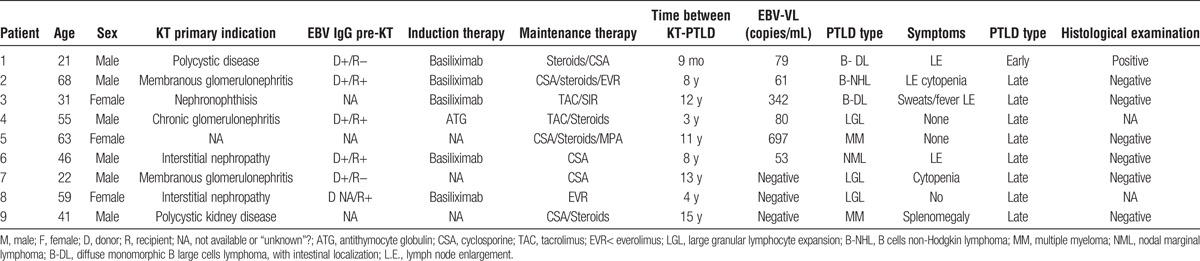
Nine (1.7%) of 523 patients developed a PTLD (Table 5). Only one (11%) was an early PTLD case. The incidence rate of PTLD in the KT population was 0.19/100 PYFU (95% CI, 0.09-0.37). The incidence rate of PTLD in patients tested for EBV-VL was 0.50/100 PYFU (95% CI, 0.23-0.95). Three (33.3%) of 9 patients had a negative EBV-VL, while the other six had a positive EBV-VL less than 4000 copies/mL. Table 5 describes the characteristics of patients with PTLD. The only patient with early PTLD was a high-risk patient and presented with bowel perforation and peritonitis 9 months after KT. The histological examination of the perforated ileal wall showed a monomorphic posttransplant lymphoproliferative disease consistent with diffuse B large cells lymphoma positive for CD20/L26+, bcl2+, MUM1+, CD30/BERH2+/− and negative for CD10−, bcl6−, and CD3−. Two (22.2%) of 9 PTLD patients had a graft failure. Regarding the 2 patients with graft failure, the first one underwent renal graft biopsy for progressive worsening of renal function in January 1999. Unfortunately, the sample was inadequate for analysis; no biopsies were subsequently performed. Notwithstanding the lack of histological diagnosis, the patient was treated with a course of steroid with a significant improvement of renal function. Based on these findings, a diagnosis of chronic allograft failure was suggested. In March 2007, a diagnosis of multiple myeloma stage I was made. In October 2009, the patient experienced graft failure with haemodialysis replacement therapy. The second patient had a diagnosis of PTLD with a large granular lymphocyte expansion in March 2000. In July 2009, the patient underwent renal graft biopsy that showed acute cellular rejection in the background of chronic allograft failure. Treatment consisted of 3 (125 mg) boluses of steroids and addition of sirolimus to the immunosuppressive drug regimen. In 2011, the patient experienced graft loss with hemodialysis replacement therapy.
TABLE 5.
Characteristics of 9 kidney transplant patients with PTLD
Remarkably, no patients with PTLD died. No putative risk factors evaluated in the univariate analysis were associated with PTLD occurrence: age (incidence rate ratio [IRR], 1.01; 95% CI, 0.96-1.06), sex (men vs women: IRR, 0.92; 95% CI, 0.23-3.69), creatinine value (IRR, 1.08; 95% CI, 0.75-1.56), and induction therapy (basiliximab/daclizumab versus thymoglobulin IRR, IRR, 2.59; 95% CI, 0.29-23.21). Maintenance therapy was also evaluated: cyclosporine (IRR, 1.05; 95% CI, 0.23-4.69), sirolimus (IRR, 2.17; 95% CI, 0.26-18.05), everolimus (IRR, 1.39; 95% CI, 0.16-11.58), tacrolimus (IRR, 3.03; 95% CI, 0.58-15.60), and steroid (IRR, 0.64; 95% CI, 0.15-2.89).
DISCUSSION
Our study shows that most EBV-VL assays performed in low-risk KT patients or after the first year post-KT seem to lack clinical significance for diagnosing PTLD. The suspicion driven by disease symptoms and clinician experience appears to be the keystone for PTLD diagnosis and treatment. The utility of routinely performing EBV-VL in adult solid organ transplant recipients to avoid PTLD development is still debated.2 Indeed, despite international guidelines2,16 suggesting EBV-VL monitoring only in high-risk recipients, in real life, the majority of European clinicians routinely use EBV-VL to guide both diagnostic workup and preemptive therapy.18
In our center, EBV monitoring has been used beyond guideline indications. In fact, more than a third of the kidney transplant patients of our cohort had at least one quantitative EBV-VL performed. Of note, only 7.6% of tested patients were at high-risk for PTLD development. These findings gave us the opportunity to discuss the usefulness of EBV-VL monitoring in real-life clinical practice in our center. Despite the high number of tests performed, no patients with PTLD had an EBV-VL superior to 4000 copies/mL, and the diagnosis was guided mostly by clinical suspicion with no important diagnostic role of EBV-VL.
A limitation to this finding is that, due to the observational study design, we are not able to assess if some preemptive treatments performed in patients with EBV-VL persistently superior to 4000 copies/mL avoided PTLD development because of the preemptive treatment with EBV-specific CTLs.
In our cohort, no risk factors for PTLD were identified. This result is consistent with a recent Irish National Observational Study on PTLD in adult KT population. O'Regan and colleagues11 identified 31 late-onset PTLD and no early-onset PTLD in almost 2000 adult KTs. Recipient EBV status was not found to be an independent risk factor. The population considered in the Irish National Study was like ours, since all patients were adult and the rate of EBV IgG positivity in the pre-KT period was high (92.4% and 94%, respectively). They concluded that the lack of EBV naive recipients in the study could have contributed to the paucity of early-onset disease. The preponderance of late-onset diseases in both studies is the most probable explanation to the lack of significance of the recipient EBV status as risk factor for PTLD. The percentage of PTLD in our population was 1.7% consistent with the percentage reported in literature where it is described a posttransplant PTLD risk between 1% and 3%.2,11,14
Concerning EBV-DNA positivity, 67.4% of our population had at least 1 EBV positivity. Morton et al25 showed EBV-DNA positivity up to 46% in renal transplant recipients, using a cutoff of 1000 copies/mL. Since we used a different cutoff (225 copies/mL), this could explain the difference between the 2 percentages. Kanakry et al20 showed that EBV-DNA was detected in the plasma and/or peripheral blood mononuclear cells in the absence of EBV disease in 25% of transplant patients with a cutoff of 50 copies/mL but transplant type were not described.
The percentage of retransplant was higher in EBV-positive patients than in EBV-negative patients, because retransplant is a known risk factor for an increased immunosuppression.
An elevated age and the use of mTORi resulted as risk factors associated with EBV positivity in the univariate analysis. If the association with an elevated age was expected, the use of mTORi as a risk factor for a viral reactivation was a little bit unexpected since literature data consider its use protective for viral reactivations and infections, in particular regarding CMV and human herpes virus 8.26-28 Sirolimus for example has immunostimulatory effects on the generation of memory virus-specific CD8 T cells and this molecular pathway can represent a significant barrier against viral infection.29 The protective role of mTORi regarding EBV is less clear in literature. Regarding PTLD, in vitro studies showed that the mTORi might exert a protective effect against PTLD both by suppressing growth of cells derived from PTLD at allograft-protecting doses30 and by inhibiting the IL-10 signal transduction pathway and the growth of EBV B cell lymphomas.31 On the contrary, different in vivo studies28 showed no reduction in PTLD development in mTORi use.
The use of MPA resulted as a risk factor for EBV reactivation with EBV-VL superior to 4000 copies/mL and these data are consistent with the literature.32
This study has several methodological limitations. First, due to the retrospective, observational, single-center study design, and the long observation period, some data considered relevant as risk factors for EBV reactivation and PTLD were lacking. We were not able to compare the 190 included patients to the rest of the whole cohort. Furthermore, in a consistent number of patients, the pretransplant EBV serology was not available. In our clinic, the pretransplant serology protocol is active since 2000 and 47 (24.74%) of 190 patients were transplanted before 2000 when EBV serology data were not available. Second, we included in the analysis only patients that were evaluated an EBV-VL in the posttransplant period at least once (36% of the total KT cohort). As mentioned before, in our center, EBV-VL assay is usually performed in high-risk patients, in case of signs and symptoms suggestive for EBV-related disease or primary infection, and in patients considered at risk for viral reactivation. For this reason, the analyzed population is probably not representative of the entire cohort, having a higher percentage of some characteristics (eg, retransplantation and acute rejection). However, no cases of PTLD were found in the nontested population, once more suggesting that the monitoring based on clinical suspicion may be sufficient to screen patients. Third, our cohort was relatively small to describe PTLD events. Our results necessitate further studies with a larger sample size.
In conclusion, our data confirm that although posttransplant lymphoproliferative disorder is one of the most devastating complications of organ transplantation, renal transplant recipients are at relatively low risk for PTLD. Our study suggests that, in line with guidelines, EBV-VL assays may be avoided in low-risk patients/period in the absence of a strong clinical PTLD suspicion without increasing patients' risk of developing the disease. This represents a safe and cost-saving clinical strategy for our center.
Footnotes
Published online 26 June, 2017.
The authors declare no funding or conflict of interest.
Each author has participated in the article preparation with conception or design, or analysis and interpretation of data, drafting the article or revising it, and providing intellectual content of critical importance to the work described. All the authors gave final approval of the version to be published. In particular, E.F., P.C., F.F., M.C., M.L., G.C., and C.M. participated in research design. E.F., L.P., I.C.G., and C.M. participated in the writing of the article. J.P., A.S., M.D., F.F., W.G., and G.A., participated in the performance of the research. S.Z. and G.G. participated in data analysis.
REFERENCES
- 1.Dolcetti R. B lymphocytes and Epstein–Barr virus: the lesson of post-transplant lymphoproliferative disorders. Autoimmun Rev. 2007;7:96–101. [DOI] [PubMed] [Google Scholar]
- 2.Allen UD, Preiksaitis JK. AST Infectious Diseases Community of Practice. Epstein-Barr virus and posttransplant lymphoproliferative disorder in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):107–120. [DOI] [PubMed] [Google Scholar]
- 3.Allen UD. The ABC of Epstein-Barr Virus Infections. In: Hot Topics in Infection and Immunity in Children II. Boston, United States: Springer US; 2005. [Google Scholar]
- 4.De Paschale M, Agrappi C, Manco MT, et al. Seroepidemiology of EBV and interpretation of the “isolated VCA IgG” pattern. J Med Virol. 2009;81:325–331. [DOI] [PubMed] [Google Scholar]
- 5.Leogrande G, Jirillo E. Studies on the epidemiology of child infections in the Bari area (south Italy). VII. Epidemiology of Epstein-Barr virus infections. Eur J Epidemiol. 1993;9:368–372. [DOI] [PubMed] [Google Scholar]
- 6.Cohen JI. Epstein-Barr virus infection. N Engl J Med. 2000;343:481. [DOI] [PubMed] [Google Scholar]
- 7.Straus SE, Cohen JI, Tosato G, et al. NIH conference. Epstein-Barr virus infections: biology, pathogenesis, and management. Ann Intern Med. 1993;118:45–58. [DOI] [PubMed] [Google Scholar]
- 8.Morton M, Coupes B, Roberts SA, et al. Epidemiology of posttransplantation lymphoproliferative disorder in adult renal transplant recipients. Transplantation. 2013;95:470–478. [DOI] [PubMed] [Google Scholar]
- 9.Quinlan SC, Pfeiffer RM, Morton LM, et al. Risk factors for early-onset and late-onset post-transplant lymphoproliferative disorder in kidney recipients in the United States. Am J Hematol. 2011;86:206–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dharnidharka VR, Lamb KE, Gregg JA, et al. Associations between EBV serostatus and organ transplant type in PTLD risk: an analysis of the SRTR national Registry Data in the United States. Am J Transplant. 2012;12:976–983. [DOI] [PubMed] [Google Scholar]
- 11.O'Regan JA, Prendeville S, McCaughan JA, et al. Posttransplant lymphoproliferative disorders in Irish renal transplant recipients. Transplantation. 2017;101:657–663. [DOI] [PubMed] [Google Scholar]
- 12.Cockfield SM. Identifying the patient at risk for post-transplant lymphoproliferative disorder. Transplant Infect Dis. 2001;3:70–78. [DOI] [PubMed] [Google Scholar]
- 13.Vereide DT, Sugden B. Lymphomas differ in their dependence on Epstein-Barr virus. Blood. 2011;117:1977–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendez JC, Dockrell DH, Espy MJ, et al. Human beta-herpesvirus interactions in solid organ transplant recipients. J Infect Dis. 2001;183:179–184. [DOI] [PubMed] [Google Scholar]
- 15.Lautenschlager I, Lappalainen M, Linnavuori K, et al. CMV infection is usually associated with concurrent HHV-6 and HHV-7 antigenemia in liver transplant patients. J Clin Virol. 2002;25 Suppl 2:S57–S61. [DOI] [PubMed] [Google Scholar]
- 16.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–S155. [DOI] [PubMed] [Google Scholar]
- 17.Stevens SJ, Verschuuren EA, Pronk I, et al. Frequent monitoring of Epstein-Barr virus DNA load in unfractionated whole blood is essential for early detection of posttransplant lymphoproliferative disease in high-risk patients. Blood. 2001;97:1165–1171. [DOI] [PubMed] [Google Scholar]
- 18.San-Juan R, Manuel O, Hirsch HH, et al. Current preventive strategies and management of Epstein-Barr virus-related post-transplant lymphoproliferative disease in solid organ transplantation in Europe. Results of the ESGICH Questionnaire-based Cross-sectional Survey. Clin Microbiol Infect. 2015;21:604.e1–604.e9. [DOI] [PubMed] [Google Scholar]
- 19.Kimura H, Ito Y, Suzuki R, et al. Measuring Epstein-Barr virus (EBV) load: the significance and application for each EBV-associated disease. Rev Med Virol. 2008;18:305–319. [DOI] [PubMed] [Google Scholar]
- 20.Kanakry JA, Hegde AM, Durand CM, et al. The clinical significance of EBV DNA in the plasma and peripheral blood mononuclear cells of patients with or without EBV diseases. Blood. 2016;127:2007–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowe DT, Webber S, Schauer EM, et al. Epstein-Barr virus load monitoring: its role in the prevention and management of post-transplant lymphoproliferative disease. Transplant Infect Dis. 2001;3:79–87. [DOI] [PubMed] [Google Scholar]
- 22.Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117:5019–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calarota SA, Chiesa A, Zelini P, et al. Detection of Epstein-Barr virus-specific memory CD4 + T cells using a peptide-based cultured enzyme-linked immunospot assay. Immunology. 2013;139:533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Comoli P, Labirio M, Basso S, et al. Infusion of autologous Epstein-Barr virus (EBV)-specific cytotoxic T cells for prevention of EBV-related lymphoproliferative disorder in solid organ transplant recipients with evidence of active virus replication. Blood. 2002;99:2592–2598. [DOI] [PubMed] [Google Scholar]
- 25.Morton M, Coupes B, Roberts SA, et al. Epstein-Barr virus infection in adult renal transplant recipients. Am J Transplant. 2014;14:1619–1629. [DOI] [PubMed] [Google Scholar]
- 26.Ponticelli C. Herpes viruses and tumours in kidney transplant recipients. The role of immunosuppression. Nephrol Dial Transplant. 2011;26:1769–1775. [DOI] [PubMed] [Google Scholar]
- 27.Webster AC, Lee VW, Chapman JR, et al. Target of rapamycin inhibitors (sirolimus and everolimus) for primary immunosuppression of kidney transplant recipients: a systematic review and meta-analysis of randomized trials. Transplantation. 2006;81:1234–1248. [DOI] [PubMed] [Google Scholar]
- 28.Ekberg H, Tedesco-Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562–2575. [DOI] [PubMed] [Google Scholar]
- 29.Araki K, Turner AP, Shaffer VO, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majewski M, Korecka M, Joergensen J, et al. Immunosuppressive TOR kinase inhibitor everolimus (RAD) suppresses growth of cells derived from posttransplant lymphoproliferative disorder at allograft-protecting doses. Transplantation. 2003;75:1710–1717. [DOI] [PubMed] [Google Scholar]
- 31.Nepomuceno RR, Balatoni CE, Natkunam Y, et al. Rapamycin inhibits the interleukin 10 signal transduction pathway and the growth of Epstein Barr virus B-cell lymphomas. Cancer Res. 2003;63:4472–4480. [PubMed] [Google Scholar]
- 32.Bamoulid J, Courivaud C, Coaquette A, et al. Subclinical Epstein-Barr virus viremia among adult renal transplant recipients: incidence and consequences. Am J Transplant. 2013;13:656–662. [DOI] [PubMed] [Google Scholar]


