Abstract
Background
Tumor volume doubling time (TVDT) is relatively important for breast cancer diagnosis and prognosis evaluation. This study aimed to analyze the related factors that may affect the TVDT of breast cancer by three-dimensional ultrasound (3D-US).
Material/Methods
A total of 69 breast cancer patients were selected. 3D-US was applied to measure the volume of breast lumps diagnosed as BI-RADS-US 4A by conventional ultrasound. TVDT was calculated according to the formula TVDT=ΔT×log2/log(V2/V1). Multiple linear regression analysis was performed to analyze the factors influencing breast cancer TVDT.
Results
The mean and median TVDT were 185±126 (range 66–521) and 164 days, respectively. TVDT showed no statistical significance according to regular shape, coarse margin, spicule sign, peripheral hyperechoic halo, microcalcification, and different posterior echo characteristics (P>0.05). Patients grouped by age, axillary lymphatic metastasis, histological differentiation, and Nottingham prognostic index (NPI) score exhibited significantly different TVDT (P<0.05). On the contrary, patients with different menstrual conditions, breast cancer family history, or pathological types presented similar TVDT (P>0.05). TVDT was obviously different in breast cancer with different ER, PR, Ki-67, and molecular subtyping but not HER2 expression. Multivariate analysis revealed that NPI score, axillary lymphatic metastasis, Ki-67, and molecular subtyping were risk factors of TVDT in breast cancer (P<0.05).
Conclusions
Breast cancer TVDT was significantly correlated with NPI score, axillary lymphatic metastasis, Ki-67, and molecular subtyping. Triple-negative breast cancer exhibited the most rapid growth.
MeSH Keywords: Imaging, Three-Dimensional; Multivariate Analysis; Tumor Burden
Background
Breast cancer is a malignant tumor characterized by various clinicopathological features, recurrence, and survival [1–3]. In addition to traditional pathological indicators, it can be classified by immunohistochemistry detection of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expressions [4]. The molecular subtyping shows good effect in predicting prognosis and treatment response [5]. Early diagnosis and treatment exhibit significant impacts on breast cancer patients. Tumors detected through screening are more likely to be ER-positive type instead triple-negative type. Moreover, triple-negative tumors usually present benign or indeterminate characteristics on ultrasound and mammography [6–8].
Tumor volume doubling time (TVDT) reflects the natural growth rate and the biology of malignancy. It not only determines the follow-up interval but also drives decisions about therapeutic schedule [9,10]. Breast lumps in breast imaging reporting and data system for ultrasonography (BI-RADS-US) 4A grade need biopsy [11,12]. However, its application is limited by various adverse impacts, such as unnecessary fear, anxiety, discomfort, and pain [13]. Patients who refuse biopsy are recommended to come in for review after 2~6 months [11], which provides the possibility for breast cancer TVDT study. In recent years, three-dimensional ultrasound (3D-US) has been widely applied in the evaluation of various diseases [14,15]. The present study used 3D-US technology to continuously measure the breast lump volume, calculate TVDT, and analyze the factors influencing breast cancer TVDT through multiple linear regression analysis. We believe this is the first report on breast cancer TVDT using 3D-US.
Material and Methods
Object of study
The patients received breast ultrasound examination in Wuxi People’s Hospital were enrolled between Feb 2012 and May 2016. 3D-US technology was used to measure breast lump volume judged as BI-RADS 4A by conventional ultrasound. Exclusion criteria: (1) diameter >3 cm on any side of the lump; (2) capsule solid mass; (3) high echo neoplasm; and (4) cystic mass. Inclusion criteria: (1) volume data measured 2 times by consecutive 3D-US; (2) time interval >2 months; and (3) no biopsy puncture or clinical treatment in the time interval. This research was approved by the Ethics Committee of Wuxi People’s Hospital and all subjects provided signed informed consent.
Ultrasound scanning
The patients were first scanned by conventional ultrasound examination using a Philips iU-Elite diasonograph with L12-5 probe and frequency at 5~12 MHz or VL13-5 probe and frequency at 5~13 MHz. BI-RADS-US descriptor was applied to evaluate the acquired image, including shape, coarse margin, spicule sign, peripheral hyperechoic halo, microcalcification, and different posterior echo characteristics. 3D-US scan was performed on breast lumps using the VL-13-5 probe. The patients held their breath for about 20 s during scanning.
TVDT measurement
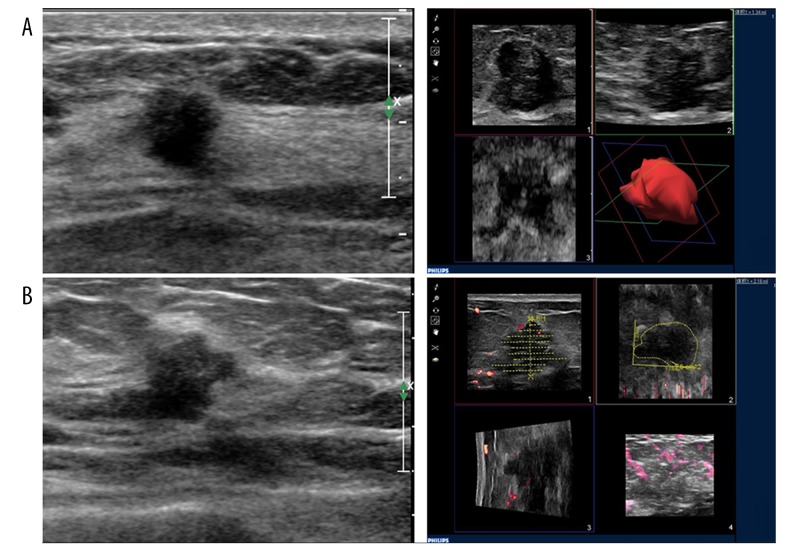
The obtained image was analyzed by Qlab software according to the manual. The tumor was equally divided into multiple levels, and the tumor boundary on each section was depicted on sagittal view. After the boundary was depicted on all the layers, the tumor volume was assumed to grow exponentially and was automatically calculated by the system and stored on a hard disk. Each tumor image was collected 3 times to calculate the average value (Figure 1). Breast cancer TVDT=ΔT×log2/log(V2/V1). ΔT, time interval. V1, the volume detected in the first time. V2, the tumor volume detected in the second time [10].
Figure 1.
(A) Triple-negative type breast cancer patient with TVDT of 133 days. (A) First examination, volume=1.34 ml. (B) Second examination after 93 days, volume=2.18 ml. Left, primary ultrasound image. Right, TVDT calculation module.
Pathological examination
Pathological examination contained traditional indicators and prognostic molecular indicators. The former included the pathological type, tumor size, histological grade, and lymph node status from patients who accepted biopsy later. Nottingham prognostic index (NPI) was calculated according to the formula NPI=size (cm)×0.2 + lymph node staging (1~3) + histologic classification (1~3) [16]. The prognostic molecular indictors were ER, PR, HER2, and Ki-67. Judgment criteria for immunohistochemistry were [17]: ER and PR positively expressed in nucleus as brown granules, and positive cell number ≥10% was considered as positive. Ki-67 positively located in nucleus and positive cell number ≥14% was judged as positive. HER2 was positively expressed on the cell membrane as clear brown staining. The cases with “1+” or “–” were considered as negative. The patients with “++” received fluorescence in situ hybridization (FISH) technique to test HER-2 gene amplification, and those without amplification were defined as negative.
Molecular subtyping
Breast cancer was classified based on immunohistochemistry indicators. Luminal subtype A: ER and/or PR positive, HER2 negative, Ki-67 <14%; Luminal type B: ER and/or PR positive, HER2 positive and/or Ki-67 ≥14%; (3) HER2 overexpression type: ER negative, PR negative, and HER2 positive; and triple-negative type: ER negative, PR negative, and HER2 negative.
Statistical analysis
All data analysis was performed on SPSS 13.0 software. Measurement data are depicted as mean ± standard deviation and compared by t test or analysis of variance. The influence factors of breast cancer TVDT was analyzed by multiple linear regression analysis. A statistical significance was presented as P<0.05.
Results
A total of 69 female breast cancer patients were enrolled, with a median age of 52 (26~71) years. The mean initial tumor volume was 0.91 ± 0.33 ml and the mean time interval was 182±81.9 days. The mean and median TVDT were 185±126 (range 66~521) and 164 days, respectively. There were 29 cases of luminal subtype A, 12 cases of luminal subtype B, 10 cases of HER2 overexpression type, and 18 cases of triple-negative type.
TVDT showed no statistical significance according to regular shape, coarse margin, spicule sign, peripheral hyperechoic halo, microcalcification, and different posterior echo characteristics (P>0.05) (Table 1).
Table 1.
The relationship between breast cancer TVDT and first time ultrasonographic features (χ±s, d, n=69).
| Item | Cases | TVDT | t value/F value | P value |
|---|---|---|---|---|
| Shape | ||||
| Circular or elliptical | 39 | 202±121 | 2.179 | 0.206 |
| Irregular | 30 | 187±158 | ||
| Coarse margin | ||||
| No | 58 | 200±165 | 1.084 | 0.784 |
| Yes | 11 | 184±124 | ||
| Spicule sign | ||||
| No | 62 | 208±136 | 0.351 | 0.109 |
| Yes | 7 | 175±88 | ||
| Peripheral hyperechoic halo | ||||
| No | 59 | 197±122 | −2.826 | 0.061 |
| Yes | 10 | 246±109 | ||
| Microcalcification | ||||
| No | 62 | 197±115 | −2.243 | 0.218 |
| Yes | 7 | 248±221 | ||
| Posterior echo characteristics | ||||
| No change | 54 | 204±149 | 1.026* | 0.090 |
| Attenuation | 7 | 230±176 | ||
| Enhancement | 8 | 189±124 | ||
F value.
Patients grouped by age, axillary lymphatic metastasis, histological differentiation, and NPI score exhibited significantly different TVDTs (P<0.05). On the contrary, patients with different menstrual conditions, breast cancer family history, or pathological types presented similar TVDTs (P>0.05) (Table 2).
Table 2.
The relationship between breast cancer TVDT and traditional pathological indicators (χ±s, d, n=69).
| Item | Cases | TVDT | t value/F value | P value |
|---|---|---|---|---|
| Age | ||||
| <52 | 37 | 167±89 | −3.959 | 0.042 |
| ≥52 | 32 | 225±109 | ||
| Menstruation | ||||
| Premenopause | 36 | 185±136 | −2.543 | 0.204 |
| Postmenopause | 33 | 209±121 | ||
| Breast cancer family history | ||||
| Yes | 16 | 175±64 | −2.426 | 0.147 |
| No | 53 | 214±102 | ||
| Pathological type | ||||
| Invasive carcinoma | 38 | 174±87 | −2.819 | 0.103 |
| Ductal carcinoma in situ | 31 | 199±54 | ||
| Axillary lymph node | ||||
| Metastasis | 10 | 131±63 | −4.641 | 0.033 |
| Non-metastasis | 59 | 226±134 | ||
| Histological grade | ||||
| I | 15 | 225±143 | 2.595* | 0.116 |
| II | 42 | 201±156 | ||
| III | 12 | 169±90 | ||
| NPI score | ||||
| <3.4 | 16 | 257±121 | 10.157* | 0.019 |
| 3.4~5.4 | 39 | 198±108 | ||
| >5.4 | 14 | 135±72 | ||
F value.
TVDT was obviously different in breast cancer with different ER, PR, Ki-67, and molecular subtyping but not HER2 expression (P<0.05). In addition, no significant difference in TVDT was observed in patients with different HER2 expression (P>0.05) (Table 3).
Table 3.
Comparison of breast cancer TVDT with prognostic molecular indicators and molecular subtyping (χ±s, d, n=69).
| Item | Cases | TVDT | t value/F value | P value |
|---|---|---|---|---|
| ER | ||||
| Positive | 41 | 221±156 | 8.513 | 0.031 |
| Negative | 28 | 160±86 | ||
| PR | ||||
| Positive | 40 | 231±143 | 5.351 | 0.048 |
| Negative | 29 | 165±96 | ||
| HER2 | ||||
| Positive | 14 | 184±71 | −0.628 | 0.739 |
| Negative | 55 | 195±112 | ||
| Ki-67 | ||||
| Negative (<14%) | 33 | 224±136 | 11.317 | 0.018 |
| Positive (≥14%) | 36 | 145±87 | ||
| Molecular subtyping | ||||
| Luminal subtype A | 29 | 257±185 | 13.751* | 0.013 |
| Luminal subtype B | 12 | 211±116a | ||
| HER2 overexpression | 10 | 184±71ab | ||
| Triple negative | 18 | 127±48abc | ||
F value.
P<0.01, vs. luminal subtype A;
P<0.01, vs. luminal subtype B;
P<0.01, vs. HER2 overexpression type.
Factors that may affect breast cancer TVDT were imported to a multiple linear regression model. Multivariate analysis revealed that NPI score, axillary lymphatic metastasis, Ki-67, and molecular subtyping were the risk factor of TVDT in breast cancer (P<0.05) (Table 4).
Table 4.
Multiple linear regression analysis of the relevant factors on breast cancer TVDT (n=69).
| Factors | Regression coefficient | Standard error | t value | P value |
|---|---|---|---|---|
| Constant | 3.514 | 2.574 | 1.216 | 0.221 |
| NPI | −0.468 | 0.234 | 6.238 | 0.021 |
| Axillary lymphatic metastasis | −0.132 | 0.059 | 4.543 | 0.034 |
| Ki-67 | −0.171 | 0.087 | 6.327 | 0.019 |
| Molecular subtyping | −0.189 | 0.129 | 8.502 | 0.002 |
Discussion
High-frequency ultrasound technology greatly increased the detection rate of breast cancer, but its sensitivity and specificity are not high. BI-RADS-US 4A grade presented malignant possibility in 20~40% of breast lumps. Therefore, regular radiographic follow-up is extremely important to observe the dynamic changes of the lesions when the patients refuses biopsy. The degree of lesion enlargement is an important index, of which TVDT is widely used in the observation of tumor growth. As an index of lesion enlargement, TVDT allowed us to separate the poor outcomes associated with screening women [10]. Förnvik calculated the mean TVDT of breast cancer as 282 (46~749) days through mammography [16]. Eun Bi Ryu reported the average TVDT of breast cancer was 141 (46~825) days by two-dimensional ultrasound [17]. All of these studies used the elliptic sphere empirical formula to estimate the tumor volume, which has a variety of deficiencies. Firstly, since the tumor shape is irregular, the estimated volume is inaccurate and the repeatability of measurement is poor. Secondly, limited by unapparent resolution and poor repeatability, the short-term maximum diameter measured has difficulty in objectively and accurately evaluating subtle changes in the nodule. The volume may be twice as large when the measured diameter value was larger than 26% [10]. Thus, inaccurate measurement greatly restricts breast cancer TVDT investigation. Inconvenience of use and radioactivity of MRI or mammography also limited their application for tumor volume calculation and follow-up [18]. This study used 3D-US to measure the breast lump volume; it can delineate the mass boundary at each level, resulting in more accurate TVDT compared with the ellipsoid empirical formula. The present study calculated the mean breast cancer TVDT as 184 (66–521) days by 3D-US.
In-depth breast cancer research shows that breast cancer is a kind of molecular disease with high heterogeneity. Breast cancer patients with similar clinical pathological features often present different outcomes and prognoses, and they also exhibit divergent response to the same therapeutic schedule. Thus, this study investigated the correlation of breast cancer TVDT with multiple factors, including ultrasonographic characteristics, traditional pathological indexes, and prognostic molecular indicators.
We found no statistically significant difference in TVDT according to ultrasonographic image, menstruation, breast cancer family history, histological type, and HER2 expression. On the contrary, TVDT was obviously different among patients with different age, axillary lymphatic metastasis, histological grade, and NPI score. Our findings agree with previous studies reporting that breast cancer patients with younger age, axillary lymphatic metastasis, high histological grade, and NPI score exhibited faster breast cancer cell growth [16,17]. Moreover, TVDT revealed significant differences in breast cancer with different ER, PR, Ki-67, and molecular subtyping. ER (−), PR (−), and Ki-67 (+) breast cancer grows faster than that with ER (+), PR (+), and Ki-67 (−) expression. The tumor growth rate is closely correlated with cell proliferation. It was reported that about 70% of ER (−) breast cancer patients overexpressed phosphate dehydrogenase (PHGDH) [19]. It was confirmed that cancer cells may change the metabolism to maintain rapid growth, while a high level of PHGDH may promote such changes. It was found that suppression of PHGDH protein production in a breast cancer cell line stopped cancer cell proliferation [20]. PR expression was positively correlated with ER and negatively correlated with epidermal growth factor receptor (EGFR) [21]. EGFR-overexpressed cancer cells exhibited worse differentiation and stronger division. Moreover, EGFR overexpression may suppress cell apoptosis and promote neovascularization in tumors. Ki-67 is a type of cell proliferation-related protein that is considered as a marker to evaluate the proliferative activity of cancer cells. Ki-67 expression is related to tumor differentiation, invasion, and metastasis. It was confirmed that Ki-67 overexpression is a risk factor for breast cancer, and 58% of patients with local recurrence showed Ki-67-positive expression [22].
Multivariate analysis showed that TVDT was negatively correlated with NPI score, axillary lymphatic metastasis, Ki-67 expression, and molecular subtyping. NPI score is classic and objective, as the information is mainly from pathological grading and tumor stage. Ki-67 is considered to be the most powerful univariate factor to predict the growth rate [23]. Breast cancer TVDT in luminal subtype A was 257±185 days, in luminal subtype B it was 211±116 days, in HER2 expression type it was 184±71 days, and in triple-negative type it was 127±48 days. Previous studies only investigated the relationship between molecular subtyping and prognosis. The present study reveals that breast cancer is correlated with growth speed, and the triple-negative group exhibited the fastest speed. Previous breast cancer growth models demonstrated that the peak time of recurrence in ER-positive patients was 36 months, while it was 12~24 months in HER2 overexpression type and triple-negative type [24,25]. However, these models did not consider the breast cancer TVDT changes among different molecular classifications. Our study provides data for establishing a breast cancer growth model.
There are many limitations in this research. (1) It had a small sample size. (2) Clinical and experimental observation show that malignant tumor growth follows an S-shaped or linear Gompertzian curve [26]. Gompertzian model assumes that TVDT varies according to tumor size. Many lumps with rapid growth but no second 3D-US examination data were excluded during the process of the initial false-negative breast cancer follow-up, which may affect the whole and partial TVDT data. (3) The distribution of molecular subtyping in this study was different from the other reports, which may have led to selection bias. (4) As the span of TVDT is large, there were few patients with rapidly-developing tumors enrolled in this study. Therefore, it is necessary to carry out further multicenter prospective study in the future.
Conclusions
Breast cancer TVDT was significantly correlated with NPI score, axillary lymphatic metastasis, Ki-67, and molecular subtyping. Triple-negative breast cancer exhibited the most rapid growth.
Footnotes
Source of support: This study was supported by the Research Project for the Women’s and Children’s Health of Jiangsu Province National Health and Family Planning Commission (F201567), the Key Research Project of Medical Research in Wuxi Hospital Management Center (YGZXZ1509), and the Research Project for Women’s and Children’s Health of the Wuxi National Health and Family Planning Commission (FYKY201502)
Conflict of interest
None.
References
- 1.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 3.Lin NU, Vanderplas A, Hughes ME, et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118:5463–72. doi: 10.1002/cncr.27581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Ronde JJ, Hannemann J, Halfwerk H, et al. Concordance of clinical and molecular breast cancer subtyping in the context of preoperative chemotherapy response. Breast Cancer Res Treat. 2010;119:119–26. doi: 10.1007/s10549-009-0499-6. [DOI] [PubMed] [Google Scholar]
- 5.Dawson SJ, Duffy SW, Blows FM, et al. Molecular characteristics of screen-detected vs. symptomatic breast cancers and their impact on survival. Br J Cancer. 2009;101:1338–44. doi: 10.1038/sj.bjc.6605317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dogan BE, Gonzalez-Angulo AM, Gilcrease M, et al. Multimodality imaging of triple receptor-negative tumors with mammography, ultrasound, and MRI. Am J Roentgenol. 2010;194:1160–66. doi: 10.2214/AJR.09.2355. [DOI] [PubMed] [Google Scholar]
- 7.Ko ES, Lee BH, Kim HA, et al. Triple-negative breast cancer: Correlation between imaging and pathological findings. Eur Radiol. 2010;20:1111–27. doi: 10.1007/s00330-009-1656-3. [DOI] [PubMed] [Google Scholar]
- 8.Boisserie-Lacroix M, Macgrogan G, Debled M, et al. Triple-negative breast cancers: associations between imaging and pathological findings for triple-negative tumors compared with hormone receptor-positive/human epidermal growth factor receptor-2-negative breast cancers. Oncologist. 2013;18:802–11. doi: 10.1634/theoncologist.2013-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackintosh JA, Marshall HM, Yang IA, et al. A retrospective study of volume doubling time in surgically resected non-small cell lung cancer. Respirology. 2014;19:755–62. doi: 10.1111/resp.12311. [DOI] [PubMed] [Google Scholar]
- 10.Bailey SL, Sigal BM, Plevritis SK. A simulation model investigating the impact of tumor volume doubling time and mammographic tumor detectability on screening outcomes in women aged 40–49 years. J Natl Cancer Inst. 2010;102:1263–71. doi: 10.1093/jnci/djq271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brancato B, Crocetti E, Bianchi S, et al. Accuracy of needle biopsy of breast lesions visible on ultrasound: Audit of fine needle versus core needle biopsy in 3233 consecutive samplings with ascertained outcomes. Breast. 2012;21:449–54. doi: 10.1016/j.breast.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Mei J, Ni M, Gao YS, Wang ZY. Femur performed better than tibia in autologous transplantation during hemipelvis reconstruction. World J Surg Oncol. 2014;12:1. doi: 10.1186/1477-7819-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphrey KL, Lee JM, Donelan K, et al. Percutaneous breast biopsy: Effect on short-term quality of life. Radiology. 2014;270:362–68. doi: 10.1148/radiol.13130865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dragan E, Guillaume OA, Haba D, Olszewski R. Three-dimensional evaluation of implant positioning in the maxillary sinus septum: A retrospective study. Med Sci Monit. 2015;21:2666–71. doi: 10.12659/MSM.894403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi J, Xia J, Wei Y, et al. Three-dimensional virtual reality simulation of periarticular tumors using Dextroscope reconstruction and simulated surgery: A preliminary 10-case study. Med Sci Monit. 2014;20:1043–50. doi: 10.12659/MSM.8897770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fornvik D, Lang K, Andersson I, et al. Estimates of breast cancer growth rate from mammograms and its relation to tumour characteristics. Radiat Prot Dosimetry. 2016;169:151–57. doi: 10.1093/rpd/ncv417. [DOI] [PubMed] [Google Scholar]
- 17.Ryu EB, Chang JM, Seo M, et al. Tumour volume doubling time of molecular breast cancer subtypes assessed by serial breast ultrasound. Eur Radiol. 2014;24:2227–35. doi: 10.1007/s00330-014-3256-0. [DOI] [PubMed] [Google Scholar]
- 18.Luczynska E, Heinze-Paluchowska S, Hendrick E, et al. Comparison between breast MRI and contrast-enhanced spectral mammography. Med Sci Monit. 2015;21:1358–67. doi: 10.12659/MSM.893018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Possemato R, Marks KM, Shaul YD, et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–50. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unterlass JE, Basle A, Blackburn TJ, et al. Validating and enabling phosphoglycerate dehydrogenase (PHGDH) as a target for fragment-based drug discovery in PHGDH-amplified breast cancer. Oncotarget. :2016. doi: 10.18632/oncotarget.11487. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan J, Liu XL, Han LZ, et al. Relation between Ki-67, ER, PR, Her2/neu, p21, EGFR, and TOP II-alpha expression in invasive ductal breast cancer patients and correlations with prognosis. Asian Pac J Cancer Prev. 2015;16:823–29. doi: 10.7314/apjcp.2015.16.2.823. [DOI] [PubMed] [Google Scholar]
- 22.Yip C, Bhoo-Pathy N, Daniel J, et al. Roles of Ki67 in breast cancer – important for management? Asian Pac J Cancer Prev. 2016;17:1077–82. doi: 10.7314/apjcp.2016.17.3.1077. [DOI] [PubMed] [Google Scholar]
- 23.Gandini S, Guerrieri-Gonzaga A, Pruneri G, et al. Association of molecular subtypes with Ki-67 changes in untreated breast cancer patients undergoing pre-surgical trials. Ann Oncol. 2014;25:618–23. doi: 10.1093/annonc/mdt528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park S, Koo JS, Kim MS, et al. Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast. 2012;21:50–57. doi: 10.1016/j.breast.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Lee JA, Kim KI, Bae JW, et al. Triple negative breast cancer in Korea-distinct biology with different impact of prognostic factors on survival. Breast Cancer Res Treat. 2010;123:177–87. doi: 10.1007/s10549-010-0998-5. [DOI] [PubMed] [Google Scholar]
- 26.Alzahrani EO, Asiri A, El-Dessoky MM, Kuang Y. Quiescence as an explanation of Gompertzian tumor growth revisited. Math Biosci. 2014;254:76–82. doi: 10.1016/j.mbs.2014.06.009. [DOI] [PubMed] [Google Scholar]