Abstract
Background
Commonly used anesthetics have been shown to disrupt neurodevelopment in preclinical models. It has been proposed that such anesthesia-induced neurotoxicity is mediated by apoptotic neurodegeneration in the immature brain. Low dose carbon monoxide (CO) exerts cytoprotective properties and we have previously demonstrated that CO inhibits isoflurane-induced apoptosis in the developing murine brain. Here we utilized anti-apoptotic concentrations of CO to delineate the role of apoptotic neurodegeneration in anesthesia-induced neurotoxicity by assessing the effect of CO on isoflurane-induced defects in neurodevelopment.
Methods
C57Bl/6 mouse pups underwent 1-hour exposure to 0 ppm (air), 5 ppm, or 100 ppm CO in air with or without isoflurane on postnatal day 7. Cohorts were evaluated 5–7 weeks post exposure with T-maze cognitive testing followed by social behavior assessment. Brain size, whole brain cellular content, and neuronal density in primary somatosensory cortex and hippocampal CA3 region were measured as secondary outcomes 1-week or 5–7 weeks post exposure along with 7-day old, unexposed controls.
Results
Isoflurane impaired memory acquisition and resulted in abnormal social behavior. Low concentration CO abrogated anesthetic-induced defects in memory acquisition, however, also resulted in impaired spatial reference memory and social behavior abnormalities. Changes in brain size, cellular content, and neuronal density over time related to the age of the animal and were unaffected by either isoflurane or CO.
Conclusions
Anti-apoptotic concentrations of CO incompletely prevented isoflurane-induced defects in neurodevelopment, lacked concentration-dependent effects, and only provided protection in certain domains suggesting that anesthesia-related neurotoxicity is not solely mediated by activation of the mitochondrial apoptosis pathway.
Keywords: anesthesia, isoflurane, neurotoxicity, carbon monoxide, spatial reference memory, social behavior, brain, development, apoptosis
Introduction
Postnatal exposure to the most commonly used anesthetic agents has been shown to disrupt neurodevelopment in preclinical models, resulting in cognitive impairments and behavioral abnormalities later in life.1–6 Such toxicity has been demonstrated in many different animal species, including nonhuman primates, using a variety of exposure paradigms.1–9 Although a causal relationship has yet to be established in children, several retrospective studies have suggested that anesthesia exposure at a young age is associated with subsequent defects in learning and scholastic performance.10–13 Despite over a decade of rigorous experimentation, however, the exact mechanisms of anesthesia-induced neurotoxicity remain undefined.
Seminal work demonstrated widespread apoptotic neurodegeneration in the immature rodent brain following exposure to MK801 and ethanol, raising concern for the neurodevelopmental consequences of perinatal drug abuse and providing a potential explanation for the reduction in brain mass and behavioral abnormalities seen with fetal alcohol syndrome.14,15 As an extension of these findings, it was found that exposure to anesthetics also triggered widespread apoptotic neuronal cell death at a critical period during neurodevelopment.1 Although the upstream mechanisms are unknown, it has been proposed that such anesthesia-induced neurotoxicity may be mediated by the oxidative stress-associated mitochondrial apoptosis pathway.16–20
Programmed neuronal cell death is a natural developmental process, necessary for selective elimination of excess neurons and aberrant connections.21–23 During development, inhibition of the mitochondrial pathway of apoptosis or innate defects in physiologic programmed neuronal cell death result in excess number of neurons and megalencephaly while pathologic apoptotic neurodegeneration can decrease the pool of neurons and diminish the size of the forebrain. 21,24,25 As an example of the latter, ethanol exposure on postnatal day 7 (P7) has been shown to reduce total murine brain volume by 10–13%, reduces neocortical volume and surface area, and leads to a significant loss of GABAergic neurons.26 Although early work suggested anesthesia-induced neuronal deletion in the developing rat brain, more recent studies indicate that postnatal anesthetic exposure does not result in a decrease in neuron numbers or neuron density despite widespread induction of apoptosis.27–29 Unlike ethanol toxicity, lack of a measureable effect of anesthetics on the net number of neurons and brain morphology raises questions about the mechanistic role of apoptotic neurodegeneration in anesthesia-induced neurotoxicity.
Low concentrations of carbon monoxide (CO) have been shown to exert cytoprotective and anti-apoptotic properties in a variety of tissues including the brain.30–37 We have previously demonstrated that 3-hour exposure to low concentration CO inhibits natural apoptosis in the developing murine brain on P10, leading to measurable increases in neuronal content and neuron numbers, detectable increases in relative brain size, and quantifiable defects in memory, learning, and social behavior.24 One-hour exposure to the same CO concentrations on P7 had variable effects on programmed cell death, however, modulated oxidative stress in the developing murine brain during isoflurane exposure and inhibited isoflurane-induced apoptosis in a dose-dependent manner.38,39 Based on these findings, we aimed to test the hypothesis that apoptotic neurodegeneration is causally linked to anesthesia-induced impairments in neurodevelopment. We hypothesized that low concentration CO would prevent isoflurane-induced defects in memory, learning, and social behavior following postnatal exposure. As secondary outcome measures, we assessed brain size, relative cellular content, and estimated neuronal density following neonatal isoflurane exposure. Postnatal isoflurane exposure induced subtle, but detectable impairments in neurocognition and socialization, but had no effect on murine brain weight or volume, cellular content, or neuron density. Low concentration CO abrogated anesthetic-induced defects in memory acquisition, however, resulted in impaired spatial reference memory and social behavior abnormalities following postnatal exposure with or without isoflurane. The data suggest anesthesia-mediated defects in neurodevelopment are not completely prevented by cytoprotective doses of CO despite prevention of isoflurane-induced apoptosis. Therefore, anesthesia-induced neurotoxicity may not be mediated solely by activation of the mitochondrial apoptosis pathway.
Methods
Animal exposures
The care of the animals in this study was in accordance with NIH and Institutional Animal Care and Use Committee guidelines. Study approval was granted by the Children’s National Medical Center and Columbia University Medical Center. Six to eight week old breeding pairs of C57Bl/6 mice (20–30 grams) were acquired (Charles River, Wilmington MA) to yield newborn pups. A total of 24 dams were used to complete the study. On P7, we exposed male and female C57Bl/6 mouse pups to air (0 ppm CO), 5 ppm CO in air, or 100 ppm CO in air with and without isoflurane (2 vol%) for 1 hour in a 7-liter Plexiglas chamber (25 cm × 20 cm × 14 cm). The chamber had a port for fresh gas inlet and a port for gas outlet which was directed to a fume hood exhaust using standard suction tubing. Specific concentrations of CO in air (premixed gas H-cylinders, Air Products, Camden, NJ) were verified using an electrochemical sensing CO detector (Monoxor III, Bacharach, Anderson, CA). Designated CO mixtures were delivered through a variable bypass isoflurane vaporizer and exposure chamber at a flow rate of 4 liters per minute. Inhaled concentration of anesthetic was determined within the chamber (RGM 5250; Datex-Ohmeda Inc., Louisville, CO) and was maintained at 2% isoflurane for the duration of exposure. Mouse body temperature was maintained between 36 – 37 °C with an infrared heating lamp (Cole-Parmer, Court Vernon Hills, IL). An equal number of male and female mice were evaluated and were randomly exposed to a designated CO concentration with or without isoflurane. Littermates were assigned to different treatment groups. Following exposure, pups were returned to their respective dams. P7 was chosen because synaptogenesis peaks at day 7 in rodents and is completed by the second or third week of life.22,40 One-hour exposure to 2% isoflurane has previously been shown to induce neuronal degeneration in 7 day old mice and represents a brief anesthetic exposure.38,41 Furthermore, 1-hour exposure to such concentrations of CO inhibited isoflurane-induced apoptosis in various regions of mouse forebrain in a dose-dependent manner.38
Brain weight and volume
Separate cohorts of mice were evaluated 1-week post exposure or 5–7 weeks post exposure. An unexposed, naïve cohort was also evaluated on P7. Following euthanasia with pentobarbital (150 mg/kg, ip), body weight was measured with a calibrated electronic scale. Fresh whole brain was dissected, dura removed, and medulla severed just distal to the cerebellum. Brain weight was measured with an analytical balance. Brain volume was determined from the amount of saline displaced by fresh whole brain based on Archimede’s principle.42 Brain weight-to-body weight ratios and brain volume-to-body weight ratios were calculated.
Immunoblot analysis
10µg samples of homogenized whole brain protein were subjected to SDS-acrylamide gel electrophoresis and immunoblotting. Blots were labeled with a primary polyclonal antibody to bovine NeuN (Molecular Probes, Eugene, Oregon, USA), a primary monoclonal antibody to rabbit S100β (Cell Signaling, Danvers, MA, USA), a primary polyclonal antibody to rabbit adenomatous polyposis coli (APC) (Abcam, Cambridge, MA, USA), a primary monoclonal antibody to rabbit ionized calcium binding adapter molecule 1 (Iba1) (Abcam, Cambridge, MA, USA), and a primary monoclonal antibody to mouse β-Actin (Thermo Fisher Scientific, Waltham, MA, USA) and secondarily exposed to goat anti-bovine IgG or donkey anti-rabbit IgG (Santa Cruz Biotechnology Inc., Santa Cruz, California) or horse anti-mouse IgG (Cell Signaling, Danvers, MA, USA), as appropriate. Signal was detected with enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech, Piscataway, New Jersey, USA), and density was measured using scanning densitometry.
Estimate of neuronal density
At the time of euthanasia, following pentobarbital injection (150 mg/kg, ip), mice were perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) via left ventricle injection for 30 min and the brain was post-fixed in additional fixative solution for 24 h at 4°C. Paraffin embedded whole brain was cut into 6-µm sections in the coronal plane through the cerebral hemispheres, slide mounted, and stained with cresyl violet for 30 minutes. Cresyl violet-positive neurons with a clear nucleus and nucleoli in the primary somatosensory cortex and in the pyramidal layer of the CA3 region of the hippocampus were counted by a blinded observer (K.K.) using ImageJ software on images captured with a computer-based CCD camera (Nikon Eclipse e800). Starting from the first section (bregma −1.7 mm, interaural 2.1 mm for P56 mice; bregma −1.0 mm, 4.7 mm from the most rostral section for P7 mice; bregma −1.0 mm, 6.0 mm from most rostral section for P14 mice), counts were taken from 3 different, non-adjacent coronal sections at 0.135 mm increments in 100,000 µm2 fields in both hemispheres per mouse.43 Brain regions were defined in accordance with brain atlases for adult and developing mice.44–47
Memory and learning assessment
Cognitive assessment was conducted using a water T-maze test. A beige-colored plastic T-maze was constructed such that each side arm measured 7.5 cm (width) × 32 cm (length) × 17 cm (height) (San Diego Instruments, San Diego, CA). During testing, the maze was filled with tap water (25°C) to a depth of 7 cm. A clear escape platform (7.5 cm × 7.5 cm) was submerged below the water surface. The T-maze location within the behavior laboratory remained constant throughout the evaluation period. Overhead lighting and the surrounding environment provided visual spatial cues. Cognitive evaluation was performed as previously described.47–49
Prior to testing, in order to identify any potential turning bias, mice were exposed to the T-maze without the platform for one day. Mice were placed in the starting arm and allowed to explore the maze for eight consecutive 30-second trials, separated by 15 minutes. If a turning bias was present during pretesting (defined as at least five entries into the same arm), then the platform was placed in the non-preferred arm during testing. Otherwise the platform was randomly placed in either the right or left arm.
During testing, eight daily trials were conducted with a 15-minute inter-trial interval. During each trial, mice were placed in the starting arm and the trial was deemed complete when the mouse either reached the platform, remaining there for two seconds or when 60 seconds had elapsed. If the test subject did not reach the platform after 60 seconds, it was gently guided to it. Once each trial was complete, the mice were left on the platform for 15 seconds and subsequently returned to their respective containers. The correct response in each trial was defined as reaching the platform from the starting arm without entering the opposite arm. The criterion for attaining spatial learning was defined as achieving at least seven correct responses during 8 trials for three consecutive days.
In order to examine cognitive flexibility, after mice achieved criterion, a reversal learning test was initiated on the following day. During reversal learning evaluation, all testing procedures were similar to initial testing except that the platform was placed in the opposite arm of the T-maze. The criterion for reversal learning was also defined as reaching at least seven correct responses out of eight trials for three consecutive days. In addition to days to criterion, latency time to find the platform was measured during each trial on the first three days of spatial and reversal testing.
Social behavior assessment
Social behavior was examined using a three-chambered apparatus (Dold Labs and Engineering, Seguin, TX) as previously described.50 The socialization apparatus included photocells that automatically recorded entry into to each chamber. Mice were first placed in the center chamber and allowed to explore it for 10 minutes for habituation. Each mouse was then permitted to freely explore all three chambers for 10 additional minutes. After the exploratory period, a wire cage (novel object) was placed in one of the side chambers and an unfamiliar B6129SF2/J mouse (novel mouse) of similar age and sex was placed inside an identical wire cage in the opposite side chamber. Social investigation between either the stimulus mouse or the inanimate object was tested. Videos of study animals were recorded for 10 minutes and the number of entries and time spent inside each chamber were determined with computerized software (Dold Labs and Engineering Seguin, TX). An investigator (L.W.) blinded to the animal’s experimental cohort reviewed the video recordings and measured total time each study mouse spent interacting with the “novel mouse” and “novel object”.
Statistical Analysis
Sample size was determined based primarily on the number of mice required to detect a 20% change in days to achieve criteria with T-maze testing and secondarily on the number of mice required to detect a 20% change in NeuN content. A sample size of 8 animals per cohort provided a power of 80 based on an α of .05 for both outcome measures. Mice were randomly assessed either 1-week post exposure (P14) or 5 to 7 weeks post exposure (P42–56). Equal number of males and females were assessed for each experiment. Ten animals per exposure cohort per time point were utilized for measurement of brain size and immunoblot analysis (providing a power of 90), while 4 animals per cohort per time point were used for slide mounted sections (providing a power of 80). A total sample size of 14 animals per exposure cohort per time point in the P42–56 cohort underwent cognitive assessment at 6 to 7 weeks of age followed by social behavior testing at 7 to 8 weeks of age. A cohort of 10 (equal number of males and females) healthy, unexposed 7 day old mice were also assessed as naïve controls but did not undergo neurodevelopmental testing. Six of these mice were utilized for brain size assessment and immunoblot analysis while 4 were used for slide mounted sections.
Data was assessed for normality by examining histograms and box plots. Parametric or non-parametric tests were applied based on assumptions of normal distribution or failure to meet requirements. Statistical significance was assessed using two-way ANOVA and post hoc Tukey’s test for brain size, cell-specific protein densities, and social behavior testing (time spent inside each chamber and interaction times). Regression models were utilized to determine significance of sex for each outcome measure. Unpaired two-tailed Student’s t-test was used to assess cell-specific protein densities between sexes in 7-day old, unexposed naïve controls. For latency escape times with T-maze testing, significance was assessed with two-way ANOVA with repeated measures and post hoc Tukey’s test. Kruskal-Wallis test with Dunn’s multiple comparison post hoc test was utilized for days to achieve criteria and neuronal density values. Data are presented as mean with standard deviation and significance was set at P < .05.
Results
Cognitive and social behavioral effects of postnatal isoflurane and CO exposure
Impaired cognition and behavior are important features of animal models of anesthesia-induced neurotoxicity.51 Although exposure to isoflurane for 1-hour has previously been shown to induce apoptosis in the developing rodent brain on P7, neurodevelopmental outcome of such a brief, clinically relevant postnatal exposure has not been previously tested.38,41 With regard to CO, 3-hour exposure to low concentration CO on P10 has been shown to impair murine memory and learning and socialization later in life.24 However, the effect of CO on neurodevelopment in the context of anesthetic-induced neurotoxicity is unknown. Thus, we assessed spatial reference memory using T-maze testing and social behavior using a three-chambered socialization apparatus 5–7 weeks following 1-hour exposure to isoflurane (2%), CO (0 ppm [air], 5 ppm, or 100 ppm), or to a combination of gases (isoflurane with CO) on P7.
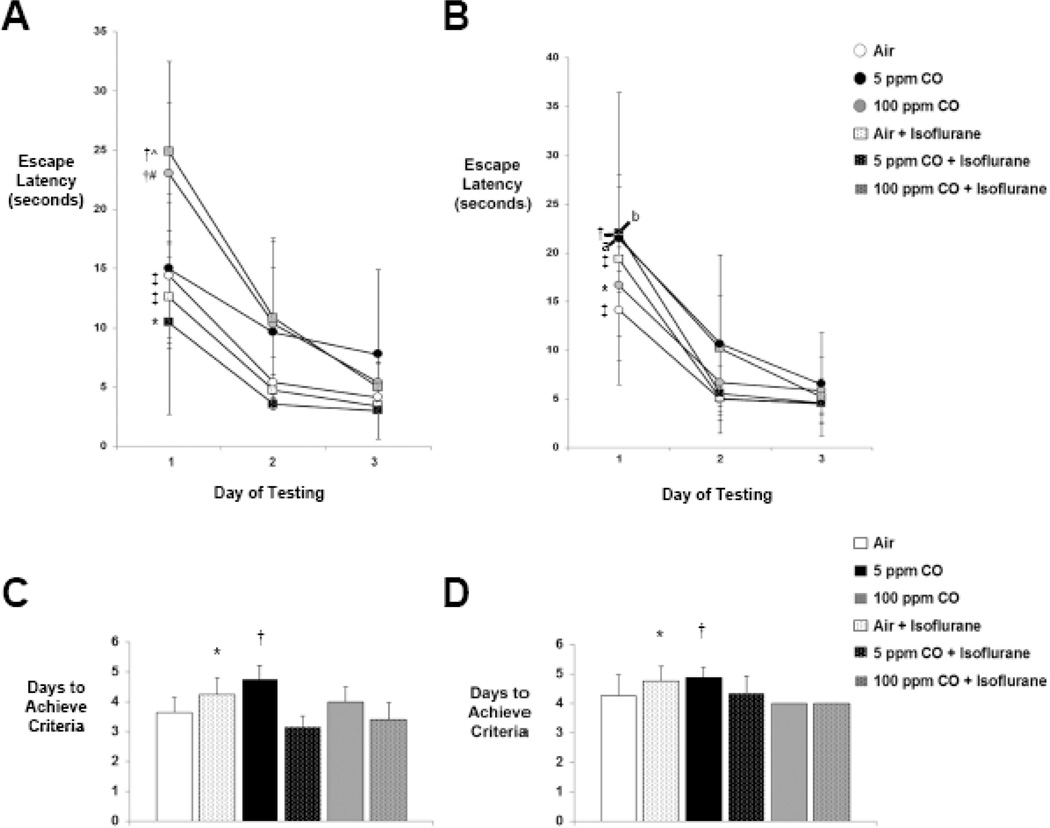
With T-maze testing, each cohort except 5 ppm CO-exposed mice learned to escape the maze over the first 3 days of testing as evidenced by significantly shortened latency over time (Figure 1A). With reversal testing, all cohorts demonstrated significantly reduced latency times by day 3 of testing compared to day 1, indicating flexibility with learning (Figure 1B). The only differences in escape latency between exposure cohorts were seen on the first day of testing (Figure 1A). Animals exposed to 100 ppm CO with isoflurane demonstrated significantly longer latency escape times compared to air-exposed controls and latency times in both 100 ppm CO-exposed cohorts (with and without isoflurane) were significantly longer than the other two isoflurane-exposed cohorts (air with isoflurane and 5 ppm CO with isoflurane) (Figure 1A). There were no significant differences in escape latency between cohorts with reversal testing and there was no significant effect of sex with either testing paradigm on any day of testing (Day 1, P = 0.39 [95% CI 2.6-(−6.4)]; Day 2, P = 0.70 [95% CI 2.5-(−3.8)]; Day 3, P = 0.49 [95% CI 3.2-(−1.6)]; Reversal Day 1, P = 0.41 [95% CI 11.1-(−4.7)]; Reversal Day 2, P = 0.11 [95% CI 8.5-(−1.0)]; Reversal Day 3, P = 0.08 [95% CI 5.2-(−0.4)]) (Figure 1B).
Figure 1. Spatial reference memory and cognitive flexibility following exposure.
A. Latency escape times from the T-maze during the first 3 days of spatial and B. reversal testing are shown. Values from the six experimental cohorts are expressed as means +/− standard deviation. *P < 0.05 Day 1 vs. Day 2 and Day 3 of testing within the cohort, †P < 0.01 Day 1 vs. Day 2 and P < 0.001 Day 1 vs. Day 3 of testing within the cohort, ‡P < 0.001 Day 1 vs. Day 2 and Day 3 of testing within the cohort, ^P < 0.05 vs. air-exposed controls and P < 0.01 vs. air + isoflurane and 5 ppm CO + isoflurane on Day 1 of testing, #P < 0.05 vs. air + isoflurane and P < 0.01 vs. 5 ppm CO + isoflurane on Day 1 of testing. aP < 0.05 Day 1 vs. Day 3 of testing within the cohort, bP < 0.01 Day 1 vs. Day 2 and Day 3 of testing within the cohort. C. Days to achieve criteria during memory acquisition testing are demonstrated. Values from the six experimental cohorts are expressed as means plus standard deviation. *P < 0.05 vs. air-exposed controls, P < 0.001 vs. 5 ppm CO + isoflurane, and P < 0.01 vs. 100 ppm CO + isoflurane. †P < 0.01 vs. air-exposed controls and 5 ppm CO + isoflurane. D. Days to achieve criteria during reversal testing are demonstrated. *P < 0.05 vs. 100 ppm CO + isoflurane. †P < 0.01 vs. 100 ppm CO-exposed cohort. N = 14 animals per cohort.
Mice exposed to air with isoflurane demonstrated impaired acquisition of memory by requiring significantly greater time to reach criteria with T-maze testing compared to air-exposed controls (Figure 1C). However, combined exposure to either concentration of CO with isoflurane resulted in significantly less number of days to reach criteria versus exposure to isoflurane alone (Figure 1C). With reversal testing, the isoflurane-exposed cohort required significantly greater time to reach criteria compared to mice exposed to 100 ppm CO with isoflurane (Figure 1D). Animals exposed to 5 ppm CO also demonstrated impaired memory acquisition by requiring significantly more time to reach criteria compared to air-exposed mice (Figure 1C). This delay in achieving criteria was also significant compared to mice exposed to 5 ppm CO with isoflurane (Figure 1C). The 5 ppm CO-exposed cohort also demonstrated relative cognitive inflexibility as evidenced by a significantly greater number of days to achieve criteria with reversal testing versus the 100 ppm CO-exposed cohort (Figure 1D). Interestingly, the number of days to achieve criteria for both cohorts that underwent combined exposure to CO with isoflurane approximated that of air-exposed controls in standard and reversal T-maze testing (Figure 1C, D). There was no significant effect of sex in any cohort with either testing paradigm (P = 0.27 [95% CI 0.52-(−0.15)]; Reversal P = 0.11 [95% CI 0.63-(−0.07)]).
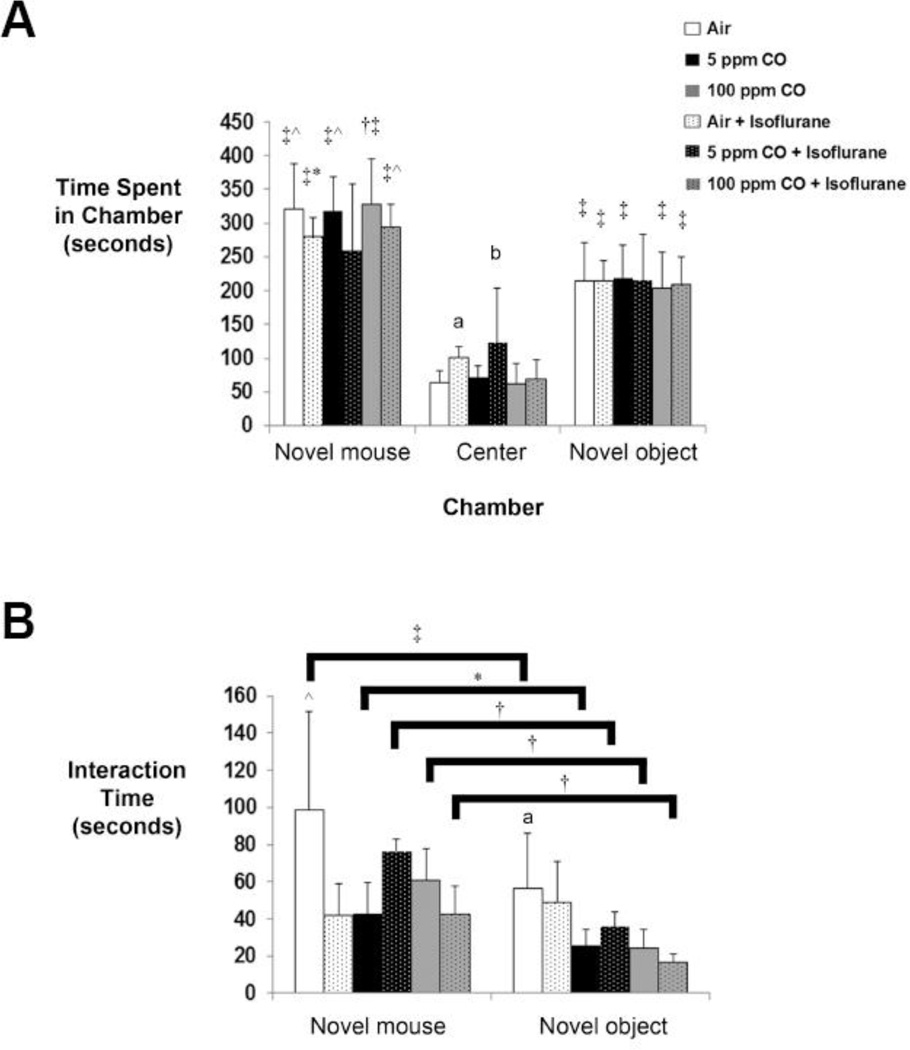
With social behavior testing, the amount of time each test mouse interacted with the novel mouse or novel object was quantified. All cohorts, except for those exposed to 5 ppm CO with isoflurane, spent significantly greater time in the chamber with the novel mouse versus the other two chambers (novel object and center chamber) and in the chamber with the novel object versus the center chamber (Figure 2A). However, there was no significant difference between exposure cohorts in the duration of time that each animal spent in either the chamber with the novel mouse or the chamber with the novel object (Figure 2A). Interestingly, the isoflurane-exposed cohort and animals exposed to 5 ppm CO with isoflurane spent significantly longer time in the center chamber compared to air-exposed controls, suggesting ambivalent behavior (Figure 2A).52 The increase in time spent in the center chamber by mice exposed to 5 ppm CO with isoflurane was also significantly greater than those exposed to 5 ppm CO alone and 100 ppm CO with isoflurane (Figure 2A).
Figure 2. Social behavior following exposure.
A. Time spent in each chamber of the apparatus is depicted. The side chambers contained either the novel mouse or novel object while the center chamber lacked the presence of a stimulus mouse or inanimate object. Values from the six experimental cohorts are expressed as means plus standard deviation. *P < 0.05 vs. center chamber within the cohort. †P < 0.01 vs. novel object within the cohort. ‡P < 0.001 vs. center chamber within the cohort. ^P < 0.001 vs. novel object within the cohort. aP < 0.05 vs. air-exposed controls in center chamber, bP < 0.01 vs. air-exposed controls and P < 0.05 vs. 5 ppm CO-exposed and 100 ppm CO + isoflurane cohorts in center chamber. B. Interaction time with either the novel mouse or novel object is depicted. Values from the six experimental cohorts are expressed as means plus standard deviation. *P < 0.05 vs. novel object within the same cohort. †P < 0.01 vs. novel object within the same cohort. ‡P < 0.001 vs. novel object within the same cohort. ^P < 0.05 vs. air + isoflurane, 5 ppm CO-exposed, and 100 ppm CO + isoflurane cohorts with novel mouse. aP < 0.05 vs. 5 ppm CO-exposed, 100 ppm CO-exposed, and 100 ppm CO + isoflurane cohorts with novel object. N = 14 animals per cohort.
With regard to interaction time, each experimental group except for the isoflurane-exposed cohort spent significantly more time interacting with the novel mouse versus the novel object indicating an anesthesia-induced abnormality (Figure 2B). Compared to air-exposed controls, animals exposed to air with isoflurane, 5 ppm CO alone, or 100 ppm CO with isoflurane demonstrated significantly less interaction time with the novel mouse (Figure 2B). Furthermore, cohorts exposed to either concentration of CO alone and 100 ppm CO with isoflurane interacted significantly less with the novel object versus air-exposed mice (Figure 2B). There were no significant differences based on sex of the animal in any cohort with regard to time spent in any chamber or interaction time (Time in chamber: novel mouse, P = 0.31 [95% CI 71.5-(−23.9)]; center, P = 0.13 [95% CI 5.5-(−39.4)]; novel object, P = 0.99 [95% CI 40.6-(−41.4)]; Interaction time: novel mouse, P = 0.09 [95% CI 20.4-(−1.7)]; novel object, P = 0.80 [95% CI 10.7-(−8.3)]). The data indicate that social behavior was impacted by exposure to isoflurane, CO, or combined exposure to isoflurane with CO.
Brain size following postnatal exposure to isoflurane and CO
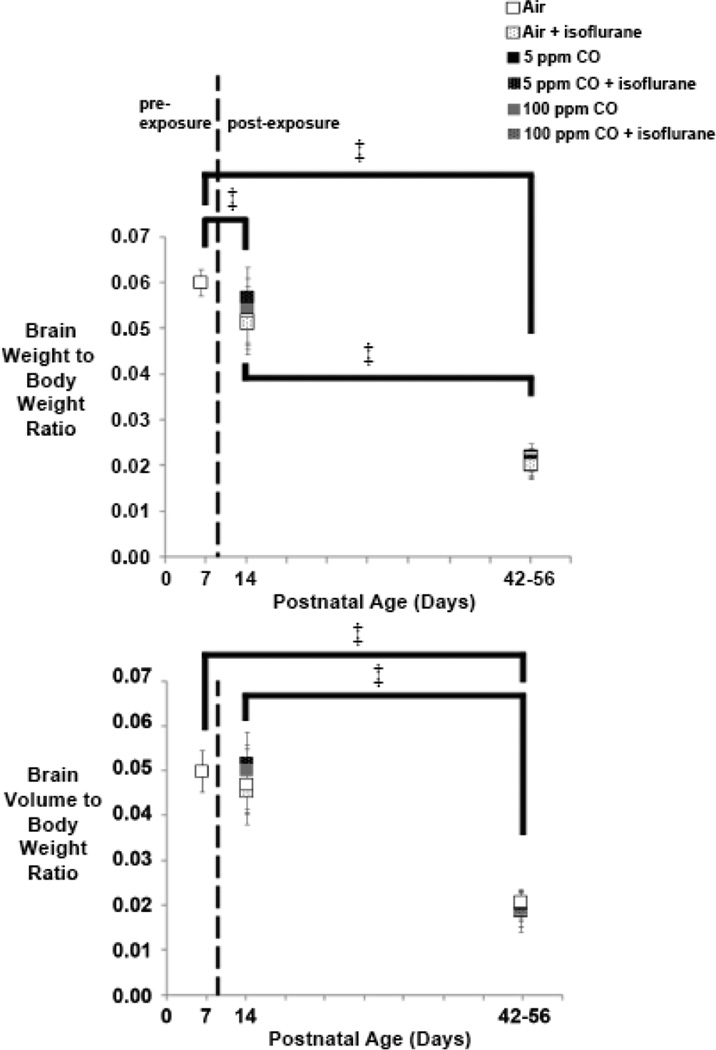
Exposure to isoflurane for 1-hour on P7 is known to induce widespread apoptosis within the murine forebrain.38,41 Simultaneous exposure to low concentration CO inhibits such isoflurane-induced apoptosis in a dose-dependent manner.38 Alternatively, neonatal exposure to CO alone for 3-hours has been shown to inhibit natural programmed cell death within the brain, resulting in megalencephaly 1-week post-exposure.24 Because pathologic apoptotic neurodegeneration has the potential to reduce brain mass, we crudely assessed brain size in mice following 1-hour exposure on P7 to isoflurane (2%), CO (0 ppm [air], 5 ppm, or 100 ppm), or to a combination of gases (isoflurane with CO) by measuring brain weight and brain volume normalized to body weight. Exposure cohorts were assessed 1-week or 5–7 weeks post exposure and compared to unexposed, 7-day old naïve controls.
Consistent with normal growth, brain weight-to-body weight ratios and brain volume-to-body weight ratios decreased over time in all exposure cohorts (Figure 3). Brain weight-to-body weight ratios were significantly less at both time points following exposure compared to 7-day old unexposed controls (Figure 3). In addition, brain weight-to-body weight ratios differed significantly within exposure cohorts between the post exposure time points (Figure 3). With regard to normalized brain volume, brain volume-to-body weight ratios were significantly less 5–7 weeks post exposure compared to 7-day old unexposed controls and were significantly less than animals within exposure cohorts 1-week post exposure (Figure 3). There was no significant difference between cohorts based on type of exposure or sex of the animal (Figure 3) (see Figure 1 in Ref #53). Thus, changes in brain weight and volume over time were based solely on the age of the animal.
Figure 3. Normalized brain weight and volume following exposure.
Brain weight-to-body weight and brain volume-to-body weight ratios are depicted for each cohort 1-week (postnatal day 14) and 5–7 weeks (postnatal days 42–56) post exposure. Values for unexposed, naïve controls on postnatal day 7 are also shown. Dotted line separates pre- and post-exposed cohorts. N = 10 animals for each exposed cohort. N = 6 animals for unexposed controls. Values are expressed as means ± standard deviation. ‡ P < 0.001. Significance post-exposure was within exposure cohort only.
Relative cellular content in the developing brain following exposure to isoflurane and CO
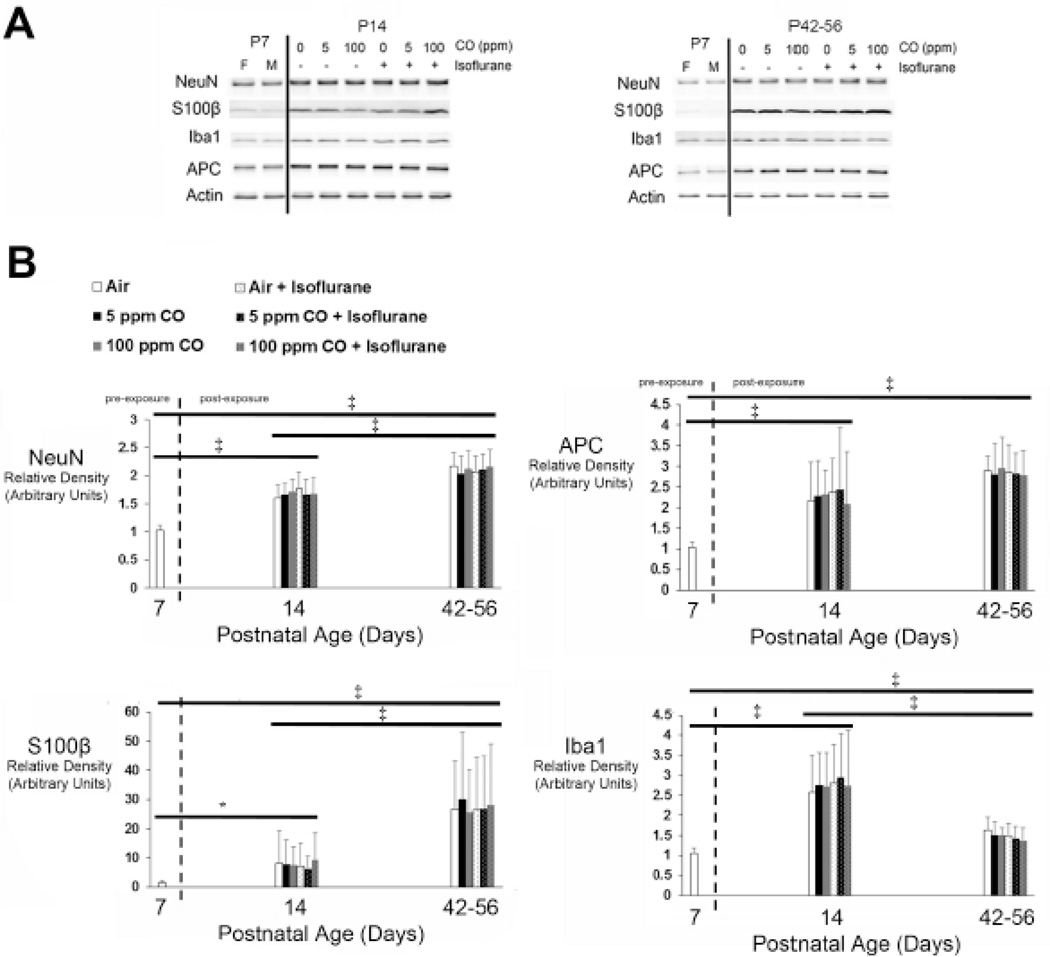
Toxicological processes that induce large scale neurodegeneration pathologically reduce the number of neurons in the developing brain and have the potential to alter the content of non-neuronal cells.55,55 On the other hand, inhibition of developmental apoptosis can result in increased neuronal content and excess number of neurons in the immature brain.24,25 Thus, we estimated the content of neurons, astrocytes, oligodendrocytes, and microglia in whole brain following 1-hour exposure on P7 to isoflurane (2%), CO (0 ppm [air], 5 ppm, or 100 ppm), or to a combination of gases (isoflurane with CO) using immunoblot analysis for neuron specific antigen (NeuN), S100β, adenomatous polyposis coli (APC), and ionized calcium binding adapter molecule 1 (Iba1), respectively.56 Exposure cohorts were assessed 1-week or 5–7 weeks post exposure and compared to unexposed, 7-day old naïve controls.
To determine if there were baseline differences in cellular content in the developing brain due to sex, we first assessed steady-state protein levels in healthy, unexposed male and female 7-day old mice. Densities were normalized to actin and values from 7-day old naïve males were arbitrarily set to 1. Comparison between unexposed male and female mice demonstrated no significant differences in steady-state levels of whole brain NeuN, S100β, APC, or Iba1 (Supplemental Figure 1). The findings indicate that there were no sex-based disparities in the cellular content of neurons, astrocytes, oligodendrocytes, and microglia in the developing brain on P7.
Steady-state whole brain protein levels were next determined in each exposure cohort 1-week and 5–7 weeks following exposure. Seven-day old naïve female #3 and male #1 mice were arbitrarily chosen as representative unexposed controls to permit age- and sex-based comparison and 7-day old male values were set to equal a relative density of 1. With regard to NeuN, S100β, and APC, steady-state levels increased significantly over time in all exposure cohorts (Figure 4). Iba1 levels increased significantly 1-week post exposure in each cohort compared to naïve controls, then decreased significantly 5–7 weeks following exposure (Figure 4). However, steady-state Iba1 levels at the 6–8-week time point remained significantly higher than 7-day old unexposed control values in all cohorts (Figure 4). Importantly, there were no significant differences in protein levels between cohorts based on type of exposure or sex of the animal (Figure 4)(see Figure 2 in Ref #53). The data indicate that neuron-, astrocyte-, and oligodendrocyte-specific protein content increased in the developing brain over time and was not affected by type of exposure or sex. The changes in microglia-specific protein were also age-dependent without any effect due to type of exposure or sex.
Figure 4. Cell-specific protein content in whole brain following exposure.
Steady-state levels of neuron-, astrocyte-, oligodendrocyte-, and microglial-specific protein were quantified in whole brain 1-week (postnatal day 14) or 5–7 weeks (postnatal days 42–56) post exposure. Seven-day old naïve female #3 and male #1 mice were arbitrarily chosen as representative unexposed controls to permit age-based comparison. A. Representative immunoblots of neuron specific antigen (NeuN), S100β, adenomatous polyposis coli (APC), and ionized calcium binding adapter molecule 1 (Iba1) are depicted for postnatal day 14 (P14) and postnatal days 42–56 (P42–56) time points. Actin was used as a loading control. Exposure cohorts are indicated by concentration of carbon monoxide (CO) (0 parts per million (ppm) [air], 5 ppm, or 100 ppm) with (+) or without (−) isoflurane. P7 female (F) and male (M) are indicated. B. Graphical representation of relative densities of NeuN, S100β, APC, and Iba1 are shown. Dotted line separates pre- and post-exposed cohorts. Values are expressed as means plus standard deviation. Unexposed, naïve P7 male values were arbitrarily set to 1. N = 10 animals for each exposed cohort per time point. *P < 0.05, ‡ P < 0.001. Significance post-exposure was within exposure cohort only.
Estimate of neuron density following postnatal exposure to isoflurane and CO
Postnatal neuronal apoptosis is a natural process in the developing brain that is necessary for proper neuronal networking and patterning.21 The role of physiologic cell death is to eliminate excess and aberrant neurons.21 Impaired or inhibited neuronal apoptosis results in pathological persistence of neurons while enhanced apoptotic neurodegeneration leads to a net loss of neurons.21,24,25 Thus, we estimated the density of neurons in the primary somatosensory cortex and in the pyramidal layer of the CA3 region of the hippocampus using cresyl violet staining of slide mounted brain sections from unexposed, 7-day old naïve controls and mice 1-week or 5–7 weeks following 1-hour exposure on P7 to isoflurane (2%), CO (0 ppm [air], 5 ppm, or 100 ppm), or to a combination of gases (isoflurane with CO). Primary somatosensory cortex was chosen because it contains the greatest number of neurons within the murine cerebral cortex and the CA3 subfield was chosen because it encodes spatial and episodic memory and is susceptible to neurodegenerative processes.57,58 The number of mice evaluated per cohort provided a power of 80 to detect a 15% difference in the number of neurons per brain region.
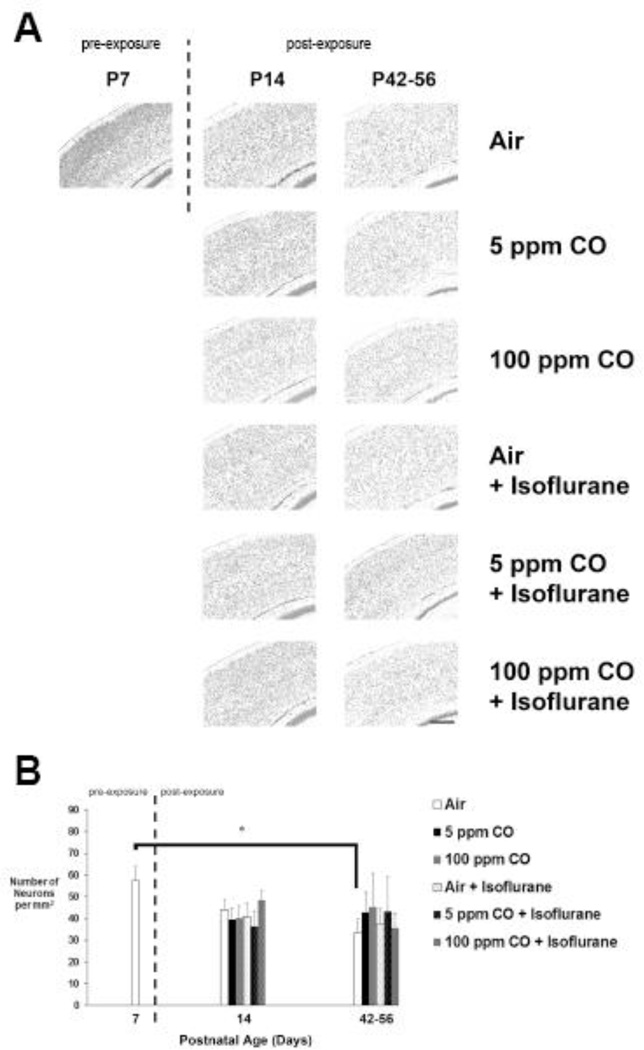
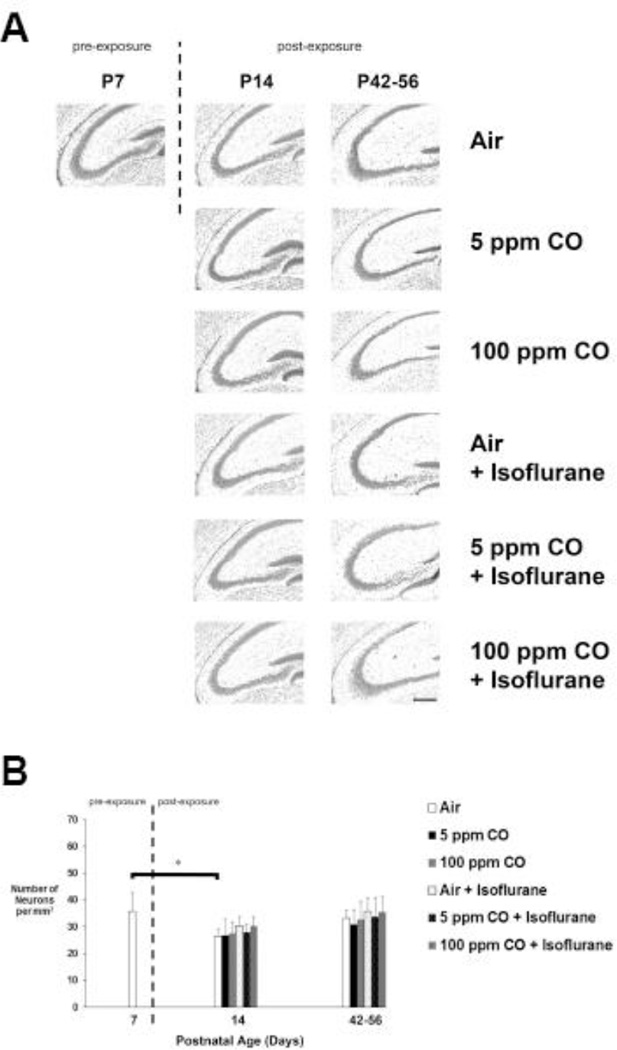
Neuronal density in the primary somatosensory cortex decreased significantly in the air-exposed cohort 5–7 weeks following exposure compared to 7-day old unexposed controls (Figure 5). There were no significant differences in cortical neuron densities following any other type of exposure compared to naïve controls or between cohorts at any time point (Figure 5). Furthermore, no significant effect of sex was identified in any cohort at any time point (See Figure 3 in Ref #53). With regard to the CA3 region of the hippocampus, neuronal density decreased significantly 1-week post exposure in air-exposed controls compared to 7-day old unexposed controls (Figure 6). However, there was no significant differences in hippocampal neuron densities following any other type of exposure compared to naïve controls or between cohorts at any time point (Figure 6). As with cortical neuron density, no significant effect of sex was identified in any cohort at any time point (See Figure 3 in Ref #53).
Figure 5. Estimated neuron density in primary somatosensory cortex following exposure.
A. Representative cresyl violet stained coronal sections obtained at 20X magnification are depicted for each cohort 1-week (postnatal day 14) and 5–7 weeks (postnatal days 42–56) post exposure as well as for unexposed, naïve controls on postnatal day 7 (P7). Dotted line separates pre- and post-exposed cohorts. Scale bar, 200 µm. B. Quantification of the number of neurons (in thousands) in primary somatosensory cortex is demonstrated. Values are expressed and means plus standard deviation. N = 4 animals per cohort per time point. *P < 0.01.
Figure 6. Estimated neuron density in CA3 region of hippocampus following exposure.
A. Representative cresyl violet stained coronal sections obtained at 20X magnification are depicted for each cohort 1-week (postnatal day 14) and 5–7 weeks (postnatal days 42–56) post exposure as well as for unexposed, naïve controls on postnatal day 7 (P7). Dotted line separates pre- and post-exposed cohorts. Scale bar, 200 µm. B. Quantification of the number of neurons (in thousands) in CA3 region is demonstrated. Values are expressed and means plus standard deviation. N = 4 animals per cohort per time point. *P < 0.001.
The data indicate physiologic, age-dependent neuron elimination in air-exposed controls within the cortex and hippocampus. Lack of significant decreases in density in the hippocampus at the late time point may have been due to neurogenesis.59–61 Importantly, there were no isoflurane or CO-mediated effects on neuronal density in either brain region and significant isoflurane-induced neuron loss was not observed.
Discussion
In prior work, we demonstrated that 1-hour exposure to isoflurane on P7 induced widespread apoptosis in the developing murine forebrain.38 Here we demonstrate that such an exposure impaired memory acquisition and led to subtle abnormalities in social behavior later in life. Thus, postnatal exposure to isoflurane for 1-hour resulted in neurotoxicity. Utilizing low concentrations of CO that inhibit isoflurane-induced apoptosis in a dose-dependent manner, we aimed to delineate the role of apoptotic neurodegeneration in anesthesia-induced neurotoxicity by assessing the effect of CO on isoflurane-induced defects in neurodevelopment.38
Our findings demonstrated that both concentrations of CO attenuated the prolonged duration of time required to reach criteria caused by isoflurane while, in reversal testing, 100 ppm CO reduced the number of days required to reach criteria relative to the anesthesia-exposed cohort. Though devoid of a clear dose-response, these effects suggest that apoptosis may have partially caused or contributed to isoflurane-mediated deficits in memory acquisition and cognitive flexibility. In contrast, however, exposure to 100 ppm CO with isoflurane increased latency escape time on Day 1 of testing, implying an acquired impairment in spatial reference memory. Furthermore, combined exposure to CO with isoflurane failed to limit or prevent anesthesia-mediated impairments in social behavior and, in fact, resulted in defects in socialization. Because anti-apoptotic concentrations of CO incompletely prevented isoflurane-induced defects in neurodevelopment, lacked concentration-dependent effects, and only provided protection in certain domains suggests that anesthesia-related neurotoxicity is not solely mediated by activation of the mitochondrial apoptosis pathway and that other pathologic processes and pathways are likely involved.
With regard to the neurodevelopmental effects of CO, both concentrations independently impaired spatial reference memory as evidenced by a lack of improvement in escape latency over time in the 5 ppm CO-exposed cohort and prolonged escape latency in the 100 ppm CO-exposed group. As with isoflurane, neonatal exposure to 5 ppm CO impaired memory acquisition and resulted in relative cognitive inflexibility. In addition, all CO exposures resulted in some defect in socialization. These findings are consistent with the known neurodevelopmental consequences of a 3-hour postnatal CO exposure.24 Thus, as a limitation of the current work, it is possible that CO confounded the neurobehavioral outcomes following combined exposure with isoflurane. However, because 1-hour postnatal CO exposure variably affects programmed cell death, such CO-mediated neurotoxicity likely involved alternative mechanisms, strengthening the case for the role of non-apoptosis pathways in this model.38
When considering the prolonged escape latency times in the 100 ppm CO-exposed cohorts, the data suggest impaired reference memory despite preserved or enhanced memory acquisition. The coexistence of these two seemingly contradictory aspects of cognitive function has been previously reported in murine models, however, alternative explanations of longer escape latency may exist.62 For example, lack of motivation, sensorimotor abnormalities, and anxiety can lengthen escape times independent of defects in memory.63 Anesthetic agents have been shown to impair motivation in neonatal non-human primates while CO reduced exploratory behavior in transgenic mice that overexpress heme oxygenase.64,65 Thus, it is possible that such effects may have contributed to the longer latency times seen on the first day of testing in the two higher CO concentration-exposed cohorts. Of note, the escape times were not significantly different from results of the other cohorts on the subsequent days of testing and approximated control values by Day 3, suggesting that potential defects were transient and could be overcome.
With regard to secondary outcome measures, isoflurane had no net effect on neuron density, cellular protein content, or on brain weight and volume. These findings differ from an early report suggesting that neuronal deletion occurred in the developing rat brain following a 6-hour exposure to an anesthesia “cocktail” (isoflurane, nitrous oxide, midazolam).27 Although this disparity could be due to differences in duration of exposure or use of a single anesthetic versus combined agents, our findings are consistent with more recent studies demonstrating that prolonged midazolam or isoflurane exposure did not reduce neuron numbers or neuron density despite widespread induction of apoptosis.28,29 The lack of a measureable effect of anesthetics on the net number of neurons and brain morphology during development suggests differences in neuron elimination in the immature brain following anesthetic exposure versus more macroscopic losses seen with ethanol toxicity.26 The reasons for this discrepancy are not known but may relate to the underlying mechanisms or degree of toxicity. Of note, neuronal density, cellular content, and brain size were also unaffected by low concentration CO. These findings differed from the transient megalencephaly and increases in neuronal content seen following a 3-hour exposure on P10 and probably were due to a shorter duration of exposure or the age of the mouse at the time of exposure.24
Taken together, the data suggest that the major determinant of changes in brain size and cellular content over time was the age of the animal. Increases in whole brain NeuN content coincided with the timing of the second phase of neocortical neurogenesis and ongoing increases in neuron numbers in the cerebellum.66 In addition, changes in glia-specific protein content were consistent with the known age-related changes in gliogenesis and increases in non-neuronal cell content.56,66 With regard to neuron density, the decreases over time in the primary somatosensory neocortex and CA3 region in air-exposed controls likely reflected physiologic neuron elimination in addition to reductions in neuron size with age in these regions.66 Lack of significant decreases in neuron density observed in the hippocampus at the 6–8-week time point may have been due to exercise- or learning-mediated effects on neuron density and number in the CA3 region given that each cohort had undergone T-maze testing 1–2 weeks prior.59–61
Based on our findings, alternative mechanisms of anesthetic toxicity, in addition to apoptosis, must be considered. It is well known that anesthetic agents cause oxidative stress in the developing brain.18–20 Specifically, free radicals are generated by mitochondrial sources during anesthetic exposure and accumulate within mitochondria.18,20 In prior work, we demonstrated that isoflurane and low concentration CO independently cause lipid peroxidation within murine forebrain mitochondria following a 1-hour exposure on P7.39 However, combined exposure to isoflurane with low concentration CO paradoxically limited such oxidative stress.35 Interestingly, the memory acquisition findings in the various exposure cohorts in the current study bear striking similarity to this pattern of oxidative stress.39 For example, when comparing the number of days that each experimental cohort required to reach criteria with T-maze testing, the magnitude and relative directionality closely follow the degree of lipid peroxidation seen within murine forebrain mitochondria following such exposures on P7.39 This suggests that certain neurocognitive consequences following neonatal exposure to each gas or their combination may be associated with the degree of reactive oxygen species generation within the developing brain.
Free radicals can damage cellular lipids, proteins, and DNA, and can lead to mitochondrial dysfunction.67 Because the immature brain is uniquely vulnerable to environmental insults during critical time points during development, it is possible that anesthesia-induced oxidative stress could disrupt fundamental processes (i.e., migration, myelination) required for normal brain maturation.40 Interruption of any of the critical developmental processes could result in functionally significant developmental abnormalities later in life.40 Future work will be necessary to test this concept and define the downstream pathways affected in order to demonstrate a causative link with oxidative stress.
Finally, our findings may have clinical relevance given that infants and children are commonly exposed to low concentrations of CO during low-flow anesthesia when re-breathing is permitted.68,69 In the current study, we found that low dose CO preserved memory acquisition and cognitive flexibility when co-administered with isoflurane. However, low dose CO may have also resulted in impaired spatial reference memory and disrupted social behavior. This dichotomy between CO-mediated neuroprotection and neurotoxicity is known to exist in nature with a variety of biologically active agents and our findings exemplify these divergent properties.70–72 Because low concentrations of CO can interfere with many different critical neurodevelopmental processes, further investigation of the safety and efficacy of CO exposure in the setting of an anesthetic is necessary.73 Such studies could have implications for the practice of low-flow anesthesia in infants and children. Importantly, investigating the various pathways that underlie CO-mediated toxicity and cytoprotection may permit us to better understand how the immature brain responds to toxicological insults at critical times during development and help to elucidate other potential mechanisms of anesthesia-induced neurotoxicity.
Supplementary Material
Highlights.
Anesthetics induce widespread apoptosis in the immature brain.
Low dose carbon monoxide (CO) exerts anti-apoptotic properties in the brain.
CO inhibits isoflurane-induced apoptosis in a dose-dependent manner.
CO incompletely prevented isoflurane-induced defects in neurodevelopment.
Anesthesia-induced neurotoxicity may not be mediated solely by apoptosis.
Acknowledgments
Supported by NIH/NIGMS R01GM103842-01 (RJL), NIH/NIEHS P30 ES009089 (RJL), FAER MSARF (WWS)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
References
- 1.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stefovska VG, Uckermann O, Czuczwar M, Smitka M, Czuczwar P, Kis J, Kaindl AM, Turski L, Turski WA, Ikonomidou C. Sedative and anticonvulsant drugs suppress postnatal neurogenesis. Ann Neurol. 2008;64:434–445. doi: 10.1002/ana.21463. [DOI] [PubMed] [Google Scholar]
- 3.Istaphanous GK, Loepke AW. General anesthetics and the developing brain. Curr Opin Anaesthesiol. 2009;22:368–373. doi: 10.1097/aco.0b013e3283294c9e. [DOI] [PubMed] [Google Scholar]
- 4.Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, Dissen GA, Creeley CE, Olney JW. Isoflurane-induced Neuroapoptosis in the Neonatal Rhesus Macaque Brain. Anesthesiology. 2010;112:834–841. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Istaphanous GK, Howard J, Nan X, Hughes EA, McCann JC, McAuliffe JJ, Danzer SC, Loepke AW. Comparison of the Neuroapoptotic Properties of Equipotent Anesthetic Concentrations of Desflurane, Isoflurane, or Sevoflurane in Neonatal Mice. Anesthesiology. 2011;114:578–587. doi: 10.1097/ALN.0b013e3182084a70. [DOI] [PubMed] [Google Scholar]
- 6.Rizzi S, Ori C, Jevtovic-Todorovic V. Timing versus duration determinants of anesthesia-induced developmental apoptosis in the young mammalian brain. Ann N Y Acad Sci. 2010;1199:43–51. doi: 10.1111/j.1749-6632.2009.05173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schenning KJ, Noguchi KK, Martin LD, Manzella FM, Cabrera OH, Dissen GA, Brambrink AM. Isoflurane exposure leads to apoptosis of neurons and oligodendrocytes in 20- and 40-day old rhesus macaques. Neurotoxicol Teratol. 2016;S0892:30141–6. doi: 10.1016/j.ntt.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raper J, Alvarado MC, Murphy KL, Baxter MG. Multiple Anesthetic Exposure in Infant Monkeys Alters Emotional Reactivity to an Acute Stressor. Anesthesiology. 2015;123:1084–92. doi: 10.1097/ALN.0000000000000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu F, Rainosek SW, Frisch-Daiello JL, Patterson TA, Paule MG, Slikker W, Jr, Wang C, Han X. Potential Adverse Effects of Prolonged Sevoflurane Exposure on Developing Monkey Brain: From Abnormal Lipid Metabolism to Neuronal Damage. Toxicol Sci. 2015;147:562–72. doi: 10.1093/toxsci/kfv150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol. 2009;21:286–91. doi: 10.1097/ANA.0b013e3181a71f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, Sprung J, Weaver AL, Schroeder DR, Warner DO. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128:e1053–e1061. doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Psaty BM, Platt R, Altman RB. Neurotoxicity of generic anesthesia agents in infants and children an orphan research question in search of a sponsor. JAMA. 2015;313:1515–6. doi: 10.1001/jama.2015.1149. [DOI] [PubMed] [Google Scholar]
- 14.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vöckler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–4. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 15.Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Hörster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–60. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- 16.Olney JW, Young C, Wozniak DF, Ikonomidou C, Jevtovic-Todorovic V. Anesthesia-induced developmental neuroapoptosis. Does it happen in humans? Anesthesiology. 2004;101:273–275. doi: 10.1097/00000542-200408000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;135:815–827. doi: 10.1016/j.neuroscience.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 18.Bai X, Yan Y, Canfield S, Muravyeva MY, Kikuchi C, Zaja I, Corbett JA, Bosnjak ZJ. Ketamine Enhances Human Neural Stem Cell Proliferation and Induces Neuronal Apoptosis via Reactive Oxygen Species-Mediated Mitochondrial Pathway. Anesth Analg. 2013;116:869–880. doi: 10.1213/ANE.0b013e3182860fc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boscolo A, Milanovic D, Starr JA, Sanchez V, Oklopcic A, Moy L, Ori CC, Erisir A, Jevtovic-Todorovic V. Early Exposure to General Anesthesia Disturbs Mitochondrial Fission and Fusion in the Developing Rat Brain. Anesthesiology. 2013;118:1086–1097. doi: 10.1097/ALN.0b013e318289bc9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Dong Y, Wu X, Lu Y, Xu Z, Knapp A, Yue Y, Xu T, Xie Z. The mitochondrial pathway of anesthetic isoflurane-induced apoptosis. J Biol Chem. 2010;285:4025–4037. doi: 10.1074/jbc.M109.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanderhaeghen P, Cheng HJ. Guidance molecules in axon pruning and cell death. Cold Spring Harb Perspect Biol. 2010;2:a001859. doi: 10.1101/cshperspect.a001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanno H, Shen X, Kuru N, Bormuth I, Bobsin K, Gardner HA, Komljenovic D, Tarabykin V, Erzurumlu RS, Tucker KL. Control of postnatal apoptosis in the neocortex by RhoA-subfamily GTPases determines neuronal density. J Neurosci. 2010;30:4221–31. doi: 10.1523/JNEUROSCI.3318-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan WY, Lorke DE, Tiu SC, Yew DT. Proliferation and apoptosis in the developing human neocortex. Anat Rec. 2002;267:261–76. doi: 10.1002/ar.10100. [DOI] [PubMed] [Google Scholar]
- 24.Cheng Y, Thomas A, Mardini F, Bianchi SL, Tang JX, Peng J, Wei H, Eckenhoff MF, Eckenhoff RG, Levy RJ. Neurodevelopmental Consequences of Sub-clinical Carbon Monoxide Exposure in Newborn Mice. PLoS One. 2012;7:e32029. doi: 10.1371/journal.pone.0032029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng Y, Corbin JG, Levy RJ. Programmed cell death is impaired in the developing brain of Fmr1 mutants. Dev Neuroscience. 2013;35:347–58. doi: 10.1159/000353248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smiley JF, Saito M, Bleiwas C, Masiello K, Ardekani B, Guilfoyle DN, Gerum S, Wilson DA, Vadasz C. Selective reduction of cerebral cortex GABA neurons in a late gestation model of fetal alcohol spectrum disorder. Alcohol. 2015;49:571–80. doi: 10.1016/j.alcohol.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikizad H, Yon JH, Carter LB, Jevtovic-Todorovic V. Early exposure to general anesthesia causes significant neuronal deletion in the developing rat brain. Ann N Y Acad Sci. 2007;1122:69–82. doi: 10.1196/annals.1403.005. [DOI] [PubMed] [Google Scholar]
- 28.Loepke AW, Istaphanous GK, McAuliffe JJ3rd, Miles L, Hughes EA, McCann JC, Harlow KE, Kurth CD, Williams MT, Vorhees CV, Danzer SC. The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg. 2009;108:90–104. doi: 10.1213/ane.0b013e31818cdb29. [DOI] [PubMed] [Google Scholar]
- 29.Osterop SF, Virtanen MA, Loepke JR, Joseph B, Loepke AW, Vutskits L. Developmental stage-dependent impact of midazolam on calbindin, calretinin and parvalbumin expression in the immature rat medial prefrontal cortex during the brain growth spurt. Int J Dev Neurosci. 2015;45:19–28. doi: 10.1016/j.ijdevneu.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Kim HP, Wang X, Zhang J, Suh GY, Benjamin IJ, Ryter SW, Choi AM. Heat shock protein-70 mediates the cytoprotective effect of carbon monoxide involvement of p38 beta MAPK and heat shock factor-1. J Immunol. 2005;175:2622–2629. doi: 10.4049/jimmunol.175.4.2622. [DOI] [PubMed] [Google Scholar]
- 31.Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol. 1999;276:L688–L694. doi: 10.1152/ajplung.1999.276.4.L688. [DOI] [PubMed] [Google Scholar]
- 32.Hoetzel A, Dolinay T, Vallbracht S, Zhang Y, Kim HP, Ifedigbo E, Alber S, Kaynar AM, Schmidt R, Ryter SW, Choi AM. Carbon monoxide protects against ventilator-induced lung injury via PPAR-gamma and inhibition of Egr-1. Am J Respir Crit Care Med. 2008;177:1223–1232. doi: 10.1164/rccm.200708-1265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavitrano M, Smolenski RT, Musumeci A, Maccherini M, Slominska E, Di Florio E, Bracco A, Mancini A, Stassi G, Patti M, Giovannoni R, Froio A, Simeone F, Forni M, Bacci ML, D’Alise G, Cozzi E, Otterbein LE, Yacoub MH, Bach FH, Calise F. Carbon monoxide improves cardiac energetics and safeguards the heart during reperfusion after cardiopulmonary bypass in pigs. FASEB J. 2004;18:1093–1095. doi: 10.1096/fj.03-0996fje. [DOI] [PubMed] [Google Scholar]
- 34.Vieira HL, Queiroga CS, Alves PM. Pre-conditioning induced by carbon monoxide provides neuronal protection against apoptosis. J Neurochem. 2008;107:375–84. doi: 10.1111/j.1471-4159.2008.05610.x. [DOI] [PubMed] [Google Scholar]
- 35.Mahan VL, Zurakowski D, Otterbein LE, Pigula FA. Inhaled carbon monoxide provides cerebral cytoprotection in pigs. PLoS One. 2012;7:e41982. doi: 10.1371/journal.pone.0041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Queiroga CS, Tomasi S, Widerøe M, Alves PM, Vercelli A, Vieira HL. Preconditioning triggered by carbon monoxide (CO) provides neuronal protection following perinatal hypoxia-ischemia. PLoS One. 2012;7:e42632. doi: 10.1371/journal.pone.0042632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang B, Cao W, Biswal S, Doré S. Carbon monoxide-activated Nrf2 pathway leads to protection against permanent focal cerebral ischemia. Stroke. 2011;42:2605–10. doi: 10.1161/STROKEAHA.110.607101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng Y, Levy RJ. Subclinical carbon monoxide limits apoptosis in the developing brain after isoflurane exposure. Anesth Analg. 2014;118:1284–92. doi: 10.1213/ANE.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng Y, Mitchell-Flack MJ, Wang A, Levy RJ. Carbon monoxide modulates cytochrome oxidase activity and oxidative stress in the developing murine brain during isoflurane exposure. Free Radic Biol Med. 2015;86:191–9. doi: 10.1016/j.freeradbiomed.2015.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson SA, Young C, Olney JW. Isoflurane-induced Neuroapoptosis in the Developing Brain of Nonhypoglycemic Mice. J Neurosurg Anesthesiol. 2008;20:21–28. doi: 10.1097/ANA.0b013e3181271850. [DOI] [PubMed] [Google Scholar]
- 42.Karlen SJ, Krubitzer L. Effects of bilateral enucleation on the size of visual and nonvisual areas of the brain. Cereb Cortex. 2009;19:1360–71. doi: 10.1093/cercor/bhn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwon MS, Lee JK, Park SH, Sim YB, Jung JS, Won MH, Kim SM, Suh HW. Neuroprotective Effect of Visnagin on Kainic Acid-induced Neuronal Cell Death in the Mice Hippocampus. Korean J Physiol Pharmacol. 2010;14:257–63. doi: 10.4196/kjpp.2010.14.5.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinate. 3rd. San Diego: Academic Press; 2008. [Google Scholar]
- 45.Paxinos G, Watson C. Atlas of the developing mouse brain: at E17.5, PO, and P6. San Diego: Academic Press; 2007. [Google Scholar]
- 46.Allen Institute for Brain Science. [Accessed June 1, 2016];Allen Developing Mouse Brain Atlas. 2015 Available from: http://developingmouse.brain-map.org.
- 47.Tanimura Y, Yang MC, Lewis MH. Procedural learning and cognitive flexibility in a mouse model of restricted, repetitive behaviour. Behav Brain Res. 2008;189:250–256. doi: 10.1016/j.bbr.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Jiao J, Dulawa SC. Infant maternal separation impairs adult cognitive performance in BALB/cJ mice. Psychopharmacology (Berl) 2011;216:207–218. doi: 10.1007/s00213-011-2209-4. [DOI] [PubMed] [Google Scholar]
- 49.Wang L, Almeida LE, de Souza Batista CM, Khaibullina A, Xu N, Albani S, Guth KA, Seo JS, Quezado M, Quezado ZM. Cognitive and behavior deficits in sickle cell mice are associated with profound neuropathologic changes in hippocampus and cerebellum. Neurobiol Dis. 2016;85:60–72. doi: 10.1016/j.nbd.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 51.Sun L. Early childhood general anaesthesia exposure and neurocognitive development. Br J Anaesth. 2010;105(Suppl 1):61–8. doi: 10.1093/bja/aeq302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brodkin ES, Hagemann A, Nemetski SM, Silver LM. Social approach-avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain Res. 2004;1002:151–7. doi: 10.1016/j.brainres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 53.Wang L, Wang A, Supplee WW, Koffler K, Cheng Y, Quezado ZM, Levy RJ. Lack of Sex-effect on the Size, Cellular Content, and Neuronal Density of the Developing Brain in Mice Exposed to Isoflurane and Carbon Monoxide. Data in Brief. 2017 doi: 10.1016/j.dib.2017.06.028. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dribben WH, Creeley CE, Farber N. Low-level lead exposure triggers neuronal apoptosis in the developing mouse brain. Neurotoxicol Teratol. 2011;33:473–80. doi: 10.1016/j.ntt.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fechter LD, Karpa MD, Proctor B, Lee AG, Storm JE. Disruption of neostriatal development in rats following perinatal exposure to mild, but chronic carbon monoxide. Neurotoxicol Teratol. 1987;9:277–81. doi: 10.1016/0892-0362(87)90013-4. [DOI] [PubMed] [Google Scholar]
- 56.Qiu L, Zhu CL, Wang XY, Xu FL. Changes of cell proliferation and differentiation in the developing brain of mouse. Neurosci Bull. 2007;23:46–52. doi: 10.1007/s12264-007-0007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herculano-Houzel S, Watson C, Paxinos G. Distribution of neurons in functional areas of the mouse cerebral cortex reveals quantitatively different cortical zones. Front Neuroanat. 2013;7:35. doi: 10.3389/fnana.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cherubini E, Miles R. The CA3 region of the hippocampus: how is it? What is it for? How does it do it? Front Cell Neurosci. 2015;9:19. doi: 10.3389/fncel.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uysal N, Tugyan K, Kayatekin BM, Acikgoz O, Bagriyanik HA, Gonenc S, Ozdemir D, Aksu I, Topcu A, Semin I. The effects of regular aerobic exercise in adolescent period on hippocampal neuron density, apoptosis and spatial memory. Neurosci Lett. 2005;383:241–5. doi: 10.1016/j.neulet.2005.04.054. [DOI] [PubMed] [Google Scholar]
- 60.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 61.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 62.Papaleo F, Yang F, Garcia S, Chen J, Lu B, Crawley JN, Weinberger DR. Dysbindin-1 modulates prefrontal cortical activity and schizophrenia-like behaviors viadopamine/D2 pathways. Mol Psychiatry. 2012;17:85–98. doi: 10.1038/mp.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bingham D, Martin SJ, Macrae IM, Carswell HV. Watermaze performance after middle cerebral artery occlusion in the rat: the role of sensorimotor versus memory impairments. J Cereb Blood Flow Metab. 2012;32:989–99. doi: 10.1038/jcbfm.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paule MG, Li M, Allen RR, Liu F, Zou X, Hotchkiss C, Hanig JP, Patterson TA, Slikker W, Jr, Wang C. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol. 2011;33:220–30. doi: 10.1016/j.ntt.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maines MD, Polevoda B, Coban T, Johnson K, Stoliar S, Huang TJ, Panahian N, Cory-Slechta DA, McCoubrey WK., Jr Neuronal overexpression of heme oxygenase-1 correlates with an attenuated exploratory behavior and causes an increase in neuronal NADPH diaphorase staining. J Neurochem. 1998;70:2057–69. doi: 10.1046/j.1471-4159.1998.70052057.x. [DOI] [PubMed] [Google Scholar]
- 66.Bandeira F, Lent R, Herculano-Houzel S. Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proc Natl Acad Sci U S A. 2009;106:14108–13. doi: 10.1073/pnas.0804650106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim GH, Kim JE, Rhie SJ, Yoon S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp Neurobiol. 2015;24:325–40. doi: 10.5607/en.2015.24.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Levy RJ, Nasr VG, Rivera O, Roberts R, Slack M, Kanter JP, Ratnayaka K, Kaplan RF, McGowan FX. Detection of carbon monoxide during routine anesthetics in infants and children. Anesth Analg. 2010;110:747–53. doi: 10.1213/ANE.0b013e3181cc4b9f. [DOI] [PubMed] [Google Scholar]
- 69.Nasr VG, Emmanuel J, Deutsch N, Slack M, Kanter J, Ratnayaka K, Levy R. Carbon monoxide re-breathing during low-flow anaesthesia in infants and children. Br J Anaesth. 2010;105:836–41. doi: 10.1093/bja/aeq271. [DOI] [PubMed] [Google Scholar]
- 70.Fountoulakis KN, Vieta E, Bouras C, Notaridis G, Giannakopoulos P, Kaprinis G, Akiskal H. A systematic review of existing data on long-term lithium therapy neuroprotective or neurotoxic? Int J Neuropsychopharmacol. 2008;11:269–87. doi: 10.1017/S1461145707007821. [DOI] [PubMed] [Google Scholar]
- 71.Papadia S, Hardingham GE. The dichotomy of NMDA receptor signaling. Neuroscientist. 2007;13:572–9. doi: 10.1177/10738584070130060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bologov A, Gafni M, Keren O, Sarne Y. Dual neuroprotective and neurotoxic effects of cannabinoid drugs in vitro. Cell Mol Neurobiol. 2011;31:195–202. doi: 10.1007/s10571-010-9604-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Levy RJ. Carbon monoxide pollution and neurodevelopment A public health concern. Neurotoxicol Teratol. 2015;49:31–40. doi: 10.1016/j.ntt.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.