Abstract
Objective
We evaluated the safety and efficacy of vonoprazan-based amoxicillin and clarithromycin 7-day triple therapy (VAC) in comparison to proton pump inhibitor (PPI)-based (PAC) as a first-line treatment and vonoprazan-based amoxicillin and metronidazole 7-day triple therapy (VAM) in comparison to PPI-based (PAM) as a second-line treatment for the eradication of Helicobacter pylori in Japan.
Methods
We performed a non-randomized, multi-center, parallel-group study to compare first-line VAC to PAC and second-line VAM to PAM. A pre-planned subgroup analysis on CAM resistance was also performed. Safety was evaluated with an adverse effects questionnaire (AEQ), which was completed by patients during therapy.
Results
The first-line eradication rates (ER) in the intention-to-treat (ITT) and per protocol (PP) analyses were 84.9% (95% CI: 81.9-87.6%, n=623) and 86.4% (83.5-89.1%, n=612), respectively, for VAC and 78.8% (75.3-82.0%, n=608) and 79.4% (76.0-82.6%, n=603), respectively, for PAC. The ER of VAC was higher than that of PAC in the ITT (p=0.0061) and PP analyses (p=0.0013). The ERs for VAC in patients with CAM-resistant and CAM-susceptible bacteria were 73.2% (59.7-84.2%, n=56) and 88.9% (83.4-93.1%, n=180), respectively. PAC was associated with higher AEQ scores for diarrhea, nausea, headache, and general malaise. In the second-line ITT and PP analyses VAM achieved ERs of 80.5% (74.6-85.6%, n=216) and 82.4% (76.6-87.3%, n=211), respectively, while PAM achieved ERs of 81.5% (74.2-87.4%, n=146) and 82.1% (74.8-87.9%, n=145), respectively. No significant differences were observed in the ITT (p=0.89) or PP (p=1.0) analyses.
Conclusion
The ER of first-line VAC was higher than that of PAC, but still <90%. No difference was observed between second-line VAM and PAM. Vonoprazan-based triple therapy was safe and well tolerated.
Keywords: eradication, Helicobacter pylori, vonoprazan, 7-day triple therapy
Introduction
Helicobacter pylori infection accounts for 89% of all non-cardia gastric cancers, and the odds ratio for a patient being H. pylori-positive was 21.4 (95% confidence interval CI=7.1-64.4) (1). In trials, the risk for gastric cancer was reduced by 40% (2); in cases in which eradication therapy resulted in the complete and sustained absence of H. pylori infection, and in which all of the patients in the control group remained infected with H. pylori, the risk was reduced by approximately 75% (3). Thus, H. pylori eradication regimens with high first- and second-line eradication rates (ERs) [at least >90% each (4)] are important for achieving complete eradication.
In the face of rising clarithromycin (CAM) resistance, the ER of 7-day proton pump inhibitor (PPI)/amoxicillin (AMPC)/CAM ‘standard’ triple therapy for H. pylori has fallen to <80% in many countries (5). Furthermore, in Japan, where 1,401,812 H. pylori eradications were performed in 2013 (personal communication), the ER of PPI/AMPC/CAM fell from 90% in 2001 to 75.9% in 2015. The decline was inversely correlated with increasing CAM resistance, which increased from <20% in 2001 to 33% in 2015 (6). Thus, in areas of low CAM resistance, CAM-containing treatment regimens are recommended as the first-line empirical treatments. However, in areas with 15-20% or more CAM resistance, the administration of PPI-CAM-containing triple therapy without prior susceptibility testing should be abandoned (7).
In February 2015, vonoprazan (VPZ), the first of a novel class of acid suppressants (potassium-competitive acid blockers, P-CABs), was approved for H. pylori eradication in Japan. In a phase III, randomized, double-blind study, the ER of VPZ/AMPC/CAM (92.6%; n=324) was found to be non-inferior to that of lansoprazole (LPZ, a PPI)/AMPC/CAM (75.9%; n=320; p<0.001) (8). In a single-arm design, the ER of second-line treatment with VPZ/AMPC/metronidazole (MNZ) was reported to be 98% (n=50). In a subgroup analysis of CAM resistance, the ER of VPZ/AMPC/CAM (82.0%; n=100) was significantly higher (p<0.0001) than that of ER of LPZ/AMPC/CAM (40.0%; n=115), whereas no significant difference was observed in the non-CAM-resistant group [VPZ/AMPC/CAM, 97.3% (n=224) vs. LPZ/AMPC/CAM, 96.0% (n=205)] (8).
We started the present study in February 2015, when vonoprazan was approved in Japan; the difference between VPZ/AMPC/CAM and LPZ/AMPC/CAM was investigated in a CAM-resistant population; no difference was observed in a CAM-susceptible population. Furthermore, the success rate of vonoprazan-based triple therapy in the treatment of CAM-resistant infections was 82% and did not reach 90%.
We performed this study to validate the results of a phase III trial that was performed in real-world practice (8). There are several differences between this study and the phase III study: 1) the timing of the 13C urea breath test (UBT) (it was performed at -8 weeks versus just 4 weeks); 2) the subjects (all infected patients versus patients with a history of gastroduodenal ulcers); and 3) the assessment of safety [an adverse effects questionnaire (AEQ) versus a physician-based assessment].
Antibiotic resistance and compliance are the most common reasons for the failure of ‘good’ regimens. The age, presentation (e.g., functional dyspepsia versus duodenal ulcer), CagA status, and smoking have been found in studies in which resistance was not assessed (9, 10). In terms of the standard triple therapy, antibiotic resistance is the main factor in PPI-based and vonoprazan-based regimens: 88% versus 18% (PPI-based, 20 studies, meta-analysis) (11); 96% versus 40% [PPI-based and 97.3% versus 82% (vonoprazan-based)] (8). A subgroup analysis without information on antibiotic resistance can be misleading; thus, we also performed a pre-planned subgroup analysis that was limited to subjects with CAM resistance.
Materials and Methods
Study design
We performed a non-randomized, open-label, multi-center, parallel-group comparative study to evaluate the efficacy and safety of the following H. pylori eradication regimens: first-line 7-day VPZ/AMPC/CAM versus 7-day PPI [LPZ, rabeprazole (RPZ), omeprazole (OPZ)], or esomeprazole (ESO)/CAM/AMPC, and second-line 7-day VPZ/AMPC/MNZ versus 7-day PPI (LPZ, RPZ, OPZ, or ESO)/AMPC/MNZ. The study was conducted by the Yokohama Gastro Intestinal Study Group (YGISG): 12 centers in Kanagawa Prefecture, Japan participated between February 2015 and February 2016.
All of the studies were performed in accordance with the Declaration of Helsinki and the “Ethical Guidelines for Medical and Health Research Involving Human Subjects” (March 2005, Japanese Ministry of Health, Labor, and Welfare) and were registered in the UMIN-CTR, a standard registry maintained by the International Committee of Medical Journal Editors (ICMJE), with the identifier UMIN000021604. The protocol was approved by the institutional review board at each study site.
Participants
Male or female H. pylori-positive patients (described below) of ≥20 years of age were eligible for inclusion. Subjects with any of the following were excluded: 1) any patient participating in UMIN000016335 (PCAB-based H. pylori eradication study for patients with penicillin allergy), UMIN000016336 (PCAB-based H. pylori third-line eradication study), UMIN000016337 (PCAB-based H. pylori first-line eradication study), or UMIN000019628 (efficacy of UBT after PCAB-based H. pylori eradication), 2) pregnancy or lactation, 3) a history of allergy to the drugs used in this therapy, 4) severe liver dysfunction, severe renal dysfunction, severe heart dysfunction, and 5) patients who were disqualified from the study by their physicians.
The determination of the H. pylori status
The H. pylori status was determined based on the following results: 1) anti-H. pylori antibodies IgG (HpIgG), 2) a rapid urease test (RUT), 3) culture, 4) pathology (histology), or a 5) a 13C urea breath test (UBT) (Tables 1, 3). H. pylori eradication was determined primarily by UBT with UBIT 100-mg tablets (Otsuka Pharmaceutical Tokyo, Japan) using a cut-off of 2.5‰ or, in a few cases, by a stool H. pylori antigen test, both values were considered as standards (Tables 1, 3). For all participants, a follow-up UBT was performed after at least 4 weeks, and typically around 8 weeks (Tables 1, 3), after the completion of treatment to confirm the successful eradication. In the prospective study part, all subjects were asked to stop taking PPIs from the completion of treatment until the UBT. The UBT was mostly performed by an external clinical inspection agency, and partly by internal inspection units of hospitals; in all cases, they were blinded to the treatment regimens and the presence of this research.
Table 1.
First-line Patient Backgrounds.
| VAC | PAC | p | |
|---|---|---|---|
| Age | 64.3 ± 12.3 | 64.5 ± 12.7 | 0.72 |
| Male, % | 54.4 | 56.9 | 0.39 |
| Smoking | 18.4 | 17.4 | 0.65 |
| CAM 200 bid, % | 94.8 | 63.8 | <0.001 |
| Evaluation by UBT, % | 99.3 | 99.8 | 0.37 |
| Endoscopic findings, % | |||
| Gastroduodenal ulcer | 11.7 | 10.7 | |
| Gastric cancer | 7.2 | 7.5 | |
| MALT | 0.3 | 0.3 | |
| Gastritis only | 80.8 | 81.5 | 0.99 |
| Diagnosis of Infection | |||
| HpIgG | 42.4 | 46.2 | |
| RUT | 21.9 | 23.6 | |
| Culture | 25.3 | 22.6 | |
| Pathology | 3.8 | 2.9 | |
| UBT | 3.8 | 3.6 | |
| Urine, stool antigen | 2.3 | 1.0 | 0.99 |
PAC: PPI (LPZ, RPZ, OPZ, or ESO)/AMPC/CAM 1 week eradication therapy, VAC: vonoprazan/AMPC/CAM 1-week eradication therapy, smoking: smoker at the time of registration, CAM 200 bid, %: percentage of CAM 200 mg twice per day (400 mg/day) against CAM 400 mg twice per day (800 mg/day), Evaluation by UBT, %: percentage determined by 13C-urea breath test versus H.pylori stool antigen test. Drug withdrawal, weeks: period from end eradication to eradication assessment, Endoscopic findings: all participants underwent endoscopy before eradication therapy, RUT: rapid urease test, UBT: 13C-urea breath test
Table 3.
Second-line Patient Backgrounds.
| VAM | PAM | p | |
|---|---|---|---|
| Age | 67.5±11.3 | 63.9±11.7 | 0.005 |
| Male, % | 57.8 | 45.5 | 0.02 |
| Smoking | 15.5 | 15.0 | 1.0 |
| Evaluation by UBT, % | 100 | 99.3 | 0.41 |
| Endoscopic findings, % | |||
| Gastroduodenal ulcer | 11.1 | 14.5 | |
| Gastric cancer | 11.1 | 17.6 | |
| MALT | 0 | 0 | |
| Gastritis only | 77.8 | 87.9 | 0.12 |
VAM: vonoprazan/AMPC/MNZ 1-week eradication therapy, PAM: PPI (LPZ, RPZ, OPZ, or ESO)/AMPC/MNZ 1-week eradication therapy
Determination of clarithromycin resistance in H. pylori
Phenotypic antibiotic resistance was determined by the minimum inhibitory concentration on the agar dilution test. The resistance breakpoint for clarithromycin was defined as ≥1 mg/L (12).
Treatment
Four treatment groups were analyzed (two for first-line and two for second-line eradication). The treatment groups received the following treatment regimens: 1) The VAC group received first-line triple therapy with VPZ (20 mg, twice a day) in combination with AMPC (750 mg, twice a day) plus CAM (200 or 400 mg, twice a day for 1 week; 2) the PAC group received first-line triple therapy with PPI [LPZ (30 mg), RPZ (10 mg), OPZ (20 mg), or ESO (20 mg), twice a day] in combination with AMPC (750 mg, twice a day) plus CAM (200 or 400 mg, twice a day for 1 week); 3) the VAM group received second-line triple therapy with VPZ (20 mg, twice a day) in combination with AMPC (750 mg, twice a day) plus metronidazole (250 mg, twice a day for 1 week), and 4) the PAM group received second-line triple therapy with PPI [LPZ (30 mg), RPZ (10 mg) and OPZ (20 mg), or ESO (20 mg), twice a day] in combination with AMPC (750 mg, twice a day) plus MNZ (250 mg, twice a day for 1 week).
The treatments compared in the analysis were first-line VAC versus PAC and second-line VAM versus PAM. Because it has been shown that the dose of CAM does not affect the H. pylori rate (9), patients who received 200 mg and 400 mg doses of CAM (twice a day) were included in the same group in the analysis. All of the treatments were administered orally, and the subjects were then followed for an additional ≥4 weeks and their H. pylori status was determined. The treatment duration and antimicrobial doses were determined according to the approved indications in Japan for first- and second-line triple therapies for the eradication of H. pylori.
Procedures
After study participation, a physician completed the study registration form, including gender, age, active smoking, endoscopic findings (all patients undergoing H. pylori eradication therapy must undergo upper endoscopy for national insurance coverage), the number of times they had previously undergone H. pylori eradication, the regimen that was used for eradication, registration in other studies, the method by which H. pylori infection was determined, the planned regimen for eradication, the start date of eradication therapy, and the antimicrobial susceptibility testing (standard agar plate dilution method) results for CAM (if present).
After eradication therapy, the efficacy of the H. pylori eradication treatment was assessed by a UBT with UBIT (100 mg) tablets (Otsuka Pharmaceutical) using a cut-off of 2.5% or a H. pylori antigen stool test. Both tests are accepted evaluation methods after eradication treatment (13, 14). A case report form was then completed, which included the date of the UBT or H. pylori antigen stool test with an immunochromatography kit (WAKAMOTO, Tokyo, Japan), compliance with treatment, adverse events, the results of the UBT or H. pylori antigen stool test, and the confirmation of washout of vonoprazan or PPIs after eradication (until the test).
In this study, the AEQ was used in all 12 centers. The AEQ was completed by patients during therapy and was collected at the first visit after eradication therapy. The AEQ contained 13 questions (diarrhea, dysgeusia, nausea, anorexia, abdominal pain, heartburn, urticaria, headache, abdominal fullness, eructation, vomiting, fatigue, and other), and the patients chose subjective answers: none (AEQ, 0), weak (AEQ, 1), moderate (AEQ, 2), or strong (AEQ, 3). Physician-based medical assessments played no role in the patients' AEQ choices because the AEQ was collected at the start of the clinical examination and was not changed by the physician, even if the physician determined that the subjective symptoms had no medical significance. Thus, there was no reporting bias; however, the results may reflect the sensitivity of the patient to symptoms.
The primary end-points were the eradication rates in first- and second-line H. pylori eradication. The secondary end-point was safety. This was evaluated using the AEQ, which was completed by the patients during first-line H. pylori eradication therapy. A subgroup analysis of the cases in which information on CAM resistance was available (resistant vs. susceptible) was performed as a pre-planned analysis.
Statistical analysis
The categorical data were compared using the χ2 test or Fisher's exact test, as appropriate. The continuous data were compared using Student's t-test or a one-way analysis of variance. For the primary end points, the frequency and two-sided 95% CIs were calculated for each treatment group. A multivariate logistic regression analysis was also performed in the subgroups with CAM resistance information. The dependent variable was the H. pylori eradication rate, and the independent variables were age, gender, the CAM dose, smoking, and CAM resistance. All of the p values were two-tailed. p values of <0.05 were considered to indicate statistical significance. The statistical analyses were performed using the SPSS software program (ver. 11.0) and the EZR software program (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for ‘R’ (The R Foundation for Statistical Computing, Vienna, Austria). Specifically, it is a modified version of R commander, with additional statistical functions that are frequently used in biostatistics. All of the authors had access to the study data and reviewed and approved the final manuscript.
Results
Patient selection flow
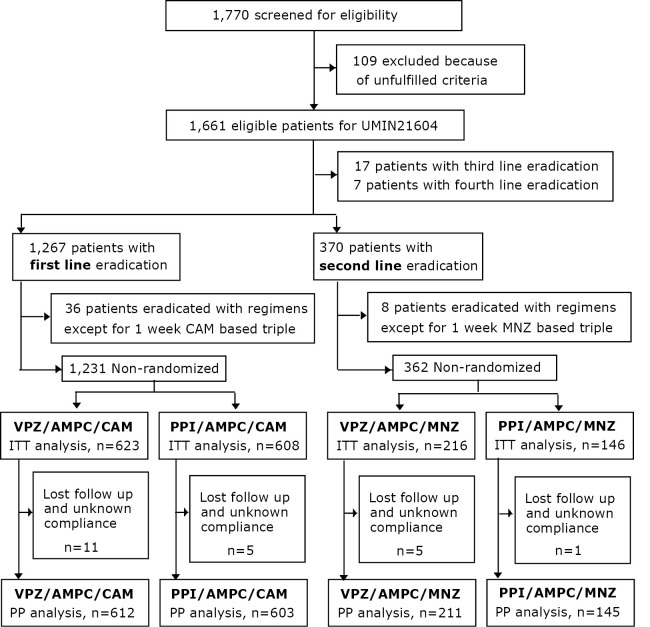
In total, 1,770 patients with H. pylori infection were screened for eligibility (Fig. 1). One hundred nine of these patients were excluded based on the inclusion and exclusion criteria; they had been enrolled in other studies performed by our group (UMIN16335, 16336, 16337, or 19628). Thus, we enrolled 1,661 patients for whom first (n=1,267), second (n=370), third (n=17), and fourth eradication treatments (n=7) were planned and who were eligible for UMIN21604. The enrollment for the prospective study of UMIN21604 was conducted between February 2015 (the date on which vonoprazan received approval in Japan) and February 2016. In the patients who underwent first-line treatment, eradication was achieved with regimens other than 1 week CAM based triple therapy in 36 patients, who were excluded from the analysis. In total, 623 patients who received VPZ/AMPC/CAM and 608 patients who received PPI/AMPC/CAM regimens were analyzed as the intention-to-treat (ITT) population. Among these patients, 11 patients who received VPZ/AMPC/CAM and 5 patients who received PPI/AMPC/CAM were lost to follow-up or had unknown compliance. Thus, 612 patients with VPZ/AMPC/CAM and 603 with PPI/AMPC/CAM were analyzed as the per-protocol (PP) population. PPI-based eradication was achieved in 513 patients who received LPZ/AMPC/CAM (LAC), 76 patients who received RPZ/AMPC/CAM (RAC), 11 patients who received ESO/AMPC/CAM (EAC) and 3 patients who received OPZ/AMPC/CAM (OAC). With regard to second-line treatment, in 8 cases, eradication was achieved with regimens other than 1-week MNZ based therapy; these cases were excluded from the analysis. Thus, 216 patients who received VPZ/AMPC/MNZ and 146 patients who received PPI/AMPC/MNZ were analyzed as the ITT population. Among these patients, 5 patients who received VPZ/AMPC/MNZ and 1 patient who received PPI/AMPC/MNZ were lost to follow-up or had unknown compliance. Thus, 211 patients who received VPZ/AMPC/MNZ and 145 patients who received PPI/AMPC/MNZ were analyzed as the PP population. With regard to the PPIs, 110 patients received LPZ/AMPC/MNZ (LAM), 28 patients received RPZ/AMPC/MNZ (RAM), 4 patients received ESO/AMPC/MNZ (EAM) and 3 patients received OPZ/AMPX/MNZ (OAM).
Figure 1.
Patient selection flow. UMIN, A registry of the International Committee of Medical Journal Editors (ICMJE). After the approval of vonoprazan (2015.2-2016.2), 1,231 first-line and 362 second-line eradication cases were analyzed in this study.
First-line eradication
The backgrounds of the patients who received first-line eradication are shown in Table 1. The baseline characteristics of the [PPI/AMPC/CAM (PAC)] and [VPZ/AMPC/CAM (VAC)] groups were similar in terms of age, gender, smoking, evaluation of eradication success by UBT, endoscopic findings before eradication, and the diagnosis of infection. Significant differences were observed in the dose of CAM (400 or 800 mg per day) and the drug withdrawal period (UBT score or H. pylori antigen stool test after the completion of treatment to confirm successful eradication). A CAM dose of 400 mg per day was administered to 94.8% of the patients in the VAC group and to 63.8% of the patients in the PAC group. This may be because physicians chose CAM (400 mg) based on the evidence of the VPZ phase III trial, which revealed that there was no significant difference in eradication rates achieved with CAM (400 mg) and CAM (800 mg). If there was an effect on eradication rate, this difference in the rate of CAM (400 mg), the use would be expected to decrease the eradication rate among patients who received VAC.
The drug withdrawal periods in the PAC and VAC groups were 7.8±3.5 weeks and 8.0±3.1, respectively. These were longer than the withdrawal period in the phase III trial (4.0 weeks).
The first-line ERs in the ITT and PP analyses were 84.9% (95% CI=81.9-87.6%, n=623) and 86.4% (83.5-89.1%, n=612), respectively, in the VAC and 78.8% (75.3-82.0%, n=608) and 79.4% (76.0-82.6%, n=603), respectively, in the PAC group. The ER in the VAC group was higher than that in the PAC group, in both the ITT (p=0.0061) and PP (p=0.0013) analyses (Fig. 2). As for the subgroup analysis of the PPI-containing regimens, the first-line ERs in the ITT and PP analyses were 78.2% (95% CI: 74.4-81.7%, n=518) and 78.9% (75.2-82.4%, n=513), respectively for LAC, while for VAC, the ERs in the ITT (p=0.0042) and PP (p=0.0011) analyses were higher in comparison to LAC. In contrast, the ER of VAC tended to be higher than that of RAC [81.6% (71.0-89.5%, n=76)], but the difference was not statistically significant. The ERs of EAC and OAC were 81.8% (48.2-97.7, n=11) and 100% (36.8-100, n=3), respectively.
Figure 2.
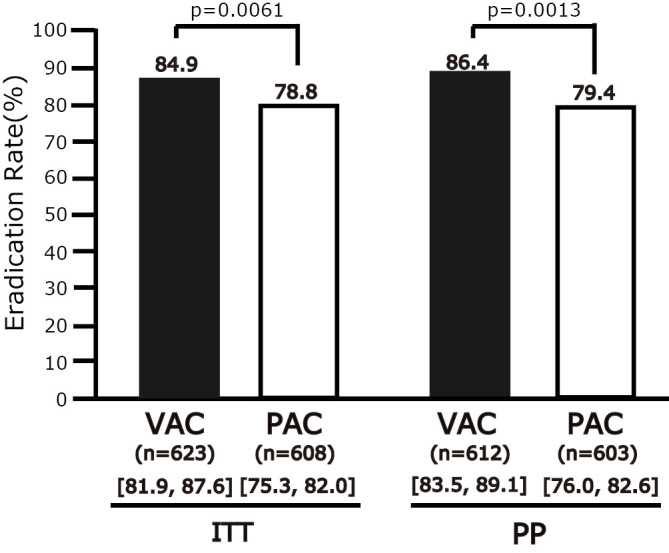
The first-line Helicobacter pylori eradication rates. CAM: clarithromycin, MIC: minimum inhibitory concentration, VAC: 1-week vonoprazan/AMPC/CAM eradication therapy, PAC: PPI (LPZ, RPZ, OPZ, or 1-week ESO)/AMPC/CAM eradication therapy, [ ]: 95% CI, ITT: intention-to-treat analysis, PP: per-protocol analysis
The rates of adverse effects during therapy, as assessed with the AEQ, are shown in Table 2. Diarrhea, general malaise, and other showed significant different AEQ scores. Additionally, with regard to the symptoms with AEQ scores of 3, there were significant differences with regard to the incidence of nausea and headache. The values of all of the significant different variables in the PAC group were higher than those in the VAC group. None of the AEQ scores in the VAC group were higher than those in the PAC group.
Table 2.
Safety of VAC versus to PAC by Questionnaire.
| Any (AEQ 1, 2, or 3) | AEQ 3 | ||||||
|---|---|---|---|---|---|---|---|
| VAC | PAC | p | VAC | PAC | p | ||
| Diarrhea | 25% | 49% | <0.001 | 3% | 2% | 0.78 | |
| Dysgeusia | 11% | 16% | 0.34 | 2% | 2% | 0.96 | |
| Nausea | 6% | 4% | 0.68 | 0% | 4% | 0.0016 | |
| Anorexia | 9% | 7% | 0.61 | 2% | 2% | 0.96 | |
| Abdominal pain | 12% | 9% | 0.61 | 0% | 2% | 0.18 | |
| Heart burn | 7% | 9% | 0.69 | 1% | 2% | 0.62 | |
| Hives | 3% | 4% | 0.48 | 1% | 2% | 0.62 | |
| Headache | 6% | 4% | 0.68 | 0% | 15% | <0.001 | |
| Abdominal fullness | 19% | 27% | 0.22 | 3% | 0% | 0.21 | |
| Belch | 16% | 20% | 0.48 | 1% | 2% | 0.62 | |
| Vomiting | 3% | 0% | 0.27 | 0% | 0% | 0.66 | |
| General malaise | 6% | 16% | 0.025 | 1% | 2% | 0.62 | |
| Other | 4% | 16% | 0.003 | 0% | 0% | 0.66 | |
The subgroup analysis with CAM resistance
The CAM resistance information for the VAC regimen was collected from some of the patients who were enrolled in this study (n=236). As shown in Fig. 3, the ERs of VAC in CAM-resistant and CAM-susceptible subjects were 73.2% (95% CI=59.7-84.2%, n=56) and 88.9% (83.4-93.1%, n=180), respectively. The ER of VAC in patients with no applicable resistance information was 87.2% (n=376). The ER of PAC in the CAM-susceptible subjects was 84.2% (95% CI=78.4-89.1, n=197). No significant difference was observed between VAC and PAC in the CAM-susceptible subjects (p=0.23).
Figure 3.
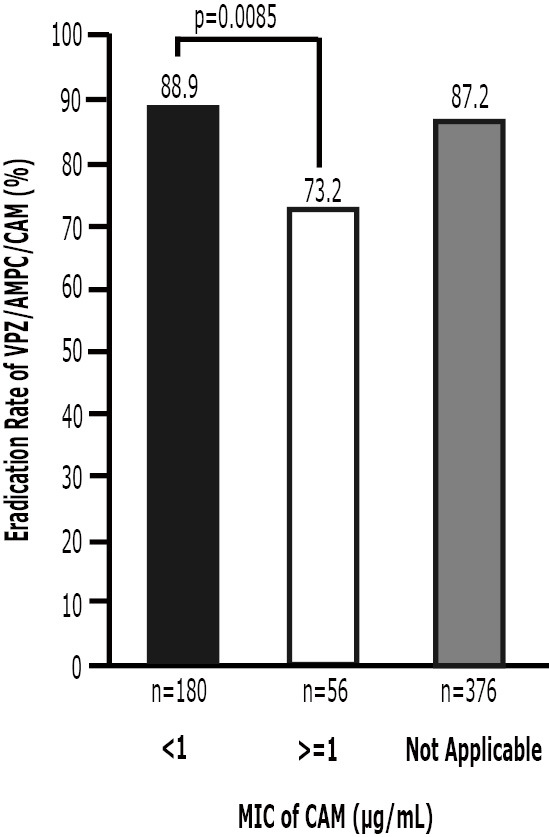
The relationship between susceptibility to CAM and the ER (VAC). VAC: 1-week vonoprazan/AMPC/CAM eradication therapy, MIC: minimum inhibitory concentration
A multivariate logistic regression analysis showed that age, gender, smoking and the CAM dose did not affect the results. CAM resistance had a significant impact on eradication failure (OR=2.86; 95% CI=1.89-4.31). The ER of VAC was significantly higher in the CAM-susceptible patients (88.9%, n=180) than it was in the CAM-resistant patients (73.2%, n=56; p=0.0085).
Second-line eradication
The backgrounds of the second-line eradication patients are shown in Table 3. The baseline characteristics of the two groups [PPI/AMPC/MNZ (PAM)], and [VPZ/AMPC/MNZ (VAM)] were similar in terms of smoking, the evaluation of eradication success by UBT, and the endoscopic findings before eradication. Differences were observed regarding the patients' age and gender. The drug withdrawal periods were 8.3±3.4 weeks in the PAM group and 8.8±3.6 weeks in the VAM group. These were longer than those observed in the phase III study (4.0 weeks); there was no significant difference between the groups (p=0.26).
The second-line ERs in the ITT and PP analyses were 80.5% (95% CI=74.6-85.6%, n=216) and 82.4% (76.6-87.3%, n=211), respectively, in the VAM group and 81.5% (74.2-87.4%, n=146) and 82.1% (74.8-87.9%, n=145), respectively, in the PAM group. No significant difference was observed in the resultso f the ITT (p=0.89) or PP (p=1.0) analysis (Fig. 4). As for the subgroup analysis by PPI, the second-line ERs in the ITT and PP analysis were 78.3% (95% CI: 69.6-85.6%, n=111) and 79.1% (70.3-86.3%, n=110) for LAM, 89.3% (71.8-97.7, n=28) and 89.3% (71.8-97.7%, n=28) for RAM, 100% (47.3-100%, n=4) and 100% (47.3-100%, n=4) for EAM, and 100% (36.8-100%, n=3) and 100% (36.8-100%, n=3) for OAM. No significant differences were observed between VAM and LAM or between VAM and RAM.
Figure 4.
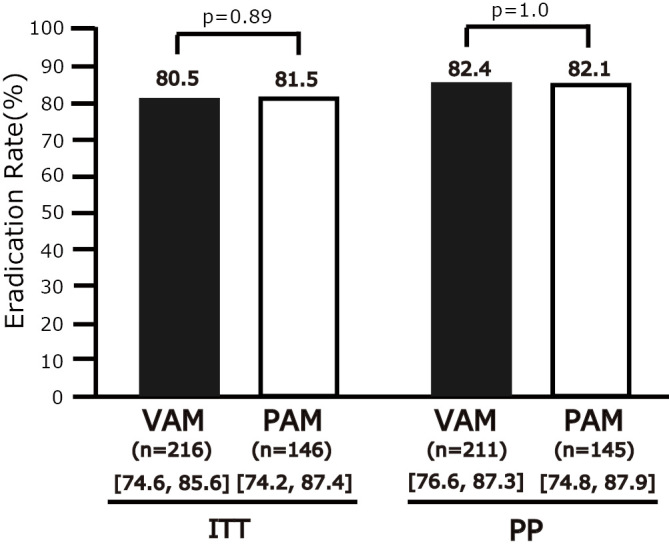
The non-superiority of VAM versus PAM for first-line eradication. VAM: 1-week vonoprazan/AMPC/MNZ eradication therapy, PAM: 1-week PPI (LPZ, RPZ, OPZ, or ESO)/AMPC/MNZ eradication therapy, [ ]: 95% CI, ITT: intention-to-treat analysis, PP: per-protocol analysis
No significant AEQ difference was observed in the CAM (any AEQ score of 2 or 3: 18%) or PAM of groups (any AEQ score of 2 or 3: 22%).
Discussion
In this multicenter cohort study, we confirmed the superiority of first-line VPZ/AMPC/CAM (VAC) versus PPI/AMPC/CAM (PAC) in real-world practice. Unexpectedly, the success rate was less than the expected value of 90%. Moreover, the ER of VAC was relatively low in comparison to the phase III VAC result. Furthermore, no significant difference was observed between second-line VPZ/AMPC/MNZ (VAM) and PPI/AMPC/MNZ (PAM) in real-world practice.
It is important to discuss the difference in the ERs, which were between 92.6% (phase III VAC, 300/324) and 86.4% (the VAC PP analysis in the present study, 529/612) or 85.2% (in the cases with CAM resistance information, VAC, 201/236). First, the difference did not occur due to the CAM-resistant population. The CAM resistance rate of 23.7% (56/236) that was reported in the present study (Fig. 3) was not higher than the rate of 30.4% (100/329) that was reported in the phase III study (8). Age, gender, and the dose of CAM do not explain the differences either. Indeed, no differences in the ERs of these subgroups were observed in the phase III trial [age <65, 93.0% (n=243) and ≥65, 91.4% (n=81); males, 93.3% (n=193) and females 91.6% (n=131), CAM (200 mg, twice per day), 93.3% (n=163) and CAM [400 mg, twice per day, 91.9% (n=161)] or in the present study (the multivariate logistic regression analysis showed that age, gender, smoking, and the dose of CAM did not affect the results). Third, it is difficult to explain the difference only by compliance, because 98.2% of participants with VAC confirmed compliance with the physician at the visit for the eradication result and they did not complete the free entry field of the AEQ. However, some compliance problems that may not be identified by the physician may still exist. This study was performed by the Yokohama Gastro Intestinal Study Group, which includes 12 centers around Yokohama, Japan, including urban and rural areas and both specialized and general hospitals. Thus, we consider our study results to represent real-world practice. Fourth, there was a difference in subjects: all of the patients in this study had an H. pylori infection, while the phase III trial included patients with a history of gastroduodenal ulcers. Clinically, H. pylori eradication therapy was administered to H. pylori-infected patients; thus our results are more useful in clinical practice. Fifth, there was a difference in the timing of the UBT: -8 weeks in this study versus just 4 weeks in the phase III trial. Thus, the results may have included some false-negatives from the 4-week UBT after eradication with VAC for CAM-resistant subjects, because the 4-6 week UBT was negative, but at 8-10 weeks, it was positive. We assessed this in another study (UMIN19628). If the difference between the ER of 82.0% (82/100) in the CAM-resistant subjects in the phase III trial and the ER of 73.2% (41/56) in the CAM-resistant subjects in this study was due to false negatives, the true ER can be calculated as 89.8% [291/324: CAM-resistant (73/100) and CAM-susceptible (218/224)], which is still under 90%. Sixth, although the resistance for AMPC was not a significant factor for ER in phase III: MIC ≤0.03 mg/L (94%, n=234) and MIC >0.03 mg/L (87.3%, n=71; p=0.073), and also no AMPC resistance data were observed, nevertheless a difference in the AMPC resistance rate may affect the ER.
The border for the CAM-resistant percentage for >90% total eradication that was calculated from the phase III results (82% in CAM-resistant and 97.3% in CAM-susceptible) was 47.7%. However, this result changes to 30.2% when the CAM-resistant results from the present study and the phase III study are included (73.2% in CAM-resistant and 97.3% in CAM-susceptible). The CAM resistance rate in Japan was 33% in 2015, with an increasing trend. To maintain an ER of >90% with VAC, further trials will be needed to optimize the duration of therapy and the dose of amoxicillin in VAC. Based on the phase III results, the ER achieved with PAC in the CAM-resistant subjects was insufficient (approximately 40%); thus PPI-based eradication therapy was only administered to small numbers of CAM-resistant subjects in the present study. To evaluate PAC in CAM-resistant subjects, we analyzed the retrospective data (one year before the approval of VPZ) of our YGISG and found that the ER of PAC in CAM-resistant subjects was 54.9% (95% CI=42.7-66.8%, n=71). Similar to the phase III results, the ER of VAC in CAM-resistant subjects was significantly higher than the ER of PAC in the CAM-resistant subjects (p=0.042).
There is no evidence to suggest that second-line VAM is very effective in real-world practice, because the good results (98%, 49/50) in the phase III trial were from a single-arm design. Our results, which show first-line superiority but no second-line superiority indicate that acid suppression is important for CAM, but not for MNZ. The mechanism is thought to involve CAM working in the growth phase (CAM inhibits protein synthesis), whereas MNZ targets DNA and, is therefore independent of the stationary or growth phase distribution (15). Vonoprazan inhibits the gastric H+,K+-ATPase in a K+-competitive and reversible manner (16). This novel mechanism enables the following: 1) a fast maximal efficacy (2-3 h for VPZ vs. 3-5 days for PPIs); 2) long-acting effects (VPZ, dose-dependent accumulation in parietal cells; PPIs, unstable under acid conditions and dependent on blood levels); and 3) low gene polymorphism (VPZ: CYP3A4, PPIs: CYP2C19 (17). AMPC also works in the growth phase (AMPC targets cell wall biosynthesis), but it is difficult to eradicate with AMPC alone in a population with first-line failure (AMPC resistance is assumed to have developed).
According to the recent meta-analysis, CYP2C19 loss-of-function variants are associated with increased H. pylori ER in patients taking PPI-based triple therapies with LPZ or OPZ, but not with RPZ or ESO (18).
As for the subgroup analysis by PPI, the ER of VAC was higher than the ER of LAC. In contrast, the ER of VAC tended to be higher in comparison to RAC [81.6% (71.0-89.5%, n=76)], but no significant difference was observed. We thought that these results might be due to the small number of patients with RAC. Thus, we performed an evaluation using retrospective data (one year before the approval of VPZ) from the YGISG, and found that the ER of VAC was also higher than that of RAC [72.4% (65.1-78.9, n=174)] with a PP (p=0.00023) analysis, suggesting that the ER of VAC was higher than that of PAC, irrespective of whether PPI was used.
In this study, first-line VAC and second-line VAM were well tolerated and patients showed good compliance. The decreased AEQ score with VAC in comparison to PAC may indicate the possibility of altering the duration of therapy and the dose of amoxicillin in VAC therapy.
Our study is associated with several limitations. First, it was a non-randomized controlled trial. Because the vonoprazan-based eradication rate was high in the phase III trial, we could not use the same design. The vonoprazan-based or PPI-based regimen depended on the physician. Second, the AEQ may reflect the sensitivity of the patient to his or her symptoms. We believe that our AEQ was good in terms of sensitivity and had no reporting bias, but was influenced by the patients' sensitivity to their symptoms. Third, in the present study the AMPC resistance information was not applied, whereas the CAM resistance information was partially applied.
In conclusion, the eradication rate of first-line VPZ/AMPC/CAM was higher than that of PPI/AMPC/CAM, but was still less than 90% in real-world practice. The second-line eradication rate of VPZ/AMPC/MNZ was not higher than that of PPI/AMPC/MNZ. None of the questionnaire scores relating to adverse effects in the VPZ/AMPC/CAM group were higher than those in the PPI/AMPC/CAM group; thus, VPZ/AMPC/CAM was safe and well tolerated. Further trials are needed to examine the possibility of using a modified VPZ/AMPC/CAM regimen in which duration of therapy and the dose of amoxicillin are optimized.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Gonzalez CA, Megraud F, Buissonniere A, et al. Helicobacter pylori infection assessed by ELISA and by immunoblot and noncardia gastric cancer risk in a prospective study: the Eurgast-EPIC project. Ann Oncol 23: 1320-1324, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Ma JL, Zhang L, Brown LM, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst 104: 488-492, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberg ER, Park JY. Effectiveness of Helicobacter pylori eradication. In: Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer IARC Working Group Report Volume 8. International Agency for Research on Cancer. Albert Thomas, Lyon Cedex, 2014: 64-71. [Google Scholar]
- 4.Yuan Y, Ford AC, Khan KJ, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev 12: CD008337, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asaka M, Sugiyama T, Kato M, et al. A multicenter, double-blind study on triple therapy with lansoprazole, amoxicillin and clarithromycin for eradication of Helicobacter pylori in Japanese peptic ulcer patients. Helicobacter 6: 254-261, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Thung I, Aramin H, Vavinskaya V, et al. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther 43: 514-533, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection: the Maastricht IV/ Florence Consensus Report. Gut 61: 646-664, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut 65: 1439-1446, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol 12: 177-186.e3; Discussion e12-13, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki T, Matsuo K, Ito H, et al. Smoking increases the treatment failure for Helicobacter pylori eradication. Am J Med 119: 217-224, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Megraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut 53: 1374-1384, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liou JM, Chen CC, Chen MJ, et al. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet 381: 205-213, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Vaira D, Holton J, Menegatti M, et al. Review article: invasive and non-invasive tests for Helicobacter pylori infection. Aliment Pharmacol Ther 14 Suppl 3: 13-22, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Gisbert JP, de la Morena F, Abraira V. Accuracy of monoclonal stool antigen test for the diagnosis of H. pylori infection: a systematic review and meta-analysis. Am J Gastroenterol 101: 1921-1930, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Sachs G, Meyer-Rosberg K, Scott DR, Melchers K. Acid, protons and Helicobacter pylori. Yale J Biol Med 69: 301-316, 1996. [PMC free article] [PubMed] [Google Scholar]
- 16.Hori Y, Imanishi A, Matsukawa J, et al. 1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438), a novel and potent potassium-competitive acid blocker for the treatment of acid-related diseases. J Pharmacol Exp Ther 335: 231-238, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Sakurai Y, Mori Y, Okamoto H, et al. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects: a randomised open-label cross-over study. Aliment Phamacol Ther 42: 719-730, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Tang HL, Li Y, Hu YF, Xie HG, Zhai SD. Effects of CYP2C19 loss-of-function variants on the eradication of H. pylori infection in patients treated with proton pump inhibitor-based triple therapy regimens: a meta-analysis of randomized clinical trials. PLoS one 8: e62162, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]