Abstract
BACKGROUND
Breast cancer survivors (BCS) face risk of recurrence and a higher risk of developing comorbidities like cardiovascular disease compared to the general population. Physical activity (PA) has been shown to reduce such risks. The present analyses sought to identify 1) unique patterns of PA among BCS, and 2) characteristics associated with level of PA.
METHODS
548 women reported PA and sociodemographic, health-related and psychosocial factors at three time points, 6 months apart, following primary treatment for breast cancer. Cancer-related factors were obtained from chart reviews. We used finite mixture modeling to examine trajectory groups of moderate-vigorous PA (MVPA) in the early post-treatment period. We then examined the characteristics associated with trajectory group membership.
RESULTS
We identified three distinct, stable patterns of PA: low (42.5%), medium (45.5%) and high MVPA (12.0%) groups. In a multivariate setting, compared to more active BCS, the least active group had higher BMI, were less likely to report alcohol consumption, were more likely to smoke cigarettes, and had worse physical functioning and vitality scores. Cancer-treatment related factors did not significantly predict group membership.
CONCLUSIONS
A large proportion of BCS remain physically inactive following treatment, suggesting the need for interventions to improve morbidity and reduce mortality in this population.
Keywords: Breast Cancer, Survivorship, Physical Activity, Quality of Life, Health Behavior
INTRODUCTION
In 2016 there were an estimated 3.56 million breast cancer survivors (BCS) in the U.S.1 and this number will increase to almost 4.57 million by 2026.1 Many BCS are at risk of subsequent comorbidities, including heart disease, diabetes, hypertension and arthritis, partly as a result of diagnosis and treatment.2,3 Regular physical activity (PA) is an effective strategy for improving the health and quality of life of BCS, and can reduce risks of recurrence and mortality.4–6 American Cancer Society guidelines recommend survivors accumulate approximately 150 minutes of moderate to vigorous intensity PA (MVPA) per week, while also decreasing inactivity.7
Previous studies have found that greater PA among post-treatment survivors is associated with younger age, white race, lower BMI, fewer depressive symptoms, and better mental health.8–11 This research, however, focuses on overall mean levels of PA and its correlates,8–13 obscuring heterogeneity in PA patterns. Examination of this heterogeneity can identify characteristics of women who might benefit from intervention.
Two recent studies identified characteristics of cancer survivors in distinct PA trajectories. The RENEW lifestyle intervention,14 which found three PA trajectories, targeted PA and diet to improve physical function in older (≥65 years), overweight, long-term cancer survivors (both men and women). Some survivors increased their PA while those reporting the least PA over time had higher BMI and lower self-efficacy for exercise at baseline.14 An observational study of recently post-treatment BCS15 found five PA trajectory groups. Higher levels of depressive symptoms and fatigue and less cancer worry were related to decreasing activity levels over time.15 Both of these studies recruited a select group of survivors (i.e., those interested in lifestyle behaviors15 or a lifestyle counseling intervention14) and one15 excluded participants with physical health concerns. Both examined a limited set of predictors.14,15
The present study uses finite mixture modeling to examine PA patterns among BCS who recently completed primary treatment and who were not specifically selected on PA-related criteria. Two objectives were: 1) to identify distinct groups of women defined by MVPA trajectories over time, and 2) to examine a large set of predictors including sociodemographic, cancer-related, health-related, and psychosocial characteristics for their association with trajectory group membership. We were particularly interested in focusing on characteristics of women with low PA levels, as these women stand to benefit the most from intervention.
MATERIALS AND METHODS
Study Population and Procedure
This is a secondary analysis of a longitudinal study of age-related differences in adjustment to breast cancer. Study design has been previously described.16 Briefly, women diagnosed with breast cancer were recruited from Memorial Sloan Kettering Cancer Center (New York, NY) and the University of Texas Southwestern Center for Breast Care (Dallas, TX) between 2002–2006 and followed until 2008. Eligibility criteria included recent (past 8 months) first-time diagnosis of stage I–III breast cancer, age ≥18 years at diagnosis (although no participants were <25 years of age), and ability to read and write English. Eligible women were mailed a baseline (T0) questionnaire to complete and return to the Coordinating Center at Wake Forest University. Follow-up questionnaires were administered 6 (T1), 12 (T2), and 18 (T3) months following baseline questionnaires. Chart reviews were conducted after completion of primary treatment to obtain cancer stage and treatment variables. All sites obtained approval from their Institutional Review Boards.
Primary Outcome
The primary outcome was self-reported MVPA (walking and/or recreational activity) at T1, T2, and T3. Women reported frequency (1 to 5+ days/week) and duration (< 20 minutes, 20–39 minutes, 40–59 minutes and 60+ minutes) of walking and other mild, moderate, and strenuous/very hard exercise over the past month. Standardized metabolic equivalent task (MET) values were assigned to each activity. For walking, intensity was assessed using speed (2 mph, 2–3 mph, 3–4 mph and >4 mph). Walking for at least 10 minutes at least 3–4 mph was considered MVPA.17 For recreational PA, moderate intensity activities included biking outdoors, use of exercise machines, calisthenics, easy swimming, and dancing. Vigorous activities were aerobics, jogging/running, tennis and swimming laps. MET hours per week (MET-h/wk) were estimated using the compendium of physical activities.18 The PA questionnaire has been used in the Women’s Health Initiative (WHI) study and is valid and reliable.19,20
Predictors
Sociodemographic, cancer-related, health-related and psychosocial variables were evaluated for their associations with MVPA trajectory group membership. Sociodemographics obtained at baseline included age at diagnosis (years), race (white, non-white), married/partnered (yes/no), educational level (≤ high school, some college, college degree, post-graduate), and ability to pay for basics (very/somewhat hard, not hard). Cancer-related variables, obtained from chart reviews, included time since diagnosis, cancer stage at diagnosis (I, II, III), type of surgery (lumpectomy only v. mastectomy), radiation therapy (yes/no), chemotherapy (none, chemotherapy with doxorubicin, chemotherapy without doxorubicin) and hormonal therapy (yes/no).
Health-related factors consisted of body mass index (BMI), self-reported weight change from pre-diagnosis to T1 (none, weight gain of >=5 lbs, weight loss of >=5 lbs), current alcohol drinker (yes/no), current smoker (yes/no), and number of comorbidities (0, 1, 2, ≥3 conditions, out of hypertension, heart disease, high cholesterol, diabetes, arthritis, COPD, asthma, lupus and Crohn’s disease). Psychosocial variables included social support, body image and depressive symptoms. Social support was assessed by the RAND Social Support Scale;21 scores range from 1–5 (coefficient alpha = 0.97). For body image, women ranked their satisfaction with their overall bodily appearance on a scale of 1–5. Depressive symptoms were measured with the Beck Depression Inventory (BDI) version BDI-1A.22 The eight SF-36 domains23 served as health-related quality of life (HRQL) measures. All TI (or baseline in the case of co-morbidities) health-related and psychosocial measures were used in analyses.
Statistical Analyses
SAS Proc Traj24 was used to conduct latent growth curve analyses with MVPA data from T1, T2, and T3. This technique identifies distinctive time-based progressions and can model variables with a censored normal distribution.25 The censored normal distribution accurately describes the distribution of MVPA MET-h in our sample, where there is a cluster of data at the lower end of the scale. The TRAJ procedure assumes that missing data are missing completely at random (MCAR).25
Trajectories of MVPA were modeled as functions of time since diagnosis. Models containing from two to seven trajectory groups were tested. Based on the recommendations of Nagin25 we used a combination of a statistical criterion (the Bayesian Information Criterion, or BIC) and subjective judgment (minimum observed group size of 10% and/or distinctively different trajectories) to select the optimal number of trajectory groups. A higher BIC indicates a better model fit.
All trajectories groups were modeled with an intercept and a linear and quadratic term for MVPA MET-h.25 When the BIC pointed to a model where the number of groups appeared higher than optimal because of repetition of visually similar trajectories, a more parsimonious model with fewer trajectories was chosen. The TRAJ procedure assigns posterior probabilities of group membership to all individuals. These probabilities measure a specific individual’s likelihood of belonging to each of the model’s trajectory groups. For each of the domains investigated, individuals were assigned to the trajectory group for which they had the maximum posterior probability. Associations between group membership and the previously described covariates were assessed using chi-square tests (for categorical variables) and F-tests (for continuous variables). Finally, a multivariable logistic regression model (reference group = low MVPA trajectory) was used to examine characteristics associated with membership in the lowest MVPA trajectory group (vs all other trajectories combined).
RESULTS
Participant Characteristics
A total of 740 surveys were mailed to eligible women; 653 completed baseline surveys for an initial response rate of 88%. A total of 565 women completed all four surveys for an overall retention rate of 86.5%. Our analytic sample consisted of women who had never or were no longer receiving chemotherapy or radiation therapy (n=548) at T1. There were 520 women at T2 and 498 at T3. The analytic sample had a mean age of 55.8 years, was predominantly white (91.6%) and well-educated (65% attended college) (Table 1). Fifty-five percent had stage I breast cancer at diagnosis, and 65% received a lumpectomy only. Participants were an average of 10.6 months from diagnosis at T1 (range = 6 to 17 months).
Table 1.
Sample Characteristics (N=548)
| Characteristics | Mean or (%) | SD |
|---|---|---|
| Sociodemographica | ||
| Age at diagnosis (mean) | 55.8 | 12.63 |
| Race (white) | 91.6 | |
| Married/partnered (yes) | 73.0 | |
| Attended college (yes) | 65.2 | |
| Difficulty paying for basics | ||
| Not hard | 83.9 | |
| Somewhat or very hard | 16.1 | |
| Cancer-relatedb | ||
| Time since diagnosis (mean)c | 10.6 | 1.32 |
| Cancer stage | ||
| I | 55.3 | |
| II | 38.5 | |
| III | 6.2 | |
| Lumpectomy only (yes) | 65.3 | |
| Radiation (yes) | 70.6 | |
| Chemotherapy | ||
| No chemotherapy | 37.4 | |
| Chemotherapy with doxorubicin | 47.6 | |
| Chemotherapy no doxorubicin | 15.0 | |
| Hormone therapy (yes) | 74.3 | |
| Health-relatedc | ||
| Comorbiditya | ||
| 0 | 41.0 | |
| 1 | 28.7 | |
| 2 | 16.9 | |
| 3 or more | 13.4 | |
| BMI (mean)c | 25.8 | 5.41 |
| No change in self-reported weight d | 42.2 | |
| Self-reported weight gain (±5lbs)d | 31.5 | |
| Self-reported weight loss(±5lbs)d | 26.3 | |
| Physical activity (MET-h/wk MVPA)(mean)c | 11.0 | 14.55 |
| Current smokerc | 4.7 | |
| Current alcohol drinkerc | 63.7 | |
Abbreviations: MVPA, Moderate to Vigorous Physical Activity; BMI, Body Mass Index.
Sociodemographics & comorbidities were assessed at T0.
Cancer related variables assessed following completion of treatment via chart review.
Variables assessed at T1.
Change in weight calculated from pre-diagnosis weight subtracted from weight at T1visit.
Trajectory Model Selection
Table 2 displays the BIC for the evaluated trajectory models, along with the estimated percentages of the sample that fell into each trajectory groups resulting from the model. These estimated percentages are based on sums of posterior probabilities, and thus differ slightly from those reported in Figure 1, which are based on the actual number of women assigned to the groups. Although the largest BIC (i.e., the smallest in absolute value of the negative BICs) was associated with the four-trajectory model, the increase in information (i.e., the change in BIC) is largest with the increase from two to three trajectories. Changes in the BIC after the three trajectory model are small in comparison. Further, visual comparison between the 3 and 4-group models showed little substantive added information (as confirmed by the comparatively small change in BIC from the 3 to 4 group model.)
Table 2.
Model Selection Results
| # different groups | BIC | Estimated probability (estimated % in each group)
|
||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| 2 | −4599.7 | 75.7 | 24.3 | |||||
| 3 | −4520.3 | 42.8 | 44.9 | 12.3 | ||||
| 4 | −4514.7 | 30.7 | 43.1 | 17.8 | 8.4 | |||
| 5 | −4515.0 | 30.6 | 42.2 | 18.0 | 0.6 | 8.5 | ||
| 6 | −4515.5 | 30.1 | 40.7 | 0.8 | 18.3 | 4.7 | 5.4 | |
| 7 | −4522.2 | 29.6 | 39.7 | 19.0 | 4.5 | 0.9 | 5.3 | 1.1 |
Abbreviations: BIC, Bayesian Information Criterion.
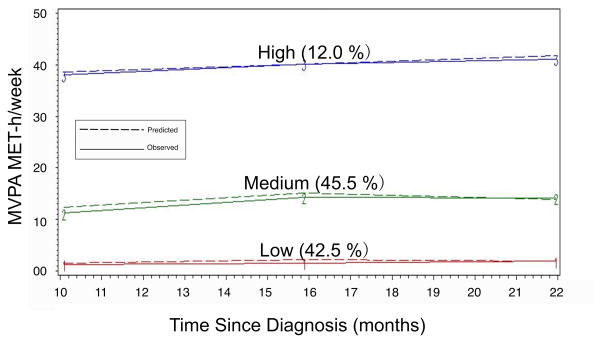
Figure 1.
Trajectory Groups of MVPA
The posterior probabilities associated with group assignment in the chosen three-trajectory model ranged from 0.50 to 1.0, with 82% of all participants having their posterior probability of group assignment above 0.75. Fifty-six percent of the sample had a posterior probability >= 0.95.
Description of MVPA Trajectories
Figure 1 shows the three distinct and stable trajectories of MVPA that emerged. Group 1, the low MVPA group, consisted of 42.5% (N=233) of the sample. This group had a consistently low level of MVPA over 12 months with a mean level of MVPA of 1.1 MET-h/week at T1, which did not increase over time. Group 2, the medium MVPA group, was the largest and consisted of 45.5% (N=249) of the sample. At T1 this group engaged in a mean level of 12.7 MET-h/week—a level of MVPA above the PA guidelines (~8.0 MET-h/week).17 Group 3, the high MVPA group, consisted of 12% of our sample (N=66). BCS in this group engaged in a mean weekly level of 39.4 MET-h/week at T1. None of the groups increased or decreased in PA over the observed time period.
Characteristics of MVPA Trajectory Group Membership
For our second objective, we examined characteristics associated with trajectory group membership. In bivariate analyses, across the three trajectory groups, women who had lower activity levels were significantly older at diagnosis, less likely to be married or partnered, and less likely to have attended college than women who had higher activity levels. Less active women also reported more difficulty paying for basics and had more comorbidities and higher BMI than more active women (Table 3). They were less likely to drink alcohol, more likely to smoke, and had less social support, worse body image, greater depressive symptoms and lower HRQL on all domains compared to more active women. Post-hoc contrasts between groups revealed significant differences between groups 1 vs. 2 and groups 2 vs. 3 on most of the predictors (except for sociodemographics) suggesting a monotonic relation between most of the predictors and group membership.
Table 3.
Characteristics Associated with each MVPA Trajectory Group Membership
| Characteristics | Trajectory Group
|
Contrasts
|
P value | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | |||||
| Low MVPA (N=233) | Medium MVPA (N=249) | High MVPA (N=66) | |||||
| % or Mean (SE) | % or Mean (SE) | % or Mean (SE) | 1 v 2 | 1 v 3 | 2 v 3 | ||
| Sociodemographica | |||||||
| Age at diagnosis (years) | 58.1 (.82) | 54.0 (.79) | 54.2 (1.54) | .01 | .02 | .88 | <.01 |
| Race (% white) | 89.3 | 92.0 | 98.5 | .06 | |||
| Married/partnered (yes) | 66.1 | 77.9 | 78.8 | <.01 | .05 | .87 | <.01 |
| Attended college (yes) | 56.7 | 69.9 | 77.3 | <.01 | <.01 | .23 | <.01 |
| Difficulty paying for basics | .04 | <.01 | .08 | <.01 | |||
| Somewhat or very hard | 21.0 | 14.1 | 6.1 | ||||
| Not hard | 79.0 | 85.9 | 93.9 | ||||
| Cancer-relatedb | |||||||
| Time since diagnosis (months)c | 10.5 (.09) | 10.6 (.08) | 10.4 (.17) | 0.46 | |||
| Cancer stage | 0.17 | ||||||
| I | 51.9 | 58.6 | 54.6 | ||||
| II | 39.1 | 37.4 | 40.9 | ||||
| III | 9.0 | 4.0 | 4.6 | ||||
| Chemotherapy | 0.37 | ||||||
| None | 40.3 | 34.1 | 39.4 | ||||
| Chemo w/ doxorubicin | 45.5 | 48.6 | 51.5 | ||||
| Chemo w/no doxorubicin | 14.2 | 17.3 | 9.1 | ||||
| Radiation (% yes) | 70.4 | 72.3 | 65.2 | 0.52 | |||
| Hormone therapy (% yes) | 73.4 | 73.5 | 80.3 | 0.49 | |||
| Type of surgery (lumpectomy only) | 66.1 | 64.9 | 63.6 | 0.45 | |||
| Health-relatedc | |||||||
| Comorbiditiesa | <.01 | <.01 | .70 | <.01 | |||
| 0 | 29.4 | 49.4 | 50.0 | ||||
| 1 | 28.1 | 27.9 | 33.3 | ||||
| 2 | 20.8 | 15.0 | 10.6 | ||||
| 3 or more | 21.7 | 7.7 | 6.1 | ||||
| BMI (kg/m2)c | 27.8 (.34) | 24.5 (.33) | 23.3 (.64) | <.01 | <.01 | .09 | <.01 |
| Weight change | .08 | .05 | .05 | 0.03 | |||
| No change in self-reported weightd | 38.7 | 42.0 | 55.7 | ||||
| Self-reported weight gain (±5lbs)d | 30.2 | 35.9 | 19.7 | ||||
| Self-reported weight loss(±5lbs)d | 31.1 | 22.1 | 24.6 | ||||
| % Meeting MVPA guidelines | 4.9 | 64.99 | 100 | <.01 | <.01 | <.01 | <.01 |
| MVPA (MET-h/wk)c | 1.1 (.56) | 12.7 (.55) | 39.4 (1.05) | <.01 | <.01 | <.01 | <.01 |
| Current alcohol drinker (% yes)c | 52.4 | 69.8 | 80.0 | <.01 | <.01 | .10 | <.01 |
| Current smoker (% yes)c | 7.1 | 3.7 | 0 | .10 | .03 | .11 | .038 |
| Psychosocialc | |||||||
| Social support | 4.0 (.05) | 4.2 (.05) | 4.4 (.09) | <.01 | <.01 | .13 | <.01 |
| Body image | 3.0 (.08) | 3.3 (.08) | 3.8 (.15) | <.01 | <.01 | <.01 | <.01 |
| Depressive symptoms (BDI) | 8.5 (.42) | 7.1 (.40) | 4.2 (.78) | .02 | <.01 | <.01 | <.01 |
| Health-Related Quality of Lifec | |||||||
| SF-36 Physical Functioning | 43.7 (.60) | 49.5 (.58) | 53.9 (1.13) | <.01 | <.01 | <.01 | <.01 |
| SF-36 Role Physical | 43.4 (.75) | 46.4 (.73) | 50.9 (1.41) | <.01 | <.01 | <.01 | <.01 |
| SF-36 Bodily Pain | 48.1 (.63) | 51.8 (.61) | 55.0 (1.18) | <.01 | <.01 | .02 | <.01 |
| SF-36 General Health | 48.1 (.59) | 51.5 (.57) | 55.5 (1.10) | <.01 | <.01 | <.01 | <.01 |
| SF-36 Vitality | 46.4 (.69) | 51.3 (.67) | 56.7 (1.29) | <.01 | <.01 | <.01 | <.01 |
| SF-36 Social Functioning | 46.9 (.64) | 49.9 (.62) | 53.6 (1.19) | <.01 | <.01 | <.01 | <.01 |
| SF-36 Role Emotional | 45.9 (.75) | 48.3 (.73) | 51.9 (1.40) | .02 | <.01 | .02 | <.01 |
| SF-36 Mental Health | 47.8 (.64) | 51.0 (.63) | 54.1 (1.20) | <.01 | <.01 | .02 | <.01 |
Abbreviations: MVPA, Moderate to Vigorous Physical Activity; BMI, Body Mass Index; BDI, Beck Depression Inventory.
Sociodemographics & comorbidities were assessed at T0.
Cancer related variables assessed following completion of treatment via chart review.
Variables assessed at T1.
Change in weight calculated from pre-diagnosis weight subtracted from weight at T1 visit.
At T1, BCS in the low MVPA trajectory group were very inactive. Fewer than 5% were meeting the PA guidelines while 81% reported 0 MET-h/week of MVPA. Examination of their light activity showed 60% of BCS in this group were doing less than 2 MET-h/week of light activity (data not shown).
Multivariable logistic regression was used to identify significant predictors of membership in the low trajectory group (inactive women). The model included all significant predictors from the bivariate analyses. Age, comorbidities, BMI, psychosocial and HRQL variables were entered as continuous variables, so that reported odds ratios (Table 4) for these independent variables represent the odds ratio associated with a 1-unit increase in the value of the independent variable. Women with a higher BMI, who smoked, consumed less alcohol, and had lower scores on physical functioning and vitality were more likely to be in the low MVPA trajectory group. A higher role-physical score was also significantly predictive of being in the low trajectory group.
Table 4.
Multivariable Logistic Regression Predicting Low MVPA Trajectory Group Membership
| Variables | Odds Ratio | 95% Confidence Intervals | P value |
|---|---|---|---|
| Sociodemographica | |||
| Age at diagnosis (years) | 1.01 | 0.99–1.03 | .49 |
| White (yes) | 1.07 | 0.49–2.37 | .86 |
| Married/partnered (yes) | 0.62 | 0.37–1.03 | .06 |
| Attended college (yes) | 0.97 | 0.61–1.55 | .90 |
| Difficulty paying for basics | 0.62 | 0.32–1.18 | .14 |
| Health-relatedc | |||
| Comorbiditiesa | 1.23 | 0.96–1.56 | .10 |
| BMI (kg/m2) | 1.10 | 1.05–1.16 | <.01 |
| Self-reported weight gain (±5lbs)d | 0.84 | 0.49–1.42 | .51 |
| Self-reported weight loss(±5lbs)d | 1.20 | 0.72–2.02 | .49 |
| Alcohol (any) | 0.53 | 0.34–0.83 | <.01 |
| Smoking (any) | 2.83 | 1.09–7.39 | .03 |
| Psychosocialc | |||
| Social support | 0.92 | 0.68–1.24 | .59 |
| Body image | 0.97 | 0.79–1.19 | .76 |
| Depressive symptoms (BDI) | 0.97 | 0.92–1.02 | .19 |
| Health-Related Quality of Lifec | |||
| SF-36 Physical Functioning | 0.94 | 0.92–.98 | <.01 |
| SF-36 Role Physical | 1.04 | 1.01–1.07 | <.01 |
| SF-36 Bodily Pain | 1.01 | 0.98–1.04 | .53 |
| SF-36 General Health | 1.00 | 0.97–1.04 | .80 |
| SF-36 Vitality | .95 | 0.93–0.98 | <.01 |
| SF-36 Social Functioning | 1.00 | 0.96–1.03 | .76 |
| SF-36 Role Emotional | .99 | 0.97–1.01 | .35 |
| SF-36 Mental Health | .98 | 0.94–1.01 | 0.14 |
Abbreviations: MVPA, Moderate to Vigorous Physical Activity; BMI, Body Mass Index; BDI, Beck Depression Inventory.
Sociodemographics & comorbidities assessed at T0.
Cancer related variables assessed following completion of treatment via chart review.
Variables assessed at T1.
Change in weight calculated from pre-diagnosis weight subtracted from weight at T1 visit.
DISCUSSION
Consistent with other research, we identified multiple distinct trajectories of MVPA. Just over forty-two percent of the sample was consistently inactive with a low level of MVPA throughout the follow-up period. The remaining BCS had medium (45.5% of the sample) or high (12.0% of the sample) levels of MVPA.
The percentage of BCS in the inactive group was considerably higher than that found in an observational study of Canadian BCS (n=199) interested in lifestyle behaviors. That study identified 5 distinct trajectory groups over 12 months, including a group that increased (10.6%) and one that decreased (9.5%) their activity.15 The consistently inactive group in this study was especially small (N=11; 5.5%). Further, this study recruited volunteers interested in lifestyle behaviors, and, importantly, participants with physical health concerns were excluded.
The percentage of women in our inactive group was higher than in either the intervention or waitlist arms of the RENEW trial at baseline. In both arms, three patterns of PA were apparent: low, medium and high PA. Both high and medium groups increased their PA in response to intervention. The RENEW trial included older participants with breast, prostate and colorectal cancer, and included men (45% of sample) who are usually more active than women.26 In addition, participants were recruited to participate in a lifestyle counseling program.14 The Women’s Health Initiative Study of over 92, 000 post-menopausal women aged 50–79 found a much larger percentage of inactive women in 8 years of follow-up among a diverse cohort. The largest proportion of women were consistently minimally active (74%), followed by moderately (22%) and highly active groups (4%).27
The number and patterns of specific trajectories across studies will vary based on sample characteristics and time frame studied, making direct comparisons difficult and not particularly meaningful. The important message is that these studies all show heterogeneity in patterns of PA over time. The present study is the only study to our knowledge that is based on a sample of BCS who were not recruited for an intervention trial or had exclusions related to physical health concerns. Given the benefits of even light intensity activity,28 and the poor outcomes associated with inactivity, the large proportion of inactive women in our study is concerning. Similar to the WHI study,27 we found only stable patterns of PA, suggesting that outside of intervention trials or selected samples, BCS are unlikely to change their PA behavior.
As with previous research8–11 several health-related and psychosocial characteristics were significantly associated with PA. In multivariable analyses, having a higher BMI, not drinking alcohol, and having lower physical functioning and lower vitality scores were significantly associated with the low MVPA group. (It is likely that the positive relationship between higher scores on the role-physical domain of the SF-36 and low PA is due to collinearity in the regression model as the low PA group had worse role-physical scores in bivariate analyses.) These characteristics suggest several avenues for possible intervention. Our results suggest that higher BMI, greater fatigue and lower levels of physical functioning are contributing factors to inactivity. Interventions to increase PA might target overweight or obese survivors with weight-loss or weight-management strategies either prior to or concurrently with PA interventions.29 Although BCS experiencing fatigue may find it harder to be physically active, a meta-analysis has shown that exercise can reduce fatigue.30 Interventions might emphasize this point with potential recruits. For BCS with compromised physical functioning, physical therapy or rehabilitation may be needed prior to starting an exercise program. The timing of these interventions is an area for future research. While not significant in multivariable analyses, smoking cessation is also critical for long-term health.31 Although several other psychosocial variables (e.g., social support, depressive symptoms, body image) were related to PA in bivariate analyses, they were no longer significant in multivariate analyses. This is likely due to collinearity, as most of the psychosocial variables, particularly BDI, were highly correlated with many of the SF-36 subscales. Consistent with Brunet and colleagues,15 we did not find cancer- or treatment-related factors to be associated with group membership, suggesting interventions may not necessarily need to be tailored based on these characteristics unless safety is a concern.
Strengths and Limitations
This study has several limitations. First, MVPA was assessed via self-report and respondents may have over- (or under-) reported their PA. Second, categorizing women into ordinal levels of time spent doing PA could have grouped women with slightly different observed PA into the same average category. While both of these limitations likely led to some misclassification, it is not likely that this led to substantial error at the level of trajectory group membership. Third, because MVPA prior to diagnosis was not assessed, we are unable to determine whether, and for how many women, PA levels changed as a result of diagnosis/treatment. Fourth, while the trajectory modeling procedure assumes data are missing completely at random, such “clean” missingness usually is not found. Based on our high retention proportion we believe that the potential impact of data not being missing completely at random in our sample is likely to be small. Finally, our sample consisted of predominantly well-educated white women.
Strengths of this study include a large sample of BCS of different ages who were not selected on factors related to PA, and a wide variety of variables measured over 12 months during the early post-treatment period. We found that a high percentage of BCS maintain relative inactivity in the two years following their diagnosis, suggesting that without intervention BCS do not increase their PA post-diagnosis. Interventions might focus on reducing sedentary behaviors in this group and/or concurrently targeting weight and symptom management. Future research should include pre-diagnosis PA to examine how cancer and its treatment impact PA.
Acknowledgments
Funding: National Cancer Institute R25 CA122061 and Department of Defense grant DAMD17-01–1-0447.
Footnotes
The authors have no conflicts of interest to report.
Author contributions: NEA acquired the funding that supported this publication. ARL, NEA and BL conceptualized the study. BL conducted the formal analysis of data, with ARL and NEA providing additional interpretation of the data. ARL, BL and NEA wrote the original draft, reviewed and edited the final version.
References
- 1.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285(7):885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 3.Sarfati D, Koczwara B, Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer J Clin. 2016;66(4):337–350. doi: 10.3322/caac.21342. [DOI] [PubMed] [Google Scholar]
- 4.Speed-Andrews AE, Courneya KS. Effects of Exercise on Quality of Life and Prognosis in Cancer Survivors. Curr Sports Med Rep. 2009;8(4):176–181. doi: 10.1249/JSR.0b013e3181ae98f3. [DOI] [PubMed] [Google Scholar]
- 5.Kohler LN, Garcia DO, Harris RB, Oren E, Roe DJ, Jacobs ET. Adherence to Diet and Physical Activity Cancer Prevention Guidelines and Cancer Outcomes: A Systematic Review. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1018–1028. doi: 10.1158/1055-9965.EPI-16-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 7.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 8.Emery CF, Yang HC, Frierson GM, Peterson LJ, Suh S. Determinants of physical activity among women treated for breast cancer in a 5-year longitudinal follow-up investigation. Psychooncology. 2009;18(4):377–386. doi: 10.1002/pon.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Littman AJ, Tang MT, Rossing MA. Longitudinal study of recreational physical activity in breast cancer survivors. J Cancer Surviv. 2010;4(2):119–127. doi: 10.1007/s11764-009-0113-2. [DOI] [PubMed] [Google Scholar]
- 10.Irwin ML, McTiernan A, Bernstein L, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36(9):1484–1491. [PMC free article] [PubMed] [Google Scholar]
- 11.Huy C, Schmidt ME, Vrieling A, Chang-Claude J, Steindorf K. Physical activity in a German breast cancer patient cohort: one-year trends and characteristics associated with change in activity level. Eur J Cancer. 2012;48(3):297–304. doi: 10.1016/j.ejca.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Andrykowski MA, Beacham AO, Jacobsen PB. Prospective, longitudinal study of leisure-time exercise in women with early-stage breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(3):430–438. doi: 10.1158/1055-9965.EPI-06-0735. [DOI] [PubMed] [Google Scholar]
- 13.Harrison S, Hayes SC, Newman B. Level of physical activity and characteristics associated with change following breast cancer diagnosis and treatment. Psychooncology. 2009;18(4):387–394. doi: 10.1002/pon.1504. [DOI] [PubMed] [Google Scholar]
- 14.Morey MC, Blair CK, Sloane R, Cohen HJ, Snyder DC, Demark-Wahnefried W. Group trajectory analysis helps to identify older cancer survivors who benefit from distance-based lifestyle interventions. Cancer. 2015;121(24):4433–4440. doi: 10.1002/cncr.29684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunet J, Amireault S, Chaiton M, Sabiston CM. Identification and prediction of physical activity trajectories in women treated for breast cancer. Ann Epidemiol. 2014;24(11):837–842. doi: 10.1016/j.annepidem.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Avis NE, Levine B, Naughton MJ, Case LD, Naftalis E, Van Zee KJ. Age-related longitudinal changes in depressive symptoms following breast cancer diagnosis and treatment. Breast Cancer Res Treat. 2013;139(1):199–206. doi: 10.1007/s10549-013-2513-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: U.S. Department of Health and Human Services; 2008. [DOI] [PubMed] [Google Scholar]
- 18.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 19.Johnson-Kozlow M, Rock CL, Gilpin EA, Hollenbach KA, Pierce JP. Validation of the WHI brief physical activity questionnaire among women diagnosed with breast cancer. Am J Health Behav. 2007;31(2):193–202. doi: 10.5555/ajhb.2007.31.2.193. [DOI] [PubMed] [Google Scholar]
- 20.Meyer AM, Evenson KR, Morimoto L, Siscovick D, White E. Test-retest reliability of the Women’s Health Initiative physical activity questionnaire. Med Sci Sports Exerc. 2009;41(3):530–538. doi: 10.1249/MSS.0b013e31818ace55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 22.Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and-II in Psychiatric Outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 23.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 24.Jones B. [Accessed July 2016];Traj: group-based modeling of longitudinal data. 2010 https://www.andrew.cmu.edu/user/bjones/index.htm.
- 25.Nagin D. Group-based modeling of development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 26.Kaplan MS, Newsom JT, McFarland BH, Lu L. Demographic and psychosocial correlates of physical activity in late life. Am J Prev Med. 2001;21(4):306–312. doi: 10.1016/s0749-3797(01)00364-6. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen HQ, Herting JR, Kohen R, et al. Recreational physical activity in postmenopausal women is stable over 8 years of follow-up. J Phys Act Health. 2013;10(5):656–668. doi: 10.1123/jpah.10.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stamatakis E, Rogers K, Ding D, et al. All-cause mortality effects of replacing sedentary time with physical activity and sleeping using an isotemporal substitution model: a prospective study of 201,129 mid-aged and older adults. Int J Behav Nutr Phys Act. 2015;12:121. doi: 10.1186/s12966-015-0280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demark-Wahnefried W, Campbell KL, Hayes SC. Weight management and its role in breast cancer rehabilitation. Cancer. 2012;118(8 Suppl):2277–2287. doi: 10.1002/cncr.27466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cramp F, Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2008;(2):CD006145. doi: 10.1002/14651858.CD006145.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Weaver KE, Foraker RE, Alfano CM, et al. Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal, and gynecologic cancers: a gap in survivorship care? J Cancer Surviv. 2013;7(2):253–261. doi: 10.1007/s11764-013-0267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]