Abstract
Transplantation of bone marrow stromal cells (BMSCs) is regarded as a promising candidate for the spinal cord injury (SCI). In the present study, we investigated whether treadmill exercise potentiate the effect of BM-SCs transplantation on the functional recovery in the SCI rats. The spinal cord contusion injury applied at the T9–T10 level using the impactor. Cultured BMSCs were transplanted into the lesion at 1 week after SCI induction. Treadmill exercise was conducted for 6 weeks. Basso-Beattie-Bresnahan (BBB) scale for locomotor function was determined. Sprouting axons in the lesion cavity were detected by immunofluorescence staining for neurofilament-200. Brain-derived neurotrophic factor (BDNF) and synapsin-I expressions were analyzed using western blotting. BMSCs transplantation improved BBB score and increased expressions of neurofilament-200, BDNF, and synapsin-I in the SCI rats. Treadmill exercise potentiated the improving effect of BMSCs transplantation on BBB score in the SCI rats. This potentiating effect of treadmill exercise could be ascribed to the enhancement of BDNF expression in the SCI rats.
Keywords: Bone marrow stromal cells, Spinal cord injury, Treadmill exercise, Locomotor function, Brain-derived neurotrophic factor, Synapsin-I
INTRODUCTION
Transplantation of bone marrow stromal cells (BMSCs) is regarded as a promising candidate for the spinal cord injury (SCI), and BMSCs are readily and safely applicable as an autologous transplant in clinical cases (Nakano et al., 2013; Tetzlaff et al., 2011). BMSCs supported the survival of the host tissue by secreting some neuroprotective factors, including vascular endothelial growth factor, fibroblast growth factor-2, hepatocyte growth factor, nerve growth factor, and brain-derived neurotrophic factor (BDNF) (Yamaguchi et al., 2006). BMSCs partly differentiated into neurons (Yano et al., 2006), and cultured BMSCs promoted axonal regeneration and improved motor function in the SCI rats (Chiba et al., 2009).
BDNF is a plasticity-related molecule which is implicated in neuronal activity, survival, and remodeling. BDNF mediated a variety of essential morphological changes including dendritic arborization, axonal and dendritic remodeling, synaptogenesis, and synaptic efficacy (Liu et al., 2009; Sallert et al., 2009; Yacoubian and Lo, 2000). Exercise-induced neuroplasticity is closely associated with BDNF level in the neuromuscular system (Gómez-Pinilla et al., 2002). Synapsin-I is implicated in the synaptogenesis and modulation of neurotransmitter release, suggesting that synapsin-I plays a potential role in several neuropsychiatric diseases (Kafitz et al., 1999). Synapsin-I expression was strongly correlated with the spontaneous recovery of hindlimb locomotion after spinal cord hemisection (Gulino et al., 2007).
Treadmill training improved behavioral recovery after partial incision of the spinal cord in rats (Fouad et al., 2000). Physical activity after spinal contusion improved sensory function and this improvement was positively correlated with BDNF level in the injured spinal cord (Hutchinson et al., 2004). Step training facilitated locomotor recovery after complete spinal lesion (Frigon and Rossignol, 2006). Physical exercise improved motor ability and enhanced BDNF expression, thereby contributed to neuronal integrity (Macias et al., 2009). Forced treadmill exercise improved movement pattern, facilitated axonal growth and sprouting, enhanced synaptic formation, and suppressed muscle atrophy after spinal cord hemisection (Goldshmit et al., 2008). Treadmill exercise facilitated locomotor recovery through axonal regeneration via BDNF expression in the SCI rats (Jung et al., 2016).
In the present study, we investigated whether treadmill exercise potentiate the effect of BMSCs transplantation on functional recovery in the spinal cord contusion injury using rats.
MATERIALS AND METHODS
Animals and treatments
Forty adult male Sprague-Dawley rats (180±10 g, 6 weeks old) were used in this study. The experimental procedures were performed in accordance with the animal care guidelines of the National Institute of Health and the Korean Academy of Medical Sciences. The animals were housed under controlled temperature (23°C±2°C) and lighting (8:00 a.m. to 8:00 p.m.) conditions with food and water available ad libitum. The animals were randomly divided into four groups (n=10 in each group): sham-operation group, SCI group, SCI and BMSCs transplantation group, and SCI and BMSCs transplantation with treadmill exercise group.
Spinal cord contusion injury
Spinal cord contusion injury was induced according to the previously described method (Shin et al., 2016). The rats were anesthetized with Zoletil 50 (10 mg/kg, intraperitoneally; Vibac Laboratories, Carros, France). Laminectomy was performed at the T9–T10 level, exposing the cord beneath without disrupting the dura. A contusion injury was created using the New York University Impactor System (NYU impactor, New York, NY, USA) by dropping a 10 g impactor from 2.5-cm height onto the exposed dura. The rats in the sham-operation group received laminectomy without weight-drop contusion injury.
BMSCs culture and transplantation
BMSCs were obtained by perfusion through femur and tibia of 4-week-old rats, as the previously described method (Shin et al., 2016). Cells were cultured in Dulbecco’s modified Eagle’s medium with 20% fetal calf serum. Cultured BMSC at a density of 5×106 in 50-μL phosphate-buffered saline (PBS) was injected into the SCI site using a micro syringe with a 30-gauge needle. For the control, PBS without BMSCs was injected as the same manner.
Treadmill exercise protocol
One week after transplantation, the rats in the exercise group performed treadmill running at a speed of 8 m/min for 30 min once a day, 6 days a week, for 6 weeks.
BBB locomotor scale
Functional recovery was assessed using the Basso-Brestti-Brenahan (BBB) locomotor scale once a week for 6 weeks, as the previously described method (Jung et al., 2016). BBB locomotor scale is a standard method for assessing hindlimb locomotion in open field. Open field locomotion was conducted by using the 21-point BBB locomotion scale. The rats were placed in an open field (80 cm×130 cm×30 cm) with a pasteboard covered nonslippery floor. In each testing session, the animals were observed individually for 3 min. The scoring range from 0 (flat paralysis) to 21 (normal gait) was graded by features of limb movement, paw placement, gait, and coordination.
Tissue preparation
The animals were fully anesthetized using Zoletil 50 (10 mg/kg, intraperitoneally). The anesthetized rats were transcardially perfused with 50-mM PBS, and fixed with a freshly prepared solution consisting of 4% paraformaldehyde in 100-mM phosphate buffer at pH 7.4. The spinal cords were dissected, postfixed in the same fixative overnight, and transferred to 30% sucrose for cryoprotection. Transverse sections of 10-μm thickness were made with a freezing microtome (Leica, Nussloch, Germany).
Immunofluorescence
Immunofluorescence for neurofilament was performed according to the previously described method (Jung et al., 2016). The sections were washed 3 times with PBS, and then exposed to 0.1% bovine serum albumin solution containing 0.1% Tween 20 in PBS for 4 hr at room temperature. Next, the sections were incubated overnight with a solution containing primary antibodies as antineurofilament rabbit polyclonal antibody (1:400, Sigma Chemical Co., St. Louis, MO, USA) for axons, After washing, the sections were incubated 2 hr with secondary antibodies as rhodamine-goat anti-rabbit secondary antibodies (1:800; Molecular, Probes, Eugene, CA, USA) for neurofilament. The sections were mounted and examined by a fluorescence microscope (Nikon Eclipse 50i, Nikon Inc., Melville, OR, USA).
Western blot analysis
Western blot for BDNF and synapsin-I was performed according to the previously described method (Jung et al., 2016). The spinal cord tissues covering approximately 1 cm of the rostral and caudal spinal cord at the injury area were prepared and washed with ice-cold PBS and sonicated in 400–600 mL of Triton lysis buffer. Protein separation was performed using a 10% polyacrylamide with 0.05% bis-acrylamide. Proteins were then transferred to nitrocellulose and the blots were probed with anti-BDNF rabbit polyclonal antibody (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-synapsin-I rabbit monoclonal antibody (1:1,000, Cell Signaling Technology, Beverly, MA, USA), and anti-β-actin mouse monoclonal antibody (1:3,000, Santa Cruz Biotechnology). Peroxidase anti-rabbit IgG (1:5,000; 1:5,000 Vector Laboratories, Burlingame, CA, USA), and peroxidase anti-mouse IgG (1:5,000, Vector Laboratories) were used as the secondary antibodies. Immunoreactivity was detected by enhanced chemiluminescence (Santa Cruz Biotechnology).
Data analysis
To compare the relative expressions of proteins, the detected bands were calculated densitometrically using Molecular Analyst, version 1.4.1 (Bio-Rad, Hercules, CA, USA). Statistical analysis was performed using one-way analysis of variance followed by Duncan’s post hoc test, and the results were expressed as the mean± standard error of the mean. Significance was set as P<0.05.
RESULTS
BBB score
BBB score is presented in Fig. 1. BBB score was decreased after induction of SCI, but transplantation of BMSCs increased BBB score 3 weeks after SCI induction. Performing treadmill exercise with BMSCs transplantation more enhanced BBB score in the SCI rats.
Fig. 1.
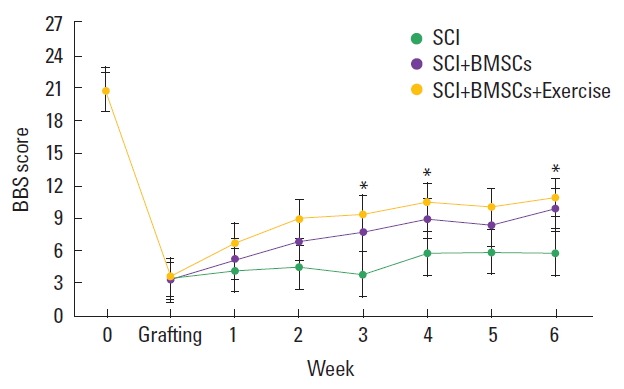
Basso-Beattie-Bresnahan (BBB) score in each group. SCI, spinal cord injury group; SCI+BMSC, spinal cord injury and bone marrow stromal cells transplantation group; SCI+BMSC+EX, spinal cord injury and bone marrow stromal cells transplantation with treadmill exercise group. Values are presented as mean±standard error of the mean. *P<0 .05 compared to the SCI group.
Axonal sprouting within the cavity
Axonal regeneration in the cavity is presented in Fig. 2. Immunostaining for neurofilament-200 (NF-200) was performed to evaluate the axonal sprouting within the cavity. The intensity of NF-200-positive cells was decreased after induction of SCI, but transplantation of BMSCs increased intensity of NF-200-positive cells. Performing treadmill exercise with BMSCs transplantation more enhanced intensity of NF-200-positive cells than BMSCs transplantation alone in the SCI rats.
Fig. 2.
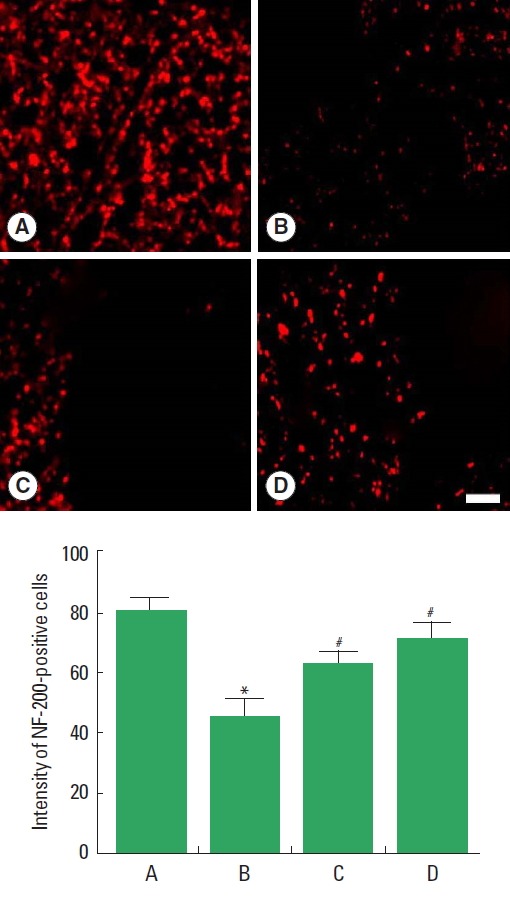
Axonal regeneration in the cavity after SCI. Upper panel: Photomicrographs showing the expression of neurofilament-200 (NF-200) in the injured spinal cord. The scale bar represents 100 μm. Lower panel: Intensity of NF-200. A, sham-operation group; B, spinal cord injury group; C, spinal cord injury and bone marrow stromal cells transplantation group; D, spinal cord injury and bone marrow stromal cells transplantation with treadmill exercise group. Values are presented as mean±standard error of the mean. *P<0.05 compared to the sham-operation group. #P<0.05 compared to the spinal cord injury group.
BDNF expression
BDNF expression in the spinal cord is presented in Fig. 3. BDNF expression was decreased after induction of SCI, but transplantation of BMSCs increased BDNF expression. Performing treadmill exercise with BMSCs transplantation more enhanced BDNF expression than BMSCs transplantation alone in the SCI rats.
Fig. 3.
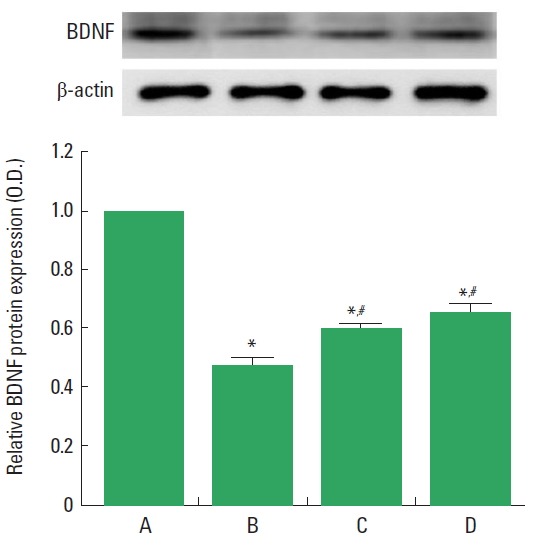
Brain-derived neurotrophic factor (BDNF) expression. Upper panel: Representative expression of the BDNF and β-actin. Lower panel: Relative BDNF expression in each group. A, sham-operation group; B, spinal cord injury group; C, spinal cord injury and bone marrow stromal cells transplantation group; D, spinal cord injury and bone marrow stromal cells transplantation with treadmill exercise group. Values are presented as mean±standard error of the mean. *P<0.05 compared to the sham-operation group. #P<0.05 compared to the spinal cord injury group.
Synapsin-I expression
Synapsin-I expression in the spinal cord is presented in Fig. 4. Synapsin-I expression was decreased after induction of SCI, but transplantation of BMSCs increased synapsin-I expression. Performing treadmill exercise with BMSCs transplantation exerted similar enhancing effect as BMSCs transplantation alone on synapsin-I expression.
Fig. 4.
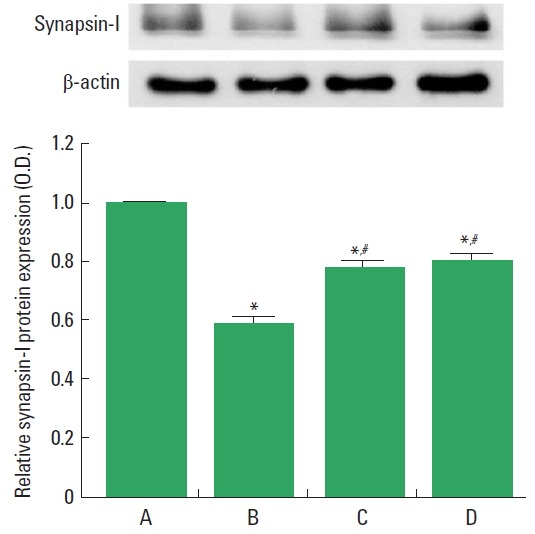
Synapsin-I expression. Upper panel: Representative expression of the synapsin-I and β-actin. Lower panel: Relative synapsin-I expression in each group. A, sham-operation group; B, spinal cord injury group; C, spinal cord injury and bone marrow stromal cells transplantation group; D, spinal cord injury and bone marrow stromal cells transplantation with treadmill exercise group. Values are presented as mean±standard error of the mean. *P<0.05 compared to the sham-operation group. #P<0.05 compared to the spinal cord injury group.
DISCUSSION
BMSCs produced BDNF mediating axon outgrowth and recovery after SCI (Neuhuber et al., 2005). BMSCs transplantation into the advanced SCI lesions facilitated tissue repair and axonal outgrowth, leading to improved locomotion in rats (Ide et al., 2010). BMSCs exerted beneficial effects on locomotor improvement as well as on axonal regeneration in both subacute and chronic SCI rats, and they suggested that BMSCs might function as the neurotrophic sources (Nakano et al., 2013).
In the present study, BMSCs transplantation improved BBB score through enhancing axonal regeneration in the SCI rats. This improving effect of BMSCs could be ascribed to the increment of BDNF and synapsin-I expressions in the SCI rats.
Voluntary wheel running elevated expressions of neurotrophin-3 and its receptor tyrosine kinase C in the spinal cord (Ying et al., 2003). Resolution of allodynia by treadmill training in the SCI rats was associated with normal BDNF mRNA level in both the lumbar spinal cord and soleus muscle (Hutchinson et al., 2004). Ying et al. (2008) suggested that exercise normalized the molecules important for synaptic function, such as cyclic AMP response element binding protein and synapsin-I in the hemisected rats. They also indicated that BDNF plays an important role in shaping the synaptic plasticity and in defining the level of recovery of locomotor performance after a cervical hemisection in rats (Ying et al., 2008). Jung et al. (2016) showed that treadmill training improved locomotor function in the SCI rats. Shin et al. (2016) demonstrated that treadmill exercise improved the therapeutic effect of BMSCs transplantation through BDNF-extracellular signal-regulated kinase pathway in the traumatic brain injury-induced rats.
In the present study, treadmill exercise potentiated the improving effect of BMSCs transplantation on BBB score in the SCI rats. This potentiating effect of treadmill exercise was achieved by facilitation of axonal regeneration through enhancing BDNF expression in the SCI rats. The present results showed that treadmill exercise may aid the therapeutic effect of BMSC transplantation on SCI.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2014 S1A5B5A02013292).
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- Chiba Y, Kuroda S, Maruichi K, Osanai T, Hokari M, Yano S, Shichinohe H, Hida K, Iwasaki Y. Transplanted bone marrow stromal cells promote axonal regeneration and improve motor function in a rat spinal cord injury model. Neurosurgery. 2009;64:991–999. doi: 10.1227/01.NEU.0000341905.57162.1D. [DOI] [PubMed] [Google Scholar]
- Fouad K, Metz GA, Merkler D, Dietz V, Schwab ME. Treadmill training in incomplete spinal cord injured rats. Behav Brain Res. 2000;115:107–113. doi: 10.1016/s0166-4328(00)00244-8. [DOI] [PubMed] [Google Scholar]
- Frigon A, Rossignol S. Functional plasticity following spinal cord lesions. Prog Brain Res. 2006;157:231–260. doi: 10.1016/s0079-6123(06)57016-5. [DOI] [PubMed] [Google Scholar]
- Goldshmit Y, Lythgo N, Galea MP, Turnley AM. Treadmill training after spinal cord hemisection in mice promotes axonal sprouting and synapse formation and improves motor recovery. J Neurotrauma. 2008;25:449–465. doi: 10.1089/neu.2007.0392. [DOI] [PubMed] [Google Scholar]
- Gómez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Gulino R, Dimartino M, Casabona A, Lombardo SA, Perciavalle V. Synaptic plasticity modulates the spontaneous recovery of locomotion after spinal cord hemisection. Neurosci Res. 2007;57:148–156. doi: 10.1016/j.neures.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Hutchinson KJ, Gómez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127(Pt 6):1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- Ide C, Nakai Y, Nakano N, Seo TB, Yamada Y, Endo K, Noda T, Saito F, Suzuki Y, Fukushima M, Nakatani T. Bone marrow stromal cell transplantation for treatment of sub-acute spinal cord injury in the rat. Brain Res. 2010;1332:32–47. doi: 10.1016/j.brainres.2010.03.043. [DOI] [PubMed] [Google Scholar]
- Jung SY, Seo TB, Kim DY. Treadmill exercise facilitates recovery of locomotor function through axonal regeneration following spinal cord injury in rats. J Exerc Rehabil. 2016;12:284–292. doi: 10.12965/jer.1632698.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafitz KW, Rose CR, Thoenen H, Konnerth A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature. 1999;401:918–921. doi: 10.1038/44847. [DOI] [PubMed] [Google Scholar]
- Liu X, Robinson ML, Schreiber AM, Wu V, Lavail MM, Cang J, Copenhagen DR. Regulation of neonatal development of retinal ganglion cell dendrites by neurotrophin-3 overexpression. J Comp Neurol. 2009;514:449–458. doi: 10.1002/cne.22016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias M, Nowicka D, Czupryn A, Sulejczak D, Skup M, Skangiel-Kramska J, Czarkowska-Bauch J. Exercise-induced motor improvement after complete spinal cord transection and its relation to expression of brain-derived neurotrophic factor and presynaptic markers. BMC Neurosci. 2009;10:144. doi: 10.1186/1471-2202-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano N, Nakai Y, Seo TB, Homma T, Yamada Y, Ohta M, Suzuki Y, Nakatani T, Fukushima M, Hayashibe M, Ide C. Effects of bone marrow stromal cell transplantation through CSF on the subacute and chronic spinal cord injury in rats. PLoS One. 2013;8:e73494. doi: 10.1371/journal.pone.0073494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhuber B, Timothy Himes B, Shumsky JS, Gallo G, Fischer I. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005;1035:73–85. doi: 10.1016/j.brainres.2004.11.055. [DOI] [PubMed] [Google Scholar]
- Sallert M, Rantamäki T, Vesikansa A, Anthoni H, Harju K, Yli-Kauhaluoma J, Taira T, Castren E, Lauri SE. Brain-derived neurotrophic factor controls activity-dependent maturation of CA1 synapses by downregulating tonic activation of presynaptic kainate receptors. J Neurosci. 2009;29:11294–11303. doi: 10.1523/JNEUROSCI.0560-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MS, Park HK, Kim TW, Ji ES, Lee JM, Choi HS, Kim MY, Kim YP. Neuroprotective effects of bone marrow stromal cell transplantation in combination with treadmill exercise following traumatic brain injury. Int Neurourol J. 2016;20(Suppl 1):S49–56. doi: 10.5213/inj.1632616.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ, Kawaja MD, Fehlings MG, Kwon BK. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28:1611–1682. doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoubian TA, Lo DC. Truncated and full-length TrkB receptors regulate distinct modes of dendritic growth. Nat Neurosci. 2000;3:342–349. doi: 10.1038/73911. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Kuroda S, Kobayashi H, Shichinohe H, Yano S, Hida K, Shinpo K, Kikuchi S, Iwasaki Y. The effects of neuronal induction on gene expression profile in bone marrow stromal cells (BMSC)--a preliminary study using microarray analysis. Brain Res. 2006;1087:15–27. doi: 10.1016/j.brainres.2006.02.127. [DOI] [PubMed] [Google Scholar]
- Yano S, Kuroda S, Shichinohe H, Seki T, Ohnishi T, Tamagami H, Hida K, Iwasaki Y. Bone marrow stromal cell transplantation preserves gammaaminobutyric acid receptor function in the injured spinal cord. J Neurotrauma. 2006;23:1682–1692. doi: 10.1089/neu.2006.23.1682. [DOI] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Edgerton VR, Gómez-Pinilla F. Voluntary exercise increases neurotrophin-3 and its receptor TrkC in the spinal cord. Brain Res. 2003;987:93–99. doi: 10.1016/s0006-8993(03)03258-x. [DOI] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Zhong H, Zdunowski S, Edgerton VR, Gomez-Pinilla F. BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience. 2008;155:1070–1078. doi: 10.1016/j.neuroscience.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]


