Abstract
Importance
Estimates of familial cancer risk from population-based studies are essential components of cancer risk prediction.
Objective
To estimate familial risk and heritability of cancer types in a large twin cohort.
Design, Setting, and Participants
Prospective study of 80 309 monozygotic and 123 382 same-sex dizygotic twin individuals (N = 203 691) within the population-based registers of Denmark, Finland, Norway, and Sweden. Twins were followed up a median of 32 years between 1943 and 2010. There were 50 990 individuals who died of any cause, and 3804 who emigrated and were lost to follow-up.
Exposures
Shared environmental and heritable risk factors among pairs of twins.
Main Outcomes and Measures
The main outcome was incident cancer. Time-to-event analyses were used to estimate familial risk (risk of cancer in an individual given a twin's development of cancer) and heritability (proportion of variance in cancer risk due to interindividual genetic differences) with follow-up via cancer registries. Statistical models adjusted for age and follow-up time, and accounted for censoring and competing risk of death.
Results
A total of 27 156 incident cancers were diagnosed in 23 980 individuals, translating to a cumulative incidence of 32%. Cancer was diagnosed in both twins among 1383 monozygotic (2766 individuals) and 1933 dizygotic (2866 individuals) pairs. Of these, 38% of monozygotic and 26% of dizygotic pairs were diagnosed with the same cancer type. There was an excess cancer risk in twins whose co-twin was diagnosed with cancer, with estimated cumulative risks that were an absolute 5% (95% CI, 4%-6%) higher in dizygotic (37%; 95% CI, 36%-38%) and an absolute 14% (95% CI, 12%-16%) higher in monozygotic twins (46%; 95% CI, 44%-48%) whose twin also developed cancer compared with the cumulative risk in the overall cohort (32%). For most cancer types, there were significant familial risks and the cumulative risks were higher in monozygotic than dizygotic twins. Heritability of cancer overall was 33% (95% CI, 30%-37%). Significant heritability was observed for the cancer types of skin melanoma (58%; 95% CI, 43%-73%), prostate (57%; 95% CI, 51%-63%), nonmelanoma skin (43%; 95% CI, 26%-59%), ovary (39%; 95% CI, 23%-55%), kidney (38%; 95% CI, 21%-55%), breast (31%; 95% CI, 11%-51%), and corpus uteri (27%; 95% CI, 11%-43%).
Conclusions and Relevance
In this long-term follow-up study among Nordic twins, there was significant excess familial risk for cancer overall and for specific types of cancer, including prostate, melanoma, breast, ovary, and uterus. This information about hereditary risks of cancers may be helpful in patient education and cancer risk counseling.
The global burden of cancer is considerable, with an estimated 12 million new cases and 8 million cancer deaths each year.1 In 2015 in the United States, 1.7 million individuals will be diagnosed with cancer and 590 000 will die of cancer, accounting for 1 in 4 deaths.2 In the Nordic countries, cancer is the leading cause of mortality, accounting for 30% of all deaths. Refinement of primary and secondary prevention strategies (ie, factors that would have the greatest influence on reducing cancer incidence and mortality) requires a detailed understanding of the contribution of genetic and environmental factors to disease pathogenesis.
Family-based studies have been helpful in describing familial aggregation of cancer.3-6 Many inherited risk loci have been identified by genome-wide association studies; however, these known loci explain only a small proportion of the variability in cancer incidence. Large twin studies of cancer can provide further insight into the relative contribution of inherited factors and characterize familial cancer risk by leveraging the genetic relatedness of monozygotic and dizygotic pairs of twins.
A study in 2000 found significant estimates of heritability (ie, the proportion of variability in disease risk in a population due to genetic factors) of 42% for prostate cancer, 35% for colorectal cancer, and 27% for breast cancer among twins from Sweden, Denmark, and Finland.7 The confidence intervals around these heritability estimates were wide, and the estimates were not interpretable for other common cancers.
To address these limitations, we undertook an analysis within the Nordic Twin Study of Cancer (NorTwinCan), including twins from nationwide registers in Denmark, Finland, Norway, and Sweden followed up for an average of 32 years for cancer incidence and mortality. Statistical methods that take into account the potential statistical bias due to censoring and competing risk of death8 were used to estimate heritability and familial risk for specific types of cancer.
Methods
Study Population
The NorTwinCan study is an international, multidisciplinary collaboration of researchers working to investigate the genetic and environmental underpinnings of cancer.9 The cohort includes 357 377 individual twins from the population-based twin registers of Denmark, Finland, Norway, and Sweden and is composed of both monozygotic and same-sex dizygotic and opposite-sex dizygotic twins. In each country, the twin registries were assembled through the nationwide identification of twins across several birth cohorts.10-13
Twins were identified through a range of methods, including review of national birth registries, church parish records, and civil registration systems. For example, the twin registry in Denmark was assembled for4 birthcohorts during the study period of 1870 through 2004. The first cohort from 1870 through 1930 was assembled retrospectively in the 1950s through review of birth register records in each of the local parishes for which births were recorded.10
Subsequent birth cohorts in Denmark were identified through review of the civil registration system and national birth registry records. Nationwide coverage of twin registries ranges between 30% for the oldest birth cohort (from 1870-1930) and 70% for the birth cohort from 1931 through 1952. Coverage was approximately 100% for later cohorts. The lower coverage in the earlier cohorts is due in large part to the fact that pairs of twins had to survive to the age of 6 years to be included in the registry.
Twin zygosity for same-sex pairs of twins is determined primarily by validated questionnaire methods that show a high degree of accuracy (>95% agreement with genetic markers; questionnaire given either to the twin member or a relative if twin diseased).14,15 We excluded data from 5376 twins with missing or inconsistent zygosity data. The ethical committees at each of the twin registries' host institutions approved this project.
Cohort Follow-up
Residents in Nordic countries each have a unique national registration number that allows for linkage of data from the Nordic twin register to national cancer registers for each country, mortality registers, and registers of the total population to glean outcome and vital status information (date of death or emigration) for each individual. For cancer diagnoses, we obtained data by linkage to the national cancer register in each country.
Physicians and pathologists are mandated by law to report every newly diagnosed malignant tumor in each of the 4 countries. In addition, the nationwide death registries send information to the cancer registry for individuals when the death certificate mentions cancer. Case reporting to each registry is close to 100% complete,16-19 the quality of the data are assessed through careful review, and all reports of cancer diagnosis are verified at national registries.
Cancer register data include diagnoses of cancer type classified according to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10). For this study, we grouped the ICD-10 codes to categories defined in comparable ways across the cancer registries using the NORDCAN (a system designed for the standardization of cancer codes across the Nordic registries) classification of cancers.20
Cohort follow-up is initiated at the start of cancer registration in each country or at a later time for birth cohorts born after the start. For example, the cancer register in Denmark was initiated in January 1943. For twins born in the cohorts to 1930, follow-up began in 1943. For Danish twins born after 1943, follow-up began in 1968 at the time when the national registration number system was started. We have follow-up through the end of 2008 in Norway, 2009 in Denmark and Sweden, and 2010 in Finland. Details of the country-specific dates of start and end of follow-up are summarized in Table 1 and additional details are provided in Hjelmborg et al.9
Table 1. Characteristics of the NorTwinCan Cohort of 203 691 Individual Twins With Follow-up for Cancer Incidence.
| Denmark | Finland | Norway | Sweden | Total | |
|---|---|---|---|---|---|
| Birth cohort date range | 1870-2004 | 1875-1957 | 1915-1979 | 1886-2000 | |
| No. of individual twins | 68 320 | 24 661 | 23 683 | 87 027 | 203 691 |
| Individual twins, No. (%) | |||||
| Same-sex dizygotic | 43 534 (64) | 16 949 (69) | 12 993 (55) | 49 906 (57) | 123 382 (61) |
| Monozygotic | 24 786 (36) | 7712 (31) | 10 690 (45) | 37 121 (43) | 80 309 (39) |
| Female | 33 330 (49) | 12 410 (50) | 12 749 (54) | 45 762 (53) | 104 251 (51) |
| Follow-up | |||||
| First date | January 1943 | Febuary 1974 | January 1964 | April 1961 | |
| End date | December 2009 | December 2010 | December 2008 | December 2009 | |
| Median (IQR), y | 41.6 (26.8-41.8) | 34.7 (29.4-34.7) | 27.9 (16.1-28.5) | 25 (5.0-37.0) | 32.2 (15.5-37.0) |
| Age at start, median (IQR), y | 12.3 (0-25.7) | 32.1 (24.4-45.8) | 29.8 (23.4-41.2) | 32.1 (21.4-42.4) | 26.4 (14.9-38.9) |
| No. of incident cancers | 8904 | 4049 | 2805 | 11 398 | 27 156 |
Abbreviations: IQR, interquartile range; NorTwinCan, Nordic Twin Study of Cancer.
Statistical Methods
After combining cancers of the head and neck (ICD-10 codes OC 00-14), 36 cancer types remained. We present results for familial risk for 23 cancer types with more than 1 concordant monozygotic and dizygotic pair, and heritability estimates for malignancies with at least 5 concordant pairs of twins. In a twin study, familial or concordance risk is defined as the risk of a specific cancer type in a twin, given that the co-twin was diagnosed with the same cancer. Comparing the conditional risk with the cumulative incidence in the population provides an estimate of the excess familial risk of a cancer.
In particular, dizygotic twins are as genetically alike as full siblings and thus familial risk among dizygotic twins can be generalized to siblings. Heritability is defined as the proportion of variance in cancer risk on the liability scale due to interindividual genetic differences in the population. For both familial risk and heritability, estimates are a function of follow-up time and the age of the cohort.
Individuals were followed up prospectively through the registries until cancer diagnosis, death or emigration during follow-up, or the end of the study. We defined the dates of entry and follow-up separately for each cohort depending on ascertainment procedures and data availability in each country (Table 1).
In statistical models, we accounted for left truncation from differing start dates of follow-up and right censoring for those censored at the end of follow-up, those censored when lost to follow-up due to emigration, or at competing risk of death. Cumulative risk of cancer was calculated using the nonparametric Aalen-Johansen estimator.13 We modeled potential competing deaths as described in Scheike et al,8 which accounted for competing causes in both the twin and co-twin.
We used quantitative genetic models to estimate the relative contribution of genetic and environmental factors with the variation in cancer risk. This approach assumes that there is a normally distributed liability to develop a genetically complex disease such as cancer. The probability that an individual will express the disease is modeled as a function of the latent unmeasured liability, and disease occurs only when an individual surpasses the threshold. This approach analyzes the disease covariance within monozygotic and dizygotic pairs and decomposes the variance into a sum of components: additive genetic effects (A), common environmental effects shared among twins (C), and individually unique environmental effects (E).
Within-pair covariance is expressed as κ × var(A)+var(C), where κ = 1 for monozygotic pairs because they share 100% of their genomes and κ = ½ for dizygotic pairs because they share on average half of their segregating genes. To test whether there is evidence of a genetic component for each of the cancer types, we compared the tetrachoric correlation for monozygotic and dizygotic twins in a model in which the marginal estimates were the same.
The biometric modeling approach is comparable with that of Lichtenstein et al,7 except that we adjusted for censoring by weighting individuals by the inverse probability of being censored at the time of follow-up using the same weights within pairs of twins.9 The probabilities of being censored were estimated using the Kaplan-Meier method stratified by zygosity and country.
We similarly used the inverse probability of weighting to estimate the median difference in age at diagnosis for the pairs of twins concordant for cancer. All analyses were performed using the R mets package version 1.1.1 (R Foundation for Statistical Computing). Two-sided P values were used with an α level of less than .05.
Results
This analysis comprised 203 691 individual twins in the cohort, 80 309 monozygotic and 123 382 same-sex dizygotic twins, of whom 104 251 were women (Table 1). During an average of 32 years of follow-up, we identified 27156 incident cancers among 23 980 individuals. In addition, 50 990 individuals died of any cause and 3804 individual twins emigrated and were lost to follow-up.
Cancer was diagnosed in both twins among 1383 monozygotic (2766 individuals) and 1933 dizygotic (2866 individuals) pairs. Of these, 38% of monozygotic pairs (n = 522) and 26% of dizygotic pairs (n = 496) were diagnosed with the same type of cancer.
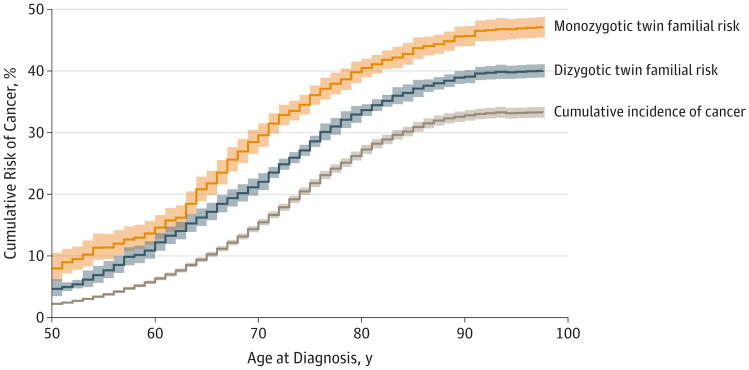
The estimated cumulative incidence of cancer in the overall cohort and the familial risk among monozygotic and dizygotic twins appear in the Figure. The estimated cumulative incidence of cancer, accounting for competing causes of death, was8% by the age of 65 years, 25% by the age of 80 years, and 32% by the age of 100 years using twins as individuals in a standard cohort analysis.
Figure. Cumulative Incidence and Familial Risk of Developing Any Cancer Over Time in the NorTwinCan Cohort.
The cumulative incidence is the risk of developing a first cancer over time within the full twin cohort, and the estimate is adjusted for censoring and competing risks of death. Familial risk is defined as the risk of developing any cancer given the twin's co-twin also developed cancer. At the start of follow-up, there were 79 980 individual monozygotic twins and 122 888 individual dizygotic twins in the risk set. The extent to which the estimate of familial risk is higher than the cumulative incidence gives a sense of the magnitude of excess risk that may be associated with familial factors. Shaded areas indicate 95% confidence intervals; NorTwinCan, Nordic Twin Study of Cancer.
These risks are similar to the nationwide rates in the Nordic populations, showing representativeness of the twins. The lifetime familial risk of any cancer for those whose co-twins were also diagnosed with cancer was 37% (95% CI, 36%-38%) among dizygotic pairs and 46% (95% CI, 44%-48%) among monozygotic pairs by the age of 100 years.
There was an excess cancer risk in twins whose co-twin was diagnosed with cancer, with estimated cumulative risks that were an absolute 5% (95% CI, 4%-6%) higher in dizygotic (37%; 95% CI, 36%-38%) and an absolute 14% (95% CI, 12%-16%) higher in monozygotic twins (46%; 95% CI, 44%-48%) whose twin also developed cancer compared with the cumulative risk in the overall cohort (32%).
The types of cancers with the highest estimated cumulative incidence in the cohort were prostate (10.5%), breast (9.4%), lung (3.2%), nonmelanoma skin (1.9%), and colon (2.9%) (Table 2). There were elevated familial risks among monozygotic and dizygotic pairs of twins for most cancer types as indicated by the familial risk estimates compared with the cumulative incidence. These risks were substantially higher for monozygotic than dizygotic pairs for cancers of the prostate and breast.
Table 2. Cumulative and Familial Risk of Cancer for Selected Malignancies Among Monozygotic and Dizygotic Twins in the NorTwinCan Cohort.
| cummulative Risk, % (95% CI)a | No. of Twin Pairsb | Familial Risk, % (95%CI)a,c | ICD-10 Code | |||||
|---|---|---|---|---|---|---|---|---|
| Monozygotic | Dizygotic | |||||||
| Concordant | Discordant | Concordant | Discordant | Monozygotic Twins | Dizygotic Twins | |||
| Overall cancerd | 32.4 (32.0-32.7) | 1383 | 5887 | 1933 | 11 461 | 45.9 (44.1-47.7) | 37.1 (35.7-38.4) | |
| Head and necke | 0.8 (0.7-0.9) | 5 | 191 | 6 | 361 | 6.0 (2.4-14.4) | 5.1 (2.2-11.3) | C00-14 |
| Esophagus | 0.4 (0.3-0.5) | 0 | 87 | 0 | 183 | C15 | ||
| Stomach | 1.1 (1.0-1.2) | 14 | 338 | 15 | 648 | 6.8 (3.9-11.4) | 4.4 (2.6-7.3) | C16 |
| Small intestine | 0.1 (0.1-0.2) | 0 | 32 | 0 | 59 | C17 | ||
| Colon | 2.9 (2.7-3.0) | 30 | 577 | 31 | 1156 | 10.9 (7.4-15.8) | 7.9 (5.4-11.4) | C18 |
| Rectum and anus | 1.9 (1.7-2.0) | 14 | 440 | 13 | 771 | 6.6 (3.7-11.4) | 5.8 (3.4-9.7) | C20-21 |
| Liver | 0.5 (0.4-0.6) | 0 | 124 | 2 | 208 | 2.1 (0-5.0) | C22 | |
| Gallbladder, extrahepatic bile duct | 0.5 (0.4-0.6) | 1 | 110 | 1 | 187 | 0.5 (0-4.7) | 0.3 (0-1.0) | C23 |
| Pancreas | 1.1 (1.0-1.1) | 4 | 234 | 6 | 508 | 4.3 (1.5-11.6) | 3.7 (1.5-8.6) | C25 |
| Nose, sinuses | 0.1 (0-0.1) | 0 | 21 | 0 | 36 | C30-31 | ||
| Larynx | 0.2 (0.2-0.2) | 2 | 53 | 1 | 113 | 8.4 (2.3-26.4) | 2.7 (1.1-6.1) | C32 |
| Lung, trachea, and bronchus | 3.2 (3.1-3.3) | 50 | 682 | 74 | 1366 | 17.5 (13.4-22.5) | 13.4 (10.8-16.6) | C34 |
| Pleura | 0.1 (0-0.1) | 1 | 22 | 0 | 38 | C38.4 | ||
| Bone | 0.1 (0-0.1) | 0 | 20 | 0 | 35 | C40-41 | ||
| Skin | ||||||||
| Melanoma | 1.2 (1.1-1.3) | 11 | 342 | 6 | 585 | 19.6 (11.5-31.3) | 6.1 (2.7-13.2) | C43 |
| Nonmelanoma | 1.9 (1.8-2.0) | 16 | 395 | 10 | 618 | 14.5 (7.5-26.2) | 4.6 (2.4-8.6) | C44 |
| Connective and soft tissues | 0.2 (0.1-0.2) | 0 | 57 | 0 | 110 | C49 | ||
| Breast | 9.4 (9.1-9.7) | 124 | 1175 | 141 | 2223 | 28.1 (23.9-32.8) | 19.9 (17.0-23.2) | C50 |
| Genital organs | ||||||||
| Cervix uteri | 1.0 (0.9-1.1) | 1 | 210 | 3 | 324 | 2.6 (0-5.6) | C53 | |
| Corpus uteri | 2.0 (1.8-2.1) | 9 | 272 | 6 | 481 | 7.0 (3.4-14.0) | 3.6 (1.6-8.0) | C54 |
| Uterus, other | 0.1 (0-0.2) | 0 | 24 | 0 | 36 | C55 | ||
| Ovary | 1.6 (1.5-1.7) | 6 | 234 | 4 | 427 | 8.7 (4.0-17.9) | 2.9 (1.1-7.4) | C57 |
| Other female | 0.4 (0.3-0.5) | 0 | 47 | 1 | 84 | |||
| Penis and other male | 0.1 (0.1-0.2) | 0 | 15 | 0 | 34 | C60,63 | ||
| Prostate | 10.5 (10.1-10.8) | 197 | 807 | 148 | 1719 | 38.0 (33.9-42.2) | 22.0 (18.8-25.7) | C61 |
| Testis | 0.5 (0.4-0.6) | 5 | 90 | 3 | 123 | 13.8 (5.7-29.6) | 6.0 (1.9-16.9) | C62 |
| Kidney | 0.8 (0.6-1.2) | 5 | 196 | 2 | 374 | 6.7 (2.8-15.1) | 1.8 (0.4-6.8) | C64 |
| Bladder, other urinary organs | 2.2 (2.0-2.3) | 18 | 471 | 13 | 870 | 9.9 (6.2-15.5) | 5.5 (3.1-9.7) | C67-68 |
| Eye | 0.1 (0-0.1) | 2 | 30 | 0 | 64 | 3.4 (0-8.1) | C69 | |
| Brain, central nervous system | 0.9 (0.8-1.0) | 1 | 343 | 3 | 522 | 1.7 (0.5-6.2) | 1.8 (0.3-12.0) | C70-72 |
| Thyroid | 0.2 (0.2-0.3) | 0 | 85 | 1 | 132 | C73 | ||
| Hodgkin disease | 0.1 (0-0.1) | 0 | 57 | 0 | 69 | C81 | ||
| Multiple myeloma | 0.4 (0.4-0.5) | 0 | 114 | 0 | 174 | C90 | ||
| Non-Hodgkin lymphoma | 0.9 (0.9-1.0) | 1 | 254 | 3 | 466 | 0.9 (0-2.1) | C83 | |
| Leukemia | ||||||||
| Acute | 0.3 (0.2-0.4) | 0 | 77 | 0 | 139 | C910, 920 | ||
| Other | 0.6 (0.5-0.7) | 5 | 128 | 3 | 259 | 15.2 (6.1-33.2) | 4.1 (1.3-11.9) | C911, 921 |
Abbreviations: ICD-10, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; NorTwinCan, Nordic Twin Study of Cancer.
Calculated to age 100 years.
The numbers refer to the number of twin pairs not the individual twins.
Not calculated for cancer types with 1 or less concordant pairs.
For individuals who were diagnosed with more than 1 cancer during follow-up (n = 3176), time to first cancer was used.
Includes cancers of the lip, tongue, salivary glands, mouth, and pharynx.
Some of the strongest familial associations were observed for somewhat less common cancers. For testicular cancer, for which the cumulative risk in the cohort was 0.5%, the risk was substantially higher when his co-twin was also diagnosed with testicular cancer; for dizygotic twins, the familial risk estimate was 6% (95% CI, 2%-17%) and for monozygotic twins it was 14% (95% CI, 6%-30%) if his co-twin had previously been diagnosed with testicular cancer.
Familial cancer risk was also high for melanoma of the skin, with familial risks of 6% (95% CI, 3%-13%) for dizygotic twins and almost 20% (95% CI, 12%-31%) for monozygotic twins compared with a cumulative risk of 1.2% for the overall cohort. Familial risk of nonmelanoma skin cancer was also evident, although less than for melanoma. Familial risk of ovarian cancer for women whose twins also had ovarian cancer was similarly greater in monozygotic (9%; 95% CI, 4%-18%) than dizygotic (3%; 95% CI, 1%-7%) twins.
Results from the quantitative genetic modeling used to decompose the familial associations into the genetic and shared environmental components that affect twins the same way appear in Table 3 and in the eTable in the Supplement. The heritability for cancer overall was 33% (95% CI, 30%-37%), with no evidence of a shared environmental component.
Table 3.
Estimates of Heritability and Shared Environment for Specific Types of Cancer in the NorTwinCan Cohorta
| Familial Risk, % (95% CI) | ||
|---|---|---|
| Heritability | Shared Environment | |
| Overall cancer | 33 (30-37) | 0 |
| Head and neck | 9 (0-60) | 26 (0-65) |
| Stomach | 22 (0-55) | 6 (0-31) |
| Colon | 15 (0-45) | 16 (0-38) |
| Rectum and anus | 14 (0-50) | 10 (0-38) |
| Lung | 18 (0-42) | 24 (7-40) |
| Skin | ||
| Melanoma | 58 (43-73) | 0 |
| Nonmelanoma | 43 (26-59) | 0 |
| Breast | 31 (11-51) | 16 (0-31) |
| Corpus uteri | 27 (11-43) | 0 |
| Ovary | 39 (23-55) | 0 |
| Prostate | 57 (51-63) | 0 |
| Testis | 37 (0-93) | 24 (0-70) |
| Kidney | 38 (21-55) | 0 |
| Bladder, other urinary organs | 30 (0-67) | 0 |
| Leukemia, other | 57 (0-100) | 0 |
Abbreviation: NorTwinCan, Nordic Twin Study of Cancer.
Not calculated for cancer types with less than 5 concordant pairs.
A high estimate of heritability of 57% (95% CI, 51%-63%) was found for prostate cancer.9 For breast cancer, 31% of variability may be associated with genetic factors and 16% with shared environmental factors. The strong familial effect noted for testicular cancer among monozygotic and dizygotic twins was associated with both significant genetic (37%) and shared environmental (24%) factors. Moreover, we found significant heritability estimates for cancer of the kidney (38%), skin melanoma (58%), and skin nonmela-noma (43%).
Lung cancer had one of the highest estimates for shared environmental factors (24%). Heritability estimates for gastrointestinal cancers of the colon (15%; 95% CI, 0%-45%), rectum (14%;95%CI, 0%-50%), and stomach (22%;0%-55%) were smaller relative to other malignancies.
Data on the median difference in age at diagnosis among concordant pairs of twins for selected cancer types appear in Table 4. For all cancer cases combined, the median age difference was slightly shorter in monozygotic pairs (8.0 years) than in dizygotic pairs (9.3 years). With the exception of prostate cancer, none of these differences in median age differed between monozygotic and dizygotic pairs for 11 cancer types.
Table 4. Difference in Age at Diagnosis Among Monozygotic and Dizygotic Pairs of Twins for Specific Types of Cancer.
| No. Concordant Pairsa | Age Difference at Diagnosis, Median (95% CI), y | P Valueb | |||
|---|---|---|---|---|---|
| Monozygotic | Dizygotic | Monozygotic | Dizygotic | ||
| Overall cancer | 1383 | 1933 | 8.0 (0.4-22.8) | 9.3 (0.3-33.9) | .002 |
| Head and neck | 5 | 6 | 6.8 (3.3-12.3) | 10.1 (3.5-17.8) | .65 |
| Stomach | 14 | 15 | 10.3 (5.2-15.4) | 14.2 (10.0-18.4) | .65 |
| Colon | 30 | 31 | 8.3 (5.8-11.1) | 6.1 (3.8-11.7) | .62 |
| Rectum, anus | 14 | 13 | 10.3 (6.0-15.3) | 5.3 (2.7-11.6) | .44 |
| Lung | 50 | 74 | 7.8 (6.1-9.5) | 7.7 (5.4-9.4) | .94 |
| Skin | |||||
| Melanoma | 1 | 6 | 8.9 (7.5-21.8) | 15.8 (3.4-23.6) | .72 |
| Nonmelanoma | 16 | 10 | 6.1 (3.5-11.9) | 7.4 (2.4-7.7) | .66 |
| Breast | 124 | 141 | 9.3 (6.9-12.0) | 10.5 (9.1-12.2) | .55 |
| Corpus uteri | 9 | 6 | 12.4 (6.7-17.2) | 9.3 (3.6-10.5) | .72 |
| Prostate | 197 | 148 | 3.7 (4.4-6.1) | 6.1 (4.6-7.9) | .008 |
| Bladder | 18 | 13 | 7.1 (4.1-9.9) | 14.3 (7.2-20.5) | .31 |
The numbers refer to the number of twin pairs not the individual twins.
For difference in median ages between twin types (monozygotic vs dizygotic).
Discussion
This prospective Nordic twin cohort study provides familial risk estimates for cancer overall, for 23 types of cancer, and for relatively rare cancer types. Overall, there was a significantly excess familial risk of developing any cancer, 37% in dizygotic pairs and 46% in monozygotic pairs compared with 32% in the whole twin cohort. The data provide strong evidence of an excess familial risk for 20 of the 23 cancer types, as shown by the comparison of familial risks for those cancers with the cumulative risk in the twin cohort overall.
Testicular cancer and nonmelanoma and melanoma skin cancers showed substantial excess familial risks, particularly among monozygotic pairs. Although the excess familial risk for breast, prostate, and other cancer types were more modest, the absolute differences in risk were considerable.
These estimates of familial risk are in line with other family- and twin-based cohort studies.6,21 Precise estimates of familial cancer risk from population-based studies are essential components of accurate cancer risk prediction,22 and could be used in clinical practice to guide genetic counseling. Dizygotic pairs of twins are as genetically similar as siblings.
As such, familial risk estimates among dizygotic pairs are relevant for siblings who are born at separate times. Risk estimates among monozygotic pairs (who share nearly 100% of their inherited genomes) can be used to derive an upper bound on the ability of genetic studies to discriminate individuals who will experience different disease outcomes.23
Shared environmental factors can include parental factors, such as socioeconomic status, lifestyle, and occupation and experiences and exposures shared by siblings during childhood and adolescence, and screening patterns in adult life. For many cancer types, we did not observe evidence of shared environmental associations, even though our models specifically allowed for estimation of these environmental factors that members of a family share.
Lung cancer had one of the highest shared environmental components, likely due to shared smoking habits of pairs of twins. Testicular and breast cancer also had significant estimates of shared environment, which may reflect in part the hypothesized in utero origins of these cancer types.24,25 It is possible that the shared environment components may be somewhat higher in twins than the estimates expected for siblings because twins may share a more similar child-raising experience.
Most pairs of twins were discordant for a specific cancer. Indeed, among the pairs of twins in which both members developed cancer, more than two-thirds were diagnosed with a different malignancy. This finding of familial aggregation across different cancer types is in line with data from an Icelandic family-based study that showed excess familial aggregation among 17 different cancer types.26
There are novel insights from genetic epidemiology studies suggesting pleiotropy of genetic variants across multiple cancer types.27,28 A more detailed investigation of shared familial risk across cancer types may provide key insights into the underlying cancer susceptibility.
Twin studies can provide context for genome-wide association studies, many of which have identified multiple risk loci for cancer incidence.29-32 Estimates of heritability in twin studies, as well as those derived from genome-wide association studies, allow for the calculation of the extent to which cancer variability is explained by established genetic risk loci. For prostate cancer, the 100 risk loci identified to date explain approximately one-third of the genetic contribution.33,34 For breast cancer, the estimated proportion of heritability explained by the known genetic risk loci may approach up to 30%.35
Few genome-wide association studies of more rare cancers have been undertaken, but our data suggest that such studies may be essential in elucidating the etiology of certain cancer types, such as melanoma, ovarian, kidney, and testicular. A recent analysis of genome-wide association studies for 13 cancer types suggests that the identified genetic variants for most cancer types do not explain the majority of heritability.36 Moreover, the heritability estimate of 33% for developing any cancer type suggests there are shared genomic regions associated with multiple cancer types. The results from 2 family-based studies are also in line with this observation.37,38 A systematic genome-wide association study of individuals with any cancer compared with controls free of any cancer may be a powerful and feasible approach to identify novel loci given that multiple cancer type–specific data sets already exist.
The concept of heritability has its limitations39 and is often misinterpreted as an estimate of population-attributable risk. Heritability can be thought of as the proportion of the variation in cancer risk in a population that can be accounted for by interindividual genetic differences.
A high estimate of heritability for cancer does not translate to a low population-attributable risk associated with lifestyle and environmental factors, nor does a high heritability exclude the possibility of an effective preventive action. The observed genetic estimates based on family relationships for specific cancer types are due to both cancer-specific genetics and genetic contributions to cancer risk factors, such as obesity and smoking, which have a genetic component.
The interval between diagnosis of cancer among concordant pairs of twins was fairly long. The median time between cancer diagnoses in concordant pairs of twins ranged from 4 to 15 years. The difference in pairs of twins with any cancer was similar to the specific numbers for cancer type.
These data suggest that there maybe unique environmental factors that influence the timing of disease development or diagnosis. Moreover, cancer likely involves stochastic processes inherent in the carcinogenic process that may ultimately influence the timing of cancer initiation and diagnosis.
Our study has some strengths and limitations to consider in interpreting the study results. To our knowledge, this is the largest familial study of cancer to date and includes more than 3 decades of follow-up. In prior studies, the number of concordant pairs with cancer was small, leading to imprecise estimates of familial risk and heritability for common cancer types and uninterpretable estimates of heritability for less common cancer types. The large number of cancer cases and long follow-up in the cohort allowed us to provide more precise estimates of familial risk and heritability for several malignancies.
Not with standing the large size of the cohort and long-term follow-up, we were unable to provide estimates of familial risk or heritability for some of the more rare cancer types, including many of the hematological malignancies. The linkage with national population-based registers and the high quality of cancer case registration allowed for complete follow-up of the study population.
The study is based on twins from the Nordic countries, primarily a white population. For the majority of cancer types, the cumulative incidence of cancer in the twin cohort was similar to that of the entire country, suggesting these data can be generalized to the Nordic countries. However, it is unclear the extent to which these data can be generalized to multiethnic populations.
Given the older age at which many cancer types occur, long-term follow-up provides greater clarity on estimates of familial risk and heritability by allowing the cohort to attain sufficient age at which most cancer types occur. On a related note, some individuals at risk of developing cancer may also be at higher risk of dying from another chronic condition, thus influencing the estimates.
Our statistical approach addressed this challenge and accounted for differential follow-up time, censoring, and competing causes of death. The twin modeling estimates of heritability assume that there are similar shared environments between monozygotic and dizygotic pairs of twins, which cannot be formally tested in this setting.
Conclusions
In this long-term follow-up study among Nordic twins, there was significant excess familial risk for cancer overall and for specific types of cancer, including prostate, melanoma, breast, ovary, and uterus. This information about hereditary risks of cancers may be helpful in patient education and cancer risk counseling.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by funding from the Ellison Foundation (awarded to Drs Mucci and Adami at the Harvard School of Public Health) and the Nordic Cancer Union (awarded to Dr Kaprio). The Finnish Twin Cohort was supported by grants 213506, 129680, 265240, and 263278 from the Academy of Finland and grant agreement HEALTH-F4-2010-261433 from US BioSHaRE-EU. The Swedish Twin Registry was supported by the Ministry for Higher Education. The Danish Twin Cohort was supported by the Odense University Hospital AgeCare program of the Academy of Geriatric Cancer Research. Data collection and research stemming from the Norwegian Twin Registry is supported, in part, from the European Union's Seventh Framework Programme and grant agreement HEALTH-F4-2010-261433 from US BioSHaRE-EU. Dr Mucci is a Prostate Cancer Foundation young investigator. Dr Adami received distinguished professor award 2368/10-221 from the Karolinska Institutet. Dr Graff is supported by National Cancer Institute training grants R25 CA098566 and R25 CA112355.
Role of the Funder/Sponsors: The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation of the manuscript; or decision to submit the manuscript for publication. The sponsors reviewed and approved the manuscript
Footnotes
Author Contributions: Drs Mucci and Hjelmborg had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs Mucci and Hjelmborg contributed equally to this article and share first authorship. Drs Adami and Kaprio contributed equally to this article and share last authorship.
Study concept and design: Mucci, Hjelmborg, Harris, Penney, Koskenvuo, Holm, Pukkala, Kaprio.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Mucci, Hjelmborg, Graff, Holst, Möller, Unger, Nuttall, Brandt, Kaprio.
Critical revision of the manuscript for important intellectual content: Mucci, Hjelmborg, Harris, Czene, Havelick, Scheike, Holst, Möller, Unger, McIntosh, Penney, Hartman, Kraft, Parmigiani, Christensen, Koskenvuo, Holm, Heikkilä, Pukkala, Skytthe, Adami.
Statistical analysis: Mucci, Hjelmborg, Scheike, Holst, Möller, Kraft, Parmigiani, Skytthe, Kaprio.
Obtained funding: Mucci, Harris, Czene, Havelick, Koskenvuo, Holm, Adami, Kaprio.
Administrative, technical, or material support: Hjelmborg, Harris, Havelick, Graff, Unger, Nuttall, Brandt, Christensen, Holm, Heikkilä, Pukkala, Skytthe, Adami.
Study supervision: Mucci, Hjelmborg, Holst, Christensen, Koskenvuo, Pukkala.
Conflict of Interest Disclosures: The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Kaprio reported receiving personal fees from Pfizer for serving as a consultant on nicotine dependence from 2012 to 2014. No other disclosures were reported
Group Information: The following individuals are additional collaborators within the NorTwinCan collaboration: Norwegian Institute of Public Health: Julia Isaeva and Thomas Nilsen. University of Helsinki: Tellervo Korhonen. University of Southern Denmark: Ulrich Halekoh.
Additional Contributions: We are grateful to the participants of the twin registries in Denmark, Finland, Norway, and Sweden. We recognize the key contribution of Kristina Glimsjo (Karolinska Institutet) for editorial assistance. Ms Glimsjo received no compensation for her contribution.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts & Figures 2012. Atlanta, GA: American Cancer Society; 2012. [Google Scholar]
- 3.Coté ML, Liu M, Bonassi S, et al. Increased risk of lung cancer in individuals with a family history of the disease: a pooled analysis from the International Lung Cancer Consortium. Eur J Cancer. 2012;48(13):1957–1968. doi: 10.1016/j.ejca.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheurer ME, Etzel CJ, Liu M, et al. GLIOGENE Consortium. Familial aggregation of glioma: a pooled analysis. Am J Epidemiol. 2010;172(10):1099–1107. doi: 10.1093/aje/kwq261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannon-Albright LA, Thomas A, Goldgar DE, et al. Familiality of cancer in Utah. Cancer Res. 1994;54(9):2378–2385. [PubMed] [Google Scholar]
- 6.Goldgar DE, Easton DF, Cannon-Albright LA, Skolnick MH. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J Natl Cancer Inst. 1994;86(21):1600–1608. doi: 10.1093/jnci/86.21.1600. [DOI] [PubMed] [Google Scholar]
- 7.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343(2):78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 8.Scheike TH, Holst KK, Hjelmborg JB. Estimating heritability for cause specific mortality based on twin studies. Lifetime Data Anal. 2014;20(2):210–233. doi: 10.1007/s10985-013-9244-x. [DOI] [PubMed] [Google Scholar]
- 9.Hjelmborg JB, Scheike T, Holst K, et al. The heritability of prostate cancer in the Nordic Twin Study of Cancer. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2303–2310. doi: 10.1158/1055-9965.EPI-13-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skytthe A, Kyvik KO, Holm V, Christensen K. The Danish Twin Registry. Scand J Public He alth. 2011;39(7) 1:75–78. doi: 10.1177/1403494810387966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaprio J, Koskenvuo M. Genetic and environmental factors in complex diseases: the older Finnish Twin Cohort. Twin Res. 2002;5(5):358–365. doi: 10.1375/136905202320906093. [DOI] [PubMed] [Google Scholar]
- 12.Nilsen TS, Brandt I, Magnus P, Harris JR. The Norwegian Twin Registry. Twin Res Hum Genet. 2012;15(6):775–780. doi: 10.1017/thg.2012.57. [DOI] [PubMed] [Google Scholar]
- 13.Lichtenstein P, Sullivan PF, Cnattingius S, et al. The Swedish Twin Registry in the third millennium: an update. Twin Res Hum Genet. 2006;9(6):875–882. doi: 10.1375/183242706779462444. [DOI] [PubMed] [Google Scholar]
- 14.Lichtenstein P, De Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. J Intern Med. 2002;252(3):184–205. doi: 10.1046/j.1365-2796.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- 15.Christiansen L, Frederiksen H, Schousboe K, et al. Age- and sex-differences in the validity of questionnaire-based zygosity in twins. Twin Res. 2003;6(4):275–278. doi: 10.1375/136905203322296610. [DOI] [PubMed] [Google Scholar]
- 16.Mattsson B, Wallgren A. Completeness of the Swedish Cancer Register: non-notified cancer cases recorded on death certificates in 1978. Acta Radiol Oncol. 1984;23(5):305–313. doi: 10.3109/02841868409136026. [DOI] [PubMed] [Google Scholar]
- 17.Cancer Society of Finland. Cancer Incidence in Finland 2000 and 2001. Helsinki: Cancer Society of Finland; 2000. [Google Scholar]
- 18.Association of the Nordic Cancer Registries. Survey of Nordic Cancer Registries: Technical Report. Copenhagen, Denmark: Danish Cancer Society; 2001. [Google Scholar]
- 19.Barlow L, Westergren K, Holmberg L, Talbäck M. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol. 2009;48(1):27–33. doi: 10.1080/02841860802247664. [DOI] [PubMed] [Google Scholar]
- 20.Engholm G, Ferlay J, Christensen N, et al. NORDCAN—a Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010;49(5):725–736. doi: 10.3109/02841861003782017. [DOI] [PubMed] [Google Scholar]
- 21.Braun MM, Caporaso NE, Page WF, Hoover RN. A cohort study of twins and cancer. Cancer Epidemiol Biomarkers Prev. 1995;4(5):469–473. [PubMed] [Google Scholar]
- 22.Jostins L, Barrett JC. Genetic risk prediction in complex disease. Hum Mol Genet. 2011;20(R2):R182–R188. doi: 10.1093/hmg/ddr378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts NJ, Vogelstein JT, Parmigiani G, Kinzler KW, Vogelstein B, Velculescu VE. The predictive capacity of personal genome sequencing. Sci Transl Med. 2012;4(133):133ra58. doi: 10.1126/scitranslmed.3003380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trichopoulos D. Is breast cancer initiated in utero? Epidemiology. 1990;1(2):95–96. [PubMed] [Google Scholar]
- 25.McGlynn KA, Trabert B. Adolescent and adult risk factors for testicular cancer. Nat Rev Urol. 2012;9(6):339–349. doi: 10.1038/nrurol.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amundadottir LT, Thorvaldsson S, Gudbjartsson DF, et al. Cancer as a complex phenotype: pattern of cancer distribution within and beyond the nuclear family. PLoS Med. 2004;1(3):e65. doi: 10.1371/journal.pmed.0010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng I, Kocarnik JM, Dumitrescu L, et al. Pleiotropic effects of genetic risk variants for other cancers on colorectal cancer risk: PAGE, GECCO and CCFR consortia. Gut. 2014;63(5):800–807. doi: 10.1136/gutjnl-2013-305189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Zhu B, Zhang M, et al. Imputation and subset-based association analysis across different cancer types identifies multiple independent risk loci in the TERT-CLPTM1L region on chromosome 5p15.33. Hum Mol Genet. 2014;23(24):6616–6633. doi: 10.1093/hmg/ddu363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghoussaini M, Fletcher O, Michailidou K, et al. Netherlands Collaborative Group on Hereditary Breast and Ovarian Cancer (HEBON); Familial Breast Cancer Study (FBCS); Gene Environment Interaction of Breast Cancer in Germany (GENICA) Network; kConFab Investigators; Australian Ovarian Cancer Study Group. Genome-wide association analysis identifies three new breast cancer susceptibility loci. Nat Genet. 2012;44(3):312–318. doi: 10.1038/ng.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haiman CA, Chen GK, Vachon CM, et al. Gene Environment Interaction and Breast Cancer in Germany (GENICA) Consortium. A common variant at the TERT-CLPTM1L locus is associated with estrogen receptor-negative breast cancer. Nat Genet. 2011;43(12):1210–1214. doi: 10.1038/ng.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kote-Jarai Z, Olama AA, Giles GG, et al. UK Genetic Prostate Cancer Study Collaborators/British Association of Urological Surgeons' Section of Oncology; UK ProtecT Study Collaborators, The Australian Prostate Cancer BioResource; PRACTICAL Consortium. Seven prostate cancer susceptibility loci identified by a multi-stage genome-wide association study. Nat Gen et. 2011;43(8):785–791. doi: 10.1038/ng.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters U, Hutter CM, Hsu L, et al. Meta-analysis of new genome-wide association studies of colorectal cancer risk. Hum Genet. 2012;131(2):217–234. doi: 10.1007/s00439-011-1055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al Olama AA, Kote-Jarai Z, Berndt SI, et al. Breast and Prostate Cancer Cohort Consortium (BPC3); PRACTICAL (Prostate Cancer Association Group to Investigate Cancer-Associated Alterations in the Genome) Consortium; COGS (Collaborative Oncological Gene-environment Study) Consortium; GAME-ON/ELLIPSE Consortium. A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nat Genet. 2014;46(10):1103–1109. doi: 10.1038/ng.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amin Al Olama A, Dadaev T, Hazelett DJ, et al. PRACTICAL Consortium; COGS-CRUK GWAS-ELLIPSE (Part of GAME-ON) Initiative; Australian Prostate Cancer BioResource; UK Genetic Prostate Cancer Study Collaborators; UK ProtecT Study Collaborators. Multiple novel prostate cancer susceptibility signals identified by fine-mapping of known risk loci among Europeans. Hum Mol Gene t. 2015;24(19):5589–5602. doi: 10.1093/hmg/ddv203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Southey MC, Park DJ, Nguyen-Dumont T, et al. COMPLEXO. COMPLEXO: identifying the missing heritability of breast cancer via next generation collaboration. Breast Cancer Res. 2013;15(3):402. doi: 10.1186/bcr3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sampson JN, Wheeler WA, Yeager M, et al. Analysis of heritability and shared heritability based on genome-wide association studies for thirteen cancer types. J Natl Cancer Inst. 2015;107(12):djv279. doi: 10.1093/jnci/djv279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li WQ, Pfeiffer RM, Hyland PL, et al. Genetic polymorphisms in the 9p21 region associated with risk of multiple cancers. Carcinogenesis. 2014;35(12):2698–2705. doi: 10.1093/carcin/bgu203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bahcall OG. iCOGS collection provides a collaborative model: foreword. Nat Genet. 2013;45(4):343–347. doi: 10.1038/ng.2592. [DOI] [PubMed] [Google Scholar]
- 39.Zuk O, Hechter E, Sunyaev SR, Lander ES. The mystery of missing heritability: genetic interactions create phantom heritability. Proc Natl Acad Sci U S A. 2012;109(4):1193–1198. doi: 10.1073/pnas.1119675109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.