Abstract
Background
No studies have explored familial risks of nasopharyngeal carcinoma (NPC) in detail and quantified its lifetime risk in high-incidence populations.
Methods
We conducted a population-based case-control study of 2,499 NPC cases and 2,576 controls randomly selected in southern China in 2010–2014. We used unconditional logistic regression to estimate multivariable-adjusted odds ratios (ORs) with 95% confidence intervals (CIs) associated with family history of NPC. In addition, we compiled a reconstructed cohort comprising 40,781 first-degree relatives of cases and controls to calculate lifetime cumulative risk of NPC.
Results
Individuals with a first-degree family history of NPC were at a greater than 4-fold (OR=4.6, 95% CI: 3.5 to 6.1) risk for NPC, compared to those without, but had no excess risk of other malignancies. The excess risk was higher for a maternal than a paternal history and slightly stronger for a sibling than a parental history, and for a sororal than a fraternal history. Among relatives of cases, the cumulative risk of NPC up to age 74 was 3.7% (95% CI: 3.3% to 4.2%), whereas that among relatives of controls was 0.9% (95% CI: 0.7% to 1.2%). Cumulative risk was higher in siblings than in parents among relatives of cases, while no such difference was noted among relatives of controls.
Conclusions
People with a family history of NPC have a substantially higher risk for NPC. These relative and cumulative risk estimates can guide strategy development for early detection and clinical consultation in NPC high-incidence populations.
Keywords: case-control study, family history, nasopharyngeal carcinoma, relative and cumulative risk, southern China
INTRODUCTION
The elevated risk of nasopharyngeal carcinoma (NPC) among individuals with a first-degree family history of NPC has been well documented.1–8 Although the 2- to 20-fold9–19 excess first-degree familial risk of NPC is among the highest of any malignancy 20, inherited cancer syndromes are presumed not to account for a high proportion of cases. However, because most studies have not ascertained all first-degree relatives and are not population-based, absolute NPC risks in the general population with and without a family history in NPC-endemic geographic regions, where the great majority of NPC cases occur worldwide21, are largely unknown. This uncertainty precludes formal evaluation of whether clinical assessment is necessary for this population.
Due to their familial history, relatives of NPC patients may seek clinical consultation more frequently than the general population. However, the cumulative risk of NPC among relatives of subjects with and without NPC diagnosis, which could inform optimal, potentially relative-specific clinical assessment strategies, also has not been quantified. In a reconstructed familial cohort design, which has not previously been used in NPC-endemic areas, cumulative risks are evaluated among relatives of cases and controls.22 Such findings can guide strategies for primary prevention, early diagnosis, and clinical consultation. We therefore conducted a large, population-based case-control and reconstructed cohort study in southern China, where the world’s highest incidence rates of NPC occur.23–25 We aimed to quantify the cumulative risk of NPC and to provide evidence that can ultimately guide age- and relative-specific clinical management for the general population in NPC-endemic geographic regions.
METHODS
Study Population
We conducted a collaborative study, “NPC Genes, Environment, and EBV” (NPCGEE), in the Zhaoqing area of Guangdong Province and the Wuzhou and Guiping/Pingnan areas of Guangxi Autonomous Region. Eligible subjects were persons aged 20–74 years, officially residing in the study area at diagnosis, with no history of malignant disease, or congenital or acquired immunodeficiency. Detailed information on study design was described previously 26. Briefly, 3027 histopathologically confirmed, first incident NPC cases were identified between 2010 and 2013 through a rapid case ascertainment network. This total closely matched the anticipated number of incident NPC cases based on historical incidence rates in the region. Of the eligible cases, 2554 (84%) consented to participate. Information on histopathological subtype was not available from all cases. A total of 3202 controls, frequency matched to the 5-year age and sex distribution of the cases by residential area, were randomly selected every 6–12 months between 2010 and 2014 from computerized, continuously updated total population registries; of these, 2648 (83%) consented to participate. After exclusion of subjects with missing, ineligible, indeterminate, or poor-quality data, 2,499 cases and 2,576 controls remained in the analysis.
This study was approved by institutional/ethics review boards at all participating centers. All subjects granted written or oral informed consent.
Data Collection
An electronic structured questionnaire was administered to study participants by trained interviewers. Full details are provided in the Supplemental Material. We used two approaches to validate self-reported first-degree family history of NPC, as described in the Supplemental Material.
Statistical Analysis
We used two complementary approaches to estimate the risk of NPC associated with a positive family history of NPC or other cancers, adjusting for potential confounders.27, 28 The first approach was a traditional case-control analysis, wherein we used multivariable unconditional logistic regression models to estimate the associations between a positive family history of NPC or other cancers, overall and for specific relatives. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to estimate the relative risks (Supplemental Material). To adjust for reporting bias, we also conducted a sensitivity analysis to estimate the OR adjusted for bias as a function of the sensitivity and specificity of self-reported first-degree family history of NPC among cases and controls. In addition, to fully account for the family size and structure of each participant, and the affected relative’s age at onset, two kin cohorts were constructed based on the self-reported family information, comprising the first-degree relatives of cases (as the exposed cohort) and of controls (as the unexposed cohort). All first-degree relatives were followed from birth until date of interview, death, age 74, or diagnosis of NPC or another cancer, whichever occurred first. We used Cox proportional hazards regression models, adjusting for age (as the time scale), sex, and geographic area, and stratifying baseline hazards by familial relationship, to estimate hazard ratios (HRs) with 95% CIs. To avoid the influence of familial aggregation, we used the method described by Lee to account for intracluster dependence.29 The assumption of non-proportionality of hazards was tested using graphical assessment, and a method based on Schoenfeld residuals 30 which indicated no violation (P= 0.565). From 42,024 first-degree relatives, we excluded 1,243 with an unknown age at death or cancer diagnosis, leaving 40,781 relatives in the cohort analysis.
We again used two complementary approaches to estimate the cumulative risk of NPC. First, to quantify cumulative risks of NPC for the first-degree relatives, we multiplied their age-specific OR estimates by the age-specific NPC cumulative risks in the underlying population in 2011 (Figure S1). Only two counties in the study area with historically high NPC incidence rates have population-based cancer incidence data,23 potentially leading to biased estimates of NPC incidence over the entire study area. We therefore estimated age-specific (20–74 years) general population incidence rates by dividing the annual number of incident NPC cases identified for this study by the total person-time of the population at risk in 2011.31 Second, to account for the family size and structure of each respondent, we used the Kaplan-Meier method to estimate cumulative risk of NPC up to age 74, which is considered as the cumulative probability of NPC occurrence among relatives of cases (the exposed cohort) and of controls (the unexposed cohort) separately. The disease-specific survival curve was plotted by using the Nelson-Aalen method. We used the log-rank test to evaluate heterogeneity of cumulative risk by familial relationship and sex of relatives, treating cases and controls separately.
RESULTS
Baseline characteristics of cases and controls
Table 1 shows the distribution of baseline demographic characteristics and other potential risk factors for NPC among the 2,499 cases and 2,576 controls. Cases and controls had similar numbers of first-degree relatives (median 8 vs. 8) (Table S1). Cases with a first-degree family history of NPC were more likely to reside in Zhaoqing/Wuzhou than in Guiping/Pingnan. Otherwise, we found no significant differences in the distribution of characteristics between familial and sporadic NPC cases (Table S2).
Table 1.
Characteristics of nasopharyngeal carcinoma cases and controls.
| Characteristics | Cases (No=2499)
|
Controls (No=2576)
|
P value |
|---|---|---|---|
| No (%) | No (%) | ||
| Residential area | 0.39 | ||
| Zhaoqing | 1271 (50.9) | 1303 (50.6) | |
| Wuzhou | 674 (27.0) | 664 (25.8) | |
| Guiping/Pingnan | 554 (22.2) | 609 (23.6) | |
| Sex | 0.96 | ||
| Male | 1835 (73.4) | 1893 (73.5) | |
| Female | 664 (26.6) | 683 (26.5) | |
| Age at diagnosis/referral (years) | |||
| Mean (SD) | 48.5 (10.6) | 49.7 (18.9) | <0.001 |
| 20–29 | 86 (3.4) | 80 (3.1) | 0.004 |
| 30–39 | 417 (16.7) | 371 (14.4) | |
| 40–49 | 904 (36.2) | 880 (34.2) | |
| 50–59 | 677 (27.1) | 724 (28.1) | |
| 60–74 | 415 (16.6) | 521 (20.2) | |
| Education level (years) | 0.003 | ||
| ≤ 6 | 988 (39.5) | 922 (35.8) | |
| 7–9 | 1004 (40.2) | 1030 (40.0) | |
| 10–12 | 401 (16.1) | 483 (18.7) | |
| > 12 | 106 (4.2) | 141 (5.5) | |
| Current housing type | <0.001 | ||
| Building (concrete structure) | 1801 (72.1) | 2007 (77.9) | |
| Cottage (clay brick structure) | 688 (27.5) | 566 (22.0) | |
| Boat | 10 (0.4) | 2 (0.1) | |
| Missing | 0 | 1 (0.04) | |
| Current occupation | <0.001 | ||
| Unemployed | 78 (3.1) | 95 (3.7) | |
| Farmer | 841 (33.7) | 975 (37.9) | |
| Blue-collar | 1016 (40.7) | 895 (34.7) | |
| White-collar | 346 (13.8) | 415 (16.1) | |
| Other/unknown | 218 (8.7) | 196 (7.6) | |
| Cigarette smoking | 0.08 | ||
| Never | 1109 (44.4) | 1206 (46.8) | |
| Ever | 1390 (55.6) | 1368 (53.1) | |
| Missing | 0 | 2 (0.08) | |
| Tea drinking | <0.001 | ||
| Less than daily | 1598 (64.0) | 1502 (58.3) | |
| Daily | 898 (35.9) | 1071 (41.6) | |
| Missing | 3 (0.1) | 3 (0.1) | |
| Salt-preserved fish consumption in 2000–2002 | 0.02 | ||
| Yearly or less | 1835 (73.4) | 1890 (73.4) | |
| Monthly | 476 (19.1) | 534 (20.7) | |
| Weekly or more | 186 (7.4) | 148 (5.7) | |
| Missing | 2 (0.1) | 4 (0.2) |
SD = standard deviation.
P value was determined by a two-sided t-test. Other P values were determined by a chi-square test.
Relative risk estimates associated with a positive family history of NPC
Compared with controls, cases were 4.6 times (95% CI: 3.5 to 6.1) more likely to report a first-degree family history of NPC (10.8% of cases vs. 2.7% of controls) (Table 2). The magnitude of the association was greater for having a mother (OR=5.6) than a father (OR=3.1) with NPC, and slightly but not significantly greater for having an affected sibling (OR=5.1) than a parent (OR=4.0), and a sister (OR=5.6) than a brother (OR=4.8). NPC risk increased for those with a greater number of affected relatives; the fully adjusted ORs (95% CIs) for having one or at least two affected relatives were 4.4 (3.3, 5.9) and 6.7 (2.8, 16.0), respectively (P trend< 0.001). A history of NPC in offspring was not associated with a significantly higher risk of NPC; however, the number of affected offspring, who were aged 22–24 years on average, was small. Based on the fully adjusted OR, up to 8.6% (95% CI: 5.9%, 11.3%) of all incident NPC cases in the population are attributable to a first-degree family history of NPC.
Table 2.
Odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) for risk of nasopharyngeal carcinoma (NPC) in association with family history of NPC in first-degree relatives.
| Cases (No=2499) |
Controls (No=2576) |
Minimally adjusted OR (95% CIs) * | Fully adjusted OR (95% CIs) † | |
|---|---|---|---|---|
| Family history of NPC in first-degree relatives | ||||
| No | 2208 | 2483 | 1.0 (reference) | 1.0 (reference) |
| Yes | 270 | 69 | 4.5 (3.4 to 5.8) | 4.6 (3.5 to 6.1) |
| 1 affected | 236 | 63 | 4.3 (3.2 to 5.7) | 4.4 (3.3 to 5.9) |
| ≥2 affected | 34 | 6 | 6.6 (2.7 to 15.7) | 6.7 (2.8 to 16.0) |
| P for trend | <0.001 | <0.001 | ||
| Affected relatives ‡ | ||||
| Father | ||||
| No | 2421 | 2543 | 1.0 (reference) | 1.0 (reference) |
| Yes | 72 | 26 | 2.9 (1.8 to 4.5) | 3.1 (2.0 to 5.0) |
| Mother | ||||
| No | 2429 | 2561 | 1.0 (reference) | 1.0 (reference) |
| Yes | 58 | 11 | 5.4 (2.8 to 10.4) | 5.6 (2.9 to 10.8) |
| Parent | ||||
| No | 2357 | 2529 | 1.0 (reference) | 1.0 (reference) |
| Yes | 130 | 37 | 3.7 (2.6 to 5.4) | 4.0 (2.7 to 5.8) |
| Brother | ||||
| No | 2332 | 2478 | 1.0 (reference) | 1.0 (reference) |
| Yes | 113 | 26 | 4.7 (3.0 to 7.2) | 4.8 (3.1 to 7.4) |
| Sister | ||||
| No | 2395 | 2499 | 1.0 (reference) | 1.0 (reference) |
| Yes | 53 | 10 | 5.7 (2.9 to 11.3) | 5.6 (2.8 to 11.1) |
| Siblings | ||||
| No | 2283 | 2466 | 1.0 (reference) | 1.0 (reference) |
| Yes | 159 | 35 | 5.0 (3.5 to 7.3) | 5.1 (3.5 to 7.4) |
| 1 affected | 142 | 34 | 4.6 (3.2 to 6.8) | 4.7 (3.2 to 6.9) |
| ≥2 affected | 17 | 1 | 19.1 (2.5 to 144.1) | 18.9 (2.5 to 142.8) |
| P for trend | <0.001 | <0.001 | ||
| Offspring | ||||
| No | 2355 | 2436 | 1.0 (reference) | 1.0 (reference) |
| Yes | 3 | 2 | 1.7 (0.3 to 10.4) | 1.6 (0.3 to 9.9) |
Adjusted for age, sex, residential area, and family size (continuous; see below).
Adjusted for age, sex, residential area, family size (see below), education level, current housing type, current occupation, cigarette smoking, tea drinking, and salt-preserved fish consumption in 2000–2002.
In analyses of history of NPC in parents, fathers, or mothers, ORs were not adjusted for family size. In analyses of history of NPC in brothers or sisters, siblings, or offspring, ORs were adjusted for number of brothers, number of sisters, number of siblings, or number of offspring, respectively.
In our validation study, the number of observed (self-reported) NPC cases between ages 20 and 74 among first-degree relatives of controls was slightly higher than that expected in the general population of the overall study area in 2011 (74 observed vs. 59 expected among controls, standardized incidence ratio=1.2, P=0.07). A positive first-degree family history of NPC was confirmed based on cancer registry data and/or medical records for 39 of 41 cases and 5 of 5 controls, whereas a negative family history was confirmed for 50 of 52 cases and 45 of 48 controls in Sihui City. Among cases, the sensitivity and specificity for self-reported first-degree family history of NPC were 95% (88%, 100%) and 98% (95% CI: 94%, 100%), respectively; among controls, they were 83% (95% CI: 54%, 100%) and 100% (95% CI: 100%, 100%), respectively. After correcting for misclassification, the OR associated with having at least one affected first-degree relative was 3.1.
We also investigated the association between self-reported second-degree family history of NPC and risk of NPC. Compared with controls, cases were 5.3 times (95% CI: 3.4, 8.3) more likely to report a second-degree family history of NPC (4.8% of cases vs. 0.9% of controls) (Table S3). NPC risk was not significantly increased among those with a positive first-degree family history of any non-NPC cancer (OR=1.1, 95% CI: 0.9, 1.3) (Table S4).
The overall adjusted HR for NPC in first-degree relatives of cases versus controls was 4.2 (95% CI: 3.2, 5.5). The HR was higher for those with an affected sibling than an affected parent, and it was also slightly higher for those with an affected mother versus a father, and for those with an affected sister versus a brother (Figure S2).
Cumulative risk of NPC associated with a positive family history of NPC
The estimated cumulative risk of NPC was greater for males than females with a first-degree family history of NPC. Because only one sister aged <50 years affected with NPC was reported among male controls, the cumulative risk in this age group was not estimated. Otherwise between ages 20 and 74, the cumulative risk of NPC for males with any affected first-degree relative was 5.0% (95% CI: 3.6%, 7.0%), ranging from 4.2% with an affected father to 6.3% with an affected sister (Table 3). Because only one mother affected with NPC was reported among female controls, the cumulative risk was not estimated. Otherwise, between ages 20 and 74, the cumulative risk of NPC for females with any affected first-degree relative was 1.9% (95% CI: 1.1%, 3.2%), ranging from 0.8% with an affected father to 2.2% with an affected brother.
Table 3.
Adjusted odds ratios (ORs) and estimated cumulative risks (%), with corresponding 95% confidence intervals, of nasopharyngeal carcinoma (NPC) by type of relatives affected with NPC and by age (years) and sex of subjects *
| Males
|
Females
|
|||||
|---|---|---|---|---|---|---|
| – 50 years | – 60 years | – 74 years | – 50 years | – 60 years | – 74 years | |
| General population (2011, %) | 0.4 | 0.7 | 1.1 | 0.2 | 0.3 | 0.4 |
| Any first-degree relatives | ||||||
| Adjusted OR | 5.7 (3.5 to 9.3) | 5.3 (3.7 to 7.7) | 4.7 (3.4 to 6.5) | 4.3 (2.0 to 8.9) | 4.5 (2.5 to 8.3) | 4.6 (2.7 to 7.8) |
| Cumulative risk (%) | 2.2 (1.4 to 3.6) | 3.9 (2.7 to 5.7) | 5.0 (3.6 to 7.0) | 0.7 (0.3 to 1.5) | 1.3 (0.7 to 2.4) | 1.9 (1.1 to 3.2) |
| Father | ||||||
| Adjusted OR | 3.7 (1.7 to 7.8) | 4.5 (2.4 to 8.6) | 3.9 (2.2 to 6.8) | 2.0 (0.7 to 5.6) | 1.8 (0.7 to 4.4) | 1.9 (0.8 to 4.3) |
| Cumulative risk (%) | 1.4 (0.7 to 3.0) | 3.3 (1.8 to 6.4) | 4.2 (2.4 to 7.3) | 0.3 (1.2 to 9.5) | 0.5 (0.2 to 1.3) | 0.8 (0.3 to 1.8) |
| Mother † | ||||||
| Adjusted OR | 6.1 (2.4 to 15.9) | 4.5 (2.2 to 9.3) | 4.4 (2.2 to 8.9) | NA | NA | NA |
| Cumulative risk (%) | 2.4 (0.9 to 6.2) | 3.3 (1.6 to 6.9) | 4.7 (2.4 to 9.5) | NA | NA | NA |
| Brother | ||||||
| Adjusted OR | 5.2 (2.0 to 13.7) | 5.2 (2.7 to 9.7) | 4.6 (2.8 to 7.6) | 4.0 (1.0 to 15.5) | 4.2 (1.6 to 10.9) | 5.4 (2.3 to 12.8) |
| Cumulative risk (%) | 2.0 (0.8 to 5.3) | 3.9 (2.0 to 7.2) | 4.9 (3.0 to 8.1) | 0.7 (0.2 to 2.6) | 1.2 (0.5 to 3.2) | 2.2 (0.9 to 5.3) |
| Sister ‡ | ||||||
| Adjusted OR | NA | 6.5 (2.5 to 16.9) | 5.9 (2.6 to 13.2) | 3.1 (0.6 to 15.9) | 5.8 (1.3 to 26.5) | 4.4 (1.2 to 15.7) |
| Cumulative risk (%) | NA | 4.8 (1.9 to 12.5) | 6.3 (2.8 to 14.1) | 0.5 (0.1 to 2.7) | 1.7 (0.4 to 7.7) | 1.8 (0.5 to 6.4) |
Adjusted for age, residential area, family size (see below), education level, current housing type, current occupation, cigarette smoking, tea drinking, and salt-preserved fish consumption in 2000–2002.
In analyses of history NPC in fathers, or mothers, ORs were not adjusted for family size. In analyses of history NPC in brothers or sisters, ORs were adjusted for number of brothers or number of sisters, respectively.
Only one mother affected with NPC was reported among female controls; therefore, the cumulative risk was not estimated.
Only one sister aged <50 years affected with NPC was reported among male controls; therefore, the cumulative risk in this age group was not estimated
Table 4 shows lifetime cumulative risks of NPC up to age 74 among first-degree relatives of cases and controls in the reconstructed cohort (N=40,781). Among relatives of cases, the cumulative risk of NPC was 3.7% (95% CI: 3.3%, 4.2%), whereas that among relatives of controls was 0.9% (95% CI: 0.7%, 1.2%). The incidence rate ratio comparing age-specific risk of NPC in relatives of cases vs. controls was as high as 12.4 at age 29, and gradually decreased with advancing age to 3.7 at age 74.
Table 4.
Lifetime cumulative risks (%) and incidence rate ratios, with corresponding 95% confidence intervals (CIs), of nasopharyngeal carcinoma (NPC) among first-degree relatives of cases and controls, by age of relatives
| Age of first-degree relatives (years)
|
|||||
|---|---|---|---|---|---|
| − 29 | − 39 | − 49 | − 59 | − 74 | |
| Controls | |||||
| No. of relatives at risk | 20,624 | 15,522 | 12,542 | 8,712 | 5,678 |
| Cumulative No. of NPCs | 1 | 11 | 42 | 62 | 74 |
| Cumulative risk (%) | 0.006 | 0.1 | 0.4 | 0.6 | 0.9 |
| 95% CIs | 0.001 to 0.04 | 0.08 to 0.2 | 0.3 to 0.5 | 0.5 to 0.8 | 0.7 to 1.2 |
| Cases | |||||
| No. of relatives at risk | 20,157 | 14,871 | 11,838 | 7,994 | 5,278 |
| Cumulative No. of NPCs | 13 | 68 | 168 | 250 | 291 |
| Cumulative risk (%) | 0.08 | 0.5 | 1.5 | 2.7 | 3.7 |
| 95% CIs | 0.05 to 0.1 | 0.4 to 0.6 | 1.3 to 1.7 | 2.4 to 3.0 | 3.3 to 4.2 |
| Cases versus controls | |||||
| Incidence rate ratio | 12.4 | 5.4 | 3.2 | 4.6 | 3.7 |
| 95% CIs | 1.9 to 526.4 | 2.7 to 11.8 | 2.1 to 5.0 | 2.8 to 7.9 | 1.9 to 7.7 |
Lifetime cumulative risk (95% CI) of NPC up to age 74 among relatives of NPC cases was greater in siblings (6.3% [4.6%, 8.5%] in brothers, 3.5% [2.4%, 5.0%] in sisters) than in parents (3.4% [2.6%, 4.3%] in fathers, and 2.5% [1.9%, 3.3%] in mothers) (P < 0.001) (Figure 1). By contrast, as expected, cumulative risk of NPC among relatives of population-based controls did not differ between siblings (1.2% [0.7%, 2.0%] in brothers, and 0.6% [0.3%, 1.2%] in sisters) and parents (1.3% [0.9%, 2.0%] in fathers, and 0.5% [0.3%, 0.9%] in mothers) (P=0.76). Cumulative risk of NPC differed significantly between fathers and brothers of cases (P<0.001), but not between fathers and brothers of controls (P=0.73). No significant differences were found between mothers and sisters of cases (P=0.08) or controls (P =1.00).
Figure 1.
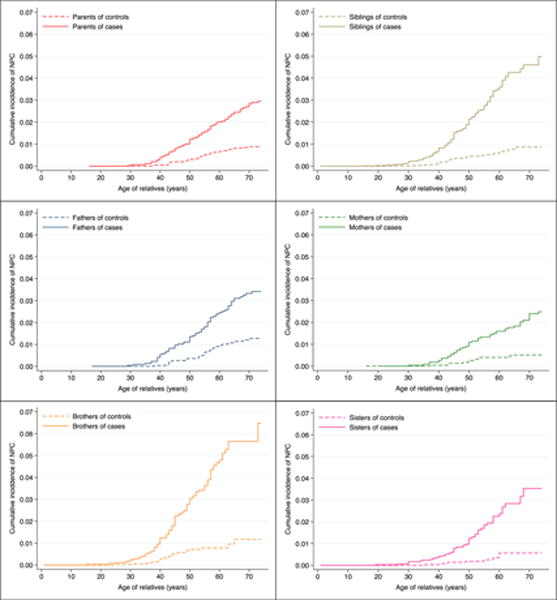
Cumulative incidence of nasopharyngeal carcinoma (NPC) among different relatives of NPC cases and controls.
DISCUSSION
In southern China, where NPC is endemic, whether family history of NPC should be considered in risk stratification or clinical management has remained uncertain, partly due to the lack of estimates of lifetime risk among individuals with a family history. Although the association between family history of NPC and NPC risk has been extensively studied in both NPC high-incidence and low-incidence populations, to the best of our knowledge, the present study is thus far the largest case-control study using a strict population-based design. Because a prospective cohort study to investigate this research question is logistically impractical, a population-based case-control study and reconstructed cohort study of relatives are the most efficient approach. Our findings show that people with a first-degree family history of NPC were at a greater than 4-fold higher risk for NPC, compared to those without, but had no excess risk of other malignancies. Among relatives of cases, the cumulative risk of NPC up to age 74 was 3.7%, whereas that among relatives of controls was 0.9%. These findings may be applicable to other populations with high NPC incidence, such as Hong Kong, Malaysia, Singapore, and Indonesia 24, 32.
We used two complementary approaches to estimate the relative risk associated with a positive family history of NPC. The more than four-fold increase of NPC risk among first-degree relatives of individuals with NPC is comparable to that reported in other studies 2–5, 9, 11, 14, 33. The restriction of the excess risk was to family history of NPC but not other non-NPC cancers is consistent with reports from Taiwan 3, 11 and one report from China 33. Yu et al. and Jia et al. reported that the increased risk of cancer in NPC families was restricted to NPC 3, 33. By contrast, studies from intermediate- 4 and low-incidence populations 5 showed that the elevated risk among first-degree relatives was not limited to NPC but extended to other cancers, such as cancer of the salivary glands and cervix uteri. These findings could be due to different frequencies of NPC susceptibility genes, variations in attributable environmental or lifestyle risk factors, or both, although they also could be explained by different study designs, misclassification, or chance. Because we did not collect information on environmental exposures among relatives in this study, we could not distinguish between the contributions of genetic susceptibility and shared environmental risk factors toward familial NPC risk. However, environmental factors alone are unlikely to account for such strong familial associations. In a community cohort study, Hsu et al. showed that the increased risk of NPC in relatives persisted, although it was attenuated, after adjustment for anti-Epstein Barr virus (EBV) antibody levels and smoking history.2 In addition, associations of NPC risk with certain human leukocyte antigen (HLA) alleles are well established.34–36 Taken together, these findings suggest a major contribution of genetic susceptibility to the occurrence of NPC, at least in high-incidence areas.
Four previous studies found a stronger association of NPC risk with a sibling history than with a parental history of NPC 11, 13, 14, 19, and three studies found a stronger association of NPC risk with a maternal history than with a paternal history of NPC 10, 12, 33. In our study, we observed a slightly, nonsignificantly higher risk of NPC among those with an affected sibling than among those with an affected parent (OR=5.1 for siblings and 4.0 for parents), and the relative risk among those with an affected mother (OR=5.6) was slightly higher still. These patterns could be explained in part by shared environmental risk factors. For example, the maternal link and sibling link may be due to similar dietary habits between mother and child, and between siblings during early childhood, as suggested by the association of early childhood exposure to salt-preserved food with NPC risk 37–39; or they could be due to chance. Our observation of slightly higher relative risks conferred by having an affected female than a male first-degree relative (i.e., a mother or sister as compared with a father or brother, respectively) may be a chance finding, but it merits further investigation into possible mechanisms.
In contrast to prior reports from China 14, 16, we found no significant modification of the association with family history of NPC by environmental risk factors. Our finding suggests that putative NPC susceptibility genes may act independently of environmental factors in NPC carcinogenesis. However, the small number of controls with a positive first-degree family history of NPC and the relatively low power for the test of heterogeneity make it difficult to draw any firm conclusion about the joint effects of family history and environmental risk factors. Pooled studies with larger sample sizes are needed to explore such interactions.
We also used two complementary approaches to estimate the cumulative risk of NPC. They showed similar results, although each approach has its strengths and limitations. Whether close clinical surveillance for NPC may be beneficial for individuals with a family history of NPC is still under debate; cost-effectiveness studies that account for family member-specific risks are needed to answer this question. Based on the case-control study design, our findings show that between ages 20 and 74 in a high-risk region, approximately 5.0% of men and 1.9% of women with a first-degree family history of NPC will develop NPC, compared with 1.1% of men and 0.4% of women in the general population without such a history. We also found that the cumulative risks are greatest among those with sisters affected with NPC. Similarly, the reconstructed kin cohort approach demonstrates that the cumulative risks associated with a family history are greater in siblings than in parents. For example, 6.3% of NPC patients’ brothers and 3.5% of sisters up to age 74 are expected to develop NPC, compared with 3.4% of fathers and 2.5% of mothers. These results could help to guide the development of clinical management strategies or targeted population-based screening programs.
The strengths of our study include its large sample size, population-based design, and high participation rates in cases and controls. We obtained information on each relative separately by using a structured interview, and we demonstrated high accuracy of the interview for the presence of NPC among first-degree relatives. For example, the observed cumulative risk of NPC up to age 74 among the first-degree relatives of controls is 0.9%, which is slightly higher than those reported from a few high incidence areas in South Asia, such as Malaysia (0.76%) and Singapore (0.67%) 24, but it is similar to other parts in southern China, such as Hong Kong (~0.9%) 32. However, the present study has a few limitations. First, we validated self-reported family history of NPC only in one county, and may not be able to generalize the findings to other study regions. Second, although we limited the primary analysis to first-degree relatives to reduce recall bias, the results of our validation study showed that ORs and cumulative risks would be overestimated. Nevertheless, the striking positive association persisted after adjustment for bias. Third, although all NPC cases were histopathologically confirmed, we lacked complete information on histopathological subtypes; however, type II/III NPC comprises the vast majority (> 95%) of NPC cases in southern China, whereas the rest are type I (keratinizing carcinoma).40, 41 Thus, our results are expected to apply mainly to type III NPC and may not be generalizable to type I NPC.
Conclusions
Our large population-based case-control study revealed a more than 4-fold increase in risk of NPC among individuals with a first-degree family history of NPC. The observed association was stronger for having a maternal than a paternal history. As the cumulative risk of NPC increases after age 30 years, the current screening strategy recommended in China focusing on population ages 30 to 59 years might be appropriate, but could be extended to age 69 years for those with a first-degree family history of NPC. Brothers of NPC cases have the highest lifetime cumulative risk of NPC among first-degree relatives, thus a higher-intensity screening strategy for this substratum could be considered. The cumulative risks quantified in this study, along with results of future studies on other risk stratification factors and cost-benefit analyses, can guide clinical consultation and development of surveillance policies for this important public health problem in NPC-endemic areas.
Supplementary Material
Condensed Abstract.
In this population based case-control study, the excess risk was higher for a maternal than a paternal history and slightly stronger for a sibling than a parental history, and for a sororal than a fraternal history. Among relatives of cases, the cumulative risk of NPC up to age 74 was 3.7%, whereas that among relatives of controls was 0.9%.
Acknowledgments
External Advisory Board: Curtis Harris and Allan Hildesheim (National Cancer Institute, US), Mary-Claire King (University of Washington, US), Xihong Lin (Harvard School of Public Health, US), Youlin Qiao (Chinese Academy of Medical Sciences, China), and Weicheng You (Peking University Health Science Center, China)
Sources of financial support: The present study was supported by grant R01 CA115873 from the National Cancer Institute at the U.S., National Institutes of Health and Swedish Research Council (2015-02625 and 2015-06268). The work from the Guiping/Pingnan area was supported by grants from the New Century Excellent Talents in University (no. NCET-12-0654), National Basic Research Program of China (no. 2011CB504300), and Guangxi Natural Science Foundation (2013GXNSFGA 019002). Karolinska Institutet Distinguished Professor Award to Prof. Hans-Olov Adami (Dnr: 2368/10-221). ZL was partly supported by a scholarship from Karolinska Institutet (KID). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Conflict of interest: None declared
Author contributions
Zhiwei Liu: Study design, data analysis, and writing of the manuscript. Ellen T. Chang: Study design and writing of the manuscript. Qing Liu: Study design and writing of the manuscript. Yonglin Cai: Study design and writing of the manuscript. Zhe Zhang: Study design and writing of the manuscript. Guomin Chen: Study design and writing of the manuscript. Qi-Hong Huang: Study design and writing of the manuscript. Shang-Hang Xie: Study design and writing of the manuscript. Su-Mei Cao: Study design and writing of the manuscript. Jian-Yong Shao: Study design and writing of the manuscript. Wei-Hua Jia: Study design and writing of the manuscript. Yuming Zheng: Study design and writing of the manuscript. Jian Liao: Study design and writing of the manuscript. Yufeng Chen: Study design and writing of the manuscript. Longde Lin: Study design and writing of the manuscript. Liming Liang: Study design and writing of the manuscript. Ingemar Ernberg: Study design and writing of the manuscript. Thomas L. Vaughan: Study design and writing of the manuscript. Hans-Olov Adami: Study design and writing of the manuscript. Guangwu Huang: Study design and writing of the manuscript. Yi Zeng: Study design and writing of the manuscript. Yi-Xin Zeng: Study design and writing of the manuscript. Weimin Ye: Study design, data analysis, and writing of the manuscript.
References
- 1.Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 2.Hsu WL, Yu KJ, Chien YC, et al. Familial tendency and risk of nasopharyngeal carcinoma in taiwan: effects of covariates on risk. Am J Epidemiol. 2011;173:292–299. doi: 10.1093/aje/kwq358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu KJ, Hsu WL, Chiang CJ, et al. Cancer patterns in nasopharyngeal carcinoma multiplex families in Taiwan. Int J Cancer. 2009;124:1622–1625. doi: 10.1002/ijc.24051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friborg J, Wohlfahrt J, Koch A, Storm H, Olsen OR, Melbye M. Cancer susceptibility in nasopharyngeal carcinoma families–a population-based cohort study. Cancer Res. 2005;65:8567–8572. doi: 10.1158/0008-5472.CAN-04-4208. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z, Fang F, Chang ET, Ye W. Cancer risk in the relatives of patients with nasopharyngeal carcinoma-a register-based cohort study in Sweden. Br J Cancer. 2015;112:1827–1831. doi: 10.1038/bjc.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams EH, de The G Letter: Familial aggregation in nasopharyngeal carcinoma. Lancet. 1974;2:295–296. doi: 10.1016/s0140-6736(74)91466-4. [DOI] [PubMed] [Google Scholar]
- 7.Zeng YX, Jia WH. Familial nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12:443–450. doi: 10.1016/s1044579x02000871. [DOI] [PubMed] [Google Scholar]
- 8.Friborg JT, Yuan JM, Wang R, Koh WP, Lee HP, Yu MC. A prospective study of tobacco and alcohol use as risk factors for pharyngeal carcinomas in Singapore Chinese. Cancer. 2007;109:1183–1191. doi: 10.1002/cncr.22501. [DOI] [PubMed] [Google Scholar]
- 9.Chen CJ, Liang KY, Chang YS, et al. Multiple risk factors of nasopharyngeal carcinoma: Epstein-Barr virus, malarial infection, cigarette smoking and familial tendency. Anticancer Res. 1990;10:547–553. [PubMed] [Google Scholar]
- 10.Yu MC, Garabrant DH, Huang TB, Henderson BE. Occupational and other non-dietary risk factors for nasopharyngeal carcinoma in Guangzhou, China. Int J Cancer. 1990;45:1033–1039. doi: 10.1002/ijc.2910450609. [DOI] [PubMed] [Google Scholar]
- 11.Ung A, Chen CJ, Levine PH, et al. Familial and sporadic cases of nasopharyngeal carcinoma in Taiwan. Anticancer Res. 1999;19:661–665. [PubMed] [Google Scholar]
- 12.Yuan JM, Wang XL, Xiang YB, Gao YT, Ross RK, Yu MC. Non-dietary risk factors for nasopharyngeal carcinoma in Shanghai, China. Int J Cancer. 2000;85:364–369. [PubMed] [Google Scholar]
- 13.Xie SH, Yu IT, Tse LA, Au JS, Lau JS. Tobacco smoking, family history, and the risk of nasopharyngeal carcinoma: a case-referent study in Hong Kong Chinese. Cancer Causes Control. 2015;26:913–921. doi: 10.1007/s10552-015-0572-x. [DOI] [PubMed] [Google Scholar]
- 14.Ren ZF, Liu WS, Qin HD, et al. Effect of family history of cancers and environmental factors on risk of nasopharyngeal carcinoma in Guangdong, China. Cancer Epidemiol. 2010;34:419–424. doi: 10.1016/j.canep.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Chen DL, Huang TB. A case-control study of risk factors of nasopharyngeal carcinoma. Cancer Lett. 1997;117:17–22. doi: 10.1016/s0304-3835(97)00182-1. [DOI] [PubMed] [Google Scholar]
- 16.Ji X, Zhang W, Xie C, Wang B, Zhang G, Zhou F. Nasopharyngeal carcinoma risk by histologic type in central China: impact of smoking, alcohol and family history. Int J Cancer. 2011;129:724–732. doi: 10.1002/ijc.25696. [DOI] [PubMed] [Google Scholar]
- 17.Guo X, Johnson RC, Deng H, et al. Evaluation of nonviral risk factors for nasopharyngeal carcinoma in a high-risk population of Southern China. Int J Cancer. 2009;124:2942–2947. doi: 10.1002/ijc.24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou J, Sun Q, Akiba S, et al. A case-control study of nasopharyngeal carcinoma in the high background radiation areas of Yangjiang, China. J Radiat Res. 2000;41(Suppl):53–62. doi: 10.1269/jrr.41.s53. [DOI] [PubMed] [Google Scholar]
- 19.Zheng X, Yan L, Nilsson B, Eklund G, Drettner B. Epstein-Barr virus infection, salted fish and nasopharyngeal carcinoma. A case-control study in southern China. Acta Oncol. 1994;33:867–872. doi: 10.3109/02841869409098448. [DOI] [PubMed] [Google Scholar]
- 20.Goldgar DE, Easton DF, Cannon-Albright LA, Skolnick MH. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J Natl Cancer Inst. 1994;86:1600–1608. doi: 10.1093/jnci/86.21.1600. [DOI] [PubMed] [Google Scholar]
- 21.Forman D, Bray F, Brewster DH, et al. Cancer Incidence in Five Continents. X. Lyon: IARC; 2013. (electronic version) [Google Scholar]
- 22.Susser E, Susser M. Familial aggregation studies. A note on their epidemiologic properties. Am J Epidemiol. 1989;129:23–30. doi: 10.1093/oxfordjournals.aje.a115119. [DOI] [PubMed] [Google Scholar]
- 23.Jia WH, Huang QH, Liao J, et al. Trends in incidence and mortality of nasopharyngeal carcinoma over a 20–25 year period (1978/1983–2002) in Sihui and Cangwu counties in southern China. BMC Cancer. 2006;6:178. doi: 10.1186/1471-2407-6-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No.11[Internet] Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr, accessed on June, 2016. [Google Scholar]
- 25.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Chang ET, Liu Q, et al. Oral Hygiene and Risk of Nasopharyngeal Carcinoma-A Population-Based Case-Control Study in China. Cancer Epidemiol Biomarkers Prev. 2016;25:1201–1207. doi: 10.1158/1055-9965.EPI-16-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang KY, Pulver AE. Analysis of case-control/family sampling design. Genet Epidemiol. 1996;13:253–270. doi: 10.1002/(SICI)1098-2272(1996)13:3<253::AID-GEPI3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 28.Zimmerman R, Pal DK, Tin A, Ahsan H, Greenberg DA. Methods for assessing familial aggregation: family history measures and confounding in the standard cohort, reconstructed cohort and case-control designs. Hum Hered. 2009;68:201–208. doi: 10.1159/000224640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin DY. Cox regression analysis of multivariate failure time data: the marginal approach. Stat Med. 1994;13:2233–2247. doi: 10.1002/sim.4780132105. [DOI] [PubMed] [Google Scholar]
- 30.Grambsch PM, Therneau TM. Proportional Hazards Tests and Diagnostics Based on Weighted Residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 31.Boyle P, Parkin DM. Cancer registration: principles and methods. Statistical methods for registries. IARC Sci Publ. 1991:148–150. [PubMed] [Google Scholar]
- 32.Cancer incidence in five continents. IARC Sci Publ. 2008;IX:1–837. [PubMed] [Google Scholar]
- 33.Jia WH, Feng BJ, Xu ZL, et al. Familial risk and clustering of nasopharyngeal carcinoma in Guangdong, China. Cancer. 2004;101:363–369. doi: 10.1002/cncr.20372. [DOI] [PubMed] [Google Scholar]
- 34.Tang M, Lautenberger JA, Gao X, et al. The principal genetic determinants for nasopharyngeal carcinoma in China involve the HLA class I antigen recognition groove. PLoS Genet. 2012;8:e1003103. doi: 10.1371/journal.pgen.1003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hildesheim A, Apple RJ, Chen CJ, et al. Association of HLA class I and II alleles and extended haplotypes with nasopharyngeal carcinoma in Taiwan. J Natl Cancer Inst. 2002;94:1780–1789. doi: 10.1093/jnci/94.23.1780. [DOI] [PubMed] [Google Scholar]
- 36.Bei JX, Li Y, Jia WH, et al. A genome-wide association study of nasopharyngeal carcinoma identifies three new susceptibility loci. Nat Genet. 2010;42:599–603. doi: 10.1038/ng.601. [DOI] [PubMed] [Google Scholar]
- 37.Ward MH, Pan WH, Cheng YJ, et al. Dietary exposure to nitrite and nitrosamines and risk of nasopharyngeal carcinoma in Taiwan. Int J Cancer. 2000;86:603–609. doi: 10.1002/(sici)1097-0215(20000601)86:5<603::aid-ijc1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 38.Zheng YM, Tuppin P, Hubert A, et al. Environmental and dietary risk factors for nasopharyngeal carcinoma: a case-control study in Zangwu County, Guangxi, China. Br J Cancer. 1994;69:508–514. doi: 10.1038/bjc.1994.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu MC. Diet and nasopharyngeal carcinoma. Prog Clin Biol Res. 1990;346:93–105. [PubMed] [Google Scholar]
- 40.Chan JKC, Bray F, McCarron P, Foo W, Lee AWM TY. Nasopharyngeal carcinoma. In: Barnes L, Eveson JW, Reichart P, D S, editors. World Health Organization Classification of Tumours Pathology & Genetics Head and Neck Tumours. Lyon: International Agency for Research on Cancer (IARC); 2005. pp. 85–97. [Google Scholar]
- 41.Liu T. Issues in the management of nasopharyngeal carcinoma. Crit Rev Oncol Hematol. 1999;31:55–69. doi: 10.1016/s1040-8428(99)00003-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


