Abstract
Background
Gastric cancer (GC) is the second leading cause of cancer-related death worldwide, but little progress has been achieved in the treatment of advanced or metastatic GC. GC is highly heterogeneous and more studies are needed to elucidate the metastatic mechanisms. Epithelial cell transforming 2 (ECT2) has been reported to be up-regulated in GC tissues, but its signaling mechanisms remain unclear.
Material/Methods
In this study, we used Western blot analysis to compare the expression level of ECT2 in 2 GC cell lines: MKN1 and MKN45. Mutagenesis and transfections were conducted to investigate the oncogenic mechanisms of ECT2 in GC cells.
Results
ECT2 was expressed at higher levels in MKN1 than in MKN45. Immunoblotting results showed that MKN1 expression was suppressed by p53-WT but was enhanced by p53-mutant. In addition, in vitro experiments showed that ECT2 positively regulated the proliferation and invasion of GC cells. To better explore the mechanisms of ECT2 in promoting GC progression, we introduced site-directed mutants of ECT2, and found that the phosphor-mimic mutant T359D enhanced its oncogenic activity. In contrast, activation of RhoA was inhibited in cells transfected with ECT2 phosphor-deficient mutant T359A. We found that the epithelial cell biomarker E-cadherin was down-regulated by ECT2-T359D, highlighting the role of phosphorylation in regulating epithelial-mesenchymal transition.
Conclusions
Our results identified p53 as a novel up-stream signaling molecule of ECT2 in GC cells, and the post-translational modifications of ECT2 play important roles in regulating cancer development and progression.
MeSH Keywords: Neoplasm Invasiveness, Phosphorylation, Stomach Neoplasms, Tumor Suppressor Protein p53
Background
Despite a remarkable decline in the incidence of gastric cancer (GC) in recent decades, it remains one of the most common malignancies and is the second leading cause of cancer-related death worldwide [1]. Over 70% of new cases and deaths occur in developing countries [2]. GC is often diagnosed at an advanced stage due to its aggressive metastasis [3]. Importantly, GC is highly heterogeneous and patients have different prognoses even at the same clinical stage [4]. Despite the development of anticancer drugs and treatments, GC cannot be cured, and the prognosis for patients in advanced stages is poor [5]. One of the most important research topics is explaining the metastatic mechanism of GC and subsequently developing novel targeted therapy drugs.
Epithelial cell transforming 2 (ECT2) is a guanine nucleotide exchange factor (GEF) that catalyzes the exchange of GDP for GTP, thereby activating Rho family members of small GTPases, such as RhoA, RhoC, Rac1, and Cdc42 [6,7]. ECT2 contains 2 tandem BRCT (breast cancer gene 1 C-terminus) domains, and both are required for the intramolecular interaction [8]. The subcellular location of ECT2 is dynamic; it is sequestered in the nucleus during interphase and dispersed to the cytoplasm during mitosis [9,10]. By regulating Rho family proteins, ECT2 plays roles in signal transduction pathways involved in the regulation of cytokinesis [11] and malignancies [12].
Due to its dynamic subcellular locations and role in activating GTPases, dysregulation of ECT2 has been discovered in numerous tumor types. High protein expression of ECT2 was reported in prostate cancer [13], lung cancer [14], colorectal cancer [15], and gastric cancer [16]. Mislocalization of ECT2 was found in ovarian cancer [17], osteosarcoma [12], and cervical cancer cells [18]. The post-translational modifications of ECT2 also regulate its protein level and oncogenic functions, including ubiquitination [19] and phosphorylation [20]. As one of the most important post-translational modifications, phosphorylation plays critical roles in regulating signaling pathways [21]. Several phosphorylation sites on human ECT2 protein have been identified by mass spectrometry method, including Thr359, Ser367, Thr373, Ser376, Thr444, and Ser716 [22–24].
Although the down-stream signaling pathways of ECT2 have been extensively investigated, few studies have reported its up-stream regulators. In this study, we found that the expression level of ECT2 was different in 2 GC cell lines (MKN1 and MKN45 cells), and further demonstrated that ECT2 was down-regulated by wild-type p53 protein. In contrast, the mutated p53 enhanced ECT2 protein expression. The abnormal enhanced expression of ECT2 in GC tissues has been reported from clinical studies [25], but its oncogenic role in GC has not been verified at cellular levels. Therefore, we performed overexpression and knock-down experiments in MKN1 and MKN45 cell lines, which demonstrated that ECT2 can promote cell proliferation and invasion. Finally, we constructed the mutagenesis plasmids of ECT2, and revealed that the phosphor-mimic mutant T359D enhanced its oncogenic activity. In contrast, the activation of RhoA was inhibited in cells transfected with ECT2 phosphor-deficient mutant T359A, while the epithelial cell biomarker E-cadherin was down-regulated by ECT2-T59D, compared with cells overexpressing ECT2-T359A.
Material and Methods
Cell culture and reagents
Human GC cell lines, MKN1 and MKN45, were obtained from Feiya Biotechnology (Nanjing, Jiangsu, China). Cells were grown in DMEM (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS, Invitrogen), 100 IU/ml penicillin (Sigma, St. Louis, MO, USA), and 100 μg/ml streptomycin (Sigma). Cells were cultured on cell culture dishes and were passaged every 2–3 days using 0.25% trypsin (Invitrogen). Antibodies (ECT2, p53, GTP-RhoA, total-RhoA, E-cadherin, and β-actin) were all purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Western blot
Protein extraction was performed by using RIPA lysis buffer. Extracted total proteins were separated by SDS-PAGE gels and transferred to polyvinylidene difluoride (PVDF) membranes. After blocking with 5% non-fat milk for 1 h, membranes were incubated with primary antibodies overnight at 4°C. Horseradish peroxidase-conjugated secondary antibodies were then added for incubations at room temperature for 1 h. After washing 3 times with phosphate-buffered saline, membranes were then exposed to enhanced chemiluminescence reagents (Merck, Darmstadt, Germany) for visualization of immuno-bands.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Total cell RNA was extracted using TRIzol reagent (Invitrogen) from MKN1 and MKN45 cells. Single-stranded cDNA was reversely synthesized by use of a TransScript First-Strand cDNA Synthesis SuperMix Kit (TransGen Biotech, Beijing, China) following the manufacturer’s instructions. To examine the mRNA levels, real-time PCR was performed and the fluorescence signal was detected with the StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). GAPDH was used as an internal reference to calculate the relative expression of ECT2 using the 2−ΔΔCT (CT, cycle threshold) method.
Primers were:
ECT2: 5′-ACTACTGGGAGGACTAGCTTG-3′;
5′-CACTCTTGTTTCAATCTGAGGCA-3′.
GAPDH: 5′-AATGACCCCTTCATTGACCTC-3′;
5′-TTCCATTGATGACAAGCTTCC-3′.
Plasmid constructs and siRNAs
Human wild-type (WT) ECT2 cDNA was obtained and isolated from a cDNA library generated by Hela cells with the following PCR conditions: initial denaturation at 95°C for 3 min, followed by 30 cycles at 95°C for 30 s, 55°C for 30 s, and 72°C for 2 min; a final extension step was carried out at 72°C for 5 min. The reverse-transcription PCR products were electrophoresed on an agarose gel and purified using the EasyPure®Quick Gel Extraction Kit (TransGen Biotech) and inserted into pcDNA3.0 plasmid vector. The constructed plasmids were transformed into DH5αE. coli cells and selected by ampicillin resistance. The sequences were tested by DNA sequencing via Sangon (Shanghai, China). The mutations of T359A and T359D on ECT2 were generated by site-directed Quickchange mutagenesis method [26]. The p53-WT and p53-V143A mutant constructs were purchased from Addgene. The sequence for ECT2-siRNA was 5′-CAGAGGAGAUUAAGACUAU-3′ and ordered from GenePharma (Shanghai, China). Stealth RNAi Negative Control (NC-siRNA, Invitrogen) served as control. Cell transfection was conducted using Lipo2000 transfection reagent (Invitrogen) following the manufacturer’s instructions.
Cell proliferation
Cell proliferation after transfection was analyzed using MTT assay. Briefly, 1×103 cells were seeded into a 96-well plate and incubated for 1, 2, 3, and 4 days. At the indicated time point, 20 μL of MTT (5 mg/mL) (Sigma-Aldrich) was added into each well and incubated for 4 h. Then, the supernatants were removed and 150 μL of DMSO (Sigma-Aldrich) was added. Spectrometric absorbance was read at 490 nm by a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Cell migration
To examine cell migration in vitro, a wound-healing assay was conducted. Briefly, transfected cells were seeded in 6-well tissue culture plates and grown to a density of 70–80%. Cells were scratched using 200 μL pipette tips to create streaks in the monolayer after 12-h culture. The wound closure was observed and calculated at 12, 36, and 60 h using a light microscope (Olympus, Tokyo, Japan) as described by others [27].
Cell invasion
Briefly, 1×105 cells were seeded in a Transwell chamber (Corning, USA) with Matrigel coating. After the cells were incubated for 12 h, we changed the medium in the upper chamber with serum-free medium, and medium supplemented with 10% FBS was added to the lower compartment. After incubating for 18–24 h at 37°C in 5% CO2, cells that invaded through the membrane were fixed with 4% formaldehyde and stained with 0.5% crystal violet. Cells adhering to the lower surface were counted under a microscope in 5 randomly selected visual fields.
Results
ECT2 expression was modulated by p53 protein in GC cells
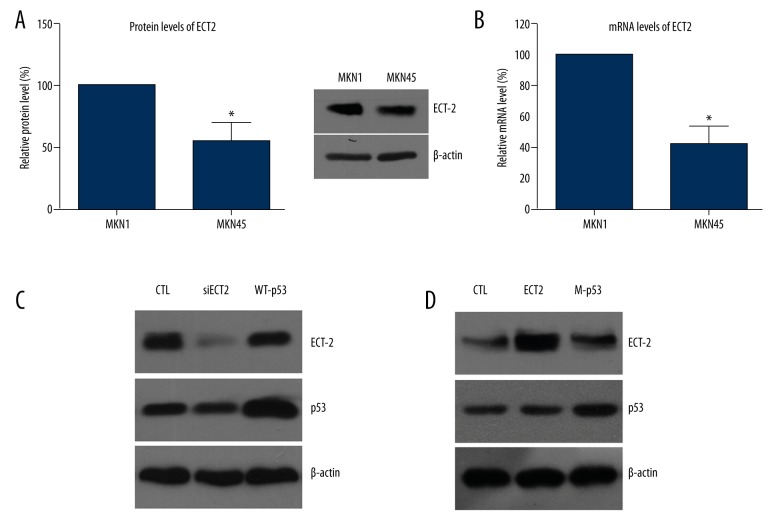
We chose 2 GC cell lines in the current study: MKN1 and MKN45 cells. We initially found that the protein expression level of ECT2 was higher in MKN1 cells than that in MKN45 cells (Figure 1A). Consistently, RT-qPCR results also showed the higher mRNA level in MKN1 cells compared with MKN45 (Figure 1B). Taking into consideration that MKN1 cells possess mutant-p53 while MKN45 is characterized with WT-p53 [28], we speculated that ECT2 levels may be affected by the p53 proteins.
Figure 1.
ECT2 expression was regulated by p53 protein. (A) The protein expression level of ECT2 was higher in MKN1 cells than that in MKN45 cells. (B) RT-qPCR results showed a higher mRNA level in MKN1 cells compared with MKN45. (C) Immunoblotting results showed that WT-p53 inhibited protein expression of ECT2. (D) Mutant-p53 increased the ECT2 protein level, indicating the oncogenic role of ECT2 in GC cells. All experiments were performed in triplicate at least 3 times.
To test our hypothesis, we transfected the MKN1 cells with WT-p53 and transfected the MKN45 cells with mutant-p53 (p53-V143A). Immunoblotting results showed that WT-p53 inhibited protein expression of ECT2 (Figure 1C), but mutant-p53 increased the ECT2 protein level (Figure 1D), indicating the oncogenic role of ECT2 in GC cells.
ECT2 promoted GC progression through up-regulating cell proliferation, migration, and invasion
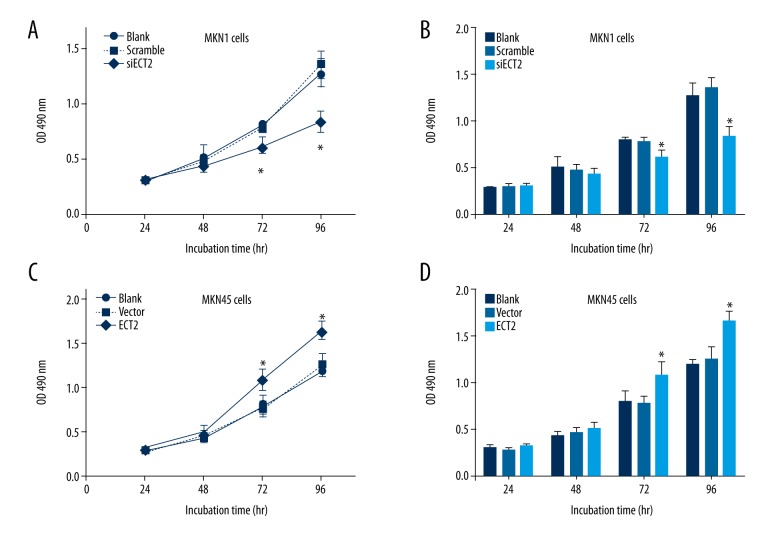
It has been recently reported that ECT2 was highly expressed in clinical GC tissues and was correlated with poor prognosis [25]. Therefore, we wanted to test its underlying oncogenic mechanisms at the cellular level. MTT experiments showed that ECT2-siRNA impaired the cell viability (Figure 2A, 2B), and ECT2-overexpression promoted cell proliferation (Figure 2C, 2D).
Figure 2.
ECT2 promoted cell proliferation of gastric cancer cells. ECT2-knockdown inhibited the cell viability as reflected by MTT assay (A, B). In contrast, ECT2-overepression enhanced cell proliferation capacity (C, D). All assays were performed in triplicate at least 3 times.
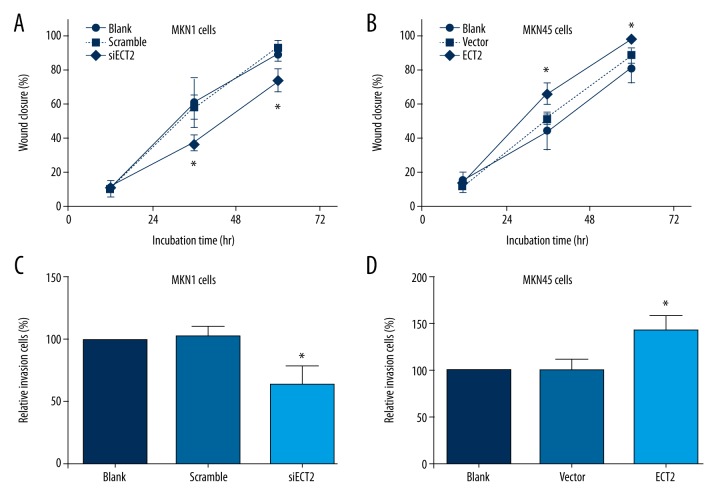
Importantly, the most aggressive characteristic of GC is the high metastasis capacity. Thus, we explored whether ECT2 affected the migration and invasion of GC cells. Wound-healing results showed that ECT2 knockdown inhibited cell migration whereas ECT2-overexpression enhanced it (Figure 3A, 3B). The invasion process of GC cells was also positively regulated by ECT2 protein (Figure 3C, 3D).
Figure 3.
ECT2 up-regulated the migration and invasion capacities of gastric cancer cells. ECT2-knockdown inhibited the cell migration as reflected by wound-healing assay (A), and ECT2-overepression enhanced cell migration (B). Similarly, the cell invasion process was also positively regulated by ECT2 (C, D). All assays were performed in triplicate at least 3 times.
Phosphorylation of Thr359 was crucial for the oncogenic functions of ECT2
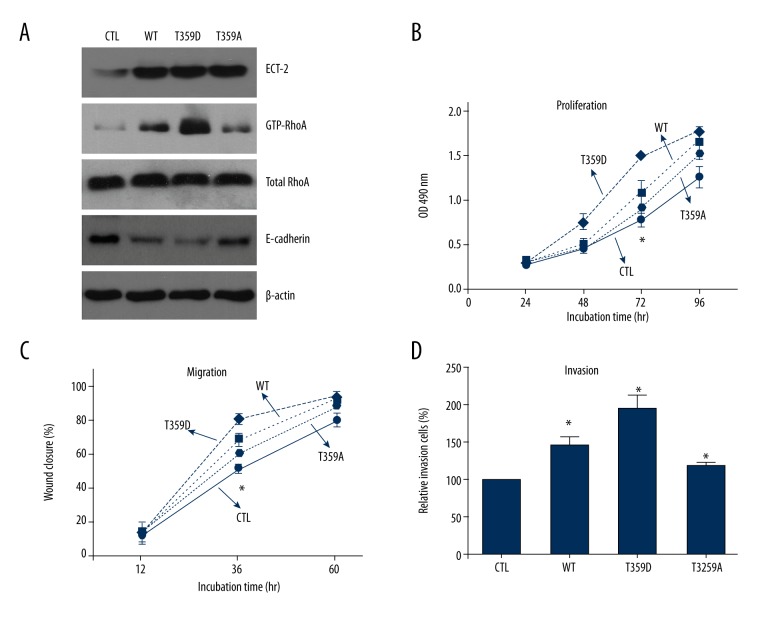
As described above, several phosphorylation sites of ECT2 have been identified, but the detailed roles of these sites have not been fully elucidated. In this study, we focused on studying the role of Thr359 in ECT2. We generated site-directed mutants of ECT2 to mimic or abolish phosphorylation status of Thr359. The Aspartic amino acid possesses a side chain, CH2COOH, which is similar to phosphate, and we chose T359D as the phosphor-mimic mutant. The Alanine has no side chain and thus T359A was selected as the phosphor-deficient mutant.
By transfecting MKN45 cells with ECT2-WT, T359D, and T359A, we demonstrated that phosphorylation of Thr359 improves its ability in activating RhoA and inhibits the expression of E-cadherin, indicating its role in promoting the epithelial-mesenchymal transition (Figure 4A). Finally, our results showed that Thr359 phosphorylation also enhanced the proliferation, migration, and invasion of GC cells (Figure 4B–4D).
Figure 4.
Phosphorylation of ECT2 modulated its oncogenic functions. (A) The phosphor-mimic mutant T359D enhanced the activation of RhoA, and inhibit the expression of E-cadherin protein, whereas ECT2 phosphor-deficient mutant T359A showed opposite effects. In addition, Thr359 phosphorylation also enhanced the proliferation (B), migration (C), and invasion (D) capacities of gastric cancer cells. All assays were performed in triplicate at least 3 times.
Discussion
Rho family small GTPases are molecular switches involved in numerous cellular functions, including cell cytoskeletal organization, differentiation, growth, and gene transcription. Three protein classes participate in regulating Rho GTPases between the active and inactive states [29]: the guanine nucleotide exchange factors (GEFs), which can promote the release of bound GDP and catalyze GTP binding [30]; the GTPase-activating proteins (GAPs), which can increase the GTPase activity and accelerate the restoration of proteins to the inactive state [31]; and the guanine nucleotide dissociation inhibitors (GDIs), which can sequester the GDP-bound form of Rho GTPases and regulate their subcellular localization [32]. The balancing between active and inactive GTPases is important in maintaining normal cell biological functions.
ECT2 is a GEF protein reported to be up-regulated in several tumor types [33]. The major oncogenic mechanism of ECT2 functions through enhancing Rho GTPase activity [6]. However, the regulators for ECT2 are largely unknown. In the current study, we demonstrated that wild-type p53 can inhibit the expression of ECT2, whereas mutant p53 can increase the ECT protein level. This phenomenon further confirmed the oncogenic role of ECT2 in tumors. Our results are consistent with a study showing that p53 repressed ECT2 gene expression via protein methyltransferases [34], although it is unknown how mutant-p53 increases ECT2 levels.
In addition, the detailed role of ECT2 in mediating the biological features of GC cells has not previously been elucidated. Our study is the first to show that overexpression of ECT2 in GC cells can significantly promote tumor cell proliferation, migration, and invasion. We demonstrated that phosphorylation status of residue Thr359 was critical in regulating the oncogenic activity of ECT2. Abolishing the phosphorylation decreased RhoA activation and epithelial-mesenchymal transition, and subsequently inhibited the malignant phenotypes of GC cells.
There are some limitations in our study. First, we did not prove the direct crosstalk between p53 and ECT2, although p53 is involved in the suppression of ECT2 in GC cells. Second, clinical studies are needed to confirm the correlations between p53 mutation and ECT2 expression. Lastly, the role of other phosphorylation sites on ECT2 should also be verified in the future.
Conclusions
In conclusion, the results of the present study suggest that ECT2 has an oncogenic role in gastric cancer cells, and determined that p53 as its up-stream modulator. The role of phosphor-Thr359 in ECT2 may also provide novel directions in drug development.
Footnotes
Source of support: Departmental sources
Conflicts of Interest
The authors have no conflicts of interest.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374(9688):477–90. doi: 10.1016/S0140-6736(09)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim L, Michael M, Mann GB, Leong T. Adjuvant therapy in gastric cancer. J Clin Oncol. 2005;23(25):6220–32. doi: 10.1200/JCO.2005.11.593. [DOI] [PubMed] [Google Scholar]
- 5.Kim HJ, Karpeh MS. Surgical approaches and outcomes in the treatment of gastric cancer. Semin Radiat Oncol. 2002;12(2):162–69. doi: 10.1053/srao.2002.30818. [DOI] [PubMed] [Google Scholar]
- 6.Tatsumoto T, Xie X, Blumenthal R, et al. Human ECT2 is an exchange factor for Rho GTPases, phosphorylated in G2/M phases, and involved in cytokinesis. J Cell Biol. 1999;147(5):921–28. doi: 10.1083/jcb.147.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J Cell Biol. 2005;170(4):571–82. doi: 10.1083/jcb.200501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J-E, Billadeau DD, Chen J. The tandem BRCT domains of Ect2 are required for both negative and positive regulation of Ect2 in cytokinesis. J Biol Chem. 2005;280(7):5733–39. doi: 10.1074/jbc.M409298200. [DOI] [PubMed] [Google Scholar]
- 9.Matthews HK, Delabre U, Rohn JL, et al. Changes in Ect2 localization couple actomyosin-dependent cell shape changes to mitotic progression. Dev Cell. 2012;23(2):371–83. doi: 10.1016/j.devcel.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotynkova K, Su KC, West SC, Petronczki M. Plasma membrane association but not midzone recruitment of RhoGEF ECT2 is essential for cytokinesis. Cell Rep. 2016;17(10):2672–86. doi: 10.1016/j.celrep.2016.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook DR, Solski PA, Bultman SJ, et al. The ect2 rho Guanine nucleotide exchange factor is essential for early mouse development and normal cell cytokinesis and migration. Genes Cancer. 2011;2(10):932–42. doi: 10.1177/1947601912437035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito S, Liu XF, Kamijo K, et al. Deregulation and mislocalization of the cytokinesis regulator ECT2 activate the Rho signaling pathways leading to malignant transformation. J Biol Chem. 2004;279(8):7169–79. doi: 10.1074/jbc.M306725200. [DOI] [PubMed] [Google Scholar]
- 13.Guo Z, Chen X, Du T, et al. Elevated levels of epithelial cell transforming sequence 2 predicts poor prognosis for prostate cancer. Med Oncol. 2017;34(1):13. doi: 10.1007/s12032-016-0872-3. [DOI] [PubMed] [Google Scholar]
- 14.Tan H, Wang X, Yang X, et al. Oncogenic role of epithelial cell transforming sequence 2 in lung adenocarcinoma cells. Exp Ther Med. 2016;12(4):2088–94. doi: 10.3892/etm.2016.3584. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Luo Y, Qin SL, Mu YF, et al. Elevated expression of ECT2 predicts unfavorable prognosis in patients with colorectal cancer. Biomed Pharmacother. 2015;73:135–39. doi: 10.1016/j.biopha.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Jin Y, Yu Y, Shao Q, et al. Up-regulation of ECT2 is associated with poor prognosis in gastric cancer patients. Int J Clin Exp Pathol. 2014;7(12):8724–31. [PMC free article] [PubMed] [Google Scholar]
- 17.Huff LP, Decristo MJ, Trembath D, et al. The role of Ect2 nuclear RhoGEF activity in ovarian cancer cell transformation. Genes Cancer. 2013;4(11–12):460–75. doi: 10.1177/1947601913514851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews HK, Delabre U, Rohn JL, et al. Changes in Ect2 localization couple actomyosin-dependent cell shape changes to mitotic progression. Dev Cell. 2012;23(2):371–83. doi: 10.1016/j.devcel.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiter LT, Seagroves TN, Bowers M, Bier E. Expression of the Rho-GEF Pbl/ECT2 is regulated by the UBE3A E3 ubiquitin ligase. Hum Mol Genet. 2006;15(18):2825–35. doi: 10.1093/hmg/ddl225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Justilien V, Jameison L, Der CJ, et al. Oncogenic activity of Ect2 is regulated through protein kinase Cι-mediated phosphorylation. J Biol Chem. 2011;286(10):8149–57. doi: 10.1074/jbc.M110.196113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao K, Liu H. “Barcode” and differential effects of GPCR phosphorylation by different GRKs. G Protein-Coupled Receptor Kinases. 2016:75–120. [Google Scholar]
- 22.Olsen JV, Vermeulen M, Santamaria A, et al. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci Signal. 2010;3(104):ra3. doi: 10.1126/scisignal.2000475. [DOI] [PubMed] [Google Scholar]
- 23.Niiya F, Tatsumoto T, Lee KS, Miki T. Phosphorylation of the cytokinesis regulator ECT2 at G2/M phase stimulates association of the mitotic kinase Plk1 and accumulation of GTP-bound RhoA. Oncogene. 2006;25(6):827–37. doi: 10.1038/sj.onc.1209124. [DOI] [PubMed] [Google Scholar]
- 24.Hara T, Abe M, Inoue H, et al. Cytokinesis regulator ECT2 changes its conformation through phosphorylation at Thr-341 in G2/M phase. Oncogene. 2006;25(4):566–78. doi: 10.1038/sj.onc.1209078. [DOI] [PubMed] [Google Scholar]
- 25.Wang HB, Yan HC, Liu Y. Clinical significance of ECT2 expression in tissue and serum of gastric cancer patients. Clin Transl Oncol. 2016;18(7):735–42. doi: 10.1007/s12094-015-1428-2. [DOI] [PubMed] [Google Scholar]
- 26.Pan C, Liu HD, Gong Z, et al. Cadmium is a potent inhibitor of PPM phosphatases and targets the M1 binding site. Sci Rep. 2013;3:2333. doi: 10.1038/srep02333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Zhang Q, Li K, et al. Prognostic significance of USP33 in advanced colorectal cancer patients: New insights into β-arrestin-dependent ERK signaling. Oncotarget. 2016;7(49):81223–40. doi: 10.18632/oncotarget.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsunemitsu Y, Kagawa S, Tokunaga N, et al. Molecular therapy for peritoneal dissemination of xenotransplanted human MKN-45 gastric cancer cells with adenovirus mediated Bax gene transfer. Gut. 2004;53(4):554–60. doi: 10.1136/gut.2003.021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moon SY, Zheng Y. Rho GTPase-activating proteins in cell regulation. Trends Cell Biol. 2003;13(1):13–22. doi: 10.1016/s0962-8924(02)00004-1. [DOI] [PubMed] [Google Scholar]
- 30.Zheng Y. Dbl family guanine nucleotide exchange factors. Trends Biochem Sci. 2001;26(12):724–32. doi: 10.1016/s0968-0004(01)01973-9. [DOI] [PubMed] [Google Scholar]
- 31.Lamarche N, Hall A. GAPs for rho-related GTPases. Trends Genet. 1994;10(12):436–40. doi: 10.1016/0168-9525(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 32.Olofsson B. Rho guanine dissociation inhibitors: Pivotal molecules in cellular signalling. Cell Signal. 1999;11(8):545–54. doi: 10.1016/s0898-6568(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 33.Fields AP, Justilien V. The guanine nucleotide exchange factor (GEF) Ect2 is an oncogene in human cancer. Adv Enzyme Regul. 2010;50(1):190–200. doi: 10.1016/j.advenzreg.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scoumanne A, Chen X. The epithelial cell transforming sequence 2, a guanine nucleotide exchange factor for Rho GTPases, is repressed by p53 via protein methyltransferases and is required for G1-S transition. Cancer Res. 2006;66(12):6271–79. doi: 10.1158/0008-5472.CAN-06-0121. [DOI] [PubMed] [Google Scholar]