Abstract
Background
Rifampin-soaked synthetic prosthetic grafts have been widely used for prevention or treatment of vascular graft infections (VGIs).
This in vitro study investigated the effect of the antibiotics daptomycin and vancomycin and the new recombinant bacteriophage endolysin HY-133 on vascular cells, as potential alternatives compared to rifampin.
Material/Methods
Primary human ECs, vascular smooth muscle cells (vSMC), and fibroblasts were cultivated in 96-well plates and incubated with rifampin, daptomycin, vancomycin, and endolysin HY-133 for 24 h. Subsequently, after washing, cell viability was determined by measuring mitochondrial ATP concentration. Antibiotics were used in their corresponding minimum and maximum serum concentrations, in decimal multiples and in maximum soaking concentration. The experiments were performed in triplicate.
Results
The 10-fold max serum concentrations of rifampin, daptomycin, and vancomycin did not influence viability of EC and vSMC (100 μg/ml, p>0.170). Higher concentrations of rifampin (>1 mg/ml) significantly (p<0.001) reduced cell viability of all cell types. For the other antibiotics, high concentrations (close to maximum soaking concentration) were most cytotoxic for EC and vSMC and fibroblasts (p<0.001). Endolysin did not display any cytotoxicity towards vascular cells.
Conclusions
Results of this in vitro study show the high cytotoxicity of rifampin against vascular cells, and may re-initiate the discussion about the benefit of prophylactic pre-soaking in high concentrations of rifampin. Further studies are necessary to determine the influence of rifampin on the restoration of vessel functionality versus its prophylactic effect against VGIs. Future use of recombinant phage endolysins for alternative prophylactic strategies needs further investigations.
MeSH Keywords: Bacterial Infections; Endothelial Cells; Myocytes, Smooth Muscle; Rifampin; Vascular Grafting
Background
Vascular grafts are used in aortic reconstructions and in default of autologous material in peripheral bypass operations. The most commonly used polymers for the production of vascular grafts are polyethylene terephthalate (“Dacron™”), polytetrafluoroethylene ethylene (e PTFE, “Teflon™”), and polyurethane (PU), with acceptable biocompatibility to replace vessels of large and medium diameter [1,2]. For bypass materials, autologous veins remain, however, superior in terms of primary patency, having the most optimal biological properties in terms of tissue acceptance [3,4]. Homografts (cryopreserved arterial allografts) are also an alternative in the treatment of infected grafts [5–7] as well as xenogenic pericardial patches [8].
Successful graft implantation requires prosthetic vascular graft healing, which is a multicellular process requiring the coordination of host immune cells activity, migration, infiltration, proliferation, and differentiation of endothelial cells (ECs), smooth muscle cells (SMCs), and their progenitors. Negative effects of exogenous factors (bacterial infection, nutrition deficiencies, and stresses) lead to synthetic graft failure due to thrombosis, anastomotic hyperplasia, and vascular graft infection (VGI), respectively [9].
Vascular graft infection occurs in 0.4–3% of cases and is associated with high morbidity and high mortality rates (10–75%) [5,10,11]. Aortic endograft infection can be eradicated by excision and in situ or extra-anatomic replacement, but is often associated with early postoperative morbidity and mortality (35%) and occasionally with a need for late removal for reinfection [5]. Prosthetic graft replacement after explanation is associated with higher reinfection and graft-related complications and decreased survival compared with autogenous reconstruction [5].
Considering that Gram-positive microorganisms typically cause aortic graft infections, pre-soaking of the graft with antibiotics seemed an ideal solution for the prevention and treatment of VGIs [11,12].
Numerous antibiotic agents (e.g., rifampin, daptomycin, and vancomycin) have been tested in vitro and in preclinical studies as potential candidates, with conflicting results [13–15]. Considering also the limitation of antibiotic resistance, bacteriophage-based enzymes, so called endolysins, have been also suggested as potential non-antibiotic impregnation agents [16]. Endolysins are phage-encoded peptidoglycan hydrolases employed by the majority of bacteriophages to enzymatically degrade the peptidoglycan layer of the host bacterium [17]. They are considered a potential new class of antimicrobial agents which could be used both for nasal decolonization of methicillin-resistant Staphylococcus aureus (MRSA) [18,19] and invasive infections, as animal models have revealed considerably reduced bacterial numbers by endolysins in murine spleens and/or protected systemically infected animals from death [17,20].
At present, common practice is the use of rifampin, due to its well-known anti-staphylococcal activity [10]. Several randomized studies assessed the efficacy of rifampin-soaked grafts to prevent VGIs after aorto-iliofemoral reconstruction, without showing any beneficial effect of the pre-soaking in terms of lower reinfection rates after 2 years [21]. While the concentration of rifampin (1 mg/ml) in these studies was low, there are several case series of graft replacement after graft infections revealing acceptable reinfection rates in which high concentrations of rifampin (60 mg/ml) were used for graft pre-soaking [12].
However, in vitro studies and animal studies revealed high cytotoxic effects on endothelial cells by concentrated rifampin solutions [13,22].
To our best knowledge, there are no data about the cytotoxic effects of the aforementioned antibiotics used for graft impregnation as well as for the bacteriophage endolysin antimicrobial effects. To inform the debate, we investigated the effect of the antibiotics daptomycin, vancomycin and the novel bacteriophage endolysin HY-133 on vascular cells, as potential impregnation alternatives compared to rifampin.
Material and Methods
Cell Culture
Primary human endothelial cells (HUVEC) were obtained from the veins of human umbilical cords of 3 healthy donors, as described before [23]. The experimental design was approved by the Ethics Committee of the Faculty of Medicine, University of Muenster. Written informed consent was obtained from all donors. Cells were cultivated in endothelial cell medium (C2210, PromoCell, Heidelberg, Germany) with the addition of 100 U/ml penicillin and 100 μg/ml streptavidin (both from Biochrom, Berlin, Germany).
Vascular smooth muscle cells (vSMC) from the coronary artery were purchased (Lonza, Cologne, Germany) and cultured in DMEM with 4.5 g/l glucose, 10% fetal bovine serum, 2 mM L-glutamine, 1 mM sodium-pyruvate, 100 U/ml penicillin, and 100 μg/ml streptavidin (Biochrom).
Fibroblasts (CCD-32Sk) were acquired from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultivated the same as vSMCs.
Immediately before reaching a confluent monolayer, cells were detached with trypsin (Biochrom). For the experiment, cells of the 2nd and 3rd passage were seeded onto 96-well plates at a cell density of 1×105 cells/cm2.
Experiment set-up
After an attachment period of 24 h, cells were incubated with different concentrations of rifampin (Eremfat®, Riemser Pharma, Greifswald, Germany), daptomycin (Cubicin®, Novartis Pharma, Nürnberg, Germany), vancomycin (Vancomycin CP, Hikma Pharmaceuticals, Terrace, Portugal), and the recombinant bacteriophage endolysin HY-133 (Hyglos GmbH, Bernried, Germany).
The antibiotics and endolysin were reconstituted in DMEM medium. Since daptomycin decreased the pH of medium, 10 mM HEPES buffer was added. Antibiotic concentrations were used in their corresponding minimum (MIN) and maximum (MAX) serum concentration, in decimal multiples and in the maximum soaking concentration of the graft in vivo, which represents the upper limit to allow the substance powder to reconstitute into solution. Additionally, at all time points, a negative control, consisting of the medium without the test compound (antibiotics / endolysin), was included. All concentrations used are listed in Table 1. HY-133 is a recombinant bacteriophage endolysin against S. aureus, which is under development and represents a further development of the recombinant bacteriophage endolysin PRF-119. The in vitro activity of PRF-119 has been reported elsewhere in detail [24]. The minimum inhibitory concentration (MIC90) of HY-133 for S. aureus was demonstrated to be 0.5 μg/ml [25]. Since this substance is not yet available as powder or as a commercial formulation for human use, there are no data on human serum concentrations of HY-133. Instead, we used HY-133 in the following concentrations: MIC90, 2×MIC90, 20×MIC90, and 100×MIC90.
Table 1.
Minimum and maximum serum concentrations of antibiotics used and MIC90 of HY-133 [24,25,54]. For each concentration and cell type, at least n=4 wells were chosen. The experiment was repeated 3 times.
| Antibiotics | MIC90 [μg/ml] | Minimum serum conc. (Min) [μg/ml] | Maximum serum conc. (Max) [μg/ml] | Max. soaking conc. [mg/ml] |
|---|---|---|---|---|
| Rifampin | 0.03 | 0.1–1.0 | 4–10 | 60 |
| Daptomycin | 1 | 4–7 | 55–60 | 100 |
| Vancomycin | 2 | 7–10 | 18–25 | 50 |
| Endolysin HY-133 | MIC90 [μg/ml] | 2×MIC90 [μg/ml] | 20×MIC90 [μg/ml] | 10×MIC90 [μg/ml] |
| Endolysin HY-133 | 0.5 | 1 | 10 | 50 |
For each concentration (including a negative control without any antibiotic/endolysin) and cell type, at least n=4 wells were chosen. The experiment was repeated 3 times.
After 24-h incubation in the solutions listed in Table 1, cells were washed with phosphate-buffered saline (PBS, Biochrom) and cell viability was determined as described below.
Cell viability measurement
Cell viability was analyzed by measuring the mitochondrial adenosine triphosphate (ATP) concentration using the CellTiter-Glo® Luminescence assay (Promega, Mannheim, Germany), as described before [26]. The number of viable cells is quantified by their amount of ATP and is directly proportional to the ATP concentration. In brief, after washing, 100 μl PBS and 100 μl Cell-TiterGlo® solution was added to each well. After an incubation period of 5 min at room temperature, the luminescent signal was recorded using a multilabel plate reader. Additionally, standard measurements with defined numbers of ECs, vSMCs, and fibroblasts were performed, and ATP standard curves were produced with defined ATP-concentrations (0.1–50 μM). The ATP measurement accuracy revealed an inter-assay-variance of 2.1 and an intra-assay variance of 8.1.
Osmolarity and pH measurement of antibiotics
In order to exclude any cytotoxic effect of the antibiotics solution due to non-physiological osmolarity or pH values, both parameters were determined before incubation of the cells.
Osmolarity of the antibiotics solutions was within the upper physiological range of the respective DMEM medium of 335±5.6 mosmol/l. The values listed for the respective antibiotics: rifampin 339±11.3 mosmol/l, vancomycin 337±7.4 mosmol/l, daptomycin 343±24.9 mosmol/l, and endolysin 336±5.3 mosmol/l (OsmoStation OM-6050, Menarini Diagnostics, Wien, Austria). The pH values were within the physiological range of 7.15–7.45 (pH meter Five Easy FE20, Mettler Toledo, Schwerzenbach, Switzerland).
Statistical analysis
The statistical analysis was performed using commercially available software (SPSS 22.0, 2013, Chicago, IL; USA). Measurement accuracy was calculated as coefficient of variability (CV) by estimating inter- and intra-assay variance. Continuous variables are presented as mean ±SD, median, and range. Before statistical testing, each continuous variable was analyzed exploratively for normal distribution (Kolmogorov-Smirnov test). The Mann-Whitney U test was used for comparison of non-parametric variables between study groups. Differences were considered significant at P<.05.
Results
Influence of rifampin
Rifampin concentrations up to the maximum serum concentration (MAX) of 10 μg/ml did not influence cell viability of endothelial cells. EC viability was decreased significantly to 44% and 20% by 10-fold and 100-fold MAX (100 and 1000 μg/ml, respectively; p≤0.001), while higher concentrations up to the maximum soaking concentration were absolutely cytotoxic (p≤0.001) (Figure 1).
Figure 1.
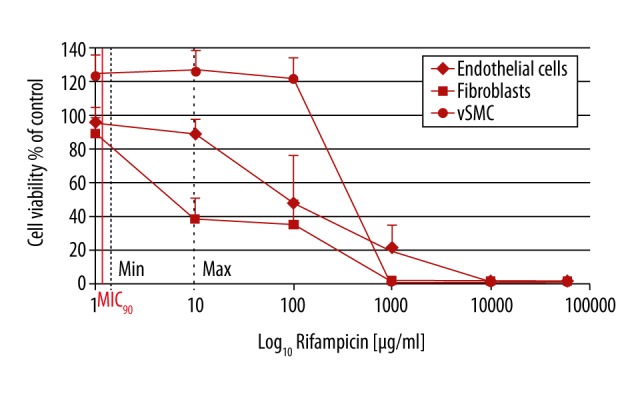
Influence of rifampin on vascular cells: EC (endothelial cells), vSMC (vascular smooth muscle cells), and fibroblasts. Cell viability of ECs and fibroblasts started to decrease at max serum concentrations of rifampin, while for SMC it occurred at 10-fold higher rifampin values. No cell survival of SMCs and fibroblasts was detected with 1000 μg/ml rifampin; the threshold was 10-fold higher for ECs. MIC90 – minimum inhibitory concentration, Min – minimum serum concentration, Max – maximum serum concentration. For each concentration and cell type, at least n= 4 wells were chosen. The experiment was repeated 3 times. Mean intra-assay coefficient of variability (CV) was 2.00±0.89.
In smooth muscle cells, viability was increased significantly (p≤0.001) with rifampin concentrations up to the 10-fold MAX (100 μg/ml) while higher concentrations up to the maximal soaking concentrations of 60 mg/ml were also maximally cytotoxic for SMCs (p≤0.001) (Figure 1).
In the presence of rifampin in its minimum serum concentration of 1 μg/ml, fibroblast viability was not influenced significantly, but concentrations of MAX and 10-fold MAX decreased fibroblast viability to 38% and 35%, respectively (p≤0.010). Higher concentrations and the maximum soaking concentrations of 60 mg/ml were also maximally cytotoxic for fibroblasts (p≤0.001) (Figure 1).
Influence of vancomycin
Vancomycin concentrations of 10 to 100 μg/ml slightly increased EC viability significantly up to 130% of control (p<0.001) while it decreased viability of SMC and fibroblasts to about 83% of the control (p≤0.003). At concentrations of 1000 μg/ml, EC viability was comparable to the controls, but SMC and fibroblast viability dropped to 52% and 45%, respectively, and were significantly lower (p≤0.003). In the maximum soaking concentration, vancomycin was maximally cytotoxic for all 3 cell types (p≤0.001) (Figure 2).
Figure 2.

Influence of vancomycin on vascular cells. Cell viability of ECs, fibroblasts, and SMCs was not affected up to a vancomycin concentration of 100 μg/ml. Compared to the negative control and to the other cell types, ECs displayed higher viability in the presence of vancomycin. We found no survival of the 3 cell types at 100 000 μg/ml vancomycin. MIC90 – minimum inhibitory concentration, Min – minimum serum concentration, Max – maximum serum concentration. For each concentration and cell type, at least n=4 wells were chosen. The experiment was repeated 3 times. Mean intra-assay coefficient of variability (CV) was 1.31±0.76.
Influence of daptomycin
All daptomycin concentrations reduced EC viability significantly (p≤0.001), even at the minimum serum concentration of 1 μg/ml. Fibroblast viability started to decrease at concentrations above the MAX: at 100 μg/ml only 80% of the cells were viable, at 1000 μg/ml 75%, and at 10 000 μg/ml only 51% (p≤0.001). At daptomycin concentrations of ≤100 μg/ml, SMC viability was even increased compared to the controls. The maximum soaking concentration of 100 mg/ml was maximally cytotoxic for all 3 cell types (p≤0.001) (Figure 3).
Figure 3.
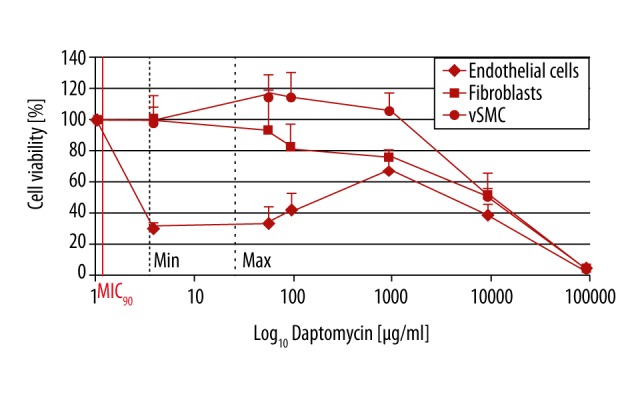
Influence of daptomycin on vascular cells. Cell viability of ECs already decreased with daptomycin concentration at MIC90. Viability of fibroblast and SMC was not affected up to 100 μg/ml daptomycin and decreased with higher concentrations. No survival of the 3 cell types could be detected at 100 000 μg/ml daptomycin. MIC90 – minimum inhibitory concentration, Min – minimum serum concentration, Max – maximum serum concentration. For each concentration and cell type, at least n=4 wells were chosen. The experiment was repeated 3 times. Mean intra-assay coefficient of variability (CV) was 2.01±0.65.
Influence of endolysin HY-133
Vascular cell viability was not decreased by endolysin concentrations of 0.5–10 μg/ml. Instead, at concentrations of 0.5 μg/ml, EC viability was increased (p=0.020). At higher endolysin concentrations (50 μg/ml), viability of ECs and SMCs was slightly decreased (p=0.025 and p=0.002, respectively), while viability of fibroblasts was comparable to the control (Figure 4).
Figure 4.
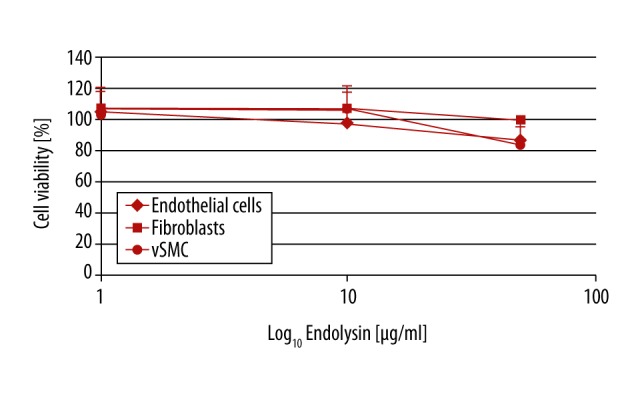
Influence of endolysin on vascular cells. Cell viability of all 3 cell types was not affected up to 100×MIC90 (50 μg/ml) endolysin. MIC90 – minimum inhibitory concentration. For each concentration and cell type, at least n=4 wells were chosen. The experiment was repeated 3 times. Mean intra-assay coefficient of variability (CV) was 1.79±0.51.
Discussion
The in vitro results of our study show the high cytotoxicity of rifampin against vascular cells already at a 10-fold maximum serum concentration. However, vancomycin and daptomycin also revealed cytotoxic effects at comparable concentrations. In summary, all antibiotics were 100% cytotoxic at their maximum re-suspending concentrations. No considerable cytotoxic effects were detected with endolysin HY-133, although very high concentrations could not be investigated.
The efficacy of the bacteriophage endolysin to remove the biofilms formed was demonstrated in several experiments [17]. Low concentrations of the endolysin LysH5 (0.15 mM) were effective to decrease viable bacteria inside biofilms formed by Staphylococcus aureus and Staphylococcus epidermidis strains. LysH5 reduced staphylococcal sessile cell counts by 1–3 log units compared with the untreated control, and sub-inhibitory concentrations of this protein did not induce biofilm formation. The results demonstrated that besides the notable activity of endolysin LysH5 against staphylococcal biofilms, persister cells were also inhibited, which raises new opportunities as an adjuvant for some antibiotics [27].
The first evidence of the potential toxicity of high rifampin concentrations for endothelial cells was provided by the use of rifampin-soaked grafts in cell culture flasks; however, cell density and actual rifampin concentration used were not defined [13]. According to the present investigation, the threshold of cell toxic rifampin concentrations seems to be the 10- to 100-fold MAX-concentration for all types of vascular cells: EC, SMC, and fibroblasts.
Preclinical experiments in a dog model with methicillin-resistant Staphylococcus aureus (MRSA) and Escherichia coli graft infections suggested that the observed necrosis at the anastomoses in 25% of grafts soaked could have been caused by rifampin toxicity against ECs [22], as the histological evaluation of the anastomoses revealed that the necrosis was not due to bacterial colonization [22].
The toxicity of vancomycin was shown in early in vitro experiments, in which release of lactate dehydrogenase (LDH) was used as a measure of cell damage. LDH concentration increased with rising vancomycin concentrations [28], but after 12-h incubation with vancomycin concentrations of 10 to 1000 μg/ml, the number of viable cells decreased slightly, and higher vancomycin concentrations of 10 000 μg/ml led to a cell survival rate of only 14.4% [28].
The first clinical indications of cytotoxicity of vancomycin were discovered after paravasal injection of vancomycin, which caused profound tissue necrosis [29]. Furthermore, vancomycin is known to cause renal toxicity, which complicates and limits clinical applications of this antimicrobial agent [30,31]. Therapeutic drug monitoring is recommended for patients receiving vancomycin to ensure its safe and effective use [32]. However, there is a lack of data on systemic exposure to vancomycin in case of vascular graft impregnation with high concentrations of this drug. In addition, tissue concentrations of vancomycin and other antibiotics, which can be achieved after graft impregnation, are insufficiently studied.
In 2000, Earnshaw suggested 3 main indications for use of the rifampin- impregnated graft: as a routine prophylaxis against infection whenever prosthetic material is inserted; selectively in high-risk situations (such as the insertion of a prosthetic graft through potentially infected sites); and as in situ replacement of established graft infection [21].
There have been 3 RCTs from 1991 to 1998 – an Italian study with 600 patients [33], a European study with 2400 patients [34], and a Joint Vascular Research Group study with 155 patients [35,36] – exploring the effect of prophylactic pre-soaking of synthetic prosthesis with rifampin on graft infection after prosthetic aorto-iliofemoral replacement. In all of these RCTs, a rifampin soaking concentration of 1 mg/ml was used. Although there was a positive influence of rifampin pre-soaking on early wound infections, with significantly lower infection rates at 3 months in 1 study (3.2% vs. 5.0% in R+ vs. R− patients, respectively), there were no significant advantages for rifampin bonding at 12 and 24 months [36,37].
Animal experiments with ovine carotid grafts infected with 108 CFU S. epidermidis or MRSA S. aureus revealed that higher rifampin concentrations of 10 mg/ml were preferable to concentrations of 1.2 mg/ml only for S. epidermidis, while there were no differences between the groups for MRSA S. aureus [38].
Adsorptive bondage of rifampicin (13 mg/ml) to collagen-impregnated Dacron grafts had antibacterial activity lasting 3 weeks in vitro on blood agar plates inoculated with 107 CFU/ml S. aureus [39]. In vivo, rifampin protected Dacron grafts from bacterial infection of 1×108 CFU of S. aureus up to 7 days after implantation [40] or with low-dose 1×103 CFU of MRSA S. aureus [41].
For in situ replacement of established graft infections, numerous case series with a total of more than 150 patients with graft infections reported low reinfection rates with synthetic grafts pre-soaked in high concentrations of rifampin (60 mg/ml) [12,37,42–44]. Adjuvant modalities like omental or muscle flap wrapping were often used [45].
Many case series with infected vascular grafts and essential graft replacement with synthetic grafts pre-soaked with high concentrations of rifampin had low reinfection rates [12,42]. Therefore, 2 of the main indications for use of rifampin-impregnated grafts mentioned – high-risk situations and replacement after established infection – seem to be recommendable and inevitable. However, the in vitro results of the present study revealed a high cytotoxicity of rifampin against vascular cells. The use of rifampin as prophylaxis whenever prosthetic material is used (Earnshaw’s first indication for rifampin) should be re-assessed. At present, no recommendation for any indication of prophylactic use of rifampin can be provided due to the lack of any evidence about its efficacy [46].
Vancomycin and daptomycin were used as additional antibiotics in our study. Both antimicrobial agents are broadly used for systemic treatment of vascular graft infections [47,48]. Furthermore, both antibiotics were suggested for preventing graft infections by their local release, including impregnation and soaking techniques [13,15,49–52]. We therefore considered it worthwhile to include vancomycin and daptomycin in our comprehensive evaluation.
A limitation of our study is the use of a statical in vitro model containing an antibiotic-dosage for 24 h. For example, in vivo, rifampin would be released more continuously over 3 days with a peak dosage in the first hours (half-life in serum is 3 h) [53]. Hypothetically, after application of the peak dosage, it would take more than 36 h for the residual rifampin concentration to decrease below the MAX serum concentration. Another issue is that an in vitro model cannot represent the vascular cell-cell and the vascular tissue interaction; therefore, the impact of antibiotics in high concentrations on the tissue can only be speculated.
Conclusions
The in vitro results of the present study display high cytotoxicity of rifampin against vascular cells and may re-initiate the discussion about the advantages of prophylactic pre-soaking with high concentrations of rifampin. Further in vivo studies are necessary to determine the influence of rifampin on the restoration of vessel functionality also including long-term patency, especially in small caliber prosthesis versus its preventive character for graft infections. The absence of serious cytotoxic effects by HY-133 warrants further investigations on the use of recombinant phage endolysin as an alternative prophylactic strategy.
Acknowledgement
We thank Ms. H. Segbert from the Department of Cranio-Maxillofacial Surgery for culturing of endothelial cells and Dr. M. Fobker from the Center of Laboratory Medicine for the osmolarity measurements, both from University Hospital Muenster, Germany.
Footnotes
Source of support: This work was supported by the fund “Innovative Medical Research“ of the University Münster Medical School (ID 111317) and in part by the BMBF-DZIF (German Center for Infection Research, TTU 08.807)
Conflict of interest
None.
References
- 1.Rychlik IJ, Davey P, Murphy J, O’Donnell ME. A meta-analysis to compare Dacron versus polytetrafluroethylene grafts for above-knee femoropopliteal artery bypass. J Vasc Surg. 2014;60:506–15. doi: 10.1016/j.jvs.2014.05.049. [DOI] [PubMed] [Google Scholar]
- 2.Zilla P, Bezuidenhout D, Human P. Prosthetic vascular grafts: wrong models, wrong questions and no healing. Biomaterials. 2007;28:5009–27. doi: 10.1016/j.biomaterials.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Dorigo W, Pulli R, Piffaretti G, et al. Results from an Italian multicentric registry comparing heparin-bonded ePTFE graft and autologous saphenous vein in below-knee femoro-popliteal bypasses. J Cardiovasc Surg. 2012;53:187–94. [PubMed] [Google Scholar]
- 4.Neville RF, Capone A, Amdur R, et al. A comparison of tibial artery bypass performed with heparin-bonded expanded polytetrafluoroethylene and great saphenous vein to treat critical limb ischemia. J Vasc Surg. 2012;56:1008–14. doi: 10.1016/j.jvs.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Chaufour X, Gaudric J, Goueffic Y, et al. A multicenter experience with infected abdominal aortic endograft explantation. J Vasc Surg. 2017;65:372–80. doi: 10.1016/j.jvs.2016.07.126. [DOI] [PubMed] [Google Scholar]
- 6.Smeds MR, Duncan AA, Harlander-Locke MP, et al. Treatment and outcomes of aortic endograft infection. J Vasc Surg. 2016;63:332–40. doi: 10.1016/j.jvs.2015.08.113. [DOI] [PubMed] [Google Scholar]
- 7.Bisdas T, Bredt M, Pichlmaier M, et al. Eight-year experience with cryopreserved arterial homografts for the in situ reconstruction of abdominal aortic infections. J Vasc Surg. 2010;52:323–30. doi: 10.1016/j.jvs.2010.02.277. [DOI] [PubMed] [Google Scholar]
- 8.McMillan WD, Leville CD, Hile CN. Bovine pericardial patch repair in infected fields. J Vasc Surg. 2012;55:1712–15. doi: 10.1016/j.jvs.2011.11.139. [DOI] [PubMed] [Google Scholar]
- 9.Shikanov A, Zhang Z, Xu M, et al. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng Part A. 2011;17:3095–104. doi: 10.1089/ten.tea.2011.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fatima J, Duncan AA, de Grandis E, et al. Treatment strategies and outcomes in patients with infected aortic endografts. J Vasc Surg. 2013;58:371–79. doi: 10.1016/j.jvs.2013.01.047. [DOI] [PubMed] [Google Scholar]
- 11.Erb S, Sidler JA, Elzi L, et al. Surgical and antimicrobial treatment of prosthetic vascular graft infections at different surgical sites: a retrospective study of treatment outcomes. PLoS One. 2014;9:e112947. doi: 10.1371/journal.pone.0112947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lew W, Moore W. Antibiotic-impregnated grafts for aortic reconstruction. Semin Vasc Surg. 2011;24:211–19. doi: 10.1053/j.semvascsurg.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Bisdas T, Beckmann E, Marsch G, et al. Prevention of vascular graft infections with antibiotic graft impregnation prior to implantation: In vitro comparison between daptomycin, rifampin and nebacetin. Eur J Vasc Endovasc Surg. 2012;43:448–56. doi: 10.1016/j.ejvs.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 14.Atahan E, Gul M, Ergun Y, Eroglu E. Vascular graft infection by Staphylococcus aureus: Efficacy of cefazolin, teicoplanin and vancomycin prophylaxis protocols in a rat model. Eur J Vasc Endovasc Surg. 2007;34:182–87. doi: 10.1016/j.ejvs.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Cirioni O, Mocchegiani F, Ghiselli R, et al. Daptomycin and rifampin alone and in combination prevent vascular graft biofilm formation and emergence of antibiotic resistance in a subcutaneous rat pouch model of staphylococcal infection. Eur J Vasc Endovasc Surg. 2010;40:817–22. doi: 10.1016/j.ejvs.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Bisdas T, Bagaev E, Burgwitz K, et al. In-vitro-Effektivität der Imprägnierung von Gefäß prothesen mit keimspezifischen Bakteriophagen für die Prävention von Protheseninfektionen. Gefässchirurgie. 2011;16:387–94. [in German] [Google Scholar]
- 17.Schmelcher M, Donovan DM, Loessner MJ. Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 2012;7:1147–71. doi: 10.2217/fmb.12.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenton M, Casey PG, Hill C, et al. The truncated phage lysin CHAP(k) eliminates Staphylococcus aureus in the nares of mice. Bioeng Bugs. 2010;1:404–7. doi: 10.4161/bbug.1.6.13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta R, Prasad Y. P-27/HP endolysin as antibacterial agent for antibiotic resistant Staphylococcus aureus of human infections. Curr Microbiol. 2011;63:39–45. doi: 10.1007/s00284-011-9939-8. [DOI] [PubMed] [Google Scholar]
- 20.Gu J, Xu W, Lei L, et al. LysGH15, a novel bacteriophage lysin, protects a murine bacteremia model efficiently against lethal methicillin-resistant Staphylococcus aureus infection. J Clin Microbiol. 2011;49:111–17. doi: 10.1128/JCM.01144-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Earnshaw JJ. The current role of rifampicin-impregnated grafts: Pragmatism versus science. Eur J Vasc Endovasc Surg. 2000;20:409–12. doi: 10.1053/ejvs.2000.1197. [DOI] [PubMed] [Google Scholar]
- 22.Schneider F, O’Connor S, Becquemin JP. Efficacy of collagen silver-coated polyester and rifampin-soaked vascular grafts to resist infection from MRSA and Escherichia coli in a dog model. Ann Vasc Surg. 2008;22:815–21. doi: 10.1016/j.avsg.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Annussek T, Szuwart T, Kleinheinz J, et al. In vitro inhibition of HUVECs by low dose methotrexate – insights into oral adverse events. Head Face Med. 2014;10:19–25. doi: 10.1186/1746-160X-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Idelevich EA, von Eiff C, Friedrich AW, et al. In vitro activity against Staphylococcus aureus of a novel antimicrobial agent, PRF-119, a recombinant chimeric bacteriophage endolysin. Antimicrob Agents Chemother. 2011;55:4416–19. doi: 10.1128/AAC.00217-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Idelevich EA, Schaumburg F, Knaack D, et al. The recombinant bacteriophage endolysin HY-133 exhibits in vitro activity against different african clonal lineages of the Staphylococcus aureus complex, including Staphylococcus schweitzeri. Antimicrob Agents Chemother. 2016;60:2551–53. doi: 10.1128/AAC.02859-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ickert I, Herten M, Vogl M, et al. Opioids as an alternative to amide-type local anaesthetics for intra-articular application. Knee Surg Sports Traumatol Arthrosc. 2015;23:2674–81. doi: 10.1007/s00167-014-2989-2. [DOI] [PubMed] [Google Scholar]
- 27.Gutierrez D, Ruas-Madiedo P, Martinez B, et al. Effective removal of staphylococcal biofilms by the endolysin LysH5. PLoS One. 2014;9:e107307. doi: 10.1371/journal.pone.0107307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muhl E, Gatermann S, Iven H, et al. Local application of vancomycin for prophylaxis of graft infection: release of vancomycin from antibiotic-bonded Dacron grafts, toxicity in endothelial cell culture, and efficacy against graft infection in an animal model. Ann Vasc Surg. 1996;10:244–53. doi: 10.1007/BF02001890. [DOI] [PubMed] [Google Scholar]
- 29.Simon C, Stille W. Antibiotika-Therapie in Klinik und Praxis. Stuttgart: Schattauer; 1989. pp. 390–93. [in German] [Google Scholar]
- 30.Bamgbola O. Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab. 2016;7:136–47. doi: 10.1177/2042018816638223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stefano GB, Samuel J, Kream RM. Antibiotics may trigger mitochondrial dysfunction inducing psychiatric disorders. Med Sci Monit. 2017;23:101–6. doi: 10.12659/MSM.899478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66:82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 33.D’Addato M, Curti T, Freyrie A. Prophylaxis of graft infection with rifampicin-bonded Gelseal graft: 2-year follow-up of a prospective clinical trial. Italian Investigators Group. Cardiovasc Surg. 1996;4:200–4. doi: 10.1016/0967-2109(96)82315-5. [DOI] [PubMed] [Google Scholar]
- 34.D’Addato M, Curti T, Freyrie A. The rifampicin-bonded gelseal graft. Eur J Vasc Endovasc Surg. 1997;14(Suppl A):15–17. doi: 10.1016/s1078-5884(97)80146-3. [DOI] [PubMed] [Google Scholar]
- 35.Braithwaite BD, Davies B, Heather BP, Earnshaw JJ. Early results of a randomized trial of rifampicin-bonded Dacron grafts for extra-anatomic vascular reconstruction. Joint Vascular Research Group. Br J Surg. 1998;85:1378–81. doi: 10.1046/j.1365-2168.1998.00878.x. [DOI] [PubMed] [Google Scholar]
- 36.Earnshaw JJ, Whitman B, Heather BP. Two-year results of a randomized controlled trial of rifampicin-bonded extra-anatomic dacron grafts. Br J Surg. 2000;87:758–59. doi: 10.1046/j.1365-2168.2000.01490.x. [DOI] [PubMed] [Google Scholar]
- 37.Goeau-Brissonniere O, Javerliat I, Koskas F, et al. Rifampin-bonded vascular grafts and postoperative infections. Ann Vasc Surg. 2011;25:134–42. doi: 10.1016/j.avsg.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Vicaretti M, Hawthorne W, Ao PY, Fletcher JP. Does in situ replacement of a staphylococcal infected vascular graft with a rifampicin impregnated gelatin sealed Dacron graft reduce the incidence of subsequent infection? Int Angiol. 2000;19:158–65. [PubMed] [Google Scholar]
- 39.Chervu A, Moore WS, Chvapil M, Henderson T. Efficacy and duration of antistaphylococcal activity comparing three antibiotics bonded to Dacron vascular grafts with a collagen release system. J Vasc Surg. 1991;13:897–901. doi: 10.1067/mva.1991.27349. [DOI] [PubMed] [Google Scholar]
- 40.Chervu A, Moore WS, Gelabert HA, et al. Prevention of graft infection by use of prostheses bonded with a rifampin/collagen release system. J Vasc Surg. 1991;14:521–24. discussion 524–25. [PubMed] [Google Scholar]
- 41.Javerliat I, Goeau-Brissonniere O, Sivadon-Tardy V, et al. Prevention of Staphylococcus aureus graft infection by a new gelatin-sealed vascular graft prebonded with antibiotics. J Vasc Surg. 2007;46:1026–31. doi: 10.1016/j.jvs.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 42.Bandyk DF, Novotney ML, Johnson BL, et al. Use of rifampin-soaked gelatin-sealed polyester grafts for in situ treatment of primary aortic and vascular prosthetic infections. J Surg Res. 2001;95:44–49. doi: 10.1006/jsre.2000.6035. [DOI] [PubMed] [Google Scholar]
- 43.Young RM, Cherry KJ, Jr, Davis PM, et al. The results of in situ prosthetic replacement for infected aortic grafts. Am J Surg. 1999;178:136–40. doi: 10.1016/s0002-9610(99)00146-4. [DOI] [PubMed] [Google Scholar]
- 44.Hayes PD, Nasim A, London NJ, et al. In situ replacement of infected aortic grafts with rifampicin-bonded prostheses: The Leicester experience (1992 to 1998) J Vasc Surg. 1999;30:92–98. doi: 10.1016/s0741-5214(99)70180-1. [DOI] [PubMed] [Google Scholar]
- 45.Oderich GS, Bower TC, Hofer J, et al. In situ rifampin-soaked grafts with omental coverage and antibiotic suppression are durable with low reinfection rates in patients with aortic graft enteric erosion or fistula. J Vasc Surg. 2011;53:99–106. 107 e1–7. doi: 10.1016/j.jvs.2010.08.018. discussion 106–7. [DOI] [PubMed] [Google Scholar]
- 46.Zegelman M, Assadian O, Guenther G, et al. Guidelines on infections in vascular surgery of the German Society for Vascular Surgery and Vascular Medicine. Current thoughts and comments. Gefässchirurgie. 2012;17:29–36. [Google Scholar]
- 47.Legout L, D’Elia P, Sarraz-Bournet B, et al. Tolerability of high doses of Daptomycin in the treatment of prosthetic vascular graft infection: A retrospective study. Infect Dis Ther. 2014;3:215–23. doi: 10.1007/s40121-014-0035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Revest M, Camou F, Senneville E, et al. Medical treatment of prosthetic vascular graft infections: Review of the literature and proposals of a Working Group. Int J Antimicrob Agents. 2015;46:254–65. doi: 10.1016/j.ijantimicag.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 49.Kuehn C, Graf K, Mashaqi B, et al. Prevention of early vascular graft infection using regional antibiotic release. J Surg Res. 2010;164:e185–91. doi: 10.1016/j.jss.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 50.Liu KS, Lee CH, Wang YC, Liu SJ. Sustained release of vancomycin from novel biodegradable nanofiber-loaded vascular prosthetic grafts: In vitro and in vivo study. Int J Nanomedicine. 2015;10:885–91. doi: 10.2147/IJN.S78675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morishima M, Marui A, Yanagi S, et al. Sustained release of vancomycin from a new biodegradable glue to prevent methicillin-resistant Staphylococcus aureus graft infection. Interact Cardiovasc Thorac Surg. 2010;11:52–55. doi: 10.1510/icvts.2010.232447. [DOI] [PubMed] [Google Scholar]
- 52.Sakaguchi H, Marui A, Hirose K, et al. Less-invasive and highly effective method for preventing methicillin-resistant Staphylococcus aureus graft infection by local sustained release of vancomycin. J Thorac Cardiovasc Surg. 2008;135:25–31. doi: 10.1016/j.jtcvs.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 53.Infektionsnetz.at/test/antibiotika/rifampicin. [Accessed 07. June 2016]. http://www.infektionsnetz.at/test/antibiotika/rifampicin.htm. [in German]
- 54.Breakpoint tables for interpretation of MICs and zone diameters. The European Committee on Antimicrobial Susceptibility Testing; [Accessed 06 June, 2016]. http://www.eucast.org. [Google Scholar]


