Abstract
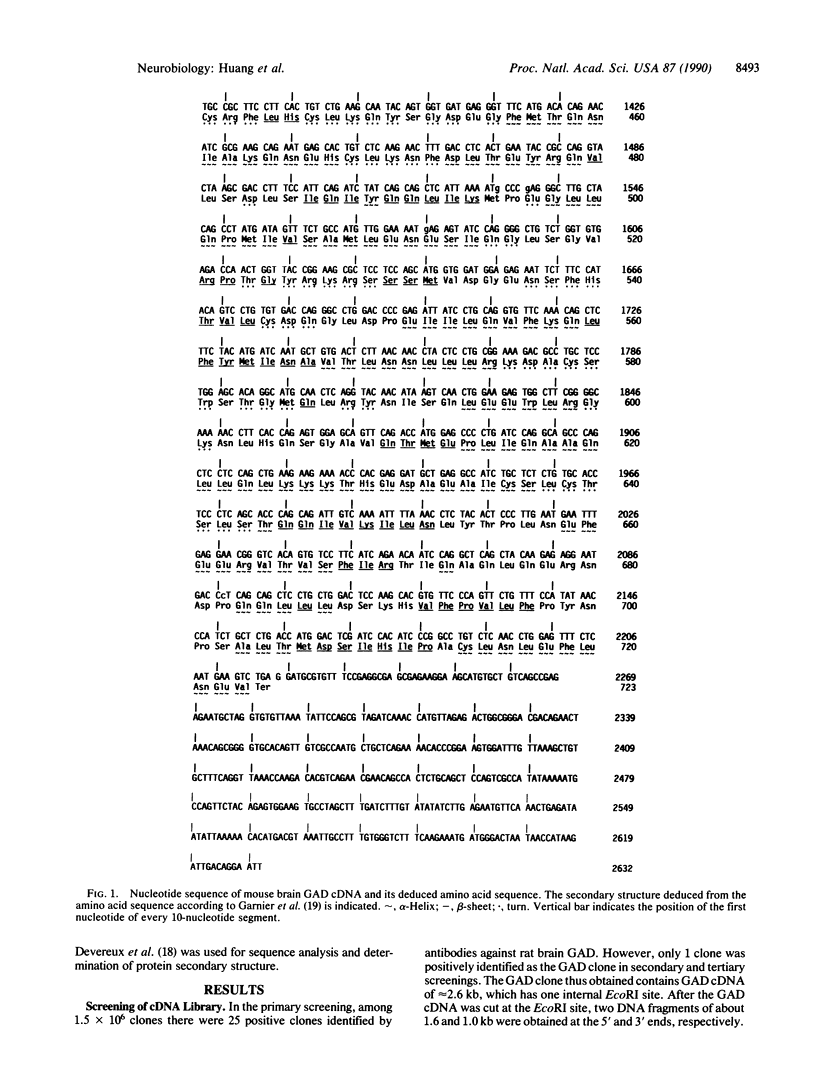
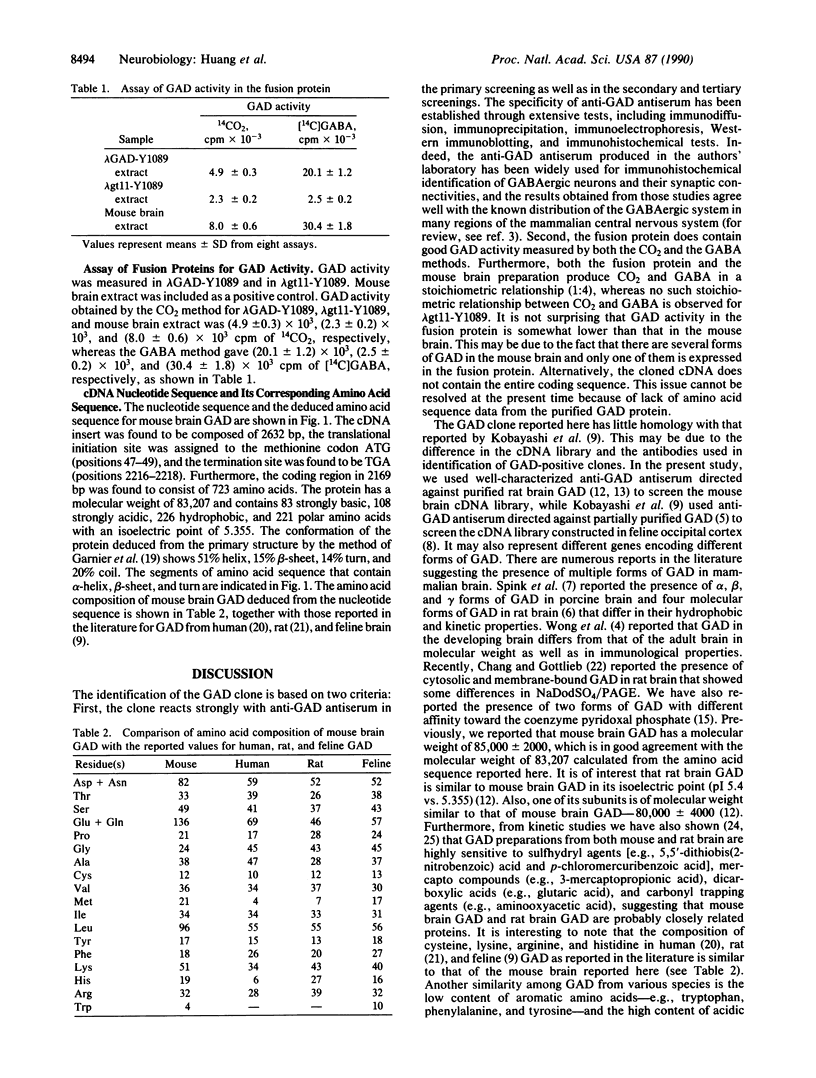
We used specific polyclonal antibodies against L-glutamate decarboxylase (GAD) to screen a mouse brain cDNA library that was constructed in the expression vector lambda gt11. We obtained 1.5 x 10(6) recombinant DNA clones in the mouse brain cDNA library. One of the clones was positively identified as a GAD clone on the basis of the following results: (i) the clone and its secondary and tertiary clones all reacted strongly with anti-GAD antibodies; (ii) the fusion protein obtained from lambda GAD-Y1089 showed good GAD enzyme activity as determined by both CO2 and gamma-aminobutyric acid methods. The GAD clone thus obtained contains GAD cDNA of approximately 2.6 kilobases that has one internal EcoRI site. After GAD cDNA was cut at the EcoRI site, two DNA fragments of about 1.6 and 1.0 kilobases were obtained at the 5' and 3' ends, respectively. The cDNA insert was found to be composed of 2632 base pairs, the translation initiation site was assigned to the methionine codon ATG, and the termination site was found to be TGA (positions 2216-2218). Furthermore, the coding region in 2169 base pairs was found to consist of 723 amino acids. The protein has a molecular weight of 83,207 and contains 83 strongly basic, 108 strongly acidic, 226 hydrophobic, and 221 polar amino acids with an isoelectric point of 5.355. The relationship of this GAD cDNA to other forms of GAD is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blindermann J. M., Maitre M., Ossola L., Mandel P. Purification and some properties of L-glutamate decarboxylase from human brain. Eur J Biochem. 1978 May;86(1):143–152. doi: 10.1111/j.1432-1033.1978.tb12293.x. [DOI] [PubMed] [Google Scholar]
- Chang Y. C., Gottlieb D. I. Characterization of the proteins purified with monoclonal antibodies to glutamic acid decarboxylase. J Neurosci. 1988 Jun;8(6):2123–2130. doi: 10.1523/JNEUROSCI.08-06-02123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chude O., Wu J. Y. A rapid method for assaying enzymes whose substrates and products differ by charge. Application to brain L-glutamate decarboxylase. J Neurochem. 1976 Jul;27(1):83–86. doi: 10.1111/j.1471-4159.1976.tb01547.x. [DOI] [PubMed] [Google Scholar]
- Denner L. A., Wei S. C., Lin H. S., Lin C. T., Wu J. Y. Brain L-glutamate decarboxylase: purification and subunit structure. Proc Natl Acad Sci U S A. 1987 Feb;84(3):668–672. doi: 10.1073/pnas.84.3.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denner L. A., Wu J. Y. Two forms of rat brain glutamic acid decarboxylase differ in their dependence on free pyridoxal phosphate. J Neurochem. 1985 Mar;44(3):957–965. doi: 10.1111/j.1471-4159.1985.tb12910.x. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Kaufman D. L., McGinnis J. F., Krieger N. R., Tobin A. J. Brain glutamate decarboxylase cloned in lambda gt-11: fusion protein produces gamma-aminobutyric acid. Science. 1986 May 30;232(4754):1138–1140. doi: 10.1126/science.3518061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Kaufman D. L., Tobin A. J. Glutamic acid decarboxylase cDNA: nucleotide sequence encoding an enzymatically active fusion protein. J Neurosci. 1987 Sep;7(9):2768–2772. doi: 10.1523/JNEUROSCI.07-09-02768.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Maitre M., Blindermann J. M., Ossola L., Mandel P. Comparison of the structures of L-glutamate decarboxylases from human and rat brains. Biochem Biophys Res Commun. 1978 Dec 14;85(3):885–890. doi: 10.1016/0006-291x(78)90626-5. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Oertel W. H., Schmechel D. E., Tappaz M. L., Kopin I. J. Production of a specific antiserum to rat brain glutamic acid decarboxylase by injection of an antigen-antibody complex. Neuroscience. 1981;6(12):2689–2700. doi: 10.1016/0306-4522(81)90113-5. [DOI] [PubMed] [Google Scholar]
- Roberts E., Kuriyama K. Biochemical-physiological correlations in studies of the gamma-aminobutyric acid system. Brain Res. 1968 Apr;8(1):1–35. doi: 10.1016/0006-8993(68)90170-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spink D. C., Porter T. G., Wu S. J., Martin D. L. Kinetically different, multiple forms of glutamate decarboxylase in rat brain. Brain Res. 1987 Sep 22;421(1-2):235–244. doi: 10.1016/0006-8993(87)91293-5. [DOI] [PubMed] [Google Scholar]
- Spink D. C., Wu S. J., Martin D. L. Multiple forms of glutamate decarboxylase in porcine brain. J Neurochem. 1983 Apr;40(4):1113–1119. doi: 10.1111/j.1471-4159.1983.tb08101.x. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Denner L. A., Wei S. C., Lin C. T., Song G. X., Xu Y. F., Liu J. W., Lin H. S. Production and characterization of polyclonal and monoclonal antibodies to rat brain L-glutamate decarboxylase. Brain Res. 1986 May 14;373(1-2):1–14. doi: 10.1016/0006-8993(86)90309-4. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Roberts E. Properties of brain L-glutamate decarboxylase: inhibition studies. J Neurochem. 1974 Oct;23(4):759–767. doi: 10.1111/j.1471-4159.1974.tb04401.x. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]



