Abstract
Creating an intestinal stoma is commonly the final aspect of an often emergent and complicated operation under difficult circumstances. While creation of a protruding, tension-free, and well-vascularized stoma is often straightforward, one must be prepared for challenging situations such as a thick abdominal wall and short, thickened mesentery. A successful stoma starts with attentive preoperative planning including site marking, thoughtful consideration of alternatives, and attention to technical detail. The tips provided in this article should facilitate the process of selecting the appropriate intestinal segment, identifying the correct stoma site, and creating a functional stoma even in the most challenging situations. Constructing a high-quality stoma will decrease complications and improve the patient's quality of life. Stoma creation is frequently the only component of an operation that the patient will have to live with for the remainder of his/her life.
Keywords: ileostomy, colostomy, surgery, stoma, surgical repair
Intestinal stomas are the surgical exteriorization of either small or large bowel through the anterior abdominal wall. While the principles behind stoma creation are typically the same, there are many different stoma configurations that are created for myriad indications. A diverting stoma must prevent the fecal stream from reaching a distal segment of small or large intestine for the purpose of either treating or preventing an anastomotic leak. Stomas may also be necessary in the setting of sacral or perineal infections that have been compromised by continuous fecal soilage. Permanent stomas are required when altered anatomy prohibits reestablishment of gastrointestinal continuity, the risks of undergoing another surgery are prohibitive due to patient comorbidities, or the functional results of a reanastomosis would adversely impact quality of life.
Regardless of stoma type, it is critical to remember that living with a stoma can have a tremendous psychological impact on patients and requires significant adjustments to activities of daily living. However, studies evaluating ostomate quality of life reveal direct correlation between ostomy function and patient satisfaction. 1 As a result, surgeons must make a concerted effort to create a properly functioning and appropriate stoma that will not cause undue complications or distress.
Successful stoma creation requires significant planning and discussion. Although not discussed here, thorough preoperative assessment is imperative. Patient preparation begins with understanding the patient's lifestyle, occupation, clothing preferences, bowel function, and any disabilities. The patient's abdomen must be carefully examined for skin folds and preexisting scars in both the supine and sitting positions. This preoperative information helps to create a healthy stoma in the correct site and using the correct segment of bowel. Unfortunately, even the best planned stoma can be difficult to construct. The goal of this article is to review general techniques of stoma creation and to identify factors which complicate stoma creation. In addition, we will discuss possible solutions to these challenges.
End Stoma Creation
There are certain key aspects to creating an ideal end ileostomy or colostomy. Among the principles:
Excise a circular skin disc approximately 2.5 cm in diameter at the previously marked site ( Fig. 1 ).
Divide the subcutaneous tissue with small retractors until the anterior rectus sheath is exposed. Do not excise or “core-out” subcutaneous tissues.
Make a vertical incision in the anterior rectus sheath approximately 3 cm in length. At the midpoint of the incision, make a perpendicular 1-cm incision laterally. The lateral counterincision keeps the stoma opening away from the midline incision ( Fig. 2 ).
Split the rectus abdominis muscle in the direction of its fibers ( Fig. 3 ).
Create a vertical incision in the posterior rectus sheath ( Fig. 4 ).
Deliver the previously divided bowel through the abdominal wall without twisting it. “Pushing” from within the abdominal cavity is preferred to “pulling” when exteriorizing the bowel ( Fig. 5 ).
Again confirm that the bowel is viable and not twisted.
For a colostomy, the colon should extend 2 cm above the skin surface. For an ileostomy, 5 cm of bowel should be pulled through. The matured colostomy should protrude 0.5 to 1 cm above the skin. The matured ileostomy should protrude 2 to 2.5 cm.
Excise the staple line at end of the bowel cleanly with a no. 10 scalpel blade. Ileostomies must be everted. Eversion of a colostomy is optional and should ideally be dictated by the stomal therapist who will be working with the patient long term. The abdominal incision should be closed before this step. However, if there is concern that the stoma is under too much tension or has questionable viability, the abdomen can be closed after stoma maturation.
Perform the enterocutaneous anastomosis with interrupted absorbable sutures that take full-thickness bites of the end of the colon and the dermal layer of the skin.
Fig. 1.
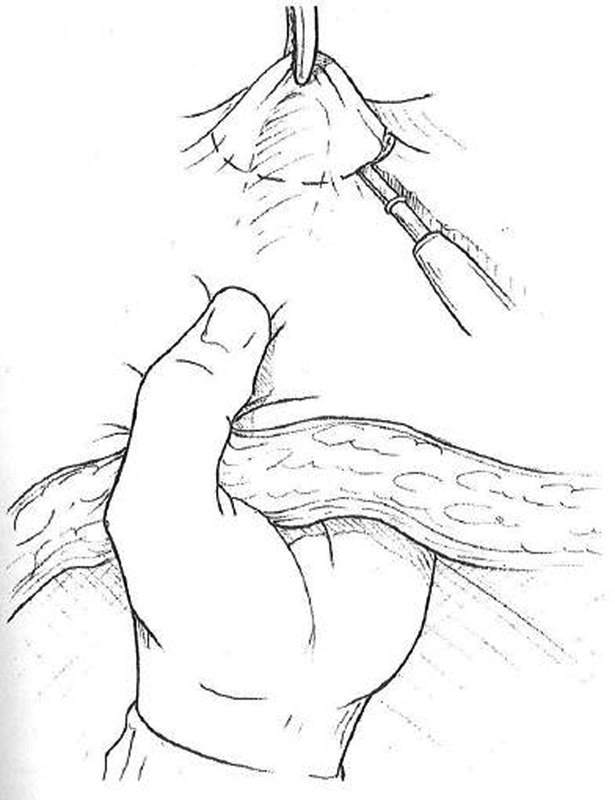
Excision of skin disc at stoma site.
Fig. 2.
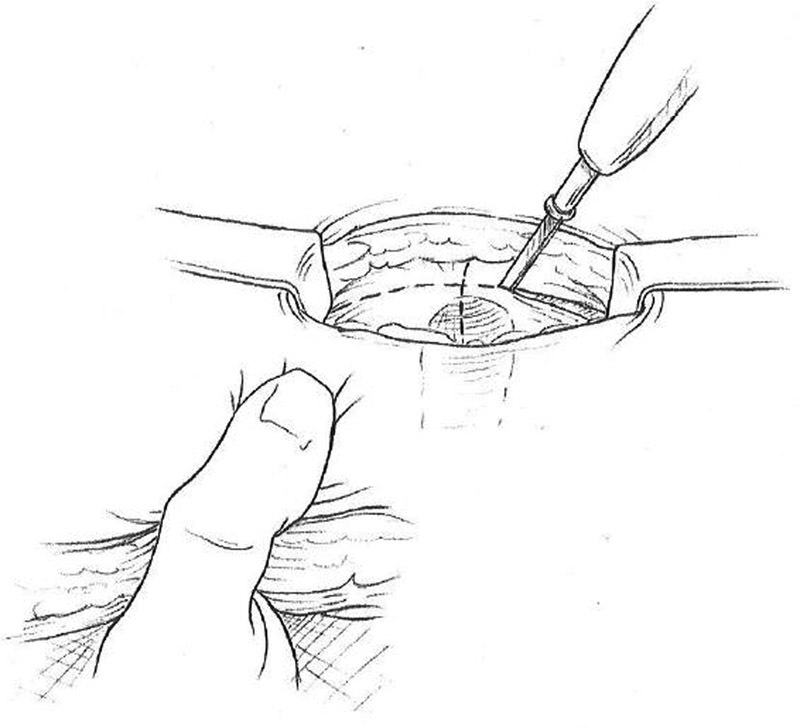
Cruciate incision in anterior fascia.
Fig. 3.
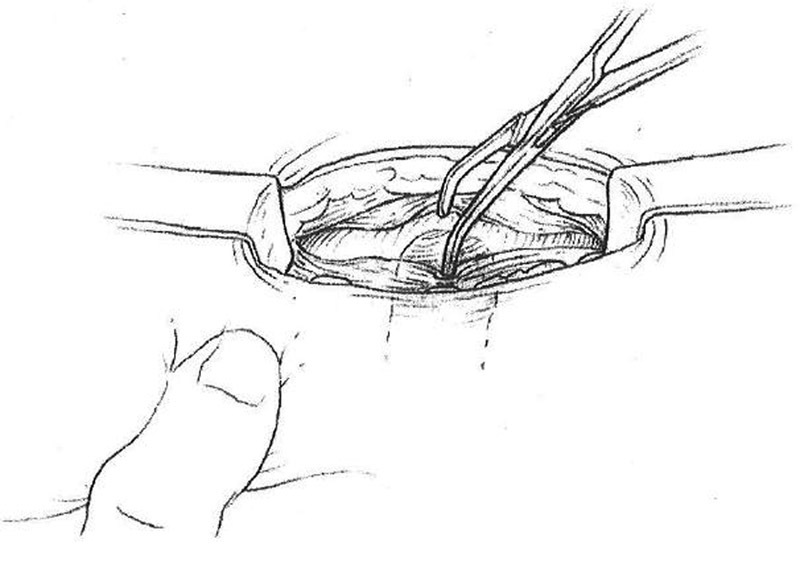
Splitting of rectus muscle in direction of fibers.
Fig. 4.
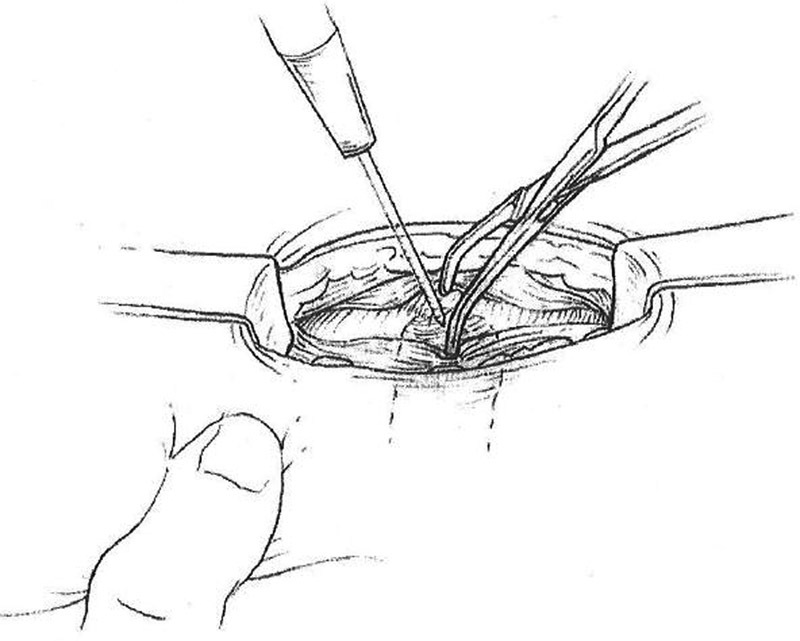
Incision of posterior rectus sheath.
Fig. 5.
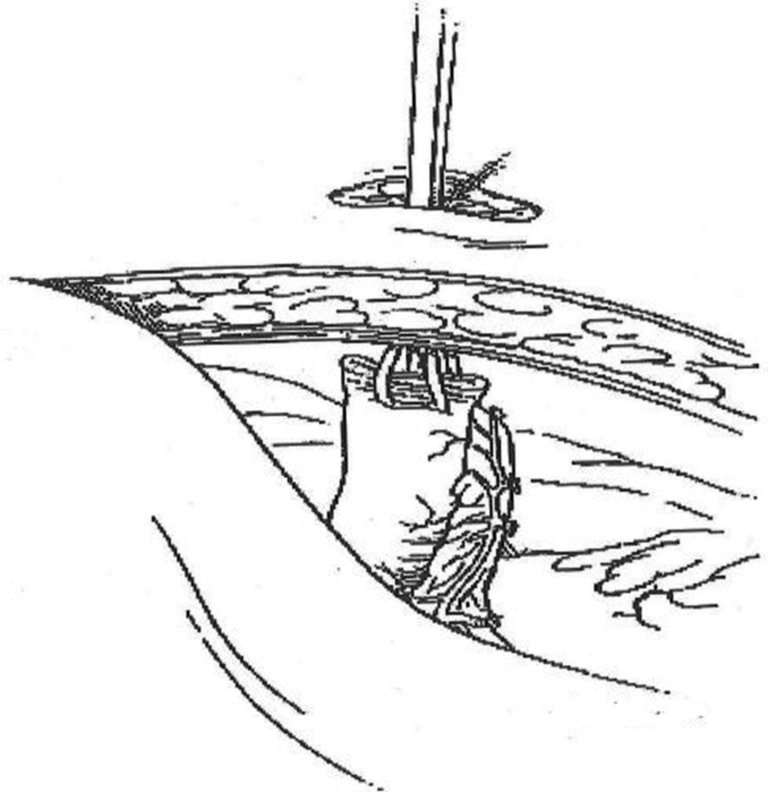
Delivery of bowel through stoma site.
Colostomies may be matured flush or can be everted similar to ileostomies. For flush creation, full-thickness bites of the terminal end of the bowel are followed by corresponding dermal bites on the stoma trephine. Sutures incorporating the epidermal skin layer may lead to “mucosal islands,” or small growths of mucosa, in the skin surrounding the stoma, and therefore should be avoided.
Ileostomies are everted by placing “triplicate” sutures. First, the suture is placed through the dermis, followed by a seromuscular bite 4 to 5 cm from the proximal to the distal end of the ileum. The final bite is passed full-thickness through the cut end of the bowel. Three everting sutures, away from the mesentery, will often evert the stoma effectively. Gaps can be closed with standard sutures between the triplicate sutures.
Loop Ileostomy Creation
When simple fecal diversion is required, a loop ileostomy is most often the procedure of choice. However, it is important to remember that a significant number of diverting stomas become permanent. Therefore, creating a well-functioning, easily reversible stoma is often the difference between reversal and life with a permanent ostomy. The purpose of a diverting stoma is most commonly to prevent fecal material from reaching a distal portion of the bowel, either because of fear of anastomotic leak or to treat a leak or injury. Diverting stomas do not decrease the incidence of anastomotic leak, per se, but instead decrease the related morbidity. 2 3 When treating pelvic infection from a colonic source or when planning diversion of a low pelvic anastomosis, the two options are transverse loop colostomy and loop ileostomy. While it is important to be aware of both the techniques, the loop ileostomy is clearly the superior procedure. 4 Loop ileostomies are created using the following technique:
Identify an appropriate loop terminal ileum that will protrude easily at the stoma site. A segment at least 20 cm from the ileocecal junction will facilitate subsequent stoma reversal. Any closer to the cecum may make a stapled anastomosis at the time of reversal more difficult. It is also important to confirm and mark the distal bowel with a suture or marker to ensure proper orientation.
Make a circular skin incision that is slightly larger than that required for an end stoma at the previously marked site and excise the skin.
Part the subcutaneous tissue with small retractors until the anterior rectus sheath is exposed. Do not excise this tissue.
Make a vertical incision in the anterior rectus sheath approximately 2 cm in length. At the midpoint of the incision, make a perpendicular 1-cm incision laterally. This will keep the stoma opening away from the midline incision.
Split the rectus abdominis muscle in the direction of its fibers.
Make a vertical incision in the posterior rectus sheath.
Deliver the bowel through the abdominal wall. This can be facilitated by placing a small Penrose drain through a defect created in the mesentery adjacent to the bowel wall. The Penrose drain can then be used as a handle to help deliver the bowel. If desired, the drain can later be exchanged for the stoma bridge. Be careful with friable bowel, as the drain (or some prefer to use an umbilical tape) can inadvertently “saw” through the bowel with excessive tension.
Placement of a plastic self-retaining wound protector (e.g., Alexis Wound Protector [Applied Medical], Covidien SurgiSleeve [Covidien]) through the stoma trephine will facilitate passage of the bowel through the subcutaneous tissue, especially in obese individuals.
Confirm that the bowel is viable and not twisted by using the previously placed suture or mark in the distal segment ( Fig. 6 ).
Transect 80% of the circumference of the antimesenteric portion of the bowel wall just above where the distal end meets the skin between two Allis clamps ( Fig. 7 ).
Peel back the edges of the bowel to reveal the two openings. Exchange the catheter for the stoma bridge, if desired. The proximal limb should still protrude approximately 2.5 cm, but the distal limb can be matured flush with the skin.
Mature the stoma (proximal end of the bowel) by eversion with standard “triplicate” sutures on either side of the mesentery and the antimesenteric border with interrupted absorbable sutures. It is best to mature the distal end without eversion using as little of the skin circumference as is practical. Complete maturation with simple sutures including terminal bowel and dermis between every suture ( Fig. 8 ).
If using a bridge or rod to support the stoma above skin level, remove it after 5 days. Of note, a bridge is rarely necessary, particularly if the loop stoma is externalized and created without tension.
Fig. 6.
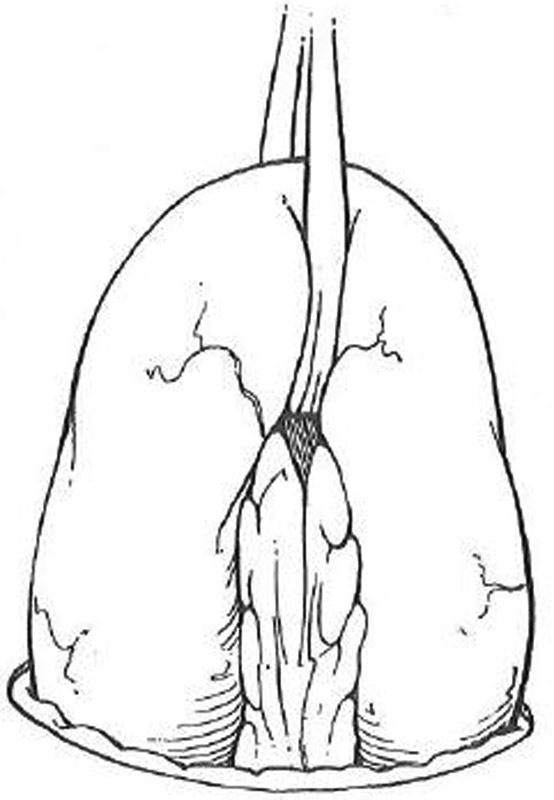
Loop ileostomy creation. Ileum is elevated through stoma site with a Penrose drain. Care is taken to avoid twisting.
Fig. 7.
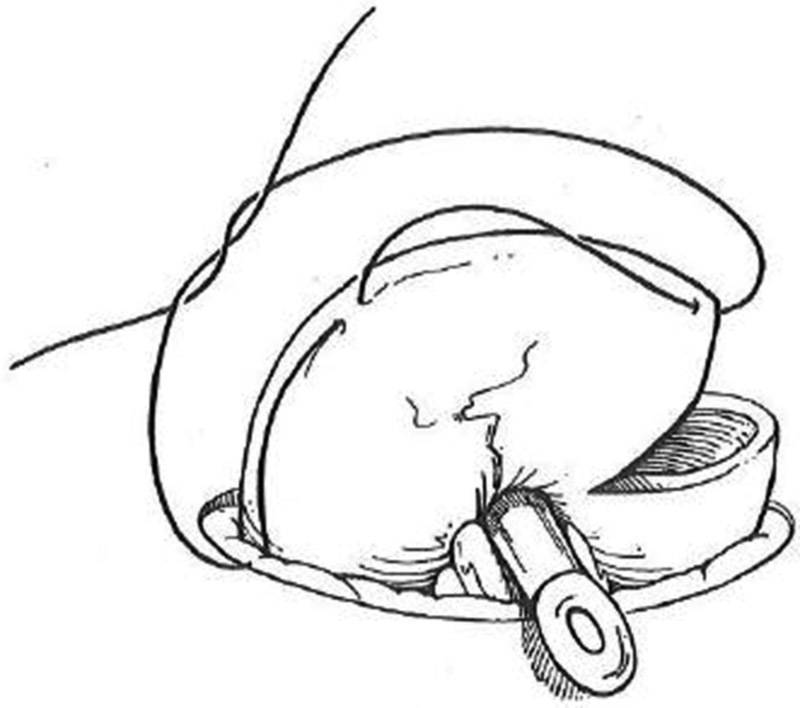
Loop ileostomy creation. Eighty percent of the antimesenteric circumference is transected between Allis clamps.
Fig. 8.
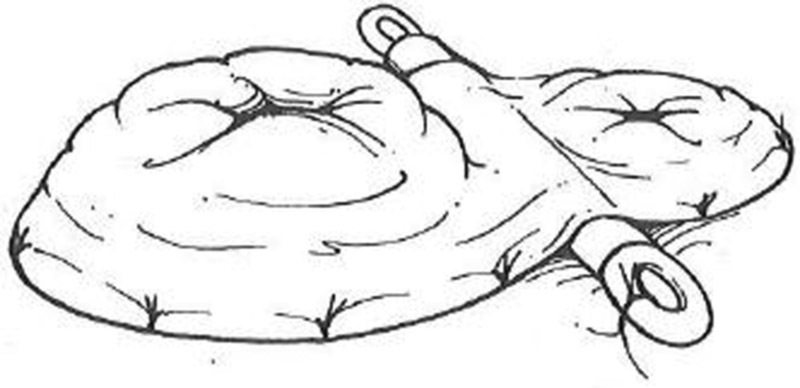
Loop ileostomy creation. Proximal end of the ileum is everted with standard three part sutures.
End-Loop Stomas
When creating a temporary stoma, it is always preferable, if possible, to bring the proximal and distal bowel loops through the same trephine in the abdominal wall. Among other advantages, this eases eventual stoma reversal by avoiding formal laparotomy. With standard loop stomas, this occurs by definition, but in other circumstances, it only occurs through proper technique and advanced planning. end-loop (or “Prasad” style) stomas can be created with remote intestinal segments following bowel resection. They consist of end-loop ileoileostomy, ileocolostomy, or colocolostomy. For example, it may be unsafe to perform a primary anastomosis after a right colectomy for trauma. An end-loop ileocolostomy (terminal ileum and transverse colon exiting through the same stoma site) is a viable alternative to an end ileostomy and long Hartmann's pouch, which would require a formal laparotomy for reversal in the future. Similar stomas can be performed following small bowel or left colon resections.
Whether using adjacent or remote intestinal segments, the technique for creating an end-loop stoma is similar. The stapled proximal end is passed through the preselected stoma site. Only the antimesenteric border of the stapled distal end is then advanced through the same trephine. The antimesenteric corner of the staple line is cut off and the small open segment of the distal bowel is matured flush to the skin using as little of the stoma circumference as possible. The proximal end is then matured in the standard fashion. A full thickness stitch between the proximal and distal ends completes the procedure ( Fig. 9 ). An end-loop sigmoid colostomy is an ideal stoma for distal fecal diversion for incontinence or in association with complex anorectal procedures. A stoma created with this technique will be easily reversible but will also function well should reversal be contraindicated.
Fig. 9.
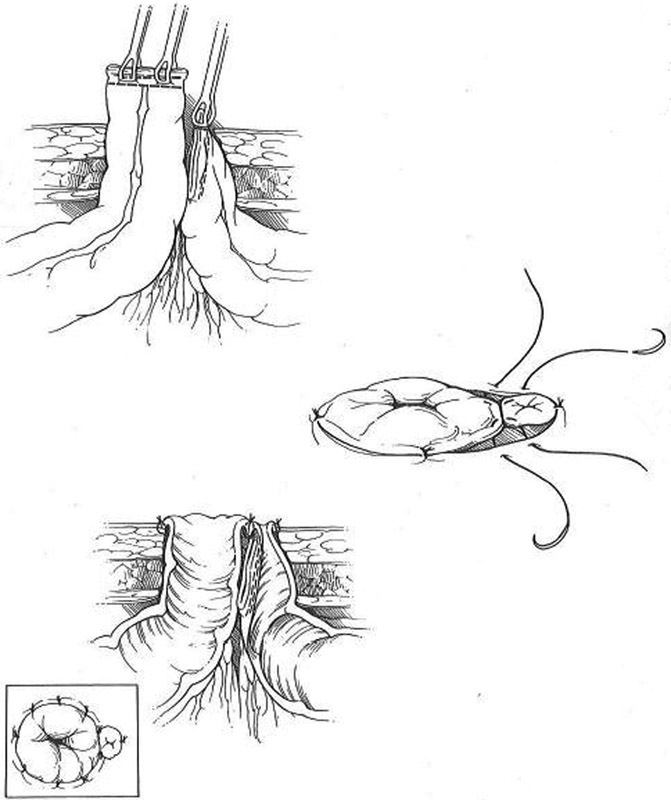
End-loop colostomy creation.
Laparoscopic Stoma Creation
Fecal diversion for unresectable cancers, severe perineal disease, or trauma is a fairly common procedure. Laparoscopic colostomy or ileostomy creation is an attractive alternative to a formal laparotomy, especially when ostomy creation is the sole purpose of the operation. As with any laparoscopic procedure, preoperative planning and patient positioning are key to success. Use of Trendelenburg positioning and right or left patient rotation, depending on the ostomy to be performed, will help expose the bowel segment targeted for stoma creation. Port placement depends upon the type of stoma undergoing creation. Using the future stoma site as a trocar site may be advantageous. Laparoscopic technique for stoma creation is detailed as follows ( Fig. 10 ):
Fig. 10.
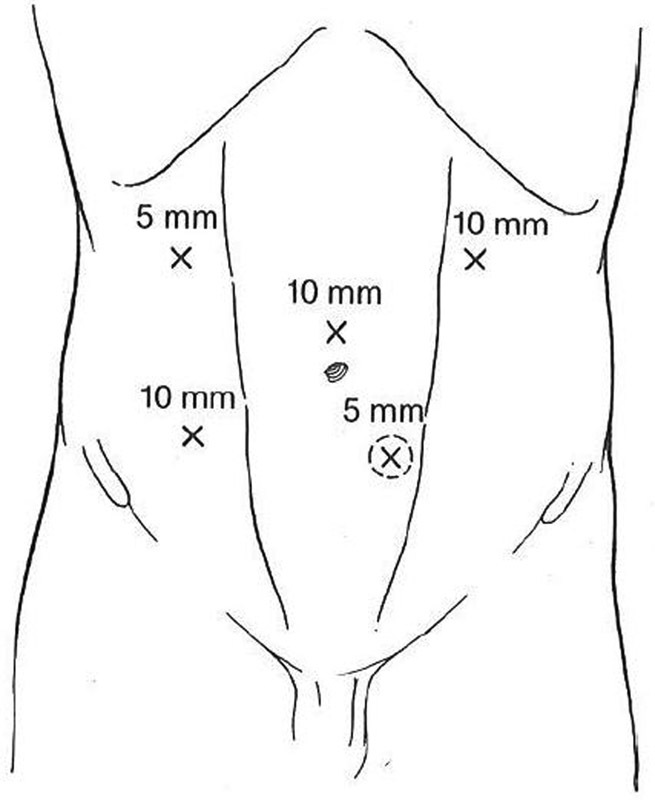
Potential port locations for laparoscopic ostomy creation.
Place the first trocar, which will accommodate the laparoscope, in the middle abdomen on the side opposite the future stoma.
If the previously marked site appears acceptable once the laparoscope is inserted, place a 5-mm port through the future stoma site.
Pass an atraumatic bowel clamp through this port, and grasp the needed segment of bowel and assess for mobility. If the desired bowel segment reaches without tension, enlarge the port site into a standard stoma trephine.
Marking the distal bowel prior to exteriorization with the felt tip of a marker held in a laparoscopic grasper facilitates proper orientation of the bowel once the stoma is matured.
If the bowel requires mobilization, which is common in sigmoid colostomy creation, place additional ports as necessary and mobilize along the white line of Toldt.
Enlarge the fascial defect as needed and deliver the bowel through the stoma site without twisting. Prior to maturing the stoma, reestablish pneumoperitoneum and confirm proximal and distal orientation of the bowel.
Mature the stoma in the standard fashion.
Prevention of Parastomal Hernias
In addition to stoma location, stomal trephine size is also important and, if created improperly, can lead to complications. Too small an opening can cause stoma ischemia and obstruction, whereas too large an opening can lead to parastomal hernia. The incidence of clinically symptomatic parastomal hernia is as high as 39% for colostomies and 6% for loop ileostomies. 5 When identified by computed tomography (CT), the incidence of parastomal hernia with a colostomy may be as high as 78%. 6 Up to one-third of these patients eventually require surgical repair due to pain, incarceration, obstruction, and poor appliance fitting. 6 Several factors may increase the risk of parastomal hernia formation, including female gender, age greater than 60 years, body mass index more than 25 kg/m 2 , hypertension, and waist circumference more than 100 cm. 7 8 Some groups propose that these patients may benefit from prophylactic mesh placement at the time of stoma creation; however, robust long-term data supporting routine use of prophylactic mesh is currently lacking.
One technique that mitigates risk of parastomal hernia is tunneling the stoma conduit through an extraperitoneal route. Such extraperitoneal tunneling has been reported during end sigmoid colostomy creation following laparoscopic abdominoperineal resection with promising results. 9 Conventional transperitoneal stomas developed more early postoperative hernias when compared with stomas created through an extraperitoneal route. A large meta-analysis of 1,071 patients also showed that extraperitoneal colostomies were associated with lower parastomal hernia rates compared with conventional colostomies. 10 Unfortunately, the aforementioned study findings are only applicable to patients undergoing permanent colostomy.
To create an extraperitoneal stoma, an incision is made in the parietal peritoneum immediately lateral to the transected end of the descending colon. A tunnel is then made that extends through the retroperitoneal tissues into the deep surface of the anterior abdominal wall. This tunnel is then connected to the stoma site on the anterior abdominal wall. Finally, the bowel is passed through the tunnel ( Fig. 11 ). 11
Fig. 11.
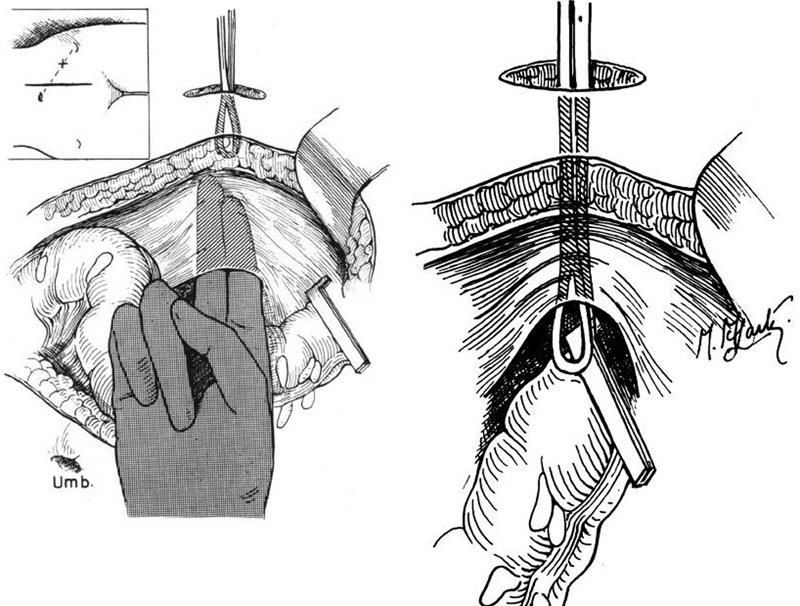
Creation of passage and tunneling of end colostomy.
When hernias do occur, mesh is often required to close or repair the defect since simple fascial repair or stoma relocation have high recurrence rates. 12 In an attempt to reduce the rate of hernias, surgeons began placing mesh prophylactically. Several small studies have shown promising results with prophylactic mesh placement. 6 Two randomized trials using a lightweight polypropylene mesh found significantly more parastomal hernias in conventional (i.e., nonmesh) group (53.7%) when compared with the mesh group (14.8%). 6 Additionally, prophylactic mesh patients who developed clinical evidence of parastomal hernia were less likely to require corrective surgery. Intriguingly, mesh-related complications were rare despite theoretic concerns of stomal bacterial contamination. Most studies examining the role of prophylactic mesh used permanent (i.e., nonabsorbable) mesh. Biological mesh has also been used prophylactically with similar results; however, the costs of some biological meshes may be prohibitive. 13 The PREVENT trial, a multicenter randomized controlled study, is currently enrolling patients to determine if prophylactic peristomal placement of a retromuscular, preperitoneal monofilament polypropylene mesh prevents parastomal herniation for patients requiring a permanent end colostomy. 6
The PREVENT trial uses a standardized technique for mesh placement. 6 First, the bowel intended for colostomy is stapled closed to minimize contamination. The trephine is created by excision of the skin-oval at the preoperatively marked ostomy site without excising any subcutaneous tissue. After exposing the anterior rectus sheath, a cruciate fascial incision is made. The rectus abdominis muscle is split (but not divided) in the direction of the fibers. A retromuscular space is created and dissected to the lateral stomal border through the midline laparotomy. The posterior fascia and peritoneum are left undisturbed. Then a 10 × 10 cm piece of mesh with a centered cruciate incision is placed between the rectus abdominis muscle and posterior rectus sheath, thereby allowing passage of the colonic conduit. The lateral corners of the mesh are fixed with absorbable monofilament sutures. The posterior fascia is opened over the trephine in the mesh, and the bowel is passed through the reinforced trephine in a stepwise manner. The running suture used to close the midline incision intentionally includes bites through the medial mesh border and the peritoneum, thus preventing contact between the mesh and the viscera. Finally, the stoma is matured ( Fig. 12 ). Preliminary data with 150 patients (72 mesh, 78 control) show that operative times are significantly longer in the mesh group with no difference in early outcomes after 3 postoperative months. 14
Fig. 12.
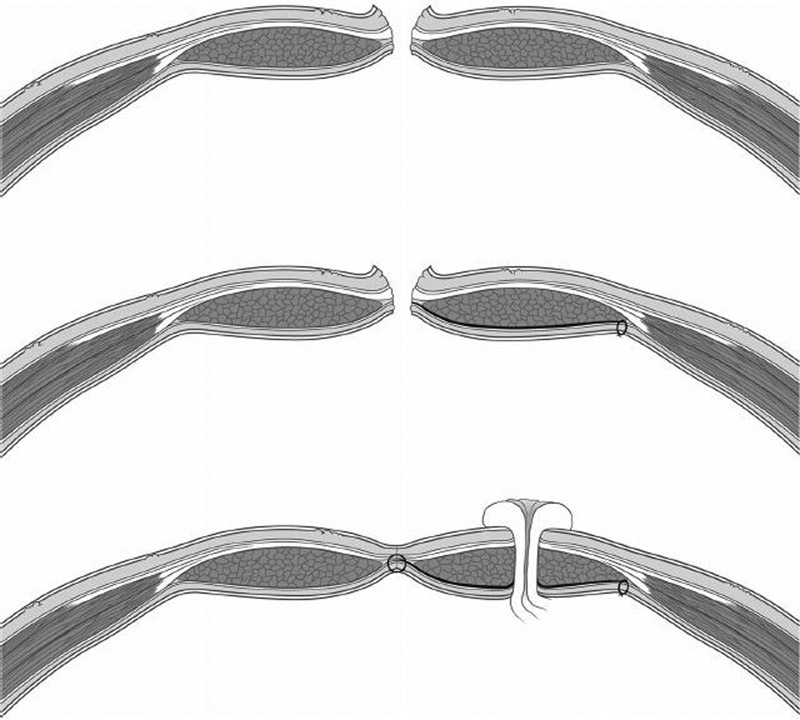
Steps in stoma creation for PREVENT trial.
Some opponents of prophylactic mesh placement argue that routine mesh use is too time-consuming and difficult to perform laparoscopically. One such group has proposed a stapled mesh stoma reinforcement technique, or SMART, to prevent parastomal hernias. 15 Following excision of the skin and soft tissue cylinder, opening of the anterior rectus sheath, and splitting of the rectus muscle, the posterior sheath/peritoneum is pierced with the tips of forceps used to grasp the anvil to a 28-mm circular stapler placed within the abdominal cavity. The main body of the stapler is preloaded with a 5-cm-diameter circular mesh and is mated with the exteriorized anvil shaft. The stapler is closed, fired, and removed to create a stapled assembly of a mesh-reinforced posterior rectus sheath and peritoneum featuring a precisely cut and stapled stoma trephine. Portion of the outer mesh circumference is sutured to the anterior rectus sheath so that it lines the trephine ( Fig. 13 ). The stoma is then fashioned using standard techniques. The SMART technique has been shown to reduce risk of parastomal hernias in high-risk patients. 16 Randomized trials are underway to determine the efficacy and cost-effectiveness of the technique in all patients undergoing permanent stoma formation.
Fig. 13.
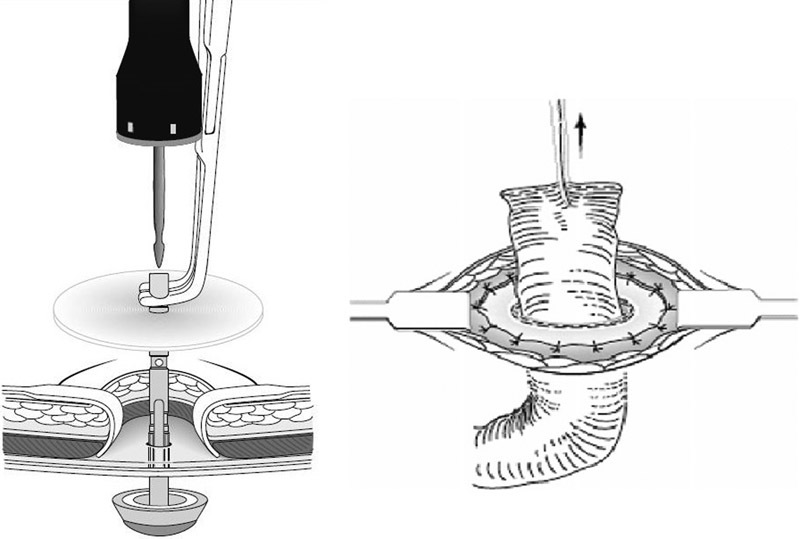
Steps in stoma creation for stapled mesh stoma reinforcement technique trial.
Prevention of parastomal hernias may also be influenced by the type of incision made in the fascia. “Stoma-Const” is a Scandinavian randomized trial for patients undergoing permanent colostomy formation. Patients will undergo colostomy formation with cruciate fascial incision, circular fascial excision, or prophylactic sublay mesh with cruciate fascial incision. 17 The study's primary endpoint will be formation of parastomal hernia at 1 year diagnosed either clinically or by CT scan. Study investigators will examine readmission rates, postoperative complications, length of hospital stay, quality of life, and mortality rates.
At the authors' institution, tunneling is rarely used, and mesh use is reserved to repair, but not prevent, parastomal hernias.
The Obese Patient
Obesity poses multiple challenges ranging from stoma siting to obtaining adequate bowel length to span subcutaneous tissues. Additionally, the mere process of bringing the bowel through the abdominal wall trephine can be difficult. Obesity is recognized as a risk factor for stoma complications, and obese patients are seven times more likely to suffer from stoma necrosis than nonobese patients. 18 Obese individuals have both a thick abdominal wall and a short, thick, and heavy mesentery that challenges construction of a tension-free and well-vascularized stoma.
Fortunately, a few techniques have been described to assist the surgeon with these difficult patients. Horwood and Hay propose the use of a surgical glove to reduce the trauma inflicted on the bowel and mesentery as it passes through the trephine. 19 Meagher et al use an Alexis Wound Protector in a similar fashion. 20 Additionally, the investigators felt that use of a plastic wound protector allows the surgeon to make a smaller defect in the abdominal wall. The authors of this article advocate that all patients, regardless of habitus, may benefit from the use of a plastic wound protector during stoma creation ( Fig. 14 ). When a thick abdominal wall inhibits creation of a tension-free stoma, subcutaneous lipectomy may be performed. 18 In this instance, the subcutaneous fat is removed and the skin affixed to the fascia. Closed suction drainage helps to obliterate any residual dead space. Similarly, Klein et al reported the removal of an elliptical segment of skin and subcutaneous tissue to facilitate the creation of a flat surface around the stoma. 21
Fig. 14.
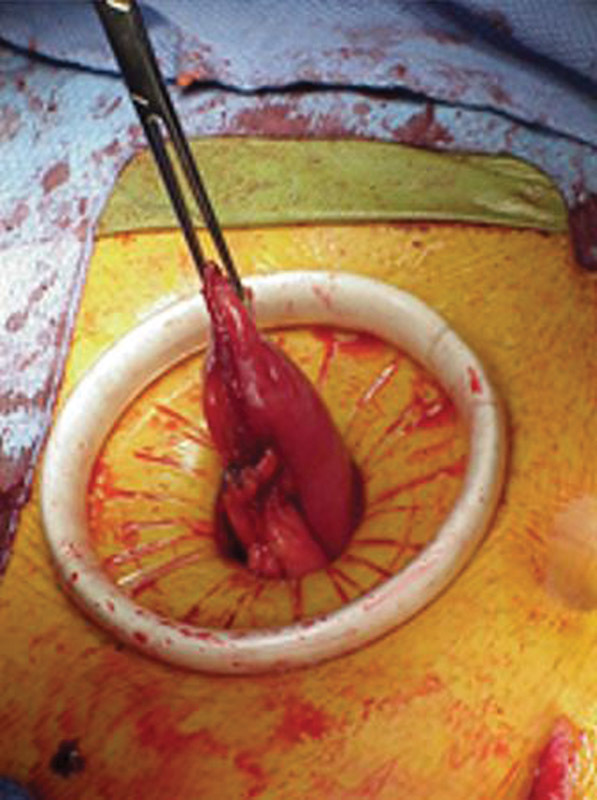
An Alexis Wound Protector facilitates passage of bowel through the abdominal wall.
While it is impossible to predict patient weight fluctuations, the surgeon should be mindful that significant postoperative weight loss can draw the stoma caudally. If the original stoma was sited remotely cephalad to a skin fold, future weight changes do not typically pose a problem. In those individuals in which weight loss has caused the stoma to migrate into a skin fold, a panniculectomy can be performed, thereby avoiding a proper stoma relocation through laparotomy. 22 For patients with smaller abdominal wall deformities that impair pouching, subcutaneous parastomal porcine collagen injections may be a suitable nonoperative option. 23
The Difficult Colostomy
The classic situation leading to a difficult colostomy occurs following emergency sigmoid resection for acute diverticulitis in an obese male with a shortened, thickened, mesentery and a very thick abdominal wall. In this situation, creation of a well-perfused protruding colostomy can be challenging. As previously mentioned, preoperative stoma site marking, particularly above the umbilicus, is invaluable. Since the abdominal pannus tends to be thinner superiorly, a well-mobilized descending colon typically protrudes through the upper abdominal wall with less tension compared with the lower abdomen. Finally, obese individuals manage supraumbilical stomas better than infraumbilical stomas due to improved line of sight to the superior portions of a pendulous pannus than the inferior dependent portions. The following tips facilitate optimal stoma creation:
Determine if there is a safe alternative to permanent colostomy creation. For example, resection and primary anastomosis with or without diverting ileostomy creation may be a better alternative than permanent stomas in some individuals.
Excise all inflamed colon and use healthy disease-free intestine, if possible, for stoma creation. The segment of colon used for the colostomy should be free of any inflamed tissue, thickening, or edema.
The left lateral peritoneal reflection should be completely divided, and the corresponding mesentery should be fully mobilized leaving the left colon attached only to its midline mesentery.
Medial peritoneal attachments at the base of the colonic mesentery should be transected if further mobility is required.
If needed, fully mobilize the splenic flexure to obtain tension-free reach of the colonic conduit.
If additional length is necessary, the inferior mesenteric artery can be ligated proximal to the takeoff of the left colic artery. Similarly, high ligation of the inferior mesenteric vein at the inferior pancreatic border can provide additional length, if needed.
Create “windows” by sequentially scoring the mesenteric peritoneum along the medial and lateral aspects of the descending mesocolon to increase length. This technique is similar to ileal–peritoneal “pie crusting,” which is sometimes used as a lengthening maneuver during an ileal pouch–anal anastomosis.
The mesentery adjacent to the terminal left colon can be trimmed provided that a 1-cm segment of mesentery containing the marginal artery remains intact along the colonic wall.
An oversized abdominal trephine will often allow passage of a thick colonic mesentery, preventing venous congestion and subsequent stomal ischemia.
The importance of a cephalad stoma site in the obese patient cannot be overstated. Selecting a superior stoma site will decrease the thickness of the abdominal wall through which the colon must pass. Using this site will also decrease tension on the stoma since mobilized left colon reaches more easily to the supraumbilical abdominal wall.
Using a self-retaining cylindrical plastic wound protector through the stoma site, and rolling it in the standard fashion will increase the diameter of the trephine, decrease the thickness of the abdominal wall, and decrease friction between the bowel and the subcutaneous tissues. All of these factors will ease stoma creation. After the bowel is passed through the abdominal wall, the inner ring of the Alexis Wound Protector is transected and removed abdominally. The outer portion is removed around the stoma.
Any stoma where length or vascularity is tenuous should be matured prior to closure of the midline incision. The closed abdominal incision acts as a psychological barrier that leads the surgeon to accept a marginally perfused stoma rather than reopen the abdominal incision.
Unfortunately, even using each of these maneuvers may not produce a tension-free and viable stoma. In this case, it may be more appropriate to create an ileostomy. When even an ileostomy is not possible, a “pseudoloop colostomy” can be created. Following the aforementioned steps, a segment of colon several centimeters proximal to the stapled portion is selected ( Fig. 15 ). An oversized trephine is created in the portion of the abdominal wall that results in the least colonic tension. Next, only the antimesenteric border of the previously selected colonic segment is passed through the abdominal wall. A small colotomy is made in the antimesenteric border of the protruding bowel and is primarily matured to the skin without eversion. This stoma will function appropriately, but will be less than ideal and may require later revision.
Fig. 15.
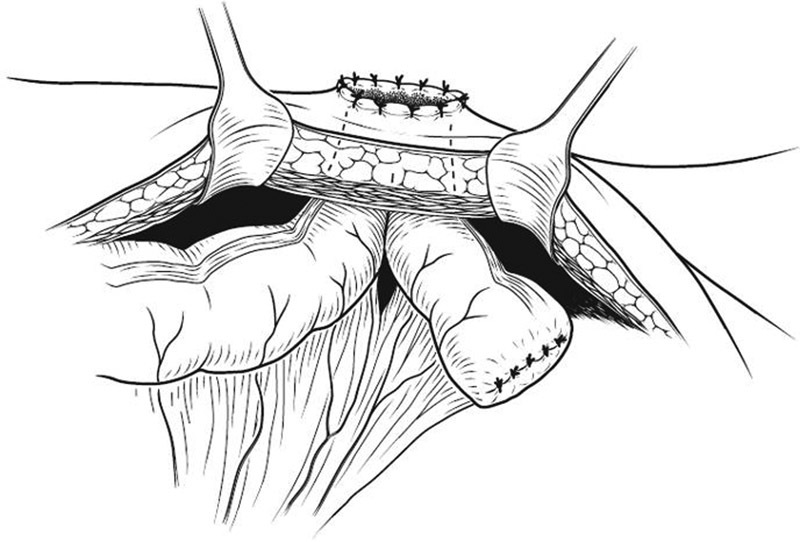
Pseudoloop colostomy creation.
The Distended Colon
Stomas are often created in cases of a large bowel obstruction where colonic distention presents three barriers to both primary anastomosis and stoma formation. First, the obstructed colon is by nature ischemic; therefore, it is difficult to assess stoma vascularity and more likely to develop clinically significant ischemia. Second, the mobility of an obstructed colonic segment is impaired, making it challenging to either create a stoma that protrudes appropriately through the abdominal wall or create a tension-free stoma. Third, the dilated colon requires a large abdominal wall trephine, which can eventually lead to a parastomal hernia once the bowel distention is relieved.
Decompression of the obstructed segment corrects all these difficulties. In extreme situations, this can be accomplished prior to passing the bowel through the abdominal wall. Although not ideal, it may be beneficial to mature the stoma prior to closing the abdominal midline incision. If possible, the colon is passed through the previously created stoma site. The staple line is resected and the bowel decompressed after protecting the operative field with towels. Once the bowel has been decompressed, it becomes more mobile and can be advanced further out of the abdomen. Its viability can then be reassessed. If the stoma's vascularity is adequate, it can be matured in the standard fashion. If not, the colon can be further mobilized with the abdomen still open, hopefully identifying a well-perfused segment appropriate for maturation. This technique does increase the risk of surgical site infection, but can be helpful in difficult situations.
The Difficult Ileostomy
While creating a functioning ileostomy is generally not as challenging as a colostomy, the obese abdominal wall or a short, thickened mesentery can produce frustration. The following guidelines should prove beneficial:
As with a colostomy, consider supraumbilical placement.
Mobilization of the small bowel mesentery to the base of the duodenum, as done for an ileal pouch–anal anastomosis, often results in substantial mobility.
High ligation of the ileocolic artery can typically be performed at its origin without causing ileal ischemia. This maneuver increases mobility of the terminal ileal mesentery and improves reach to the abdominal wall.
An oversized abdominal wall trephine is beneficial in decreasing tension and improving perfusion in thickened bowel with short mesentery.
Although not ideal, a “noneverted” ileostomy can be created in an emergency situation. This situation may eventually result in stomal stenosis, but may allow temporization in a difficult situation.
Very rarely these maneuvers will not result in a viable ostomy. In this case, a “pseudoloop” technique can also be tried, substituting the ileum for colon. The stoma will undoubtedly be difficult to manage for both the patient and care team and may require early reversal. If a permanent stoma becomes nonviable, a revision is almost always necessary.
Conclusions
Stoma creation is often the last component of a long and difficult operation and may seem trivial when compared with the essential portions of the surgery. Yet, the stoma will undoubtedly have the largest impact on the patient's quality of life in the long term. A well-made and properly sited stoma will have minimal implications once the patient has adjusted to its presence. Alternatively, a difficult or complicated stoma will plague both the patient and the surgeon.
The easiest way to avoid a difficult stoma is to avoid stoma formation. When this is not possible, careful preoperative planning can help to avoid future complications. Both eventual stoma reversal, when appropriate, and long-term patient satisfaction should always be considered at the time of stoma creation, no matter how difficult and complex the procedure. Unfortunately, even a well-planned stoma can be problematic. Prophylactic mesh placement may decrease the incidence of parastomal hernia. An experienced enterostomal therapist can assist with many issues as they arise, but sometimes the only option is stoma revision.
Unfortunately, challenging patients and situations will still arise, in which the surgeon has to make sacrifices. It is not always possible to create the ideal stoma with the ideal bowel segment in the ideal location. In these situations, it is best to remember the real-estate mantra “location, location, location.” It is “better to create an ugly stoma in a good location, than to create a pretty stoma in a bad location.” An ugly stoma may resolve into a well-vascularized and well-functioning stoma, but there is little hope for a stoma in a bad location.
References
- 1.McLeod R S, Lavery I C, Leatherman J R et al. Factors affecting quality of life with a conventional ileostomy. World J Surg. 1986;10(03):474–480. doi: 10.1007/BF01655313. [DOI] [PubMed] [Google Scholar]
- 2.Mengual-Ballester M, García-Marín J A, Pellicer-Franco E et al. Protective ileostomy: complications and mortality associated with its closure. Rev Esp Enferm Dig. 2012;104(07):350–354. doi: 10.4321/s1130-01082012000700003. [DOI] [PubMed] [Google Scholar]
- 3.Shiomi A, Ito M, Saito N et al. Diverting stoma in rectal cancer surgery. A retrospective study of 329 patients from Japanese cancer centers. Int J Colorectal Dis. 2011;26(01):79–87. doi: 10.1007/s00384-010-1036-0. [DOI] [PubMed] [Google Scholar]
- 4.Williams N S, Nasmyth D G, Jones D, Smith A H. De-functioning stomas: a prospective controlled trial comparing loop ileostomy with loop transverse colostomy. Br J Surg. 1986;73(07):566–570. doi: 10.1002/bjs.1800730717. [DOI] [PubMed] [Google Scholar]
- 5.Carne P WG, Robertson G M, Frizelle F A. Parastomal hernia. Br J Surg. 2003;90(07):784–793. doi: 10.1002/bjs.4220. [DOI] [PubMed] [Google Scholar]
- 6.Brandsma H T, Hansson B M, V-Haaren-de Haan H, Aufenacker T J, Rosman C, Bleichrodt R P. PREVENTion of a parastomal hernia with a prosthetic mesh in patients undergoing permanent end-colostomy; the PREVENT-trial: study protocol for a multicenter randomized controlled trial. Trials. 2012;13:226. doi: 10.1186/1745-6215-13-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sohn Y J, Moon S M, Shin U S, Jee S H. Incidence and risk factors of parastomal hernia. J Korean Soc Coloproctol. 2012;28(05):241–246. doi: 10.3393/jksc.2012.28.5.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Raet J, Delvaux G, Haentjens P, Van Nieuwenhove Y. Waist circumference is an independent risk factor for the development of parastomal hernia after permanent colostomy. Dis Colon Rectum. 2008;51(12):1806–1809. doi: 10.1007/s10350-008-9366-5. [DOI] [PubMed] [Google Scholar]
- 9.Hamada M, Ozaki K, Muraoka G, Kawakita N, Nishioka Y. Permanent end-sigmoid colostomy through the extraperitoneal route prevents parastomal hernia after laparoscopic abdominoperineal resection. Dis Colon Rectum. 2012;55(09):963–969. doi: 10.1097/DCR.0b013e31825fb5ff. [DOI] [PubMed] [Google Scholar]
- 10.Lian L, Wu X R, He X S et al. Extraperitoneal vs. intraperitoneal route for permanent colostomy: a meta-analysis of 1,071 patients. Int J Colorectal Dis. 2012;27(01):59–64. doi: 10.1007/s00384-011-1293-6. [DOI] [PubMed] [Google Scholar]
- 11.Elliot-Smith A, Painter N S. Experiences with extraperitoneal colostomy and ileostomy. Gut. 1961;2:360–362. doi: 10.1136/gut.2.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aquina C T, Iannuzzi J C, Probst C Pet al. Parastomal hernia: a growing problem with new solutions Dig Surg 201431(4-5):366–376. [DOI] [PubMed] [Google Scholar]
- 13.Figel N A, Rostas J W, Ellis C N.Outcomes using a bioprosthetic mesh at the time of permanent stoma creation in preventing a parastomal hernia: a value analysis Am J Surg 201220303323–326., discussion 326 [DOI] [PubMed] [Google Scholar]
- 14.Brandsma H T, Hansson B M, Aufenacker T J et al. Prophylactic mesh placement to prevent parastomal hernia, early results of a prospective multicentre randomized trial. Hernia. 2016;20(04):535–541. doi: 10.1007/s10029-015-1427-9. [DOI] [PubMed] [Google Scholar]
- 15.Williams N S, Nair R, Bhan C. Stapled mesh stoma reinforcement technique (SMART)--a procedure to prevent parastomal herniation. Ann R Coll Surg Engl. 2011;93(02):169. doi: 10.1308/003588411X12851639107313c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams N S, Hotouras A, Bhan C, Murphy J, Chan C L. A case-controlled pilot study assessing the safety and efficacy of the Stapled Mesh stomA Reinforcement Technique (SMART) in reducing the incidence of parastomal herniation. Hernia. 2015;19(06):949–954. doi: 10.1007/s10029-015-1346-9. [DOI] [PubMed] [Google Scholar]
- 17.Correa Marinez A, Erestam S, Haglind E et al. Stoma-Const--the technical aspects of stoma construction: study protocol for a randomised controlled trial. Trials. 2014;15:254. doi: 10.1186/1745-6215-15-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meguid M M, McIvor A, Xenos L. Creation of a neoabdominal wall to facilitate emergency placement of a terminal ileostomy in a morbidly obese patient. Am J Surg. 1997;173(04):298–300. doi: 10.1016/s0002-9610(96)00393-5. [DOI] [PubMed] [Google Scholar]
- 19.Horwood J, Hay D. The ‘glove cuff’ technique for difficult stomas. Ann R Coll Surg Engl. 2009;91(05):438. doi: 10.1308/rcsann.2009.91.5.438a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meagher A P, Owen G, Gett R. Multimedia article. An improved technique for end stoma creation in obese patients. Dis Colon Rectum. 2009;52(03):531–533. doi: 10.1007/DCR.0b013e31819a2441. [DOI] [PubMed] [Google Scholar]
- 21.Klein F A, Herr H W, Sogani P C, Whitmore W F., Jr Panniculectomy in conjunction with radical cystectomy in the obese patient. Surg Gynecol Obstet. 1983;156(01):31–33. [PubMed] [Google Scholar]
- 22.Zolfaghari S, Gauthier J C, Jarmuske M B, Boushey R P. Panniculectomy: an alternative approach to the revision of a difficult stoma. Colorectal Dis. 2011;13(07):e176–e177. doi: 10.1111/j.1463-1318.2010.02388.x. [DOI] [PubMed] [Google Scholar]
- 23.Arai Y, Okubo K. Correction of dermal contour defect with collagen injection: a simple management technique for difficult stomal care. J Urol. 1999;161(02):601–602. [PubMed] [Google Scholar]


