Abstract
Background:
The high prevalence of multimorbidity necessitates rethinking of the health care system. The overarching goal of the Patient-Centred Innovations for Persons with Multimorbidity program is to build on existing structures and find and evaluate patient-centred innovations relevant to multimorbidity.
Methods:
We describe the protocol for a proposed multijurisdictional (Quebec and Ontario) concurrent triangulation mixed-methods study. In both provinces, a qualitative descriptive study will be used to explore innovations in patient-centred multimorbidity care. Two randomized controlled trials, 1 in either province, will evaluate the innovations in a wait-list-controlled design using patient-reported outcomes. An additional control group, matched on age, sex, enrolment/index date (± 3 mo) and propensity score, will be created with the use of health administrative data. Patients will be 18-80 years of age and will have 3 or more chronic conditions. The innovations will have elements of relevance to multimorbidity care, patient-centred partnerships and integration of care. The primary outcome measures will be 2 patient-reported outcomes: patient education and self-efficacy. Secondary outcomes will include patient-reported health status, quality of life, psychological distress and health behaviours, and costs of care.
Interpretation:
This protocol describes a mixed-method study in 2 jurisdictions. The studies will answer the questions of what innovations work and how they work for patients, health care professionals and policy-makers. Trial registration: ClinicalTrials.gov, no NCT02789800 (Quebec Trial), NCT02742597 (Ontario Trial).
In Canada, a considerable number of innovations relevant to multimorbidity have been mounted based on the Chronic Care Model,1-3 self-management programs4 and primary care renewal,5 but very few of these have been described, and only a minority have been evaluated.6 Therefore, it is timely to identify such innovations and to evaluate whether they are effective and, if so, how they are effective.
The Patient-Centred Innovations for Persons with Multimorbidity (PACE in MM) study is a mixed-methods study in 2 Canadian jurisdictions (Quebec and Ontario) that will evaluate complex interventions to improve patient-centred outcomes of people with multimorbidity. The protocol was funded as a Team Grant in the Community-Based Primary Health Care Signature Initiative of the Canadian Institutes of Health Research (grant no. 01247-000).
The PACE in MM study will identify innovations with the following goals: to realign care toward multimorbidities rather than the single diseases, an approach known to be effective;7-18 to focus care on the patient in context versus only on the disease, an approach found to have an impact on improving patient health,19 decreasing costs20 and mitigating the deleterious effects of deprivation;21,22 and to improve integration and coordination of the health care system.23
Protocol
The PACE in MM study is a concurrent triangulation mixed-methods study24 (Appendix 1, available at www.cmajopen.ca/content/5/2/E365/suppl/DC1) in 2 Canadian jurisdictions (Quebec and Ontario) that will evaluate 2 complex innovative interventions to improve patient-centred outcomes of people with multimorbidity. The methods will comprise both a qualitative evaluation of interventions and a quantitative evaluation of interventions, each of which has different objectives. We provide methodologic details of each part separately.
Qualitative evaluation of interventions
The qualitative evaluation will answer the following research questions: What are the components of the 2 interventions to be used in the intervention study? What are the contextual factors that may influence the content and effectiveness of the interventions? What are the potential barriers to and facilitators of the interventions in their context? How do barriers and facilitators apply to population subgroups, including patients of different genders and vulnerable patient groups? How do the interventions promote patient-centred care?
Methods and design
The team will conduct a qualitative descriptive study of 2 specific interventions to explore the process of the interventions and experiences of key stakeholders involved.25 In‑depth individual interviews will be conducted by the qualitative research team, consisting of coinvestigators, doctoral students and trained research staff, with the 4 types of stakeholders involved in each intervention: decision-makers (n = 10), primary care physicians (n = 10-15), professionals conducting the intervention (n = 10-15), and a purposive sample of patients with multiple diseases participating in the intervention (n = 10) and their informal caregivers (n = 10).26 The numbers of interviews are estimates and will be guided by the saturation of data.26
Data collection
The individual interviews, lasting 30-60 minutes, will be conducted in person by the qualitative research team at a location convenient to the participant. Interviewers will conduct a semistructured interview using a guide to allow for exploration of relevant factors such as how contextual factors, barriers, facilitators and personal factors will inform program delivery, with respect to both current goals and future scalability. An example of an interview guide is presented in Appendix 2 (available at www.cmajopen.ca/content/5/2/E365/suppl/DC1); certain questions will be tailored to capture the experiences and knowledge unique to each group. All interviews will be audiotaped and transcribed verbatim.
Data analysis
We will analyze the data using an iterative and interpretative approach.27 The verbatim transcripts will be examined through both independent and team analysis, occurring concurrently, to build and develop on the emerging themes. There will be 3 phases: 1) each transcript will be independently reviewed and coded by all members of the qualitative research team to determine the key concepts emerging from the data; 2) the researchers will meet to examine their independent coding and will reach consensus on the coding template with themes and subthemes; these meetings will be repeated until all interviews have been analyzed; and 3) the research team will review the overarching themes and identify exemplar quotes illustrating the themes and subthemes. In particular, data related to patient subgroups (different genders and vulnerable populations) will be identified. The data management software NVivo 10 (QSR International) will be used to organize the coded data and identify exemplar quotes reflecting the central themes.
Quantitative evaluation of effects of interventions
The detailed version of the evaluation framework below will be made available to initiatives across Canada interested in performing their own evaluation with the guidance of the PACE in MM program.
Trial design
The design will be a randomized wait-list-controlled trial in which the patient will be the unit, with a delayed intervention assessing short-term effectiveness. There will be 2 parallel groups for each of 2 trials, 1 in Quebec and 1 in Ontario, each with equal numbers of patients in the intervention and control groups. The risk of contamination (control-group patients' experiencing part of the intervention) will be minimal because intervention-group patients will be referred by the family physician to receive the intervention, separate from usual family physician care, with a different constellation of providers. Both the intervention group and the control group will receive usual care by the family physician.
Follow-up measures with all patients will permit assessment of mid-term effects, and we will use health administrative data to assess long-term effects.
Setting
Two interventions have been identified, 1 in Quebec and 1 in Ontario, that are innovations being rolled out in primary care practices. The Quebec site will be in the Saguenay region, and the Ontario site will be in Toronto. The methods, presented below according to the SPIRIT guidelines,28 are for 1 site and will be duplicated at the second site.
Eligibility
Eligible patients will be cognitively intact and literate women and men aged 18-80 years newly referred to receive the innovative intervention by their provider because of 3 or more chronic conditions; they will never before have been exposed to the intervention. We chose the upper limit of 80 years to avoid recruiting patients at risk of being admitted to an institution or being dependent during the 2-year follow-up. For the purpose of this study, we will operationalize the definition of multimorbidity using Fortin's list of 21 chronic conditions.29
Interventions
The Quebec and Ontario complex interventions chosen for this trial will have common elements: referral by the family physician to the intervention, shared philosophy of care, team-based care, external relations with community resources, provider training and patient partnership. However, the 2 selected interventions will differ because of differing policy environments, with the Quebec intervention stressing a co‑located team, shared electronic medical record and explicit empowerment of the patient, and the Ontario intervention serving patients with more complex care needs and their presence at the interdisciplinary team meeting where the care plan will be discussed and mutually agreed on.
The Quebec intervention will be a program spreading across the Saguenay region called the Démarche intégrée en maladies chroniques région 02 for patients whose care needs are of low to moderate complexity. A nurse will coordinate interdisciplinary care by relevant providers, such as a kinesiologist, nutritionist and other primary health care professionals, including motivational and self-management support over 4 months as well as a knowledge-reactivation appointment in the following year.
The Ontario intervention will be part of the Health Links initiative for patients with care needs of moderate to high complexity called Telemedicine IMPACT Plus. A nurse will coordinate a unique constellation of providers for each patient, including several medical disciplines such as family medicine, internal medicine and psychiatry as well as providers from other interprofessional fields such as physiotherapy, occupational therapy, social work, pharmacy, dietetics and home care. The providers and the patient will meet for 1.5 to 2 hours for a case conference where issues will be discussed and a mutually agreed-on care plan constructed. Follow-up will be done by the family physician and nurse.
Data will be collected to describe the elements of the interventions that each patient experienced. Concomitant care will be expected, permitted and measured with the use of an adaptation of the Health Services Utilization Inventory of Browne and colleagues.30
Methods
Patients referred by the primary care provider will be contacted by telephone by a research assistant to assess eligibility and obtain informed consent. After completing questionnaires collecting sociodemographic data and baseline measures with the patient, the research assistant will open a sealed envelope containing the group assignment (intervention or control). The sealed envelopes will have been randomly sequenced.
Cards will be prepared, half with "Intervention" and half with "Control" printed on them. Carbon paper will be overlaid on top of each card and a layer of cardboard placed under the card, rendering the card impenetrable to light. The card will then be placed into an opaque envelope and the envelope sealed. The envelopes will be ordered according to a random-number sequence and then sequentially numbered on the outside such that the number is transferred to the allocation card. These numbers will be the participant study identification numbers. The envelopes will be opened sequentially with each enrolment only after the participant's name has been written on the envelope and subsequently transferred by carbon copy to the allocation card. In this way, simple randomization will be achieved with adequate concealment and an audit trail created.
Research staff who did not participate in preparing the envelopes or allocation sequence will confirm eligibility, enrol participants, perform informed-consent procedures, administer the baseline questionnaire, write the participant's name on the envelope, open the envelopes and reveal group assignment to participants. Randomization results will be recorded in a master list of participants. Group allocation concealment is not feasible. Blinding is neither feasible nor necessary for this trial because both patients and health care providers will need to know who is involved in each group as they both participate actively in the intervention.
Timeline
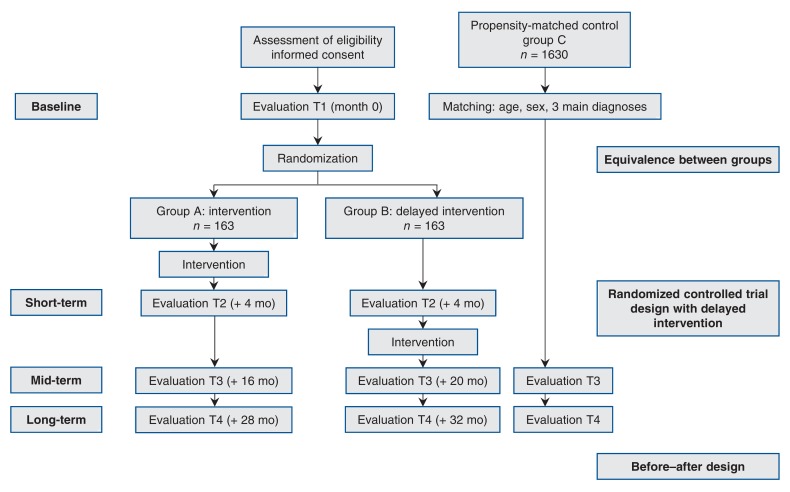
The timeline in the trial is shown in Figure 1. Eligible patients who agree to participate will complete at baseline (T1) the questionnaires collecting the sociodemographic data and baseline measures, which will be used to document equivalence between groups. We will assess the effectiveness of the interventionusing 3 strategies:
Figure 1.
Flow chart showing quantitative study design.
1. To measure short-term effects (4 mo), we will use a randomized controlled trial design with a delayed intervention (wait-list control group).31 Patients will be randomly assigned to receive the intervention either within a short period (group A) or 4 months later (group B), an equal distribution of sex in either group being ensured. Questionnaires assessing self-perceived health education impact, self-efficacy and other measures (see the following section) will be completed by telephone at baseline by all participants, 4 months after the end of the intervention by the participants in group A (T2) and 4 months after baseline by the participants in control group B (T2). This will constitute the short-term measure of effectiveness of the intervention, whose primary outcome measures are shown in Table 1. Group B will then receive the intervention (Figure 1).
Table 1: Covariables and outcome measures for the randomized controlled trial of short-term effects.
| Measure | Main covariable of interest | Psychometric properties |
|---|---|---|
| MM21 questionnaire | Multimorbidity | Validity: sensitivity 84.6%, specificity 84.3% compared with a quality of life measure; test-retest reliability: intraclass correlation coefficient 0.88, Cohen κ 0.64; interrater reliability: intraclass correlation coefficient 0.88, Cohen κ 0.7932 |
| Patient Perception of Patient Centeredness scale | Patient-centred partnership | 14-item measure of patient perception of patient centredness of visits with health care professionals. Reliability: Cronbach α 0.71 (n = 315); validity: significant associations with 36-Item Short Form Health Survey33-35 |
| Patient perceptions of transitions in care measure | Patient-centred coordination | 9-item questionnaire for patients. Items adapted from Coleman et al.36 Reliability: Cronbach α of Coleman measure 0.9336 |
| Patient-perceived primary outcome measures | ||
| Health Education Impact Questionnaire | Patient education program benefits such as empowerment | "Proxy of empowerment." Cronbach α 0.70-0.89 according to domain, ≥ 0.80 for 7 out of 8 domains37 |
| Self-Efficacy for Managing Chronic Disease scale | Self-efficacy | 6 items + 2 items from general scale. Reliability: Cronbach α 0.91 for Self-Efficacy for Managing Chronic Disease scale, 0.87 for General Self-Efficacy subscale38 |
| Patient-perceived secondary outcome measures | ||
| SF-12v2 Health Survey | Health status | Multipurpose short-form generic measure of health status. Validity: r = 0.95 and 0.96 for physical and mental components, respectively, of 36-Item Short Form Health Survey39 |
| EQ-5D | Quality of life | Intraclass correlation: EQ-5D utility 0.73, EQ-5D visual analogue scale 0.70; test-retest reliability: 0.86 for group-level coefficients, 0.90 for coefficient derived from individual correlations40 |
| Kessler Psychological Distress Scale (K6) | Psychological well-being | Internal consistency: Cronbach α 0.89; concurrent validity: r = 0.97 for correlation with its equivalent, the K10; discriminant validity: area under the receiver operating characteristic curve 0.89 (95% confidence interval 0.88-0.90); sensitivity (SE): 0.36 (0.08); specificity (SE): 0.96 (0.02)41 |
| Behavioral Risk Factor Surveillance System Questionnaire | Health behaviour | Eating habits, physical activity, smoking and alcohol consumption. Individual-level test-retest reliability: κ > 0.60 for 19 demographic and risk factors, intermediate for food consumption measure (0.40-0.76), lowest for medical examination and blood pressure measurement in previous 2 yr (κ = 0.54 and 0.23, respectively)42 |
Note: SE = standard error.
2. To measure the mid-term effects (1 yr) on participant-reported outcomes, we will conduct a repeated-measures study combining groups A and B. Twelve months after the end of the intervention (T3), the participants will complete the same questionnaires completed at baseline and at 4 months.
3. To measure mid-term (T3) and long-term (2 yr, T4) effects on use and cost of health care services, we will ask all participants (groups A and B) to provide consent to give access to their health administrative data, which we will use to collect and evaluate comprehensive information on use of health care services by participants in both groups; the data will be examined separately for the 2 provinces. We will identify a matched sample of nonparticipating patients using health administrative data. A sampling population will be created including all people who meet the study eligibility criteria and are cared for in a separate health region with similar health care system (acute, primary and secondary care) supply characteristics. We will set an index date for all patients in the sampling population based on the distribution of enrolment dates in the intervention groups. We will calculate a propensity score by merging the intervention groups with the sampling population and predicting the likelihood of enrolment in the intervention groups using age, sex, income quintile, diagnoses and use of health care services before enrolment as predictors. We will then match participants from groups A and B to an additional control group (group C) using sex, age, enrolment/index date (± 3 mo) and the (predicted) propensity score within a caliper of 0.2 of the standard deviation of the propensity score. After establishing balance on measured covariates in the intervention and propensity-score-matched control groups, we will use a differences-in-differences method to compare outcomes in the pre- versus postintervention period between the intervention and control groups.
Variables and outcome measures
Details of the short-term covariables and outcome measures are given in Table 1. The variables fall into 5 categories:
1. Sociodemographic characteristics: sex, gender, age, education, family income, marital status, occupation, housing and number of people living under the same roof.
2. Primary care context variables: type of primary care organization in which the intervention occurs (solo or group practice, Family Health Team/Family Medicine Group, community health centre, teaching unit/academic centre).
3. Main covariables of interest: i) self-reported multimorbidity (measured with the MM21 questionnaire32), ii) patient-centred partnership (measured with the Patient Perception of Patient Centeredness Scale33-35) and iii) patient-centred coordination (measured with the patient perceptions of transitions in care measure, adapted from Coleman and colleagues36).
4. Primary outcome measures: i) the Health Education Impact Questionnaire,37 which provides a broad profile of the potential impacts of patient education interventions, and ii) the 6-item Self-Efficacy for Managing Chronic Disease scale,38 which measures the level of perceived disease-management self-efficacy.
5. Secondary patient-perceived outcomes: i) health status, assessed with the SF-12 Health Survey,39 ii) quality of life, assessed with the EQ-5D,40 iii) psychological distress, assessed with the Kessler Psychological Distress Scale,41 and iv) health behaviours, assessed with the Behavioral Risk Factor Surveillance System Questionnaire.42
We will also use health administrative data as secondary outcomes to compare use and cost of health care services before and after the interventions. Health administrative data will include emergency department visits, avoidable hospital admissions, readmissions, time to first primary care visit after an emergency department visit and continuity of care. We will seek permission from the appropriate authorities in the 2 provinces to access the health administrative data.
Data collection
Three types of data will be collected: data on referral and consent, collected at the intervention sites; data on covariables and on primary and secondary outcomes, collected by research assistants; and data to allow linking with health administrative data at the Régie de l'assurance maladie du Québec in Quebec and the Institute for Clinical Evaluative Sciences in Ontario.
Data management
We will use secure file transfer, secure email or secure fax to transmit patient information on referral and consent that needs to flow between intervention settings and the PACE in MM research team. Forms that physicians use to obtain patient consent to be contacted by the PACE in MM research team will be shredded at the site once transferred. In Quebec, the referral information will be kept in the patient's research file. Nurses and other professionals in Quebec and Ontario will maintain their normal patient records for patients who participate in the intervention and will observe their institutional privacy policy for these documents.
Personal information needed to allow linking with health administrative data will include patient name, address, telephone number, date of birth, list of diagnosed conditions and Ontario Health Insurance Plan/Régie de l'assurance maladie du Québec (health card) number. This participant information and the outcome data will be stored in separate files on secure institutional drives. Hard copies of patient questionnaires, signed paper information letters and consent forms will be stored in locked filing cabinets and the data entered into protected electronic files. PACE in MM sites (Western University in Ontario and the Université de Sherbrooke/Centre intégré universitaire de santé et de services sociaux du Saguenay-Lac-Saint-Jean) are committed to providing an environment that protects the privacy and security of information and electronic resources. Information and data management terms of reference and policies are adopted in each site. Access to the files and the ability to link participant information when necessary (for health administrative data linkage and participant follow-up) will be monitored by the principal investigators and PACE in MM research coordinators.
Qualitative interviews will be recorded as audio files and transcribed verbatim using secure file transfer. Interview participants will be given a number before transcription. A master list of participant name and interview number will be held in a separate password-protected file on the server, and a hard copy will be locked in a secure cabinet.
All data will be kept for 25 years in Quebec and for 7 years in Ontario following study completion. At the end of the storage period, transcripts and any paper reports will be shredded, and electronic data files will be purged with the assistance of information services.
There will be minimal risk of release of personal health information to a third party. We will take all measures to keep this information protected. If personal health information were inappropriately released, the investigators would stop the study and contact the institutional privacy offices and research ethics boards to help manage the situation.
Local standard does not require a data monitoring committee, but as part of the overall PACE in MM project there is governance through a measurement committee along with health care provider and patient advisory groups.
All investigators will have access to complete data sets. Analyzed data and results will be made available to local sites and interested participants on publication of the results. De‑identified subsets of data will be transferred on request to health care providers at participating institutions, and they will be responsible for pursuing additional ethics approval as required.
Data analysis
We will first describe participants' baseline characteristics in each group and compare them across groups. To evaluate short-term effects, we will compare groups A and B on T2 scores with an analysis of covariance adjusted for T1 scores.43 To document mid-term effects, we will use a repeated-measures analysis of variance to study the evolution of continuous variables collected 3 times.44 We will seek advice from our collaborating biostatistician for the possible transformation of variables or the use of additional statistical analyses (e.g., other longitudinal and structural equation modelling analyses).
Analyses assessing differences by sex, gender and socioeconomic status will be performed. We will evaluate health care system costs in the intervention and control groups using amounts paid to providers based on provincial fee schedules and cost-weighted use of institutions including hospitals and long-term care facilities. Use records obtained from health administrative data will be multiplied by applicable cost weights (e.g., Canadian Institute for Health Information Resource Intensity Weights and Cost of a Standard Hospital Stay).45-47 The methods employed will model the individual patient-level costs incurred in the health care system, involving methods developed for costing with the use of administrative data.48 We will identify and cost incremental resources in the intervention group using applicable time/resource inputs and relevant wage rates following guidelines for economic evaluation in health interventions.49
Cross-jurisdictional comparisons
The duplication of the evaluation in 2 provinces at the same time will allow for cross-jurisdictional comparisons of the 2 primary outcomes as well as of the use and cost of health care services. We will adjust costs appropriately to obtain a valid comparison. Context variables related to the primary care organizations will be considered as potential confounders.
Sample size and effect size
We have estimated a sample size for each individual component of this evaluation framework. We calculated the required sample size for the randomized clinical trial for the 2 main outcome variables (Health Education Impact Questionnaire and Self-Efficacy for Managing Chronic Disease scale) with a 2-sided α = 0.05 and 80% power. First, for continuous scores, 64 participants in both the intervention group and the control group will allow detection of a medium effect size (0.5).50 However, for the Health Education Impact Questionnaire, results are typically expressed as the proportion of patients improving by at least half a standard deviation, found in a previous study to be 35%.37 With a conservative scenario of improvement of 20% in the control group, 138 patients would be required in either group to detect this 15% difference. Accounting for an anticipated drop-out rate of 15%, we will enrol 326 patients, 163 in either group, in both provinces. For assessing the long-term effects, a ratio of 5:1 for control group C will provide 1630 matched patients in either province. With a population of this magnitude, we will be able to detect at minimum a difference of 10% in use of health care services (2-sided α = 0.05 and 90% power). Moreover, since we have a limited set of characteristics for the control group, the ratio 5:1 will ensure that we have an average picture for each control subject.
Dissemination
The PACE in MM team intends to disseminate the results of this program of research widely. Qualitative and quantitative findings will be shared with patients, providers, partner decision-makers and other researchers. Three provinces other than Quebec and Ontario (British Columbia, Manitoba and Nova Scotia) are coinvestigators and will participate in a scaling-up component of the program.
Authorship
PACE in MM has an authorship policy for its coinvestigators and does not intend to use professional writers.
Ethics approval
The qualitative study and randomized trial in Quebec were approved by the Comité d'éthique de la recherche du Centre intégré universitaire de santé et de services sociaux du Saguenay-Lac-St-Jean. The qualitative study in Ontario was approved by the ethics review board of Western University, and the randomized trial in Ontario was approved by the ethics review boards of Western University, Sunnybrook Hospital, Toronto, the University Health Network, Toronto, and Toronto East General Hospital.
Ancillary care needed by participants will be provided by the family physician referrer.
Interpretation
Driving innovation in health care to better manage urgent problems such as chronic disease prevention and management is a high priority in Canada, for funders such as the Canadian Institutes of Health Research and for policy-makers. Other countries, too, are experimenting with innovations in chronic disease care, with 18 interventions representing 5 countries identified and evaluated in the latest Cochrane review.18 These interventions have shown mixed results and had low power, so the contribution of the PACE in MM program in this field has great potential.
This protocol sets out a way to identify and evaluate innovations. Because policy-makers are part of the PACE in MM research team, integrated knowledge translation will be possible.
Conclusion
Our goal is to show how to evaluate innovations in patient-centred care for patients with multimorbidity. We propose 2 concurrent triangulation mixed-methods studies, 1 of an innovation in Quebec and 1 in Ontario. Both studies have a qualitative component and a randomized controlled trial. The protocol outlines the way to achieve 2 key deliverables. The first is to illustrate cross-jurisdictional comparisons of innovative programs for chronic disease prevention and management. The second is to provide a rigorous quantitative evaluation framework that others may use and that includes both measures of patient experience, and health administrative and cost data.
The proposed randomized trials have the potential to reveal whether the innovations work in multimorbidity care. The qualitative studies have the potential to show how the innovations work for patients, health care professionals and policy-makers.
Supplemental information
For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/5/2/E365/suppl/DC1
Supplementary Material
Footnotes
Funding: The Patient-Centred Innovations for Persons with Multimorbidity Research Program is funded by a Team Grant from the Community-Based Primary Health Care Signature Initiative of the Canadian Institutes of Health Research. Moira Stewart is funded by the Dr. Brian W. Gilbert Canada Research Chair in Primary Health Care Research. Martin Fortin holds a Research Chair on Chronic Diseases in Primary Care (Fondation de ma vie, Hôpital de Chicoutimi and Université de Sherbrooke).
ICES disclaimer: This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by the ICES or the Ontario MOHLTC is intended or should be inferred.
Members of the Patient-Centred Innovations for Persons with Multimorbidity Team: Mathieu Bélanger PhD (Department of Family Medicine, Université de Sherbrooke, Sherbrooke, Que.), Louise Bestard-Denomme MA (Centre for Studies in Family Medicine, Western University, London, Ont.), Onil Bhattacharyya MD PhD (Faculty of Medicine, University of Toronto, Toronto, Ont.), Roxane Borgès Da Silva PhD (Faculté des sciences infirmières, Université de Montréal, Montréal, Que.), Tarek Bouhali MSc (Faculté de médecine et des sciences de la santé, Université de Sherbrooke, Sherbrooke, Que.), Judith Belle Brown PhD (Department of Family Medicine, Western University, London, Ont.), Jocelyn Charles MD (Division of Clinical Public Health, University of Toronto, Toronto, Ont.), Maud-Christine Chouinard RN PhD (Département des sciences humaines, Université du Québec à Chicoutimi, Chicoutimi, Que.), Valérie Émond MSc (Institut national de santé publique du Québec, Québec, Que.), Frances Gallagher RN PhD (Faculté de médecine et des sciences de la santé, Université de Sherbrooke, Sherbrooke, Que.), Richard H. Glazier MD MPH (Institute for Clinical Evaluative Sciences, Toronto, Ont.), William E. Hogg MSc MCISc MD (Department of Family Medicine, University of Ottawa, Ottawa, Ont.), Alan Katz MD MBChB MSc (Department of Community Health Sciences, University of Manitoba, Winnipeg, Man.), Christine Loignon PhD (Department of Family Medicine, Université de Sherbrooke, Sherbrooke, Que.), Pauline Pariser MASc MD (Department of Family and Community Medicine, University of Toronto, Toronto, Ont.), Thuy-Nga Pham MASc MD (Department of Family and Community Medicine, University of Toronto, Toronto, Ont.), Helena Piccinini-Vallis MD MSC (Department of Family Medicine, Dalhousie University, Halifax, NS), Sonja Reichert MD MSc (Department of Family Medicine, Western University, London, Ont.), Bridget L. Ryan PhD (Departments of Family Medicine and of Epidemiology and Biostatistics, Western University, London, Ont.), Tara Sampalli PhD (Primary Health Care, Nova Scotia Health Authority, Dalhousie University, Halifax, NS), Jonathan Sussman MD MSc (Department of Oncology, McMaster University, Hamilton, Ont.), Amardeep Thind MD PhD (Department of Epidemiology and Biostatistics, Western University, London, Ont.), Walter P. Wodchis MA MAE PhD (Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, Ont.), Sabrina T. Wong RN PhD (University of British Columbia Centre for Health Services and Policy Research, Vancouver, BC), Merrick F. Zwarenstein MBBCh MSc PhD (Department of Family Medicine, Western University, London, Ont.), Martine Couture BSc Inf MAP (Centre intégré universitaire de santé et de services sociaux du Saguenay-Lac-Saint-Jean, Chicoutimi, Que.), Paul W. Huras MSc MBA (South East Local Health Integration Network, Belleville, Ont.)
References
- 1.Nutting PA, Dickinson WP, Dickinson LM, et al. Use of chronic care model elements is associated with higher-quality care for diabetes. Ann Fam Med. 2007;5:14–20. doi: 10.1370/afm.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294:716–24. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 3.Fortin M, Soubhi H, Hudon C, et al. Multimorbidity's many challenges. BMJ. 2007;334:1016–7. doi: 10.1136/bmj.39201.463819.2C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du S, Yuan C. Evaluation of patient self-management outcomes in health care: a systematic review. Int Nurs Rev. 2010;57:159–67. doi: 10.1111/j.1466-7657.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 5.Hutchison B. A long time coming: primary healthcare renewal in Canada. Healthc Pap. 2008;8:10–24. doi: 10.12927/hcpap.2008.19704. [DOI] [PubMed] [Google Scholar]
- 6.Ryan BL, Stewart M. Environmental scan of primary care-linked chronic disease prevention and management programs in Ontario. Report to the Canadian Institutes of Health Research Community-based Primary Health Care Innovation Team, Patient-Centered Innovations for Persons with Multimorbidity. London (ON): Western University. 2012. [Google Scholar]
- 7.Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 8.van den Akker M, Muth C. How common is multimorbidity? In: Mercer SW, Salisbury C, Fortin M, editors. ABC of multimorbidity. Chichester (UK): BMJ Books (Wiley Blackwell) 2014. pp. 5–7. [Google Scholar]
- 9.Terner M, Reason B, McKeag AM, et al. Chronic conditions more than age drive health system use in Canadian seniors. Healthc Q. 2011;14:19–22. doi: 10.12927/hcq.2011.22485. [DOI] [PubMed] [Google Scholar]
- 10.How Canada compares: results from the Commonwealth Fund 2015 International Health Policy Survey of Primary Care Physicians. Ottawa: Canadian Institute for Health Information. 2016. [Google Scholar]
- 11.Asada Y, Kephart G. Equity in health services use and intensity of use in Canada. BMC Health Serv Res. 2007;7:41. doi: 10.1186/1472-6963-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyd CM, Fortin M. Future of multimorbidity research: How should understanding of multimorbidity inform health system design? Public Health Rev. 2010;32:451–74. [Google Scholar]
- 13.Fortin M, Stewart M, Poitras ME, et al. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med. 2012;10:142–51. doi: 10.1370/afm.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fortin M, Bravo G, Hudon C, et al. Relationship between multimorbidity and health-related quality of life of patients in primary care. Qual Life Res. 2006;15:83–91. doi: 10.1007/s11136-005-8661-z. [DOI] [PubMed] [Google Scholar]
- 15.Lorig KR, Ritter P, Stewart AL, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care. 2001;39:1217–23. doi: 10.1097/00005650-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Tackling Europe's major diseases: the challenges and the solutions. Fact sheet EURO/03/06 (11 September). Copenhagen: WHO Regional Office for Europe. 2006. [Google Scholar]
- 17.Stange KC. The generalist approach. Ann Fam Med. 2009;7:198–203. doi: 10.1370/afm.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith SM, Wallace E, O'Dowd T, et al. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev. 2016;3:CD006560. doi: 10.1002/14651858.CD006560.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin SJ, Kinmonth AL, Veltman MW, et al. Effect on health-related outcomes of interventions to alter the interaction between patients and practitioners: a systematic review of trials. Ann Fam Med. 2004;2:595–608. doi: 10.1370/afm.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart M, Ryan BL, Bodea C. Is patient-centred care associated with lower diagnostic costs? Healthc Policy. 2011;6:27–31. [PMC free article] [PubMed] [Google Scholar]
- 21.Mercer SW, Jani BD, Maxwell M, et al. Patient enablement requires physician empathy: a cross-sectional study of general practice consultations in areas of high and low socioeconomic deprivation in Scotland. BMC Fam Pract. 2012;13:6. doi: 10.1186/1471-2296-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLean G, Guthrie B, Mercer SW, et al. General practice funding underpins the persistence of the inverse care law: cross-sectional study in Scotland. Br J Gen Pract. 2015;65:e799–805. doi: 10.3399/bjgp15X687829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith SM, Allwright S, O'Dowd T. Effectiveness of shared care across the interface between primary and specialty care in chronic disease management. Cochrane Database Syst Rev. 2007:CD004910. doi: 10.1002/14651858.CD004910.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Creswell JW. Research design: qualitative, quantitative, and mixed methods approaches. 2nd ed. Thousand Oaks (CA): Sage Publications. 2003. [Google Scholar]
- 25.Sandelowski M. Whatever happened to qualitative description? Res Nurs Health. 2000;23:334–40. doi: 10.1002/1098-240x(200008)23:4<334::aid-nur9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 26.Kuzel AJ. Sampling in qualitative inquiry. In: Crabtree BF, Miller WL, editors. Doing qualitative research. Thousand Oaks (CA): Sage Publications. 1999. [Google Scholar]
- 27.Patton MQ. Qualitative research & evaluation methods. 3rd ed. Thousand Oaks (CA): Sage Publication. 2002. [Google Scholar]
- 28.Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholson K, Terry AL, Fortin M, et al. Examining the prevalence and patterns of multimorbidity in Canadian primary healthcare: a methodologic protocol using a national electronic medical record database. J Comorbidity. 2015;5:150–61. doi: 10.15256/joc.2015.5.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Browne GB, Arpin K, Corey P, et al. Individual correlates of health service utilization and the cost of poor adjustment to chronic illness. Med Care. 1990;28:43–58. doi: 10.1097/00005650-199001000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Moher D, Hopewell S, Schulz KF, et al. Consolidated Standards of Reporting Trials Group. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol. 2010;63:e1–37. doi: 10.1016/j.jclinepi.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Richards M, Ramond-Roquin A, Fortin M. Validation of the MM21 questionnaire developed for the measurement of multimorbidity [poster]. North American Primary Care Research Group annual meeting; Colorado Springs (CO).2016 Nov. 12. [Google Scholar]
- 33.Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49:796–804. [PubMed] [Google Scholar]
- 34.Stewart M, Brown JB, Hammerton J, et al. Improving communication between doctors and breast cancer patients. Ann Fam Med. 2007;5:387–94. doi: 10.1370/afm.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart M, Meredith L, Ryan BL, et al. The patient perception of patient-centeredness questionnaire (PPPC) no 04-1. London (ON): Western University. 2004. [Google Scholar]
- 36.Coleman EA, Mahoney E, Parry C. Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure. Med Care. 2005;43:246–55. doi: 10.1097/00005650-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Nolte S, Elsworth GR, Sinclair AJ, et al. The extent and breadth of benefits from participating in chronic disease self-management courses: a national patient-reported outcomes survey. Patient Educ Couns. 2007;65:351–60. doi: 10.1016/j.pec.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 38.Sherer M, Maddux JE, Mercandante B, et al. The Self-Efficacy Scale: construction and validation. Psychol Rep. 1982;51:663–71. [Google Scholar]
- 39.Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary steps of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Räsänen P, Roine E, Sintonen H, et al. Use of quality-adjusted life years for the estimation of effectiveness of health care: a systematic literature review. Int J Technol Assess Health Care. 2006;22:235–41. doi: 10.1017/S0266462306051051. [DOI] [PubMed] [Google Scholar]
- 41.Kessler RC, Barker PR, Colpe LJ, et al. Screening for serious mental illness in the general population. Arch Gen Psychiatry. 2003;60:184–9. doi: 10.1001/archpsyc.60.2.184. [DOI] [PubMed] [Google Scholar]
- 42.Behavioral Risk Factor Surveillance System survey questionnaire. Atlanta: Centers for Disease Control and Prevention, US Department of Health and Human Services. 2007. [Google Scholar]
- 43.Daniel WW, Cross CL. Biostatistics: a foundation for analysis in the health sciences. 9th ed. Hoboken (NJ): Wiley. 2009. [Google Scholar]
- 44.Van Breukelen GJ. ANCOVA versus change from baseline: more power in randomized studies, more bias in nonrandomized studies [corrected]. J Clin Epidemiol. 2006;59:920–5. doi: 10.1016/j.jclinepi.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 45.Canadian Hospital Reporting Project (CHRP) Ottawa: Canadian Institute for Health Information. 2012. [accessed 2016 Mar. 14]. Available https://secure.cihi.ca/free_products/HI2013_Jan30_EN.pdf.
- 46.Indicator library - cost of a standard hospital stay. Ottawa: Canadian Institute for Health Information. 2016. [accessed 2016 Mar. 30]. Available http://indicatorlibrary.cihi.ca/pages/viewpage.action?pageId=1114237.
- 47.Patient cost estimator. Ottawa: Canadian Institute for Health Information. 2016. [accessed 2016 Mar. 30]. Available https://www.cihi.ca/en/spending-and-health-workforce/spending/patient-cost-estimator.
- 48.Wodchis WP, Bushmeneva K, Nikitovic M, et al. Guidelines on person-level costing using administrative databases in Ontario. Working Paper Series vol. 1. Toronto: Health System Performance Research Network. 2013. [Google Scholar]
- 49.Drummond MF, Sculpher MJ, Torrance G, et al. Methods for the economic evaluation of health care programmes. 3rd ed. New York: Oxford University Press. 2005. [Google Scholar]
- 50.Cohen J. Statistical power analysis for the behavioural sciences. 2nd ed. Illsdale (NJ): Academic Press. 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.