Abstract
BACKGROUND
Clinical misdiagnosis, particularly at early disease stages, is a roadblock to finding new therapies for Lewy body disorders. Biopsy of a peripheral site might provide improved diagnostic accuracy. Previously, we reported, from both autopsy and needle biopsy, a high prevalence of submandibular gland synucleinopathy in Parkinson’s disease (PD). Here, we report on an extension of these studies to subjects with dementia with Lewy bodies (DLB) and other Lewy body disorders in 228 autopsied subjects from the Arizona Study of Aging and Neurodegenerative Disorders.
OBJECTIVE
To provide an estimate of the prevalence of histological synucleinopathy in the submandibular glands of subjects with PD and other Lewy body disorders.
METHODS
Submandibular gland sections from autopsied subjects were stained with an immunohistochemical method for α-synuclein phosphorylated at serine 129. Included were 146 cases with CNS Lewy-type synucleinopathy (LTS), composed of 46 PD, 28 DLB, 14 incidental Lewy body disease (ILBD), 33 Alzheimer’s disease with Lewy bodies (ADLB) and 2 with progressive supranuclear palsy and Lewy bodies (PSPLB). Control subjects included 79 normal elderly, 15 AD, 12 PSP, 2 corticobasal degeneration (CBD) and 2 multiple system atrophy (MSA).
RESULTS
Submandibular gland LTS was found in 42/47 (89%) of the PD subjects, 20/28 (71%) DLB, 4/33 (12%) ADLB and 1/9 (11%) ILBD subjects but none of the 110 control subjects.
CONCLUSIONS
These results provide support for further clinical trials of in vivo submandibular gland diagnostic biopsy for PD and DLB. An accurate peripheral biopsy diagnosis would assist subject selection for clinical trials and could also be used to verify other biomarkers.
Keywords: biopsy, diagnosis, clinical trial, biomarker, pathology, therapy
INTRODUCTION
The accuracy of the clinical diagnosis of Parkinson’s disease (PD) has been reported to range between 26% and 90% [1–6] with the higher accuracy figures being dependent on prolonged clinical observation and response to dopaminergic treatment. Misdiagnosis may occur in close to 50% of PD subjects who are within the first 5 years of symptom onset, even if responsive to dopaminergic medication [7]. The situation is even more problematic for dementia with Lewy bodies (DLB), as only 15–25% of neuropathologically-defined DLB subjects are identified as DLB during life [8]. As early diagnosis and treatment of PD and DLB are likely to be more beneficial, especially for disease-modifying treatments, it is clear that poor clinical diagnostic accuracy is a critical roadblock to clinical trials and biomarker studies as these are heavily dependent on the clinical diagnosis. Additionally, for clinical trials utilizing invasive methods, such as pallidotomy, deep brain stimulation or neural transplantation [9–17], misdiagnosis exposes considerable numbers of subjects to potentially harmful procedures.
If the characteristic Lewy-type α-synucleinopathy (LTS) seen in the brains of these cases could be sensitively and reliably detected with a biopsy of peripheral tissue, this could improve clinical diagnostic accuracy, thereby facilitating clinical trials by allowing their performance with fewer subject numbers and lower cost. Additionally, biopsy could be used as the gold-standard diagnostic test to evaluate less-invasive candidate biomarkers. In a comprehensive survey of multiple organs and tissue types [18], we found LTS to be widespread throughout the peripheral nervous system (PNS) of subjects with PD and DLB, and to be detectable, although at much sparser densities and with a more restricted distribution, in less severe forms of Lewy body disorders such as incidental Lewy body disease (ILBD) and Alzheimer’s disease with Lewy bodies (ADLB). Our survey indicated that the PNS sites with the greatest LTS prevalences and densities were the paraspinal sympathetic ganglia, vagus nerve, submandibular gland and lower esophagus. Of these peripheral locations, the submandibular gland is the most accessible and safe to biopsy and we therefore have pursued additional studies [19], including two pilot clinical trials of submandibular gland needle biopsy in people with PD [20, 21].
Here, we report on a further extension of these studies in submandibular glands from 228 autopsied subjects.
MATERIALS AND METHODS
Human subjects
The study took place at Banner Sun Health Research Institute (BSHRI), which is part of Banner Health, a non-profit healthcare provider centered in Phoenix, Arizona. BSHRI and the Mayo Clinic Arizona are the principal members of the Arizona Parkinson’s Disease Consortium. Brain necropsies and neuropathological examinations were performed on elderly subjects who had volunteered for the Arizona Study of Aging and Neurodegenerative Disorders/BSHRI Brain and Body Donation Program (BBDP) [22]. The BBDP has been approved by the designated BSHRI Institutional Review Board. The majority of BBDP subjects are clinically characterized at BSHRI with annual standardized test batteries that include movement disorders and neuropsychological components, including the Mini Mental State Examination (MMSE) and Unified Parkinson’s Disease Rating Scale (UPDRS). Additionally, private medical records are requisitioned and reviewed for each subject and a postmortem questionnaire is conducted with subject contacts to help determine the presence or absence of dementia and parkinsonism for those subjects that did not have a recent standardized antemortem evaluation.
Subjects received complete neuropathological examinations as described previously [23]. Specific consensus diagnostic criteria were used for PD [3,24], DLB and Alzheimer’s disease (AD). For the latter two conditions, cases received the diagnosis if they were classified as “intermediate” or “high” according to National Institute on Aging – Reagan Institute criteria for AD [25] or the Dementia with Lewy Bodies Consortium for DLB [26]. Progressive supranuclear palsy (PSP), corticobasal degeneration (CBD) and multiple system atrophy (MSA) were diagnosed according to conventional published criteria [27–37]. Subjects with AD or PSP who had LTS but did not otherwise meet diagnostic criteria for either PD or DLB were designated as AD with Lewy bodies (ADLB) or PSP with Lewy bodies (PSPLB). Subjects with LTS but who lacked dementia or parkinsonism were termed incidental Lewy body disease (ILBD).
Case selection was done by searching the BBDP database for all those that had died and had a full autopsy including submandibular gland evaluation with immunohistochemical staining for phosphorylated α-synuclein.
Histological methods
The process leading to the choice of immunohistochemical method has been described in a previous publication [38]. The usage of proteinase K not only results in superior epitope exposure but also may assist with the pathological specificity of the stain by digesting normal, non-aggregated α-synuclein. Using an antibody specific for α-synuclein phosphorylated at serine 129 [39–43] also helps identify stained structures as pathological since normal control subjects never have immunohistochemically-positive brain tissue elements [44,45]. Complete details of the staining procedure have been previously described [19] and so only a brief description is given here.
Formalin-fixed, paraffin-embedded 5–7 μm sections were deparaffinized and treated with 1:100 proteinase K (Enzo Life Sciences, Farmingdale, NY) at 37° C for 20 minutes, followed by suppression of endogenous peroxidase activity with 1% hydrogen peroxide for 30 minutes, incubation in primary antibody against α-synuclein phosphorylated at serine 129, diluted 1:10,000 [40–43], incubation in biotinylated secondary antibody, avidin-biotin peroxidase complex (ABC, Vector Laboratories; Burlingame, CA) and 3,3′-diaminobenzidine (DAB; Sigma, St. Louis, MO) with saturated nickel ammonium sulfate and imidazole. All solutions subsequent to proteinase K, and all wash steps, excluding DAB incubation, were carried out in 0.1 M PBS with 0.3% Triton X-100, pH 7.4. Sections were then counterstained in 1% Neutral Red. Positive neuronal perikarya and nerve fibers are bluish-black.
Two-color immunofluorescence was used to confirm the colocalization of LTS within axons. The same anti-phosphorylated α-synuclein antibody utilized for the single-label immunoperoxidase procedures was used in combination with an antibody against neurofilament (Abcam ab 7795, Cambridge, MA), along with goat anti-mouse IgG for neurofilament and goat anti-rabbit IgG for phosphorylated α-synuclein, fluorescently-conjugated with Alexa Fluor 546 and 488, respectively (Molecular Probes, Eugene, OR). Sections were viewed with conventional fluorescence microscopy (Nikon Eclipse 80i fluorescent microscope equipped with a Digital Sight DS-Ri1 camera) and laser confocal microscopy (Leica DM-25 equipped with TCS SPE confocal).
Submandibular gland tissue processing and slide evaluation
Large (approximately 1.5 cm2) segments of submandibular gland from all study subjects were placed at autopsy into standard paraffin embedding cassettes and fixed in 10% neutral buffered formalin for two days at 4 degrees C, followed by 2 × 60 minutes changes in 50% ethanol, dehydration and paraffin embedding. Sections were cut at 6 μm and stained for α-synuclein phosphorylated at serine 129 as described above. Submandibular gland sections were counted as positive for LTS only if stained elements had morphological features consistent with nerve fibers; occasionally the cytoplasm of serous gland cells or of extracellular secretion within gland contents were stained but this was regarded as non-specific, based on a previous analysis [46]. Initially, only 1–3 sections were stained and examined. In 3 PD cases that were initially negative as well as in a subset of ADLB cases and all of the ILBD cases, additional sections were immunostained: Of the 3 PD cases, 1 of these had 2 80-μm-thick free-floating sections stained and examined while for the other 2 cases, 4 additional paraffin sections were stained and examined for each case; all were positive on at least 1 of the additional sections. Nine ADLB cases had additional sections stained and examined. Of these, 5 had between 1 and 5 additional 80-μm-thick stained sections examined and 7 had an additional 4 stained paraffin sections examined (4 cases had extra sections of both types stained and examined); of these, 2 cases were positive on at least 1 of the additional sections. One ILBD case had an additional 20 paraffin sections stained and examined whereas another 4 ILBD cases each had 10 additional paraffin sections stained and examined; none of the additional sections were positive. Of the normal control subjects, 23 had one section of submandibular gland stained while 28 had two sections stained and 28 more had three sections stained.
As for the brain regions, the densities of immunoreactive fibers were graded, at 400 × total magnification, at sites of maximum density as mild, moderate, severe and very severe, according to the templates published by the Dementia with Lewy Bodies Consortium [26].
Statistical tests
Group means were compared with unpaired, 2-tailed t-tests. Groups of three or more were compared with one-way analysis of variance. Correlations were performed using Spearman’s method.
RESULTS
Description of diagnostic groups
Subjects with Lewy body disorders included 46 PD, 28 DLB, 9 ILBD, 33 ADLB and 2 PSPLB (Table 1). Control subjects, defined as those without CNS LTS, included 79 normal elderly subjects, 15 AD (ADNLB), 12 PSP, 2 with CBD and 2 with MSA. The subjects were predominantly of advanced age, with the mean age ranges for the diagnostic groups falling between 73 (CBD) and 90 (PSPLB). Of the DLB cases, all except one also met diagnostic criteria for AD. The classification of cases with LTS according to the Unified Staging System for Lewy Body Disorders [43] is given in Table 2, along with the summary scores of brain regional LTS densities and submandibular gland LTS densities. The majority of DLB and PD subjects were Stage IV (Neocortical) and Stage III (Brainstem and Limbic), while ADLB and PSPLB subjects were mostly Stage IIb (Limbic Predominant) or Stage III, and ILBD subjects were distributed more evenly between Stages I (Olfactory Bulb Only), IIa (Brainstem Predominant), IIb and III. The summary of brain regional LTS density scores was approximately 2.5-fold higher for PD and DLB as compared to ILBD, ADLB or PSPLB. The median postmortem interval, for all subjects combined, was 3.1 hours and analysis of variance showed that there were no significant differences amongst groups.
Table 1.
General characteristics of the study subjects, by neuropathologic diagnosis, age, gender, last MMSE score, last motor UPDRS score, Braak neurofibrillary stage and CERAD neuritic plaque (NP) density. Three PSP cases and one CBD case also had PD while one PSP case also had DLB and two other PSP cases had brain LTS but not meeting diagnostic criteria for either PD or DLB; all these mixed pathology cases are listed with their Lewy body disorders group only. Means and standard deviations (SD) are given. ILBD = incidental Lewy body disease; PD = Parkinson’s disease; PSP = progressive supranuclear palsy without LTS; PSPLB = progressive supranuclear palsy with Lewy bodies; CBD = corticobasal degeneration; MSA = multiple system atrophy; ADLB = Alzheimer’s disease with Lewy bodies; ADNLB = Alzheimer’s disease with no Lewy bodies; NP = neuritic plaque; N/A = not applicable.
| Diagnosis (N) | Age at Death Mean (SD) | Gender (% Male) | MMSE1 | UPDRS2 | Disease Duration (Years) | Braak Stage | CERAD NP Density |
|---|---|---|---|---|---|---|---|
| Normal (79) | 84.3 (11.9) | 56.9 | 27.7 (2.0) | 9.5 (8.3) | N/A | 3.1 (1.2) | 1.2 (1.3) |
| PD (46) | 78.9 (6.5) | 80.1 | 22.7 (6.3) | 38.8 (20.3) | 13.4 (7.6) | 3.7 (2.0) | 1.4 (1.4) |
| DLB (28) | 82.6 (7.9) | 64.3 | 11.2 (7.7) | 28.5 (20) | 9.1 (4.4) | 4.6 (1.1) | 2.9 (0.4) |
| ILBD (9) | 85.6 (6.2) | 66.7 | 28.5 (2.1) | 5.9 (4.2) | N/A | 3.3 (0.5) | 1.4 (1.5) |
| ADLB (33) | 83.6 (6.8) | 54.5 | 10.7 (7.8) | 21.7 (18.9) | 8.4 (4.2) | 5.5 (0.7) | 2.9 (0.2) |
| PSPLB (2) | 90.0 (9.9) | 0 | 17.0 (12.7) | 22 (0) | 10.0 (4.2) | 5.5 (0.7) | 3.0 (0) |
| ADNLB (15) | 84.2 (6.1) | 62.5 | 12.9 (8.3) | 16.4 (16.2) | 6.9 (5.1) | 4.5 (1.3) | 2.8 (0.4) |
| PSPNLB (12) | 83.7 (11.3) | 58.3 | 22.2 (4.4) | 30.2 (20.6) | 5.6 (5.3) | 4.4 (0.9) | 1.9 (1.3) |
| CBD (2) | 73.5 (0.7) | 50.0 | 15.0 (5.7) | 69 | 5.0 (1.4) | 5.0 (0) | 1.3 (1.5) |
| MSA (2) | 79.5 (6.4) | 50.0 | 27.5 (0.7) | 11 | 10.0 (0) | 3.5 (2.1) | 1.5 (2.1) |
MMSE scores not available for 5 PD, 2 ADLB, 1 ADNLB I ILBD and 14 Normal controls.
UPDRS scores not available for 3 PD, 1 PSPNLB, 1 PSPLB, 1 CBD, 1 MSA, 17 ADLB, 5 ADNLB, 8 DLB, 1 ILBD and 15 Normal controls.
Table 2.
Classification of subjects according to their brain distribution of phosphorylated α-synuclein Lewy-type synucleinopathy (LTS) and the Unified Staging System for Lewy Body Disorders. Number of subjects in each stage are given, along with the mean (SD) summary LTS density score for 10 standard brain regions. One PD subject was not classifiable due to missing brain regions needed for staging; 3 PD subjects did not have summary brain scores due to missing regions; 6 ADLB subjects did not have summary brain scores due to missing regions; one DLB subject was not classifiable due to missing brain regions needed for staging; 4 DLB subjects did not have summary brain scores due to missing regions;. ILBD = incidental Lewy body disease; PD = Parkinson’s disease; ADLB = Alzheimer’s disease with Lewy bodies; PSPLB = progressive supranuclear palsy with Lewy bodies.
| Diagnosis | Olf Bulb Stage I | Brainstem Stage IIa | Limbic Stage IIb | Brainstem & Limbic Stage III | Neocortical Stage IV | Summary Brain LTS Score Mean (SD) | Sub Gland LTS score Median (Mean) Range |
|---|---|---|---|---|---|---|---|
| PD | 0 | 2 | 1 | 18 | 23 | 28.0 (6.5) | 1 (1.7) 0–4 |
| DLB | 0 | 0 | 1 | 5 | 21 | 26.2 (7.7) | 1 (1.5) 0–4 |
| ILBD | 3 | 2 | 2 | 2 | 0 | 7.2 (4.0) | 0 (0.4) 0–4 |
| ADLB | 3 | 0 | 22 | 8 | 0 | 10.1 (6.3) | 0 (0.3) 0–4 |
| PSPLB | 0 | 0 | 1 | 1 | 0 | 11.5 (9.2) | 0 (0) 0 |
Submandibular gland phosphorylated α-synuclein immunoreactivity
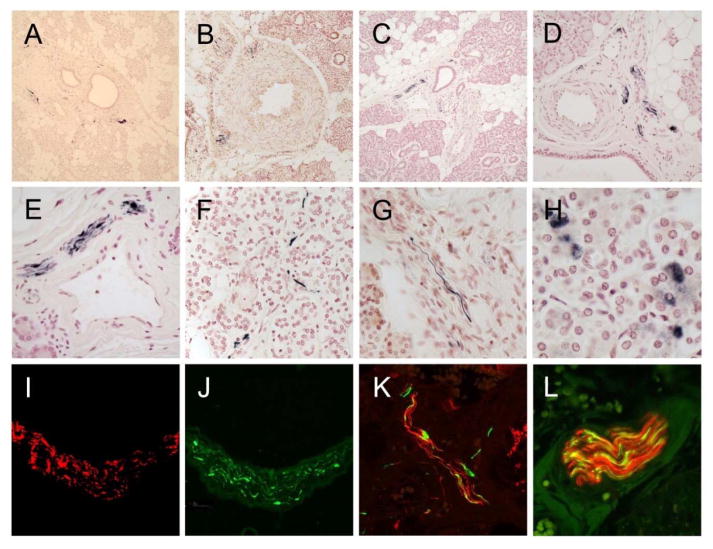
As in our previous investigations [18–21], only staining that was morphologically consistent with nerve fibers was considered to be specific for submandibular gland LTS. Immunoreactive nerve fibers were present within 42/46 PD subjects (91%), including 3 cases with co-existing PD and PSP and one case with co-existing PD and CBD, 20/28 (71%) DLB subjects, including one with co-existing DLB and PSP, 4/33 (12%) ADLB subjects and 1/9 (11%) ILBD subjects. No submandibular gland LTS was present in the 2 subjects with PSPLB and no LTS was found in the submandibular glands of the 110 non-LTS control subjects, including those classified as normal (without parkinsonism or dementia), AD, PSP, CBD or MSA. As previously reported [18, 20, 46], immunoreactive nerve fibers were most frequently found in nerve fascicles running in the connective tissue stroma (Figure 1A, C–F) but were also seen closely applied to the peripheral surface of small arteries (Figure 1B), or as single fibers interweaving amongst serous gland cells (Figure 1F). Immunoreactive nerve fibers were most often normal in appearance but occasional enlarged and distorted fibers were present.
Figure 1.
Photomicrographs of sections stained immunohistochemically for phosphorylated α-synuclein. A) Low magnification of gland from a PD subject with immunoreactive fibers in the stroma around some large ducts. B) Low magnification of gland from a DLB subject with immunoreactive fibers at the periphery of a small artery in the stroma. C – E) Low, medium and high magnification of gland from an ILBD subject with immunoreactive fibers running within nerve fascicles in the stroma. F) High magnification of single immunoreactive nerve fibers amongst serous gland cells in a PD subject. G) High magnification of gland from a DLB subject with a single immunoreactive fiber in the stroma. H) High magnification of immunoperoxidase reaction product within the cytoplasm of serous gland cells in the submandibular gland of a subject with PD. This type of staining was seen in control subjects as well as in subjects with Lewy body disorders and is therefore considered diagnostically non-specific. I and J) High magnification of a nerve fascicle in the gland stroma of a DLB subject, stained with an immunofluorescent method for neurofilament (I, red) and phosphorylated α-synuclein (J, green) and viewed using a fluorescence microscope. K) Medium magnification of a nerve fascicle in the gland stroma of an ILBD subject, stained with an immunofluorescent method for neurofilament (red) and phosphorylated α-synuclein (green) and viewed with a confocal laser microscope. L) High magnification of a nerve fascicle in the gland stroma of an ILBD subject, stained with an immunofluorescent method for neurofilament (red) and phosphorylated α-synuclein (green) and viewed with a confocal laser microscope. Merged fibers are orange.
In glands from some subjects in all diagnostic categories, the cytoplasm of some serous gland cells was positively stained (Figure 1H) and occasionally, secretory material within ducts was positively stained (not shown). As previously reported, [19] this type of staining is diagnostically non-specific as it was found in both control subjects as well as subjects with Lewy body disorders so was not counted as positive for LTS.
Two-color immunofluorescence revealed presumptive LTS-containing axons running in parallel with neurofilament-immunoreactive axons within stromal nerve fascicles of subjects with DLB and ILBD (Figure 1, I–L). Merging of the two colors showed orange-colored fibers indicative of intra-axonal colocalization (Figure 1, K and L).
Statistical analyses
The differing prevalences of LTS in submandibular gland in different categories of Lewy body disorders suggests that the spread of LTS to submandibular gland is probabilistically related to total brain LTS load, as subjects with ILBD, ADLB and PSPLB, who have much lower total brain LTS loads (Table 2) also have much lower prevalences of submandibular gland LTS. Statistical testing confirmed this, as with all Lewy body disorders subjects combined, the mean brain LTS load was significantly higher in those with than those without submandibular gland LTS (means of 28.3 and 12.3; p < 0.0001).
The LTS density scores within the submandibular gland were significantly correlated, within the combined Lewy body disorder groups, with brain LTS total score (Spearman rho = 0.62; p < 0.0001) and UPDRS motor score (Spearman rho = 0.48; p < 0.0001), while there was no significant correlation with MMSE score.
Within the PD group, there were only 4 subjects lacking submandibular gland LTS, making it difficult to determine how LTS-negative subjects might differ from LTS positive ones. Despite the small group size, however, the LTS-negative group had a significantly shorter disease duration than the 41 subjects who were positive (means of 5.2 and 14.1 years; p = 0.02). The correlation of disease duration with submandibular gland LTS density score was weak and missed the significance level (Spearman rho = 0.28; p = 0.07), suggesting that factors other than disease duration, perhaps disease severity, are important. In this respect, the submandibular gland LTS-negative PD group had a significantly lower brain LTS load (means of 21.7 and 28.3; p < 0.05).
Within the DLB category, subjects without submandibular gland LTS (N = 8) tended to have lower total brain LTS loads than subjects with submandibular LTS (N = 20); mean total brain LTS loads of those with (30.3) and without LTS (24.4) were not statistically significant in this small sample (p = 0.10). Disease duration did not differ between the two groups (means of 9.0 and 9.1 years, respectively).
DISCUSSION
The results of this investigation show that submandibular gland LTS is present in 91% of autopsy confirmed PD subjects, 71% of autopsied DLB, 12% of ADLB subjects, 11% of ILBD subjects but none of 2 with PSPLB. None of the 110 subjects without a brain-based Lewy body disorder had LTS in the submandibular gland.
This is the first reasonably comprehensive assessment of the prevalence of submandibular gland LTS in DLB subjects. We had previously reported LTS in 2 of 6 DLB subjects, based on single-section analysis within a body-wide survey [18]. In the present study we assessed multiple sections of submandibular gland (between 3 and 13) and found an LTS prevalence of 71%. This suggests that submandibular gland biopsy could improve diagnostic sensitivity for DLB, is a potential prognostic indicator, and/or could be used to validate other biomarkers. International consensus diagnostic criteria for dementia with Lewy bodies (DLB) were most recently updated in 2005 and define the characteristic clinical and neuropathological findings [26]. Despite this, a recent study of more than 30 National Institute on Aging Alzheimer’s Disease Centers found that only 15–25% of neuropathologically-defined DLB subjects were identified as DLB during life [8]. Dopaminergic functional imaging assists in the diagnosis of both PD and DLB, but does not separate them from other disorders characterized by a dopaminergic deficit [47, 48], including PSP, CBD and MSA, all of which, like PD and DLB, may be neuropathologically concurrent with AD. Until large, autopsy-confirmed studies of both diagnostic tests are performed, however, it is not possible to compare PET dopaminergic imaging with submandibular gland needle biopsy, in terms of diagnostic accuracy.
There is therefore an urgent need for sensitive and specific diagnostic biomarkers for DLB [5, 8, 26, 49–58] that are validated by histopathological examination. As it is costly and takes 2–3 years or more to assemble an adequate number of autopsied subjects, submandibular gland biopsy could be used directly as a biomarker in living subjects, or could provide a more expedient route to validate other biomarkers.
This confirms and extends previous reports of a high prevalence of submandibular gland LTS in PD subjects [19, 20, 45,59]. Biopsy of the submandibular gland could potentially provide a tissue-based and thus possibly more accurate diagnosis of PD. The study population had a mean disease duration of 13.4 yrs with a mean age of PD onset of about 65 yrs. This cohort is therefore comparable to the general population of PD subjects. We reported two clinical trials of needle biopsy of the submandibular gland, in PD subjects with both long-duration and short-duration disease, finding submandibular gland LTS in 73–75% of subjects in both categories [20, 21].
This is the largest published investigation to date of submandibular gland LTS in ILBD subjects. In our prior work [18] we had found none of 3 ILBD subjects to have submandibular gland LTS while one other group reported LTS in 2 of 3 subjects [59]. In this current work we have added an additional 6 ILBD subjects and found LTS in only 1 of the 9. There is an urgent need to find biomarkers of PD that are detectable prior to the onset of motor signs and symptoms but it appears that submandibular gland biopsy will not fulfill this need as the sensitivity is too low, at least using current methods. This relative insensitivity to ILBD, however, makes submandibular gland biopsy more useful for distinguishing PD and DLB from asymptomatic Lewy body disease, which is present in up to 25% or more of the elderly population [43]. If ILBD cases, which are 10–25% of the normal elderly population, frequently had submandibular gland LTS, then a positive needle biopsy of the gland would not necessarily be diagnostic of PD or DLB, it could merely indicate the presence of ILBD in a subject without nigral LTS, and hence a different underlying disorder, not PD or DLB. At present it is unknown what fraction of ILBD would ultimately become symptomatic, either as PD or DLB, but it is likely to be a small fraction, given that the estimated prevalences of PD and DLB in the elderly are much smaller than that of ILBD. The relatively low apparent rate of conversion of ILBD to PD or DLB may be due to the advanced age of most ILBD subjects. All subjects with Lewy body disorders must pass through an ILBD phase, however. Despite the apparently low conversion of ILBD to PD or DLB, it would appear that ILBD is not a completely benign condition, as it is associated with an approximately 50% decrease in striatal dopaminergic markers as well as the clinical biomarker of hyposmia [60–63].
Submandibular gland LTS was uncommon in subjects with ADLB, being present in only 4 of 33 subjects. ADLB is a diagnosis of exclusion, consisting of subjects meeting clinicopathological criteria for AD but lacking sufficient brain LTS spread and density to qualify for the diagnosis of DLB [25], and/or lacking a predementia presentation with parkinsonism. It is remarkable that up to 60% of subjects with AD have brain LTS [43,64]. The database of the Arizona Study of Aging and Neurodegenerative Disorders [22] currently has 673 neuropathologically-confirmed AD cases with a full brain examination for LTS. Of these, 391 (58.1%) have brain LTS, with 100 (14.8%) meeting DLB clinicopathological criteria, 60 meeting PD clinicopathological criteria and 231 classified as ADLB. Given the high population prevalence of AD, ADLB is probably the second most common Lewy body disorder (after ILBD). Although ADLB has attracted little attention from the research community, its regional brain LTS distribution suggests that a subset of ADLB cases represent early-stage DLB [43] and it appears likely that DLB subjects pass through the ADLB stage.
As LTS is likely to be widespread within the peripheral autonomic nervous system, other sites might be considered for peripheral biopsy diagnosis of Lewy body disorders. Our wide-ranging survey of multiple body locations, however, indicated that the lower esophagus and submandibular gland had the highest prevalences of LTS in subjects with Lewy body disorders [18]. We chose to pursue further studies in the submandibular gland as we thought it would be the least invasive site to biopsy. Needle biopsy has been extensively used to investigate submandibular gland neoplasms [65]. Other salivary glands, including the parotid gland, sublingual glands and minor (labial) salivary glands, might also be considered as potential biopsy sites. Of these, the parotid gland, while easily accessible, has the greatest risk of side effects due to the proximity of the facial nerve and the possibility of facial disfigurement. The sublingual glands have not yet been investigated. Three studies have determined LTS prevalence in the minor salivary glands, with 2 studies reporting a very low prevalence (1/15 cases positive in one study and 3/16 in a second study) [19, 66] while the other study found 2/3 were positive [67].
A critical question has been whether or not LTS begins in the brain or within elements of the peripheral nervous system [69–71]. The present results, as well as our previous reports [18,19], do not support the concept that the initiation of LTS is in the peripheral nervous system rather than the brain. The stimulus for this intriguing hypothesis has come largely from clinical studies of PD that have found a wide range of non-motor signs and symptoms that accompany the disease [72]. Many of these non-motor accompaniments are related to dysfunction of the peripheral autonomic system. These may predate or occur early in the motor progression [73–77]. The description of Lewy bodies within the sympathetic and parasympathetic ganglia, adrenal medulla and selected GI tract regions within autopsied subjects with PD and DLB [78–82] has shown that peripheral nervous system LTS is certainly present but there has been insufficient data regarding the findings in prodromal phases of disease. Autopsy studies of relatively small numbers of subjects with ILBD have demonstrated a high prevalence of LTS within the spinal cord, sympathetic ganglia, adrenal medulla and upper GI tract [18,78,79,84–87] but no study to date has found LTS in the spinal cord or in peripheral nervous system sites in the absence of brain involvement, with the possible exception of Fumimura et al [82] who reported one case out of 783 with adrenal medulla as the only site with LTS. One other case report exists of Lewy body pathology restricted to the heart and stellate ganglion [88]. The present work is in agreement with these prior studies as, of the 79 normal elderly subjects without brain LTS, none had submandibular gland LTS. Additionally, none of the 31 other subjects lacking brain LTS were positive for submandibular gland LTS. It would therefore appear that α-synucleinopathy likely begins in the CNS and spreads both rostrally and caudally. Given the frequency of isolated LTS in the olfactory bulb of ILBD cases, the initial onset may well be in the olfactory bulb.
In conclusion, we report here submandibular gland LTS prevalence rates of 91%, 71%, 12% and 11%, respectively, in autopsy-confirmed subjects with PD, DLB, ADLB and ILBD. Needle biopsy of the submandibular gland may prove to be useful as a diagnostic and/or progression biomarker in PD and DLB, and may provide an expeditious means with which to validate other potential biomarkers of these conditions. Further biopsy studies of larger numbers of subjects are needed. We have had unpublished confirmation of a positive LTS immunohistochemical signal in close to 90% of autopsied PD submandibular gland and absence from controls, from two separate, independent laboratories using different primary antibodies and signal development methods (personal communication, Wagner Zago, Prothena Biosciences, Inc.). These were small subject sets, however (less than 30 total subjects) and therefore further confirmation is needed. This may be forthcoming shortly, as the Michael J Fox Foundation has just started a multi-center clinical trial of submandibular gland needle biopsy, as well as sigmoid colon and skin biopsy, in 60 PD subjects and 20 controls. Additionally, it should be noted that several studies have investigated the minor salivary glands [89–91], with conflicting results, while multiple other peripheral biopsy sites [92–95] have been suggested for diagnosing PD and/or DLB; all of these latter, however, are single-center studies and will require independent replication.
Acknowledgments
This study was made possible by grants from the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research. We are grateful for the altruism of all of those who have volunteered for the Banner Sun Health Research Institute Brain and Body Donation Program. The authors thank Mr. Bruce Peterson for developing and maintaining the database.
Reference List
- 1.Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson’s disease. Neurology. 2001;57:1497–1499. doi: 10.1212/wnl.57.8.1497. [DOI] [PubMed] [Google Scholar]
- 2.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 4.Cornford ME, Chang L, Miller BL. The neuropathology of parkinsonism: an overview. Brain Cogn. 1995;28:321–341. doi: 10.1006/brcg.1995.1261. [DOI] [PubMed] [Google Scholar]
- 5.Litvan I, MacIntyre A, Goetz CG, Wenning GK, Jellinger K, Verny M, Bartko JJ, Jankovic J, McKee A, Brandel JP, Chaudhuri KR, Lai EC, D’Olhaberriague L, Pearce RK, Agid Y. Accuracy of the clinical diagnoses of Lewy body disease, Parkinson disease, and dementia with Lewy bodies: a clinicopathologic study. Arch Neurol. 1998;55:969–978. doi: 10.1001/archneur.55.7.969. [DOI] [PubMed] [Google Scholar]
- 6.Gibb WR. Neuropathology of Parkinson’s disease and related syndromes. Neurol Clin. 1992;10:361–376. [PubMed] [Google Scholar]
- 7.Adler CH, Beach TG, Hentz JG, Shill HA, Caviness JN, Driver-Dunckley E, Sabbagh MN, Sue LI, Jacobson SA, Belden CM, Dugger BN. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: clinicopathologic study. Neurology. 2014;83:406–412. doi: 10.1212/WNL.0000000000000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson PT, Jicha GA, Kryscio RJ, Abner EL, Schmitt FA, Cooper G, Xu LO, Smith CD, Markesbery WR. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol. 2010;257:359–366. doi: 10.1007/s00415-009-5324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obeso JA, Rodriguez MC, Gorospe A, Guridi J, Alvarez L, Macias R. Surgical treatment of Parkinson’s disease. Baillieres Clin Neurol. 1997;6:125–145. [PubMed] [Google Scholar]
- 10.Subramanian T. Cell transplantation for the treatment of Parkinson’s disease. Semin Neurol. 2001;21:103–115. doi: 10.1055/s-2001-13125. [DOI] [PubMed] [Google Scholar]
- 11.Master Z, McLeod M, Mendez I. Benefits, risks and ethical considerations in translation of stem cell research to clinical applications in Parkinson’s disease. J Med Ethics. 2007;33:169–173. doi: 10.1136/jme.2005.013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paluzzi A, Belli A, Bain P, Liu X, Aziz TM. Operative and hardware complications of deep brain stimulation for movement disorders. Br J Neurosurg. 2006;20:290–295. doi: 10.1080/02688690601012175. [DOI] [PubMed] [Google Scholar]
- 13.Lang AE, Houeto JL, Krack P, Kubu C, Lyons KE, Moro E, Ondo W, Pahwa R, Poewe W, Troster AI, Uitti R, Voon V. Deep brain stimulation: preoperative issues. Mov Disord. 2006;21(Suppl 14):S171–S196. doi: 10.1002/mds.20955. [DOI] [PubMed] [Google Scholar]
- 14.Tir M, Devos D, Blond S, Touzet G, Reyns N, Duhamel A, Waucquier N, Cottencin O, Dujardin K, Debailleul AM, Cassim F, Destee A, Defebvre L, Krystkowiak P. Exhaustive, one year followup of subthalamic nucleus deep brain stimulation in a large, single-center cohort of parkinsonian patients. Neurosurgery. 2007 doi: 10.1227/01.NEU.0000285347.50028.B9. [DOI] [PubMed] [Google Scholar]
- 15.Gill CE, Konrad PE, Davis TL, Charles D. Deep brain stimulation for Parkinson’s disease: the Vanderbilt University Medical Center experience, 1998–2004. Tenn Med. 2007;100:45–47. [PubMed] [Google Scholar]
- 16.Seijo FJ, varez-Vega MA, Gutierrez JC, Fdez-Glez F, Lozano B. Complications in subthalamic nucleus stimulation surgery for treatment of Parkinson’s disease. Review of 272 procedures. Acta Neurochir (Wien ) 2007;149:867–875. doi: 10.1007/s00701-007-1267-1. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez RL, Fernandez HH, Haq I, Okun MS. Pearls in patient selection for deep brain stimulation. Neurologist. 2007;13:253–260. doi: 10.1097/NRL.0b013e318095a4d5. [DOI] [PubMed] [Google Scholar]
- 18.Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White CL, III, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, Walker DG. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beach TG, Adler CH, Dugger BN, Serrano G, Hidalgo J, Henry-Watson J, Shill HA, Sue LI, Sabbagh MN, Akiyama H. Submandibular gland biopsy for the diagnosis of Parkinson disease. J Neuropathol Exp Neurol. 2013;72:130–136. doi: 10.1097/NEN.0b013e3182805c72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adler CH, Dugger BN, Hinni ML, Lott DG, Driver-Dunckley E, Hidalgo J, Henry-Watson J, Serrano G, Sue LI, Nagel T, Duffy A, Shill HA, Akiyama H, Walker DG, Beach TG. Submandibular gland needle biopsy for the diagnosis of Parkinson disease. Neurology. 2014;82:858–864. doi: 10.1212/WNL.0000000000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adler CH, Dugger BN, Hinni N, Lott D, Driver-Dunckley E, Mehta S, Serrano G, Sue LI, Duffy A, Walker DG, Beach TG. Submandibular gland needle biopsy for the diagnosis of early Parkinson’s disease. Movement Disorders. 2015 doi: 10.1002/mds.26476. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beach TG, Adler CH, Sue LI, Serrano G, Shill HA, Walker DG, Lue L, Roher AE, Dugger BN, Maarouf C, Birdsill AC, Intorcia A, Saxon-Labelle M, Pullen J, Scroggins A, Filon J, Scott S, Hoffman B, Garcia A, Caviness JN, Hentz JG, Driver-Dunckley E, Jacobson SA, Davis KJ, Belden CM, Long KE, Malek-Ahmadi M, Powell JJ, Gale LD, Nicholson LR, Caselli RJ, Woodruff BK, Rapscak SZ, Ahern GL, Shi J, Burke AD, Reiman EM, Sabbagh MN. Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology. 2015 doi: 10.1111/neup.12189. (Epublication, in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beach TG, Adler CH, Sue LI, Serrano G, Shill HA, Walker DG, Lue L, Roher AE, Dugger BN, Maarouf C, Birdsill AC, Intorcia A, Saxon-Labelle M, Pullen J, Scroggins A, Filon J, Scott S, Hoffman B, Garcia A, Caviness JN, Hentz JG, Driver-Dunckley E, Jacobson SA, Davis KJ, Belden CM, Long KE, Malek-Ahmadi M, Powell JJ, Gale LD, Nicholson LR, Caselli RJ, Woodruff BK, Rapscak SZ, Ahern GL, Shi J, Burke AD, Reiman EM, Sabbagh MN. Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology. 2015 doi: 10.1111/neup.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, Hardy J, Del TK, Wszolek ZK, Litvan I. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol. 2009;8:1150–1157. doi: 10.1016/S1474-4422(09)70238-8. [DOI] [PubMed] [Google Scholar]
- 25.Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 26.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del ST, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 27.Dickson DW, Liu W, Hardy J, Farrer M, Mehta N, Uitti R, Mark M, Zimmerman T, Golbe L, Sage J, Sima A, D’Amato C, Albin R, Gilman S, Yen SH. Widespread alterations of alpha-synuclein in multiple system atrophy. Am J Pathol. 1999;155:1241–1251. doi: 10.1016/s0002-9440(10)65226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Litvan I, Goetz CG, Jankovic J, Wenning GK, Booth V, Bartko JJ, McKee A, Jellinger K, Lai EC, Brandel JP, Verny M, Chaudhuri KR, Pearce RK, Agid Y. What is the accuracy of the clinical diagnosis of multiple system atrophy? A clinicopathologic study. Arch Neurol. 1997;54:937–944. doi: 10.1001/archneur.1997.00550200007003. [DOI] [PubMed] [Google Scholar]
- 29.Takeda A, Hashimoto M, Mallory M, Sundsumo M, Hansen L, Masliah E. C-terminal alpha-synuclein immunoreactivity in structures other than Lewy bodies in neurodegenerative disorders. Acta Neuropathol. 2000;99:296–304. doi: 10.1007/pl00007441. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida M. Multiple system atrophy: alpha-synuclein and neuronal degeneration. Neuropathology. 2007;27:484–493. doi: 10.1111/j.1440-1789.2007.00841.x. [DOI] [PubMed] [Google Scholar]
- 31.Yoshita M. Differentiation of idiopathic Parkinson’s disease from striatonigral degeneration and progressive supranuclear palsy using iodine-123 meta-iodobenzylguanidine myocardial scintigraphy. J Neurol Sci. 1998;155:60–67. doi: 10.1016/s0022-510x(97)00278-5. [DOI] [PubMed] [Google Scholar]
- 32.Dickson DW. Required techniques and useful molecular markers in the neuropathologic diagnosis of neurodegenerative diseases. Acta Neuropathol. 2005;109:14–24. doi: 10.1007/s00401-004-0950-z. [DOI] [PubMed] [Google Scholar]
- 33.Dickson DW, Rademakers R, Hutton ML. Progressive supranuclear palsy: pathology and genetics. Brain Pathol. 2007;17:74–82. doi: 10.1111/j.1750-3639.2007.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dickson DW. Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol. 1999;246(Suppl 2):II6–15. doi: 10.1007/BF03161076. [DOI] [PubMed] [Google Scholar]
- 35.Komori T. Tau-positive glial inclusions in progressive supranuclear palsy, corticobasal degeneration and Pick’s disease. Brain Pathol. 1999;9:663–679. doi: 10.1111/j.1750-3639.1999.tb00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arai T, Ikeda K, Akiyama H, Shikamoto Y, Tsuchiya K, Yagishita S, Beach T, Rogers J, Schwab C, McGeer PL. Distinct isoforms of tau aggregated in neurons and glial cells in brains of patients with Pick’s disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol (Berl ) 2001;101:167–173. doi: 10.1007/s004010000283. [DOI] [PubMed] [Google Scholar]
- 37.Trojanowski JQ, Revesz T. Proposed neuropathological criteria for the post mortem diagnosis of multiple system atrophy. Neuropathol Appl Neurobiol. 2007;33:615–620. doi: 10.1111/j.1365-2990.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- 38.Beach TG, White CL, Hamilton RL, Duda JE, Iwatsubo T, Dickson DW, Roncaroli F, Buttini M, Hladik CL, Sue LI, Noorigian JV, Adler CH. Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta Neuropathol. 2008;116:277–288. doi: 10.1007/s00401-008-0409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lue LF, Walker DG, Adler CH, Shill H, Tran H, Akiyama H, Sue LI, Caviness J, Sabbagh MN, Beach TG. Biochemical Increase in Phosphorylated Alpha-Synuclein Precedes Histopathology of Lewy-Type Synucleinopathies. Brain Pathol. 2012 doi: 10.1111/j.1750-3639.2012.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 41.Obi K, Akiyama H, Kondo H, Shimomura Y, Hasegawa M, Iwatsubo T, Mizuno Y, Mochizuki H. Relationship of phosphorylated alpha-synuclein and tau accumulation to Abeta deposition in the cerebral cortex of dementia with Lewy bodies. Exp Neurol. 2008;210:409–420. doi: 10.1016/j.expneurol.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 42.Lue LF, Walker DG, Adler CH, Shill H, Tran H, Akiyama H, Sue LI, Caviness J, Sabbagh MN, Beach TG. Biochemical increase in phosphorylated alpha-synuclein precedes histopathology of Lewy-type synucleinopathies. Brain Pathol. 2012;22:745–756. doi: 10.1111/j.1750-3639.2012.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker DG, Lue LF, Adler CH, Shill HA, Caviness JN, Sabbagh MN, Akiyama H, Serrano GE, Sue LI, Beach TG. Changes in properties of serine 129 phosphorylated alpha-synuclein with progression of Lewy-type histopathology in human brains. Exp Neurol. 2013;240:190–204. doi: 10.1016/j.expneurol.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R, Eschbacher J, Data !Lost, Akiyama H, Caviness J, Shill HA, Connor DJ, Sabbagh MN, Walker DG. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–634. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White CL, III, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, Walker DG. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010 doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beach TG, Adler CH, Dugger BN, Serrano G, Hidalgo J, Henry-Watson J, Shill HA, Sue LI, Sabbagh MN, Akiyama H. Submandibular Gland Biopsy for the Diagnosis of Parkinson Disease. J Neuropathol Exp Neurol. 2013;72:130–136. doi: 10.1097/NEN.0b013e3182805c72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoessl AJ, Halliday GM. DAT-SPECT diagnoses dopamine depletion, but not PD. Mov Disord. 2014 Dec;29(14):1705–6. doi: 10.1002/mds.26000. [DOI] [PubMed] [Google Scholar]
- 48.McCleery J, Morgan S, Bradley KM, Noel-Storr AH, Ansorge O, Hyde C. Dopamine transporter imaging for the diagnosis of dementia with Lewy bodies. Cochrane Database Syst Rev. 2015 Jan 30;1:CD010633. doi: 10.1002/14651858.CD010633.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jicha GA, Schmitt FA, Abner E, Nelson PT, Cooper GE, Smith CD, Markesbery WR. Prodromal clinical manifestations of neuropathologically confirmed Lewy body disease. Neurobiol Aging. 2010;31:1805–1813. doi: 10.1016/j.neurobiolaging.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nelson PT, Schmitt FA, Jicha GA, Kryscio RJ, Abner EL, Smith CD, Van Eldik LJ, Markesbery WR. Association between male gender and cortical Lewy body pathology in large autopsy series. J Neurol. 2010;257:1875–1881. doi: 10.1007/s00415-010-5630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dugger BN, Boeve BF, Murray ME, Parisi JE, Fujishiro H, Dickson DW, Ferman TJ. Rapid eye movement sleep behavior disorder and subtypes in autopsy-confirmed dementia with Lewy bodies. Mov Disord. 2012;27:72–78. doi: 10.1002/mds.24003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferman TJ, Boeve BF, Smith GE, Lin SC, Silber MH, Pedraza O, Wszolek Z, Data !Lost, Uitti R, Van GJ, Pao W, Knopman D, Pankratz VS, Kantarci K, Boot B, Parisi JE, Dugger BN, Fujishiro H, Petersen RC, Dickson DW. Inclusion of RBD improves the diagnostic classification of dementia with Lewy bodies. Neurology. 2011;77:875–882. doi: 10.1212/WNL.0b013e31822c9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adler CH, Hentz JG, Shill HA, Sabbagh MN, Driver-Dunckley E, Evidente VG, Jacobson SA, Beach TG, Boeve B, Caviness JN. Probable RBD is increased in Parkinson’s disease but not in essential tremor or restless legs syndrome. Parkinsonism Relat Disord. 2011;17:456–458. doi: 10.1016/j.parkreldis.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lopez OL, Litvan I, Catt KE, Stowe R, Klunk W, Kaufer DI, Becker JT, DeKosky ST. Accuracy of four clinical diagnostic criteria for the diagnosis of neurodegenerative dementias. Neurology. 1999;53:1292–1299. doi: 10.1212/wnl.53.6.1292. [DOI] [PubMed] [Google Scholar]
- 55.Verghese J, Crystal HA, Dickson DW, Lipton RB. Validity of clinical criteria for the diagnosis of dementia with Lewy bodies. Neurology. 1999;53:1974–1982. doi: 10.1212/wnl.53.9.1974. [DOI] [PubMed] [Google Scholar]
- 56.McKeith IG, Ballard CG, Perry RH, Ince PG, O’Brien JT, Neill D, Lowery K, Jaros E, Barber R, Thompson P, Swann A, Fairbairn AF, Perry EK. Prospective validation of consensus criteria for the diagnosis of dementia with Lewy bodies. Neurology. 2000;54:1050–1058. doi: 10.1212/wnl.54.5.1050. [DOI] [PubMed] [Google Scholar]
- 57.Luis CA, Barker WW, Gajaraj K, Harwood D, Petersen R, Kashuba A, Waters C, Jimison P, Pearl G, Petito C, Dickson D, Duara R. Sensitivity and specificity of three clinical criteria for dementia with Lewy bodies in an autopsy-verified sample. Int J Geriatr Psychiatry. 1999;14:526–533. [PubMed] [Google Scholar]
- 58.Mok W, Chow TW, Zheng L, Mack WJ, Miller C. Clinicopathological concordance of dementia diagnoses by community versus tertiary care clinicians. Am J Alzheimers Dis Other Demen. 2004;19:161–165. doi: 10.1177/153331750401900309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Del Tredici K, Hawkes CH, Ghebremedhin E, Braak H. Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson’s disease. Acta Neuropathol. 2010;119:703–713. doi: 10.1007/s00401-010-0665-2. [DOI] [PubMed] [Google Scholar]
- 60.Beach TG, Adler CH, Sue LI, Peirce JB, Bachalakuri J, sing-Hernandez JE, Lue LF, Caviness JN, Connor DJ, Sabbagh MN, Walker DG. Reduced striatal tyrosine hydroxylase in incidental Lewy body disease. Acta Neuropathol. 2008;115:445–451. doi: 10.1007/s00401-007-0313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Driver-Dunckley E, Adler CH, Hentz JG, Dugger BN, Shill HA, Caviness JN, Sabbagh MN, Beach TG. Olfactory dysfunction in incidental Lewy body disease and Parkinson’s disease. Parkinsonism Relat Disord. 2014;20:1260–1262. doi: 10.1016/j.parkreldis.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dickson DW, Fujishiro H, Delledonne A, Menke J, Ahmed Z, Klos KJ, Josephs KA, Frigerio R, Burnett M, Parisi JE, Ahlskog JE. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathol. 2008;115:437–444. doi: 10.1007/s00401-008-0345-7. [DOI] [PubMed] [Google Scholar]
- 63.Delledonne A, Klos KJ, Fujishiro H, Ahmed Z, Parisi JE, Josephs KA, Frigerio R, Burnett M, Wszolek ZK, Uitti RJ, Ahlskog JE, Dickson DW. Incidental Lewy body disease and preclinical Parkinson disease. Arch Neurol. 2008;65:1074–1080. doi: 10.1001/archneur.65.8.1074. [DOI] [PubMed] [Google Scholar]
- 64.Hamilton RL. Lewy bodies in Alzheimer’s disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol. 2000;10:378–384. doi: 10.1111/j.1750-3639.2000.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lussier C, Klijanienko J, Vielh P. Fine-needle aspiration of metastatic nonlymphomatous tumors to the major salivary glands: a clinicopathologic study of 40 cases cytologically diagnosed and histologically correlated. Cancer. 2000;90:350–356. [PubMed] [Google Scholar]
- 66.Folgoas E, Lebouvier T, Leclair-Visonneau L, Cersosimo MG, Barthelaix A, Derkinderen P, Letournel F. Diagnostic value of minor salivary glands biopsy for the detection of Lewy pathology. Neurosci Lett. 2013;551:62–64. doi: 10.1016/j.neulet.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 67.Cersosimo MG, Perandones C, Micheli FE, Raina GB, Beron AM, Nasswetter G, Radrizzani M, Benarroch EE. Alpha-synuclein immunoreactivity in minor salivary gland biopsies of Parkinson’s disease patients. Mov Disord. 2011;26:188–190. doi: 10.1002/mds.23344. [DOI] [PubMed] [Google Scholar]
- 69.Langston JW. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59:591–596. doi: 10.1002/ana.20834. [DOI] [PubMed] [Google Scholar]
- 70.Braak H, Rub U, Gai WP, Del TK. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003;110:517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- 71.Hawkes CH, Del TK, Braak H. Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adler CH. Nonmotor complications in Parkinson’s disease. Mov Disord. 2005;20(Suppl 11):S23–S29. doi: 10.1002/mds.20460. [DOI] [PubMed] [Google Scholar]
- 73.Kaufmann H, Nahm K, Purohit D, Wolfe D. Autonomic failure as the initial presentation of Parkinson disease and dementia with Lewy bodies. Neurology. 2004;63:1093–1095. doi: 10.1212/01.wnl.0000138500.73671.dc. [DOI] [PubMed] [Google Scholar]
- 74.Abbott RD, Ross GW, Petrovitch H, Tanner CM, Davis DG, Masaki KH, Launer LJ, Curb JD, White LR. Bowel movement frequency in late-life and incidental Lewy bodies. Mov Disord. 2007 doi: 10.1002/mds.21560. [DOI] [PubMed] [Google Scholar]
- 75.Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD, Grandinetti A, Blanchette PL, Popper JS, Ross GW. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology. 2001;57:456–462. doi: 10.1212/wnl.57.3.456. [DOI] [PubMed] [Google Scholar]
- 76.Siddiqui MF, Rast S, Lynn MJ, Auchus AP, Pfeiffer RF. Autonomic dysfunction in Parkinson’s disease: a comprehensive symptom survey. Parkinsonism Relat Disord. 2002;8:277–284. doi: 10.1016/s1353-8020(01)00052-9. [DOI] [PubMed] [Google Scholar]
- 77.Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2003;2:107–116. doi: 10.1016/s1474-4422(03)00307-7. [DOI] [PubMed] [Google Scholar]
- 78.Takeda S, Yamazaki K, Miyakawa T, Arai H. Parkinson’s disease with involvement of the parasympathetic ganglia. Acta Neuropathol. 1993;86:397–398. doi: 10.1007/BF00369454. [DOI] [PubMed] [Google Scholar]
- 79.Braak H, Sastre M, Bohl JR, de Vos RA, Del TK. Parkinson’s disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol. 2007;113:421–429. doi: 10.1007/s00401-007-0193-x. [DOI] [PubMed] [Google Scholar]
- 80.Braak H, de Vos RA, Bohl J, Del TK. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 81.Koike Y, Takahashi A. Autonomic dysfunction in Parkinson’s disease. Eur Neurol. 1997;38(Suppl 2):8–12. doi: 10.1159/000113470. [DOI] [PubMed] [Google Scholar]
- 82.Fumimura Y, Ikemura M, Sengoku R, Kanemaru K, Sawabe M, Arai T, Ito G, Iwatsubo T, Fukayama M, Mizusawa H. Analysis of the adrenal gland is useful for evaluating pathology of the peripheral autonomic nervous system in lewy body disease. J Neuropathol Exp Neurol. 2007;66:354–362. doi: 10.1097/nen.0b013e3180517454. [DOI] [PubMed] [Google Scholar]
- 83.Hishikawa N, Hashizume Y, Hirayama M, Imamura K, Washimi Y, Koike Y, Mabuchi C, Yoshida M, Sobue G. Brainstem-type Lewy body disease presenting with progressive autonomic failure and lethargy. Clin Auton Res. 2000;10:139–143. doi: 10.1007/BF02278018. [DOI] [PubMed] [Google Scholar]
- 84.den Hartog Jager WA, Bethlem J. The distribution of Lewy bodies in the central and autonomic nervous systems in idiopathic paralysis agitans. J Neurol Neurosurg Psychiatry. 1960;23:283–290. doi: 10.1136/jnnp.23.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bloch A, Probst A, Bissig H, Adams H, Tolnay M. Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol. 2006;32:284–295. doi: 10.1111/j.1365-2990.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 86.Klos KJ, Ahlskog JE, Josephs KA, Apaydin H, Parisi JE, Boeve BF, DeLucia MW, Dickson DW. Alpha-synuclein pathology in the spinal cords of neurologically asymptomatic aged individuals. Neurology. 2006;66:1100–1102. doi: 10.1212/01.wnl.0000204179.88955.fa. [DOI] [PubMed] [Google Scholar]
- 87.Minguez-Castellanos A, Chamorro CE, Escamilla-Sevilla F, Ortega-Moreno A, Rebollo AC, Gomez-Rio M, Concha A, Munoz DG. Do alpha-synuclein aggregates in autonomic plexuses predate Lewy body disorders?: a cohort study. Neurology. 2007;68:2012–2018. doi: 10.1212/01.wnl.0000264429.59379.d9. [DOI] [PubMed] [Google Scholar]
- 88.Miki Y, Mori F, Wakabayashi K, Kuroda N, Orimo S. Incidental Lewy body disease restricted to the heart and stellate ganglia. Mov Disord. 2009;24:2299–2301. doi: 10.1002/mds.22775. [DOI] [PubMed] [Google Scholar]
- 89.Gao L, Chen H, Li X, Li F, Ou-Yang Q, Feng T. The diagnostic value of minor salivary gland biopsy in clinically diagnosed patients with Parkinson’s disease: comparison with DAT PET scans. Neurol Sci. 2015;36:1575–1580. doi: 10.1007/s10072-015-2190-5. [DOI] [PubMed] [Google Scholar]
- 90.Folgoas E, Lebouvier T, Leclair-Visonneau L, Cersosimo MG, Barthelaix A, Derkinderen P, Letournel F. Diagnostic value of minor salivary glands biopsy for the detection of Lewy pathology. Neurosci Lett. 2013;551:62–64. doi: 10.1016/j.neulet.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 91.Cersosimo MG, Perandones C, Micheli FE, Raina GB, Beron AM, Nasswetter G, Radrizzani M, Benarroch EE. Alpha-synuclein immunoreactivity in minor salivary gland biopsies of Parkinson’s disease patients. Mov Disord. 2011;26:188–190. doi: 10.1002/mds.23344. [DOI] [PubMed] [Google Scholar]
- 92.Zange L, Noack C, Hahn K, Stenzel W, Lipp A. Phosphorylated α-synuclein in skin nerve fibres differentiates Parkinson’s disease from multiple system atrophy. Brain. 2015;138:2310–21. doi: 10.1093/brain/awv138. [DOI] [PubMed] [Google Scholar]
- 93.Tolosa E, Vilas D. Peripheral synuclein tissue markers: a step closer to Parkinson’s disease diagnosis. Brain. 2015;138:2120–2. doi: 10.1093/brain/awv164. [DOI] [PubMed] [Google Scholar]
- 94.Schneider SA, Boettner M, Alexoudi A, Zorenkov D, Deuschl G, Wedel T. Can we use peripheral tissue biopsies to diagnose Parkinson’s disease? A review of the literature. Eur J Neurol. 2015 doi: 10.1111/ene.12753. (in press) [DOI] [PubMed] [Google Scholar]
- 95.Gelpi E, Navarro-Otano J, Tolosa E, Gaig C, Compta Y, Rey MJ, Martí MJ, Hernández I, Valldeoriola F, Reñé R, Ribalta T. Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov Disord. 2014;29:1010–8. doi: 10.1002/mds.25776. [DOI] [PubMed] [Google Scholar]