Abstract
Background
Diabetic retinopathy (DR) and diabetic optic neuropathy are important complications of diabetes mellitus (DM) which can lead to blindness in diabetic patients. Recent studies showed that chronic low-grade inflammation is thought to be one of the important pathophysiological mechanisms in the occurrence and development of DR and diabetes optic neuropathy. This study explored the expressions of inflammatory factors HMGB-1 and TLR9.
Material/Methods
SD rats were randomly divided into a diabetic mellitus group and a control group. A DM rat model was produced by intraperitoneal injection of 1% STZ with 60 mg/Kg weight. At 4, 8, and 16 weeks after injection, the rats were sacrificed and eyeballs were enucleated for HE staining, immunohistochemistry, Western blot, and RT-PCR.
Results
We found that, compared with the control group, levels of HMGB1 and TLR9 in retinas were significantly increased in DM groups of different time courses. Furthermore, a significant correlation was found between HMGB1 and TLR9 (all P<0.05).
Conclusions
Our results demonstrated that the HMGB1-TLR9 signaling pathway may be involved in the pathogenesis of diabetic retinopathy. Blockage of HMGB1 and/or TLR9 may represent a novel approach to treating diabetic retinopathy and diabetic optic neuropathy.
MeSH Keywords: Diabetic Retinopathy, HMGB1 Protein, Toll-Like Receptor 9
Background
Diabetic retinopathy (DR) is the main microvascular complication of diabetes mellitus (DM) and one of the leading causes of blindness worldwide [1]. About one-third of individuals with diabetes have signs of retinopathy, and of these, one-third may have diabetic retinopathy and diabetic optic neuropathy [2]. It is important to understand the mechanism underlying pathological DR and diabetic optic neuropathy to identify new targets for treatment.
The basic pathological processes of DR and the diabetic optic neuropathy mechanism are still unclear, but chronic low-grade inflammation is thought to be one of the important pathophysiological mechanisms [3]. Damage to the retina and optic nerve mediated by abnormal activation of inflammation factors are important factors in the occurrence and development of DR [4].
HMGB1, also known as high mobility protein 1, is a late inflammatory factor. HMGB-1 protein is a nuclear DNA binding protein released passively from necrotic cells and actively from monocytes/macrophages and endothelial cells [5]. As a damage-associated molecular pattern (DAMP), HMGB1 is involved in physiological and pathological processes through its receptors, including the release of inflammatory cytokines, cell migration, and angiogenesis [6–8].
Research on HMGB1 receptors has mainly focused on TLR2, TLR4, and RAGE, but TLR9 has seldom been studied. This study explored the expressions of HMGB1 and TLR9 in the retinas of diabetic rats, and elucidated their possible roles in diabetic retinopathy and diabetic optic neuropathy.
Material and Methods
Animal model
A total of 80 specific pathogen-free (SPF) grade, male SD rats, body weight 240–280 g were provided by the Animal Experimental Committee of Jinzhou Medical University (Liaoning Province, China). Rats were randomly assigned to the control group (n=20) and DM group (4, 8, and 16 weeks, n=20 in each subgroup). Rats in the DM group were intraperitoneally injected with 1% streptozotocin (STZ, Sigma, USA) at 60 mg/Kg, and rats in the control group were injected with an equivalent amount of citrate buffer solution. At 72 h after intraperitoneal injection, blood glucose was measured via the caudal vein using a blood glucose tester (Bayer). Blood glucose was measured monthly.
Reagents and instruments
Rabbit polyclonal antibody against HMGB1, mouse monoclonal antibodies against TLR9, and β-actin were purchased from Abcam (UK). Streptozotocin (STZ) was purchased from Sigma (USA). All the other reagents were obtained from standard commercial suppliers unless otherwise noted.
HE staining
Circular shearing parallel to the direction of the corneal limbus 0.5 mm behind the corneal limbus was performed to gently remove the lens and vitreum. The cupula oculi were fixed again, dehydrated, embedded with soft and hard paraffin, and successively cut into 5.0-um sections. The sections were conventionally stained with HE, dehydrated, mounted, observed, and imaged under a light microscope.
Western blot analysis
Rat retinas were collected at different time points after STZ administration. For extraction of total cellular protein, tissues were lysed in RIPA buffer with PMSF. Protein concentration was quantified using the BCA kit (Pierce Biotechnology, Inc., Rockford, IL, USA). Proteins were separated and transferred to PVDF membranes. The membranes were incubated overnight at 4°C with the HMGB1, TLR9, and β-actin (1: 1000). Thereafter, the membranes were incubated with HRP-labeled anti-rabbit secondary antibodies (1: 1000) for 1 h at room temperature. The membrane was visualized by use of an enhanced chemiluminescence kit (Thermo Fisher Scientific Inc., Rockford, IL, USA).
Gene expression analysis
For reverse transcription-polymerase chain reaction analysis, RNA in the samples was extracted with TRIzol (Invitrogen, Carlsbad, CA, USA). Reverse transcription into cDNA with a reverse transcriptase kit (TaKaRa) was performed according to the manufacturer’s protocol. Primers were designed and purchased from TaKaRa Biotech Co., Ltd. (Dalian, China), and GAPDH was used as a normalizing control. The sequence of primers is shown in Table 1. We calculated the 2−ΔΔCt to perform relative quantification of gene expression.
Table 1.
Primers for reverse transcription-polymerase chain reaction.
| Gene | Primer sequences (5′-3′) | Product length (bp) | Temperature (°C) | |
|---|---|---|---|---|
| HMGB1 | F | CGAATGTGTCTTTAGCTAGCCCTGT | 71 | 64.0 |
| R | CAGACTGTACCAGGCAAGGTTAGTG | |||
| TLR9 | F | TGTTGCCTTTACTGCAGCATCTC | 148 | 64.4 |
| R | CTCTGCGCCTTATCGAACACC | |||
| GAPDH | F | GGCACAGTCAAGGCTGAGAATG | 143 | 63.3 |
| R | ATGGTGGTGAAGACGCCAGTA |
Statistical analysis
All quantitative data are presented as the mean ± standard deviation (χ̄±s). Data were analyzed using variance (ANOVA) by SPSS 20.0 statistical software. p<0.05 was considered as a significant difference.
Results
Animal model
There was no significant difference in body weight and blood glucose between the control group and DM group at baseline (p>0.05). At 72 h after STZ intraperitoneal injection, blood glucose was significantly increased to >16.7 mmol/L, suggesting a DM model success rate of up to 100%. In the experimental period, the weights of rats in the DM group were lower than in the control group (t=6.532, 7.812, 13.863, all p<0.05), but the blood glucose levels were much higher than in the control group (t= 20.253, 27.786, 21.003, all p<0.05). The details of body weight and blood glucose of different time points in the 2 groups are shown in Table 2.
Table 2.
Body weight and blood glucose at different time points.
| Times (wk) | Body weight (g) | Blood glucose (mmol/L) | ||
|---|---|---|---|---|
| Control | DM | Control | DM | |
| 0 | 210.23±8.08 | 215.23±11.09 | 5.27±0.90 | 5.12±0.68 |
| 4 | 317.50±10.88 | 270.90±12.38* | 4.52±0.68 | 20.56±0.64* |
| 8 | 420.50±25.98 | 280.55±32.34* | 5.51±0.57 | 21.07±2.26* |
| 16 | 458.62±15.74 | 316.77±12.65* | 4.97±0.71 | 21.10±0.81* |
p<0.05 compared with the control group.
HE staining of retinal sections
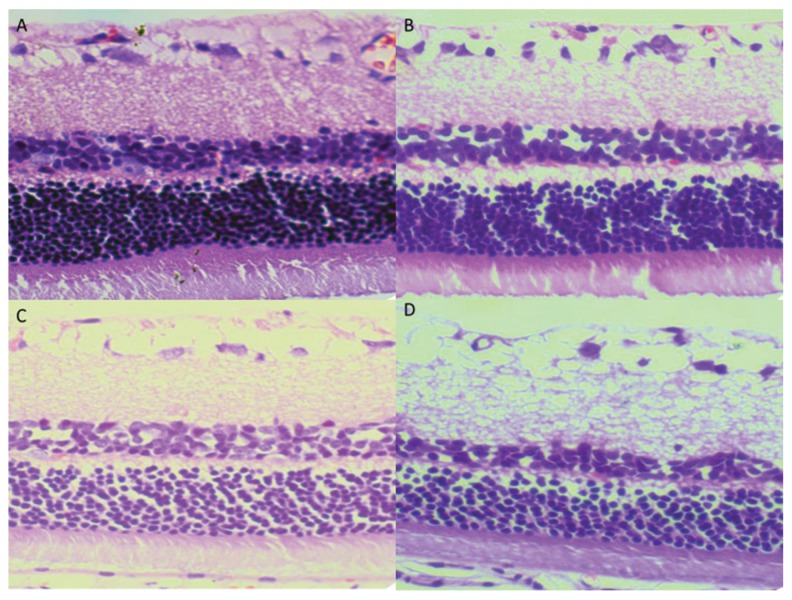
In the control group, the retina had a smooth surface, as well as neatly arranged retinal ganglion cells (RGCs) and inner and outer nuclear layers. In the DM group, the retinas had no significant morphological changes at 4 week. With the extension of the diabetes course, the arrangement retina cells became disordered and there were microvascular expansion and microvascular lesions. At 16 weeks, the arrangement of retinal ganglion cells was completely disordered, and the capillary endothelial cells were observed to protrude through the inner limiting membranes (Figure 1).
Figure 1.
Histopathological examination of the retina. (A) Normal retina in the control group; (B) DM 4 wk; (C) DM 8 wk; (D) DM 16 wk. (HE ×400).
Immunohistochemical staining result
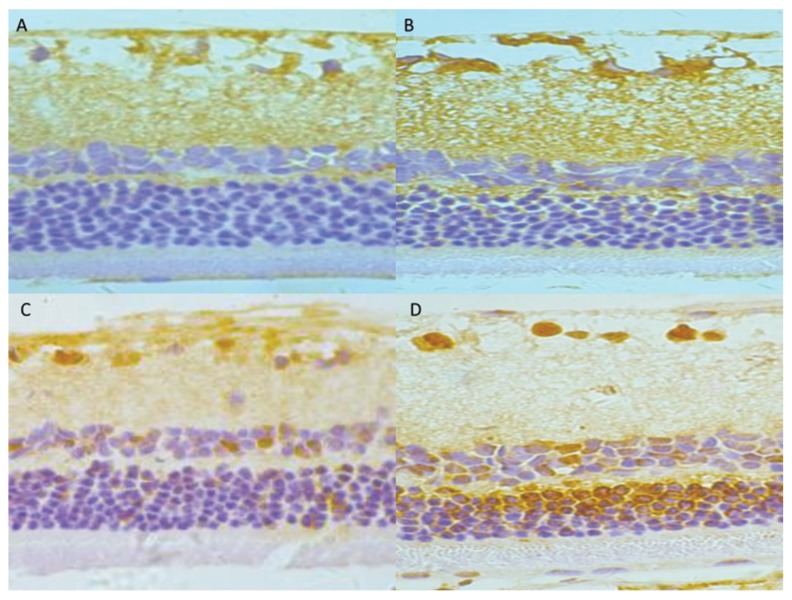
Little positive expression of HMGB1 and TLR9 was detected in the control group. Positive expressions of HMGB1 and TLR9 were observed in the RGCs layer and inner nuclear layer. The positive expression was mainly located in the nucleus of the retina, and was stained pale brown-yellow. At ≥4 week, HMGB1 and TLR9 expression was strongly detected in the RGCs layer, inner nuclear layer, and outer nuclear layer. Compared with the control group, the optical density values of HMGB1 and TLR9 positive cells in the retina of the DM group were significantly increased at various time points (p<0.05). Detailed data are shown in Figure 2 and Table 3.
Figure 2.
Immunohistochemical staining of HMGB1 protein in rat retinas. (A) Control group; (B) DM4 wk group; (C) DM 8 wk group; (D) DM 16 wk group (×400).
Table 3.
The optical density values of HMGB1 and TLR9 in retinal sections.
| N | DM 4W | DM 8W | DM 16W | |
|---|---|---|---|---|
| HMGB1 | 0.1037±0.0011 | 0.1342±0.0023* | 0.1487±0.0051* | 0.1837±0.0034* |
| TLR9 | 0.0983±0.0023 | 0.1350±0.0074* | 0.1563±0.0048* | 0.1768±0.0022* |
p<0.05 compared with the control group.
Evaluation of protein levels in retinal tissue
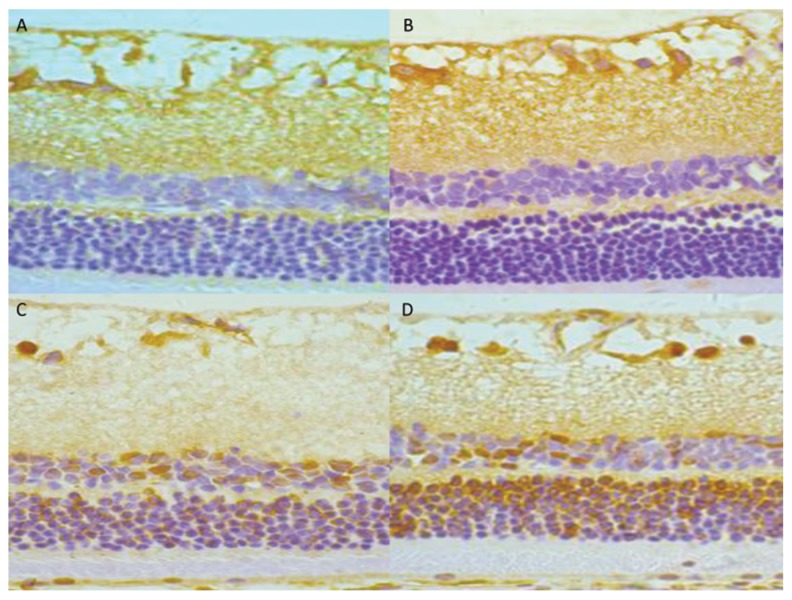
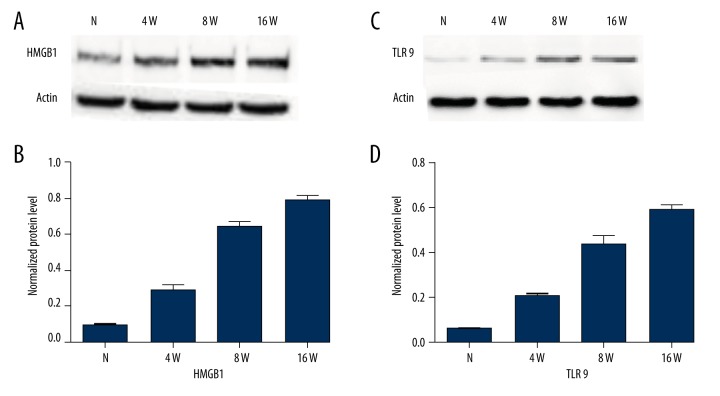
The protein levels were detected and quantified by Western blot in retinal tissues of the DM group and control group. We observed significantly elevated levels of HMGB1 and TLR9 in retinas of the DM group compared with the control group. Moreover, this rise showed obvious time dependence. The detailed data are shown in Figures 3 and 4. To determine whether they were correlated, we performed Pearson correlation analysis. By Pearson correlation analysis, r=0.991, P<0.05, the results showed that the expressions of HMGB1 and TLR9 were significantly positively correlated (Figure 5).
Figure 3.
Immunohistochemical staining of TLR9 protein in the rat retinas. (A) Control group; (B) DM 4 wk group; (C) DM 8 wk group; (D) DM 16 wk group (×400).
Figure 4.
HMGB1 and TLR9 protein expression in rat retinas by Western blot. (A) HMGB1 protein expression in retinal tissues of different groups. (A) Western blot assay for HMGB1; (B) Histogram of HMGB1 protein expression in each group; (C) Western blot assay for TLR9; (D) Histogram of TLR9 protein expression in each group. * Compared with control group, p<0.05.
Figure 5.
HMGB1 and TLR9 mRNA expression of DM group and control group. (A) Histogram of HMGB1 mRNA expression in each group. (B) Histogram of TLR9 mRNA expression in each group. * Compared with control group, p<0.05.
Evaluation of mRNA levels in retinal tissue
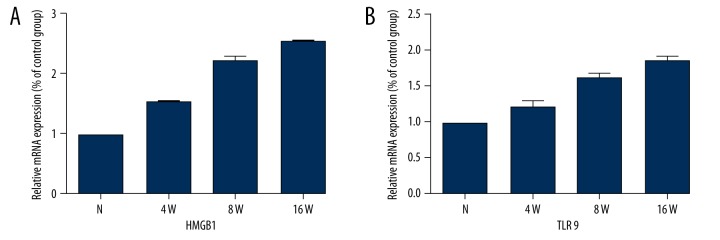
The mRNA levels of HMGB1 and TLR9 in retinal tissues were evaluated by RT-PCR. Interestingly, similar to the results obtained in the protein expression study, HMGB1 (+158%, +209%, and +257%) and TLR9 (+135%, +156%, and +189%) expression was increased in the DM group at different time points compared with the control group (all p<0.05) (Figure 6).
Figure 6.
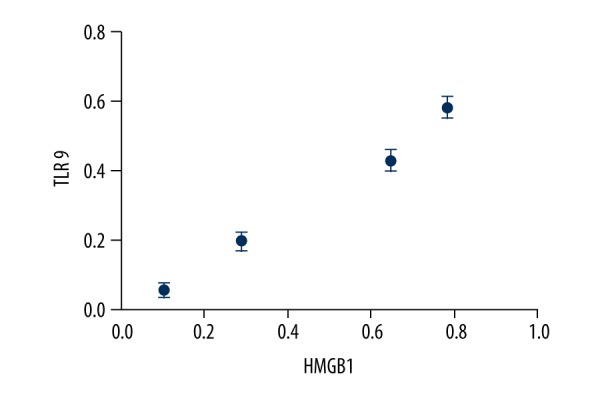
Correlation analysis chart between HMGB1 and TLR9. The value of p is 0.042, the value of r is 0.991. There was a significant positive correlation between HMGB1 and TLR9.
Discussion
HMGB1 is a nonhistone DNA-binding protein and serves as a structural component to facilitate the assembly of nucleoprotein complexes in the nucleus. Chromatin-associated HMGB1 plays multiple roles in the regulation of genome replication, recombination, mRNA transcription, and DNA repair [9–11]. HMGB1 is highly conserved in all mammals and is expressed consistently in the nucleus of almost all cell types examined [12]. It serves as a damage-associated molecular pattern (DAMP) and mediates various physical or pathological processes, including inflammatory cytokine release, cell migration, and angiogenesis, mainly through TLR2, TLR4, and RAGE [13–15]. Thus, it has been demonstrated to be a late mediator of infection involved in the pathogenesis of autoimmunity [16], cancer [17], trauma [18], and IR injury [19]. Indeed, as a DAMP molecule, HMGB1 is receiving increasing DR research attention [20]. Although higher levels of HMGB1 were observed in a previous study [21], the specific mechanism has remained unclear.
Our study found that the expressions of HMGB1 protein were significantly increased in a significantly time-dependent manner in the DM groups. Yao Yu et al. found that HMGB1 is involved in DR through binding with RAGE, and HMGB1 content is positively correlated with retinal cell apoptosis [22]. Fu D and Tian X showed that HMGB1 and VEGFA level were positively associated in diabetic patients, and they demonstrated that HMGB1 and VEGFA are key players in the ability to suppress cell viability and induce apoptosis, which shows that HMGB1 might be useful as a biomarker of DR [23]. Zhao et al. found that as a therapeutic target, HMGB1 can inhibit inflammation and promote RGCs survival to delay DR progression [24]. All of the above results indicate that HMGB1 promotes the development and progression of diabetic retinopathy and the formation of retinal neovascularization, which is similar to the results of our study. In addition, we also detected the mRNA expression of HMGB1. To our surprise, the expression of mRNA was completely consistent with the results of protein expression. Moreover, the increase in mRNA showed a significant time dependence. The results above show that the HMGB1 expression is significantly increased in both gene transcription and protein synthesis.
The specific mechanism by which HMGB1 leads to DR has been unclear, so we detected the expression of TLR9. TLR9 is a member of the Toll-like receptor family, which are major contributors to the innate immune system and mediate inflammatory responses against infectious and noninfectious pathogens by recognizing structures conserved among pathogen-associated molecular patterns (PAMP) and damage-associated molecular patterns (DAMP). Previous studies had shown that HMGB1 via TLR9 can lead to the occurrence of hepatocellular carcinoma [25], vascular injury [26], and autoimmune pathogenesis [27]. However, the role of TLR9 in DR has not been investigated. Therefore, we also detected the expression of TLR9 in our study. The results showed that the expression of TLR9 was gradually increased in a time-dependent manner, which was positively correlated with the expression of HMGB1. Our study results suggest that HMGB1 may exert its function through the TLR9 signaling pathway in the occurrence and development of diabetic retinopathy and diabetic optic neuropathy.
Conclusions
The present study confirms that HMGB1 and TLR9 play important roles in the occurrence and development of diabetic retinopathy and diabetic optic neuropathy. Research on the relationship between HMGB1 and TLR9 is relatively new, and further exploration is needed. Studying the relationship between HMGB1 and TLR9 and use of HMGB1 and/or TLR9 inhibitors can provide new strategies for DR prevention and treatment. HMGB1 serves as an endogenous mediator of inflammation via the TLR9 pathway in response to diabetic retinopathy and diabetic optic neuropathy. Blockage of HMGB1 and/or TLR9 may be a novel approach to treating diabetic retinopathy and diabetic optic neuropathy.
Footnotes
Conflicts of interest
The authors declare that they have no competing interests.
Source of support: This work was partly supported by the National Natural Science Foundation of China (No. 81570866)
References
- 1.Agarwal A, Soliman MK, Sepah YJ, et al. Diabetic retinopathy: Variations in patient therapeutic outcomes and pharmacogenomics. Pharmgenomics Pers Med. 2014;7:399–409. doi: 10.2147/PGPM.S52821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belgium: International Diabetes Federation; 2013. http://www.idf.org/diabetesatlas. [Google Scholar]
- 3.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–36. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 4.Torres PF, Kijlstra A. The role of cytokines in corneal immunopathology. Ocul Immunol Inflamm. 2001;9:9–24. doi: 10.1076/ocii.9.1.9.3978. [DOI] [PubMed] [Google Scholar]
- 5.Mudaliar H, Pollock C, Ma J, et al. The role of TLR2 and 4-mediated inflammatory pathways in endothelial cells exposed to high glucose. PLoS One. 2014;9:e108844. doi: 10.1371/journal.pone.0108844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xing Z, Liu F, Yuan J. Research advance in immunological mechanism after corneal alkali burns. Chinese Ophthalmic Research. 2010;28:6. [Google Scholar]
- 7.Sims GP, Rowe DC, Rietdijk ST, et al. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–88. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 8.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2010;29:139–62. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang W, Tang Y, Li L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine. 2010;51:119–26. doi: 10.1016/j.cyto.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 10.Thomas JO, Stott K. H1 and HMGB1: modulators of chromatin structure. Biochem Soc Trans. 2012;40:341–46. doi: 10.1042/BST20120014. [DOI] [PubMed] [Google Scholar]
- 11.Thomas JO, Travers AA. HMG1 and 2, and related ‘architectural’ DNA-binding proteins. Trends Biochem Sci. 2001;26:167–74. doi: 10.1016/s0968-0004(01)01801-1. [DOI] [PubMed] [Google Scholar]
- 12.Stros M. HMGB proteins: interactions with DNA and chromatin. Biochim Biophys Acta. 2010;1799:101–13. doi: 10.1016/j.bbagrm.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Yang H, Antoine DJ, Andersson U, Tracey KJ. The many faces of HMGB1: Molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol. 2013;93:865–73. doi: 10.1189/jlb.1212662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sims GP, Rowe DC, Rietdijk ST, et al. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–88. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 15.Kang R, Chen R, Zhang Q, et al. HMGB1 in health and disease. Mol Aspects Med. 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang W, Tang Y, Li L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine. 2010;51:119–26. doi: 10.1016/j.cyto.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 17.Tang D, Kang R, Zeh HJ, III, Lotze MT. High-mobility group box 1 and cancer. Biochim Biophys Acta. 2010;1799:131–40. doi: 10.1016/j.bbagrm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh MK, Singh L, Pushker N, Sen S, et al. Correlation of high mobility group box-1 protein (HMGB1) with clinicopathological parameters in primary retinoblastoma. Pathol Oncol Res. 2015;21:1237–42. doi: 10.1007/s12253-015-9951-6. [DOI] [PubMed] [Google Scholar]
- 19.Biscetti F, Ghirlanda G, Flex A. Therapeutic potential of high mobility group box-1 in ischemic injury and tissue regeneration. Curr Vasc Pharmacol. 2011;9:677–81. doi: 10.2174/157016111797484125. [DOI] [PubMed] [Google Scholar]
- 20.Yang S, Xu L, Yang T, Wang F. High-mobility group box-1 and its role in angiogenesis. J Leukoc Biol. 2014;95:563–74. doi: 10.1189/jlb.0713412. [DOI] [PubMed] [Google Scholar]
- 21.Abu El-Asrar AM, Nawaz MI, Kangave D, et al. High-mobility group box-1 and endothelial cell angiogenic markers in the vitreous from patients with proliferative diabetic retinopathy. Mediators Inflamm. 2012;2012:697489. doi: 10.1155/2012/697489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu Y, Yang L, Lv J, et al. The role of high mobility group box 1 (HMGB-1) in the diabetic retinopathy inflammation and apoptosis. Int J Clin Exp Pathol. 2015;8:6807–13. [PMC free article] [PubMed] [Google Scholar]
- 23.Fu D, Tian X. Effect of high mobility group box 1 on the human retinal pigment epithelial cell in high-glucose condition. Int J Clin Exp Med. 2015;8:17796–803. [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao H, Zhang J, Yu J. HMGB-1 as a potential target for the treatment of diabetic retinopathy. Med Sci Monit. 2015;21:3062–67. doi: 10.12659/MSM.894453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Yan W, Tohme S, et al. Hypoxia induced HMGB1 and mitochondrial DNA interactions mediate tumor growth in hepatocellular carcinoma through Toll Like Receptor 9. J Hepatol. 2015;63:114–21. doi: 10.1016/j.jhep.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirata Y, Kurobe H, Higashida M, et al. HMGB1 plays a critical role in vascular inflammation and lesion formation via toll-like receptor 9. Atherosclerosis. 2013;231:227–33. doi: 10.1016/j.atherosclerosis.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Tian J, Avalos AM, Mao SY, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–96. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]