Abstract
Alzheimer’s disease (AD) is an age-related neurodegenerative disorder characterized by aggregation of toxic forms of amyloid β peptide (Aβ). Treatment strategies have largely been focused on inhibiting the enzymes (β- and γ-secretases) that liberate Aβ from the amyloid precursor protein (APP). While evidence suggests that individuals who exercise regularly are at reduced risk for AD and studies of animal models demonstrate that running can ameliorate brain Aβ pathology and associated cognitive deficits, the underlying mechanisms are unknown. However, considerable evidence suggests that brain-derived neurotrophic factor (BDNF) mediates beneficial effects of exercise on neuroplasticity and cellular stress resistance. Here we tested the hypothesis that BDNF promotes non-amyloidogenic APP processing. Using a transgenic mouse model of Alzheimer’s disease and cultured human neural cells, we demonstrate that exercise and BDNF reduce production of toxic Aβ peptides through a mechanism involving enhanced α-secretase processing of APP. This anti-amyloidogenic APP processing involves subcellular redistribution of α-secretase and an increase in intracellular neuroprotective APP peptides capable of binding and inhibiting BACE1. Moreover, our results suggest that BDNF’s ability to promote neurite outgrowth is primarily exerted through pathways other than APP processing. Exercise and other factors that enhance BDNF signaling may therefore have both therapeutic and prophylactic value in the battle against AD.
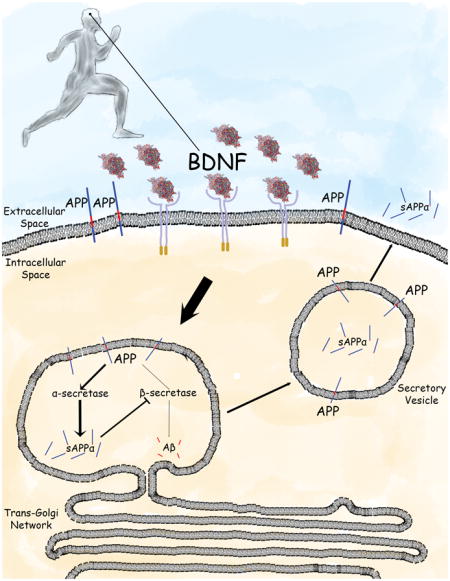
Graphical Abstract
The findings of Nigam et al. demonstrate that running wheel exercise and brain-derived neurotrophic factor (BDNF) enhance cleavage of the β-amyloid precursor protein (APP) by α-secretase to generate secreted APPα (sAPPα) in neurons. This enzymatic processing of APP occurs in an intracellular compartment where sAPPα also inhibits β-secretase, thereby preventing production of the neurotoxic amyloid β-peptide. These findings suggest a novel mechanism whereby exercise may protect the brain against Alzheimer’s disease by BDNF-mediated non-amyloidogenic processing of APP.
INTRODUCTION
Several neurodegenerative diseases, including Huntington’s, Parkinson’s and Alzheimer’s diseases are marked by accumulation of self-aggregating neurotoxic proteins (Stranahan and Mattson 2010). In Alzheimer’s disease (AD), the production of aggregation-prone amyloid β-peptide (Aβ) is thought to be an early event that promotes synaptic dysfunction, neuritic degeneration, and neuronal death (Mucke and Selkoe 2012). Accordingly, major efforts have been placed on understanding mechanisms that regulate amyloidogenic proteolytic processing of the amyloid precursor protein (APP). APP is a single-pass transmembrane protein that is transported into neurites and present in relatively high amounts in plasma and endocytic membranes (Cirrito et al. 2008). APP is cleaved by three enzymes, α-, β- and γ-secretases. Cleavage by α-secretase (ADAM10) occurs at the membrane surface within the Aβ sequence to release a secreted form of APP (sAPPα), which is believed to modulate neuronal excitability and neurite outgrowth (Chow et al. 2010). Sequential cleavages by β-secretase (BACE1) and γ-secretase (presenilin-1) at the N- and C-termini of the Aβ sequence liberates Aβ (Aβ40 and Aβ42). Efforts to develop disease-modifying treatments for AD treatment have centered largely on drugs that inhibit γ- or β-secretases, and removal of Aβ using “immunotherapy” approaches (De Strooper and Chavez Gutierrez 2015; Heneka et al. 2015). However, clinical trials using such approaches have failed. While it is known that cleavage of APP by α-secretase prevents Aβ generation and that sAPPα is neuroprotective (Mattson et al. 1993; Furukawa et al. 1996; Obregon et al. 2012), little is known of the signaling pathways that may stimulate α-secretase cleavage of APP.
Epidemiological findings suggest that regular exercise can reduce the risk of AD, and studies of mouse models of AD have demonstrated that running wheel exercise can lessen Aβ accumulation and ameliorate cognitive deficits (Adlard et al. 2005; Mattson 2012). The underlying cellular and molecular mechanisms are unknown. Exercise enhances synaptic plasticity (synapse formation and increased functional connectivity) and can stimulate neurogenesis (generation of neurons from stem cells) in the hippocampus, a brain region critical for learning and memory that is heavily affected by Aβ pathology and neuronal degeneration in AD (van Praag et al. 2014). Moreover, exercise can protect neurons against excitotoxic and metabolic stress in experimental models of relevance to AD (Reiss et al. 2009; Lau et al. 2011; Cheng et al. 2016). A large body of evidence suggests that brain-derived neurotrophic factor (BDNF) plays major roles in adaptive responses of neuronal circuits to exercise including neurogenesis, synapse formation, learning and memory, and neuronal stress resistance (Intlekofer and Cotman 2013; Marosi and Mattson 2014). Mature BDNF is generated by proteolytic cleavage of a longer pro-BDNF protein by a furin protease or plasmin. A polymorphism located at amino acid 66 in pro-BDNF (Val66Met), which may influence the amount of mature BDNF produced, may influence the risk of several major psychiatric disorders, including depression and eating disorders (Notaras et al., 2015). Levels of mature BDNF and its high affinity receptor TrkB are decreased in brain tissue samples of AD patients compared to age-matched control subjects (Hock et al. 2000; Ginsberg et al. 2006). It was also reported that BDNF can affect subcellular trafficking of APP (Rohe et al. 2009) and that inhibition of BDNF signaling increases Aβ generation (Matrone et al. 2008). However, it is not known whether exercise and BDNF affect APP processing in a manner that would be expected to reduce Aβ production and protect neurons against dysfunction and degeneration.
In the present study, we show that running wheel exercise increases the levels of sAPPα and BDNF, with associated reductions in levels of Aβ40 and Aβ42, in the hippocampus of APP/presenilin 1 double mutant transgenic mice. To test the possibility that BDNF mediates the effects of exercise on APP processing, we measured levels of sAPPα and Aβs in cultured human neuroblastoma cells exposed directly to BDNF and found that BDNF reduces Aβ peptides by enhancing α-secretase processing of APP. BDNF does not increase the expression of ADAM10 but instead causes a redistribution of ADAM10 away from the cell surface. Additional findings suggest that the ability of BDNF to induce neurite outgrowth is independent of its effects on α-secretase. Taken together, these results suggest that BDNF-mediated enhancement of non-amyloidogenic processing of APP may be one mechanism whereby exercise protects the brain against AD.
METHODS
Animals
Male APP/PS1 double mutant transgenic mice (2xTg AD mice; B6C3-Tg(APPswe,PS1Δ9)85Dbo) (Borchelt et al. 1997) were obtained from Jackson Laboratories (Bar Harbor, ME), and were maintained under a 12 h light/12 h dark cycle with food and water available ad libitum. Once aged 7–9 months, mice were screened for 48 hours to identify their propensity to participate in voluntary running on a running wheel. Mice were then randomly assigned to cages where they were housed individually either with access to a functional running wheel (runners) or with a locked running wheel (sedentary controls). After 3 weeks, mice were euthanized with an overdose of isoflurane anesthesia. The hippocampus was isolated, flash frozen on dry ice and subsequently kept at −80°C. All procedures were approved by the National Institute on Aging Animal Care and Use Committee and complied with NIH guidelines.
Antibodies and recombinant proteins
The following antibodies were used: anti-sAPPα 2B3 (IBL, Gunma, Japan; RRID:AB_1630819) and anti-ADAM10 (Santa Cruz Biotechnology, Santa Cruz, CA; RRID:AB_626635). The following recombinant proteins were used: human mature BDNF (Preprotech, Rocky Hill, NJ) and human sAPPα (Sigma Aldrich, St. Louis, MO). Note that we used mature BDNF and not pro-BDNF, and therefore the Val66Met polymorphism has no bearing on our findings.
Cell Culture
The human neuroblastoma cell line, SHSY5Y (American Type Culture Collection, Manassas, VA; catalog #CRL-2266), was cultured in Dulbecco’s modified Eagle medium (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, and 10 μM retinoic acid (RA). After 2 days, some cultures were further supplemented with 50 ng/mL BDNF and/or 5 μM batimastat (Sigma Aldrich). Cultures were maintained for an additional 3 days before harvesting cells and media. For neurite outgrowth experiments, differentiation was achieved as previously described (Encinas et al. 2000) by sequentially treating cells with retinoic acid for 2 days followed by treatment with BDNF for 5 days in serum-free media. Cells were maintained at 37°C in a saturated humidity incubator containing 5% CO2.
Biochemistry
Measurement of Aβ40, Aβ42 and sAPPα in cell culture media, cell lysates and mouse tissue lysates were performed using Mesoscale Discovery (MSD) immunoassays on a Quickplex SQ 120 (Mesoscale Discovery). The MSD multiplex ELISA platform is based on electrochemiluminescence enabling high sensitivity, specificity and a wide dynamic range. Measurement of BDNF in mouse tissue lysates was performed using BDNF ELISA immunoassays (Promega, Madison, WI) on a Synergy H1 multiplate reader (Biotek, Winooski, VT). All experiments were performed according to the manufacturer’s suggested protocol. Lysates were prepared in RIPA buffer supplemented with protease inhibitor cocktail (Sigma Aldrich) and 1 mM PMSF. Protein concentration was determined by Pierce BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA). Western blots were performed by running lysates on 10% Bis-Tris Gels using NuPage MOPS SDS running buffer (Thermo Fisher Scientific). Gels were then transferred onto 0.45 μm pore-size nitrocellulose membranes (Thermo Fisher Scientific). Membranes were incubated with Pierce ECL Western Blotting substrate (Thermo Fisher Scientific) and developed onto autoradiography film. Densitometric analysis was performed using ImageJ.
Flow Cytometry
SHSY5Y cells were harvested, pelleted and suspended in 5% NGS in PBS at a concentration of 2.0×107 cells/ml. Cell surface quantification of ADAM10 was performed using flow cytometry. Briefly, cells were pelleted and suspended in primary antibody solution for 30 minutes at 4°C followed by secondary antibody solution for 30 minutes at 4°C. Thereafter, cells were suspended in 500 μl of 2% NGS in PBS and passed through a 100 μm cell strainer before being analyzed on an LSRFortessa X-20 (BD Biosciences). For each sample, 500,000 cells were analyzed. Data analysis was performed using FlowJo software.
Immunocytochemistry
SHSY5Y cells were seeded at 25,000 cells/cm2 in tissue culture plates (BD Biosciences, San Jose, CA) and cultured as described above. Cells were fixed for 15 minutes in PBS containing 4% paraformaldehyde. After washing with PBS, cells were incubated for 1 hour with 5% normal goat serum in PBS. Cells were then incubated with primary antibody solution overnight at 4°C followed by secondary antibody solution for 1 hour at room temperature. Cells were treated with Hoechst 33342 dye (Life Technologies, Carlsbad, CA) for 5 minutes and washed with PBS. Images were acquired and analyzed using a fluorescence microscope system with ImageXpress software (Molecular Devices, Sunnyvale, CA).
BACE1 inhibition assay
Inhibition of BACE1 was evaluated using the Sensolyte 520 β-secretase activity assay (Anaspec, CA). Purified BACE1 was incubated with a FRET peptide containing a sequence derived from the Swedish APP mutation. As such, BACE1 has a preferential high affinity for the peptide. The peptide sequence is flanked by a HiLyte Fluorophore 488 nm and a QXL 520 nm quencher. Upon cleavage of the substrate, the 488 nm fluorophore was recovered and measured in a Synergy H1 plate reader (Biotek).
Neurite outgrowth assay
SHSY5Y cells were seeded at a density of 15,000 cells/cm2 and sequentially differentiated as described above. Cells were then stained with the Molecular Probes Neurite Outgrowth Staining kit (Thermo Fisher Scientific) according to the manufacturer’s suggested protocol. Briefly, media was removed from cell culture plates and cells were fluorescently stained with both a cell viability marker and a cell membrane marker. A background suppression dye was added to aid in visualization. Fluorescence was then measured using a fluorescence plate reader (Biotek). Cell membrane measurements were normalized to cell viability.
Statistics
Unless otherwise noted, statistical significance was calculated using Student’s t-test. Data values are given as mean ± SEM.
RESULTS
Running wheel exercise reduces Aβ levels and increases sAPPα levels in the hippocampus of 2xTgAD mice
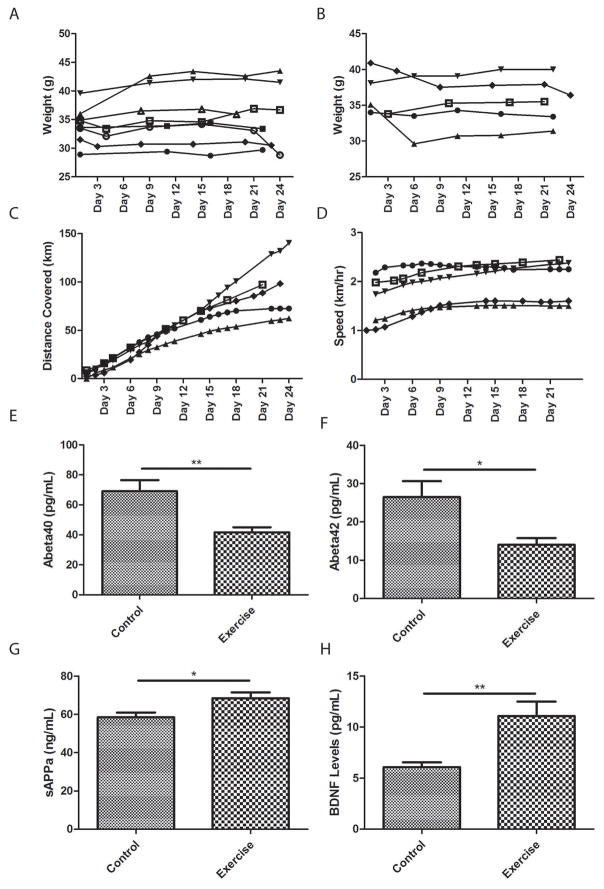
To test the role that exercise has on APP processing, 2xTgAD mice were housed individually with access to a running wheel for 3 weeks. These mice were compared to non-exercising control 2xTgAD mice whose running wheel was locked. Throughout the duration of the study, there was no difference in body weight between control (Figure 1A) and exercising mice (Figure 1B). There was a linear increase in the cumulative distance run by each mouse during the 3 week period (Figure 1C) and their average daily running speed remained stable (Figure 1D). As the hippocampus is particularly vulnerable to Aβ pathology, we measured Aβ40 and Aβ42 levels in hippocampal tissue from runner mice and sedentary mice after 3 weeks. Using the highly sensitive and specific MSD multiplex ELISA system, we found that Aβ40 and Aβ42 levels were significantly reduced in runner mice as compared to sedentary control mice (Figure 1E and F). These results demonstrate that even a relatively short 3-week period of exercise is sufficient to reduce Aβ levels in 2xTgAD mice. Moreover, we measured sAPPα levels in the same hippocampal samples and found that running resulted in a significant increase in sAPPα levels (Figure 1G). Taken together, these results suggest that exercise shifts the processing of APP from the amyloidogenic to the non-amyloidogenic pathway.
Figure 1. Exercise shifts APP processing towards the non-amyloidogenic pathway.
A and B. Body weights of APP/PS1 (2xTgAD) mice that were housed individually with either locked running wheels (A) or working running wheels (B). C and D. In runner mice, the cumulative distance ran (C) and average speed (D) were measured. E, F, G. Using the Mesoscale multiplex ELISA system, levels of hippocampal Aβ40 (E), Aβ42 (F) and sAPPα (G) were determined in both non-exercising control 2xTgAD mice and runner 2xTgAD mice. H. Hippocampal BDNF levels were determined by ELISA. (n = 5–8 mice/group). *p<0.05, **p<0.01 as determined by Student’s t-test.
BDNF decreases Aβ levels through a mechanism involving enhanced α-secretase activity
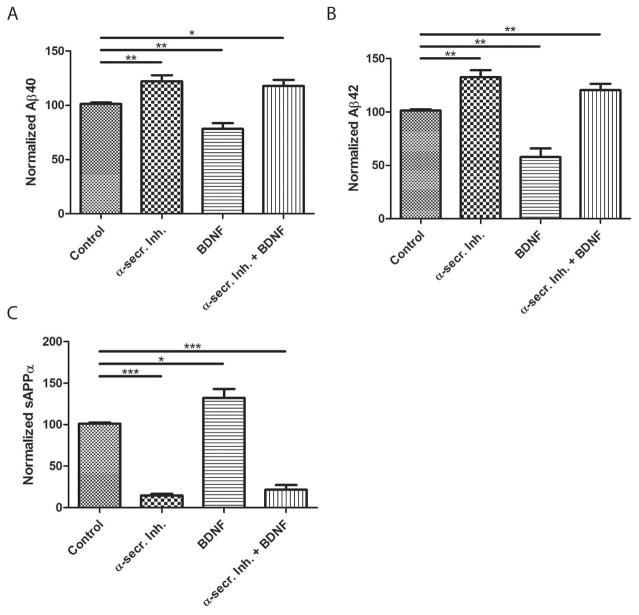
Although exercise can counteract neurodegenerative processes in animal models of AD, the underlying cellular and molecular mechanisms are unknown. One mechanism supported by data from previous studies involves up-regulation of BDNF expression (Mattson 2012; Marosi and Mattson 2014; Sleiman et al. 2016). We found that BDNF levels were significantly elevated by approximately 2-fold in hippocampus of runner compared to sedentary 2xTgAD mice (Figure 1H). To test whether BDNF decreases Aβ levels by modulating α-secretase activity, we used the inhibitor of α-secretase, batimastat (Parvathy et al. 1998). RA-differentiated human SHSY5Y neural cells were cultured in serum-supplemented media and subsequently treated with batimastat, BDNF or the combination of batimastat and BDNF. Levels of Aβ40, Aβ42 and sAPPα secreted into the culture medium were measured. Treatment with batimastat resulted in a significant increase in the generation of Aβ40 and Aβ42 compared to untreated controls. Consistent with the in vivo experiments, treatment of cultured cells with BDNF led to a significant reduction in both secreted Aβ40 and Aβ42 compared to control cultures. Importantly, when cultured neural cells were co-treated with batimastat and BDNF, levels of Aβ40 and Aβ42 secreted into the medium remained significantly higher than in control cultures (Figure 2A,B). Thus, it appears that BDNF reduces Aβ levels through a mechanism involving enhanced α-secretase activity. To further test this hypothesis, sAPPα levels were measured in culture media. As expected, treatment with batimastat significantly reduced sAPPα levels compared to control cultures. Conversely, BDNF treatment led to a significant increase in sAPPα levels compared to controls. In cultures co-treated with batimastat and BDNF, sAPPα levels remained significantly reduced compared to control (Figure 2C). Taken together, these results indicate that BDNF likely reduces Aβ levels by enhancing α-secretase activity.
Figure 2. BDNF decreases the amount of Aβ released from human neural cells in an α-secretase-dependent manner.
SHSY5Y cells were treated with the α-secretase inhibitor (α-secr.Inh.) batimastat (5 μM), BDNF (50 ng.ml) or a combination of batimastat and BDNF. A–C. Levels of Aβ40 (A), Aβ42(B) and (C) sAPPα (C) released into the culture medium during a 3 day incubation period were measured using the Mesoscale multiplex ELISA system (n = 3 separate cultures per condition). *p<0.05, **p<0.01, ***p<0.001 as determined by Student’s t-test.
BDNF reduces cell-surface ADAM10 levels
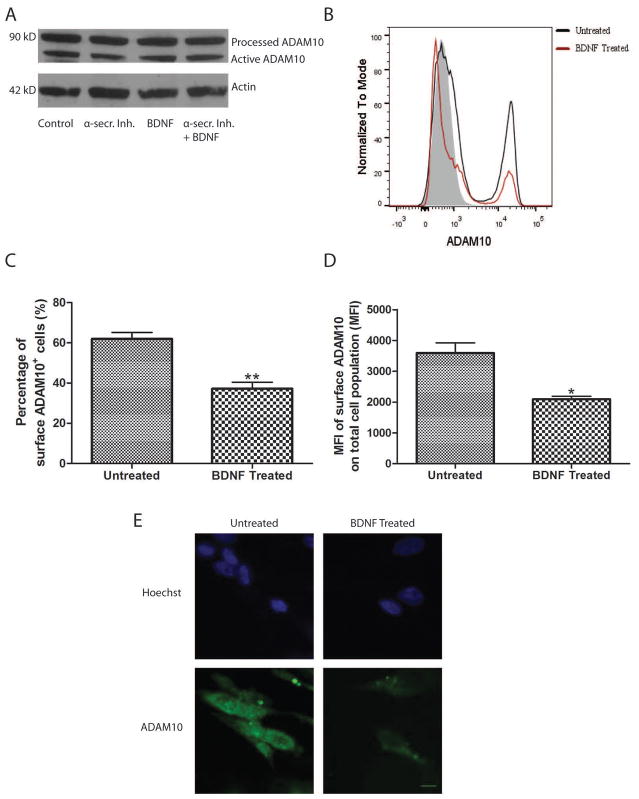
The increase in sAPPα levels upon BDNF treatment indicates an enhancement of α-secretase activity. To test the possibility that an increase in α-secretase expression drives this enhancement in activity, immunoblots were performed. ADAM10, the main active component of α-secretase (Kuhn et al. 2010), was assessed in RA-differentiated SHSY5Y cells treated with batimastat, BDNF, batimastat plus BDNF, or vehicle control. In each case, there was no difference in the levels of processed or active ADAM10 (Figure 3A). α-secretase processing is thought to primarily occur at the cell surface (Chow et al. 2010). To test the hypothesis that BDNF increases the amount of cell surface ADAM10, cells were cultured either with or without BDNF and assessed by flow cytometry for their cell surface-associated ADAM10 (Figure 3B). To our surprise, BDNF significantly reduced the percentage of cells expressing ADAM10 on their surface when compared to control cultures (Figure 3C). Moreover, the amount of ADAM10 (as indicated by mean fluorescence intensity) was significantly reduced on the surface of the total cell population (Figure 3D). In addition to these quantitative experiments, immunocytochemical labeling was also performed. Representative images are shown (Figure 3E). Taken together, these results indicate that the distribution of ADAM10 upon BDNF treatment is shifted towards intracellular accumulation. This finding is consistent with studies that suggest that regulated α-secretase activity occurs intracellularly (Skovronsky et al. 2000).
Figure 3. BDNF reduces cell-surface ADAM10 levels.
A. Intracellular ADAM10 levels are unaffected after BDNF treatment. Cell lysates from SHSY5Y cell cultures treated with α-secretase inhibitor (α-secr.Inh.) (5 μM batimastat), BDNF (50 ng/ml) or a combination of batimastat and BDNF for 72 hours were analyzed by immunoblot using an ADAM10 antibody. Actin was used as a loading control. A representative blot is shown (n = 3 separate cultures). B. Representative flow cytometry plot for cell-surface ADAM10 labeling. Negative staining control is shown in gray. Untreated cells are shown in black and BDNF-treated cells are shown in red. C and D. Results of quantification of the percentage of surface ADAM10+ cells out of the total cell population (C) and the mean fluorescence intensity (MFI) of surface ADAM10 (D) out of the total cell population measured by flow cytometry in untreated and BDNF-treated SHSY5Y cells (n = 3 separate cultures). E. Representative images of immunocytochemical ADAM10 surface labeling in untreated and BDNF-treated SHSY5Y (n= 3 separate cultures). *p<0.05, **p<0.01 as determined by Student’s t-test. Scale bar = 10 μm.
BDNF may regulate BACE1 activity via its effect on APP processing
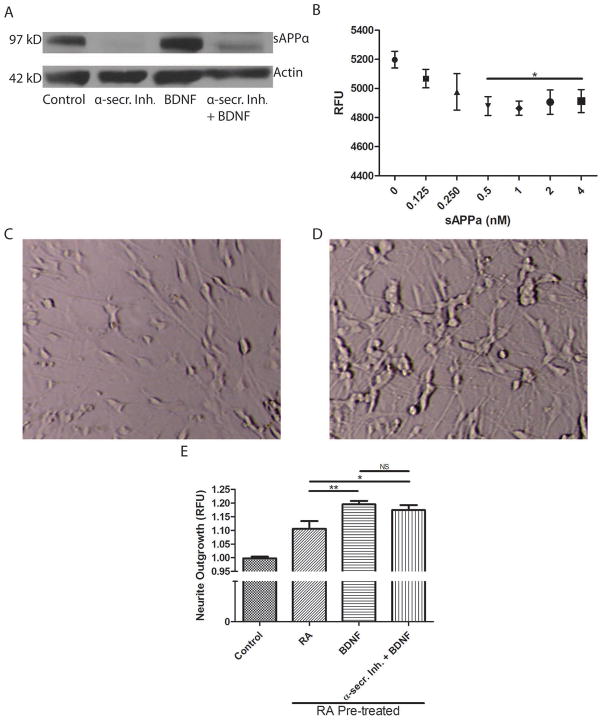
When taken together with increased secretion of sAPPα into the culture medium, the redistribution of ADAM10 upon BDNF treatment suggests that α-secretase cleavage occurs intracellularly. Using a specific sAPPα antibody, non-secreted sAPPα levels from cell lysates were measured by immunoblot (Figure 4A). Consistent with MSD measurements of sAPPα secreted in media, sAPPα levels were reduced in cell lysates from cultures treated with batimastat compared to control cultures. Notably, BDNF-treated cell cultures exhibited increased sAPPα levels in their lysates compared to control (12.5% increase; p<0.05). Co-treatment with batimastat and BDNF resulted in reduced sAPPα levels compared to control. sAPPα levels were higher in cells co-treated with batimastat and BDNF compared to cells treated only with batimastat, although the sAPPα levels did not reach the level in cells treated with BDNF alone (Figure 4A).
Figure 4. BDNF treatment produces sAPPα that inhibits BACE1, but sAPPα does not mediate BDNF-induced neurite outgrowth.
A. Cell lysates from SHSY5Y cell cultures treated with α-secretase inhibitor (α-secr.Inh.) (5 μM batimastat), 50 ng/ml BDNF or a combination of batimastat and BDNF for 72 hours were assessed by immunoblot for their cellular sAPPα levels. Actin was used as a loading control. A representative blot is shown (n = 3 separate cultures). B. A FRET-based BACE1 activity assay was used to determine the inhibitory capacity of recombinant sAPPα. Reduction in relative fluorescence units (RFU) indicates a reduced ability of BACE1 to cleave APP peptides harboring the APP Swedish mutation (n = 3 separate cultures). One-way ANOVA revealed statistically significant differences between group means (F(6,14)= 7.23, p = 0.001). Furthermore, Bonferroni-corrected t-test revealed significant differences between control and recombinant sAPPα treatment at the concentrations denoted by * (p<0.0083). Negative control resulted in fluorescence at 4400 RFU (not shown). C and D. Representative transmitted light micrographs of SHSY5Y cells in a culture treated for 7 days with vehicle (C) or treated sequentially with retinoic acid (RA) for 2 days followed by BDNF for 5 days. Note the robust neurite outgrowth in cells differentiated with RA plus BDNF compared to the control culture. E. Using a validated fluorescence assay, neurite outgrowth was determined in vehicle-treated control cultures, RA-treated cultures, BDNF-treated cultures, and cultures treated with batimastat plus BDNF. The control value was significantly different than each of the other three values (p<0.001; n = 25–50 wells in 3 separate experiments). *p<0.05, **p<0.01 as determined by Student’s t-test.
The physiological functions of secreted sAPPα are thought to include neuroprotection, neurite outgrowth, synaptogenesis and cell adhesion. However, the function of intracellular sAPPα is poorly understood. Recent evidence suggests that sAPPα may be involved in direct inhibition of BACE1 (Obregon et al. 2012). To confirm this possibility, an in vitro BACE1 activity assay was performed using recombinant sAPPα. Recombinant sAPPα inhibited BACE1 activity in a dose-dependent manner until a plateau was reached at a concentration of 0.5 nM (Figure 4B). This result indicates that sAPPα can inhibit BACE1 independently (i.e. without helper molecules or cofactors). Indeed, BDNF-treated cell cultures secreted less sAPPβ into the culture media compared to control (18% decrease; p<0.05). This finding supports the notion that BDNF may reduce amyloidogenic processing of APP through sAPPα.
BDNF promotes neurite outgrowth primarily through pathways other than APP processing
Whether BDNF promotes neurite outgrowth through enhanced sAPPα production is unclear. To test this hypothesis, SHSY5Y cells were sequentially differentiated with RA followed by BDNF in serum-free media. This sequential process yields a homogenous population of neuronally differentiated cells (Encinas et al. 2000). These differentiated cells had a visible increase in neurite outgrowth compared to untreated control cultures (Figure 4C, D). RA-differentiated, BDNF-differentiated and BDNF-differentiated cells co-treated with batimastat all had significantly more neurite outgrowth compared to control cultures as determined by a validated neurite outgrowth assay (Yeyeodu et al. 2010; Hancock et al. 2015) (Figure 4E). Moreover, BDNF treatment resulted in a significant increase in neurite outgrowth compared to cultures treated only with retinoic acid. Cultures co-treated with BDNF and batimastat also exhibited significantly more neurite outgrowth than cultures treated only with retinoic acid. Accordingly, there was no significant difference between BDNF-differentiated cultures and cultures co-treated with BDNF and batimastat (Figure 4E). This indicates that there are likely multiple mechanisms by which BDNF promotes neurite outgrowth and enhancement of α-secretase activity is likely only a minor contributor.
DISCUSSION
We found that 3 weeks of voluntary exercise resulted in reduced Aβ40 and Aβ42 levels in the hippocampus of 2xTgAD mice. This is consistent with previous studies that show that exercise can lead to reductions in soluble Aβ levels in other mice models of AD (Adlard et al. 2005; Um et al. 2008). A recent study indicated that exercise reduces Aβ through enhanced clearance (Moore et al. 2016). However, we found that running wheel exercise results in increased levels of sAPPα in the hippocampus, which was associated with reduced levels of Aβ40 and Aβ42. This suggests that exercise can serve as a prophylactic by shifting the equilibrium of APP processing away from the amyloidogenic pathway to the nonamyloidogenic pathway. Although it is difficult to determine the mechanism by which exercise exerts this effect, our findings suggest that it involves BDNF, which is a prominent mediator of effects of exercise on hippocampal neuroplasticity (Marosi and Mattson 2014). Accordingly, we demonstrate that, in SHSY5Y neural cells, BDNF reduces Aβ40 and Aβ42 levels through its regulation of α-secretase activity. Moreover, BDNF was able to increase secreted sAPPα levels. These results agree well with the notion that exercise can shift the processing of APP to the non-amyloidogenic pathway.
Our findings in cultured neural cells suggest that BDNF promotes redistribution of α-secretase from the cell surface to intracellular compartments where it is catalytically active in processing APP. This finding is consistent with previous studies that show that regulated α-secretase cleavage takes place intracellularly, including in vesicles of the trans Golgi network (Skovronsky et al. 2000). The function of intracellular sAPPα is unclear, but our findings corroborate recent evidence which suggests that sAPPα can directly inhibit BACE1 activity (Obregon et al. 2012), which is predominantly intracellular (Vassar et al. 1999), active in acidic microenvironments (Hook et al., 2002) and is rapidly internalized from the cell surface (Huse et al. 2000).
BDNF has been proposed as a therapeutic in AD primarily due to its ability to promote neuronal survival and neurite outgrowth through its interaction with tropomyosin-related kinase B (TrkB) receptors and subsequent activation of various signaling pathways including ras, PI-3, ERK, PKA, and PLCγ/PKC (Patapoutian and Reichardt 2001; Lin et al. 2010; Elliott et al. 2005). While previous studies have shown that sAPPα can enhance neurite outgrowth (Mattson 1994; Williamson et al. 1996; Small et al. 1999), our findings in human neuroblastoma cells suggest that BDNF-induced neurite outgrowth occurs primarily through pathways other than sAPPα.
Intensive efforts have been placed on targeting secretases involved in amyloidogenic APP processing (β- and γ-secretases) for treatment of AD. Several of these efforts have resulted in adverse events and, ultimately, clinical failure (De Strooper and Chavez Gutierrez 2015). Because β- and γ-secretase both have multiple substrates other than APP, they may be unattractive targets for prophylaxis. Recent studies indicate that sAPPα participates in a positive feedback loop where it can regulate its own production by directly inhibiting BACE1 (Obregon et al. 2012). Strategies aimed at promoting this endogenous autoregulatory positive feedback loop may promote the non-amyloidogenic pathway for processing APP and thereby limit off-target adverse events seen with secretase inhibitors. Along with this BACE1 inhibitory capacity, sAPPα has neurotrophic and neuroprotective properties (Mattson et al. 1993). Indeed, AD patients have lower levels of sAPPα in their cerebrospinal fluid and this correlates with impaired cognition (Almkvist et al. 1997). Thus, sAPPα holds both prophylactic and therapeutic value.
Uncontrollable psychosocial stress, sleep deprivation and depression adversely affect cognition and may increase the risk of AD (Rothman and Mattson 2010). Studies of animal models suggest that chronic elevation of glucocorticoids and suppression of BDNF expression mediate, in part, the adverse effects of uncontrollable stress on hippocampal synaptic plasticity and cognition (Bennett and Lagopoulos 2014). Moreover, chronic psychosocial stress and sleep deprivation can accelerate the accumulation of Aβ in the brains of 3xTgAD mice (Rothman et al. 2012; Rothman et al. 2013). It is not known whether a reduction of BDNF signaling contributes to the amyloidogenic processing of APP caused by chronic stress. Housing mice singly rather than in small groups can be considered a mild isolation stress and might, therefore, be expected to reduce BDNF expression and exacerbate amyloidogenesis. Consistent, with this possibility, it has been reported that environmental enrichment increases hippocampal BDNF expression, reduces Aβ accumulation and improves cognitive performance in AD mouse models (Hu et al. 2010; Stuart et al. 2016). Moreover, enrichment increases α-secretase activity in the brains of aged dogs (Pop et al. 2010). In contrast to adverse stressors, there is evidence that running wheel exercise in singly housed mice can protect the brain against a range of stressors including Aβ-peptide (Ryan and Kelly 2016). There is an extensive literature showing that voluntary running wheel exercise enhances hippocampal synaptic plasticity, neurogenesis and learning and memory in singly housed mice (for review see Voss et al. 2013). Running wheel exercise stimulates a robust increase in BDNF expression in multiple brain regions, and experimental evidence suggests that BDNF signaling via TrkB mediates many of the beneficial effects of exercise on the brain (Mattson 2012). Our findings suggest a role for BDNF in promoting exercise-induced α-secretase cleavage of APP to increase the production of neurotrophic sAPPα and reduce production of Aβ, which might counteract adverse psychological and age-related stressors.
In order to enhance generation of sAPPα, several studies have sought to identify factors which affect ADAM10, the main enzymatic component of α-secretase (Kuhn et al. 2010). These studies have found that SIRT1 overexpression (Theendakara et al. 2013), all-trans retinoic acid and acitretin (Tippmann et al. 2009) can increase expression of ADAM10. We find that BDNF can increase activity of α-secretase without altering ADAM10 levels. Because BDNF may have low blood-brain barrier penetration and has poor biostability (Song et al. 2015), it is important to identify factors that influence BDNF production in vivo. Exercise, which stimulates BDNF expression in brain cells, is therefore an attractive approach for increasing α-secretase cleavage of APP resulting in reduced generation of Aβ and simultaneous production of neuroprotective sAPPα.
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Institute on Aging.
List of abbreviations
- Aβ
amyloid β-peptide
- AD
Alzheimer’s disease
- ADAM10
a disintegrin and metalloproteinase domain-containing protein 10
- APP
β-amyloid precursor protein
- BACE1
β-secretase
- BDNF
brain-derived neurotrophic factor
- ECL
electrochemiluminescence
- ELISA
enzyme-linked immunosorbent assay
- ERK
extracellular signal regulated kinase
- FRET
fluorescence resonance energy transfer
- PBS
phosphate buffered saline
- sAPPα
PI-3, phosphatidylinositol 3
- PKA
protein kinase A
- PKC
protein kinase C
- PLC
phospholipase C; α-secretase-derived secreted form of APP
Footnotes
Conflict of interest statement: The authors declare no conflicts of interest.
References
- Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almkvist O, Basun H, Wagner SL, Rowe BA, Wahlund LO, Lannfelt L. Cerebrospinal fluid levels of alpha-secretase-cleaved soluble amyloid precursor protein mirror cognition in a Swedish family with Alzheimer disease and a gene mutation. Arch Neurol. 1997;54:641–644. doi: 10.1001/archneur.1997.00550170111022. [DOI] [PubMed] [Google Scholar]
- Bennett MR, Lagopoulos J. Stress and trauma: BDNF control of dendritic-spine formation and regression. Prog Neurobiol. 2014;112:80–99. doi: 10.1016/j.pneurobio.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–945. doi: 10.1016/s0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- Cheng A, Yang Y, Zhou Y, Maharana C, Lu D, Peng W, Liu Y, et al. Mitochondrial SIRT3 Mediates Adaptive Responses of Neurons to Exercise and Metabolic and Excitatory Challenges. Cell Metab. 2016;23:128–142. doi: 10.1016/j.cmet.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow VW, Mattson MP, Wong PC, Gleichmann M. An overview of APP processing enzymes and products. Neuromolecular Med. 2010;12:1–12. doi: 10.1007/s12017-009-8104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott E, Atlas R, Lange A, Ginzburg I. Brain-derived neurotrophic factor induces a rapid dephosphorylation of tau protein through a PI-3 Kinase signalling mechanism. Eur J Neurosci. 2005;22:1081–1089. doi: 10.1111/j.1460-9568.2005.04290.x. [DOI] [PubMed] [Google Scholar]
- Encinas M, Iglesias M, Liu Y, Wang H, Muhaisen A, Cena V, Gallego C, Comella JX. Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J Neurochem. 2000;75:991–1003. doi: 10.1046/j.1471-4159.2000.0750991.x. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Barger SW, Blalock EM, Mattson MP. Activation of K+ channels and suppression of neuronal activity by secreted beta-amyloid-precursor protein. Nature. 1996;379:74–78. doi: 10.1038/379074a0. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Che S, Wuu J, Counts SE, Mufson EJ. Down regulation of trk but not p75NTR gene expression in single cholinergic basal forebrain neurons mark the progression of Alzheimer’s disease. J Neurochem. 2006;97:475–487. doi: 10.1111/j.1471-4159.2006.03764.x. [DOI] [PubMed] [Google Scholar]
- Hancock M, Kopp L, Kaur N, Hanson BJ. A facile method for simultaneously measuring neuronal cell viability and neurite outgrowth. Curr Chem genomics Transl Med. 2015;9:6–16. doi: 10.2174/2213988501509010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, Khoury J, El Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock C, Heese K, Hulette C, Rosenberg C, Otten U. Region-specific neurotrophin imbalances in Alzheimer disease: decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch Neurol. 2000:57. doi: 10.1001/archneur.57.6.846. [DOI] [PubMed] [Google Scholar]
- Hu Y-S, Xu P, Pigino G, Brady ST, Larson J, Lazarov O. Complex environment experience rescues impaired neurogenesis, enhances synaptic plasticity, and attenuates neuropathology in familial Alzheimer’s disease-linked APPswe/PS1DeltaE9 mice. FASEB J Off Publ Fed Am Soc Exp Biol. 2010;24:1667–1681. doi: 10.1096/fj.09-136945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse JT, Pijak DS, Leslie GJ, Lee VM, Doms RW. Maturation and endosomal targeting of beta-site amyloid precursor protein-cleaving enzyme. The Alzheimer’s disease beta-secretase. J Biol Chem. 2000;275:33729–33737. doi: 10.1074/jbc.M004175200. [DOI] [PubMed] [Google Scholar]
- Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease. Neurobiol Dis. 2013;57:47–55. doi: 10.1016/j.nbd.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Kuhn P-H, Wang H, Dislich B, Colombo A, Zeitschel U, Ellwart JW, Kremmer E, Rossner S, Lichtenthaler SF. ADAM10 is the physiologically relevant, constitutive alpha-secretase of the amyloid precursor protein in primary neurons. EMBO J. 2010;29:3020–3032. doi: 10.1038/emboj.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau Y-S, Patki G, Das-Panja K, Le W-D, Ahmad SO. Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson’s disease with moderate neurodegeneration. Eur J Neurosci. 2011;33:1264–1274. doi: 10.1111/j.1460-9568.2011.07626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Shindel AW, Fandel TM, Bella AJ, Lin C-S, Lue TF. Neurotrophic effects of brain-derived neurotrophic factor and vascular endothelial growth factor in major pelvic ganglia of young and aged rats. BJU Int. 2010;105:114–120. doi: 10.1111/j.1464-410X.2009.08647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. 2014;25:89–98. doi: 10.1016/j.tem.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrone C, Ciotti MT, Mercanti D, Marolda R, Calissano P. NGF and BDNF signaling control amyloidogenic route and Abeta production in hippocampal neurons. Proc Natl Acad Sci U S A. 2008;105:13139–13144. doi: 10.1073/pnas.0806133105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Secreted forms of beta-amyloid precursor protein modulate dendrite outgrowth and calcium responses to glutamate in cultured embryonic hippocampal neurons. J Neurobiol. 1994;25:439–450. doi: 10.1002/neu.480250409. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16:706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Cheng B, Culwell AR, Esch FS, Lieberburg I, Rydel RE. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein. Neuron. 1993;10:243–254. doi: 10.1016/0896-6273(93)90315-i. [DOI] [PubMed] [Google Scholar]
- Moore KM, Girens RE, Larson SK, Jones MR, Restivo JL, Holtzman DM, Cirrito JR, Yuede CM, Zimmerman SD, Timson BF. A spectrum of exercise training reduces soluble Abeta in a dose-dependent manner in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2016;85:218–224. doi: 10.1016/j.nbd.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Mucke L, Selkoe DJ. Neurotoxicity of Amyloid β-Protein: Synaptic and Network Dysfunction. Cold Spring Harb Perspect Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notaras M, Hill R, van den Buuse M. The BDNF gene Val66Met polymorphism as a modifier of psychiatric disorder susceptibility: progress and controversy. Mol Psychiatry. 2015;20:916–930. doi: 10.1038/mp.2015.27. [DOI] [PubMed] [Google Scholar]
- Obregon D, Hou H, Deng J, Giunta B, Tian J, Darlington D, Shahaduzzaman M, et al. Soluble amyloid precursor protein-alpha modulates beta-secretase activity and amyloid-beta generation. Nat Commun. 2012;3:777. doi: 10.1038/ncomms1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvathy S, Hussain I, Karran EH, Turner AJ, Hooper NM. Alzheimer’s amyloid precursor protein alpha-secretase is inhibited by hydroxamic acid-based zinc metalloprotease inhibitors: similarities to the angiotensin converting enzyme secretase. Biochemistry. 1998;37:1680–1685. doi: 10.1021/bi972034y. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11:272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- Pop V, Head E, Hill M-A, Gillen D, Berchtold NC, Muggenburg BA, Milgram NW, Murphy MP, Cotman CW. Synergistic effects of long-term antioxidant diet and behavioral enrichment on beta-amyloid load and non-amyloidogenic processing in aged canines. J Neurosci. 2010;30:9831–9839. doi: 10.1523/JNEUROSCI.6194-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praag H, van Fleshner M, Schwartz MW, Mattson MP. Exercise, energy intake, glucose homeostasis, and the brain. J Neurosci. 2014;34:15139–15149. doi: 10.1523/JNEUROSCI.2814-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss JI, Dishman RK, Boyd HE, Robinson JK, Holmes PV. Chronic activity wheel running reduces the severity of kainic acid-induced seizures in the rat: possible role of galanin. Brain Res. 2009;1266:54–63. doi: 10.1016/j.brainres.2009.02.030. [DOI] [PubMed] [Google Scholar]
- Rohe M, Synowitz M, Glass R, Paul SM, Nykjaer A, Willnow TE. Brain-derived neurotrophic factor reduces amyloidogenic processing through control of SORLA gene expression. J Neurosci. 2009;29:15472–15478. doi: 10.1523/JNEUROSCI.3960-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SM, Herdener N, Camandola S, Texel SJ, Mughal MR, Cong WN, Martin B, Mattson MP. 3xTgAD mice exhibit altered behavior and elevated Abeta after chronic mild social stress. Neurobiol Aging. 2012;33:830, e1–12. doi: 10.1016/j.neurobiolaging.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SM, Herdener N, Frankola KA, Mughal MR, Mattson MP. Chronic mild sleep restriction accentuates contextual memory impairments, and accumulations of cortical Aβ and pTau in a mouse model of Alzheimer’s Disease. Brain Res. 2013;1529:200–208. doi: 10.1016/j.brainres.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SM, Mattson MP. Adverse Stress, Hippocampal Networks, and Alzheimer’s Disease. Neuromolecular Med. 2010;12:56–70. doi: 10.1007/s12017-009-8107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SM, Kelly AM. Exercise as a pro-cognitive, pro-neurogenic and anti-inflammatory intervention in transgenic mouse models of Alzheimer’s disease. Ageing Res Rev. 2016;27:77–92. doi: 10.1016/j.arr.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Skovronsky DM, Moore DB, Milla ME, Doms RW, Lee VM. Protein kinase C-dependent alpha-secretase competes with beta-secretase for cleavage of amyloid-beta precursor protein in the trans-golgi network. J Biol Chem. 2000;275:2568–2575. doi: 10.1074/jbc.275.4.2568. [DOI] [PubMed] [Google Scholar]
- Sleiman SF, Henry J, Al-Haddad R, Hayek L, El Abou Haidar E, Stringer T, Ulja D, et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body beta-hydroxybutyrate. Elife. 2016:5. doi: 10.7554/eLife.15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DH, Clarris HL, Williamson TG, Reed G, Key B, Mok SS, Beyreuther K, Masters CL, Nurcombe V. Neurite-outgrowth regulating functions of the amyloid protein precursor of Alzheimer’s disease. J Alzheimers Dis. 1999;1:275–285. doi: 10.3233/jad-1999-14-508. [DOI] [PubMed] [Google Scholar]
- Song J-H, Yu J-T, Tan L. Brain-Derived Neurotrophic Factor in Alzheimer’s Disease: Risk, Mechanisms, and Therapy. Mol Neurobiol. 2015;52:1477–1493. doi: 10.1007/s12035-014-8958-4. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Mattson MP. Selective Vulnerability of Neurons in Layer II of the Entorhinal Cortex during Aging and Alzheimer ’ s Disease. 2010:2010. doi: 10.1155/2010/108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strooper B, De Chavez Gutierrez L. Learning by failing: ideas and concepts to tackle gamma-secretases in Alzheimer’s disease and beyond. Annu Rev Pharmacol Toxicol. 2015;55:419–437. doi: 10.1146/annurev-pharmtox-010814-124309. [DOI] [PubMed] [Google Scholar]
- Stuart KE, King AE, Fernandez-Martos CM, Dittmann J, Summers MJ, Vickers JC. Mid-life environmental enrichment increases synaptic density in CA1 in a mouse model of Abeta-associated pathology and positively influences synaptic and cognitive health in healthy ageing. J Comp Neurol. 2016 doi: 10.1002/cne.24156. [DOI] [PubMed] [Google Scholar]
- Theendakara V, Patent A, Peters Libeu CA, Philpot B, Flores S, Descamps O, Poksay KS, et al. Neuroprotective Sirtuin ratio reversed by ApoE4. Proc Natl Acad Sci U S A. 2013;110:18303–18308. doi: 10.1073/pnas.1314145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippmann F, Hundt J, Schneider A, Endres K, Fahrenholz F. Up-regulation of the alpha-secretase ADAM10 by retinoic acid receptors and acitretin. FASEB J Off Publ Fed Am Soc Exp Biol. 2009;23:1643–1654. doi: 10.1096/fj.08-121392. [DOI] [PubMed] [Google Scholar]
- Um HS, Kang EB, Leem YH, Cho IH, Yang CH, Chae KR, Hwang DY, Cho JY. Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer’s disease in an NSE/APPsw-transgenic model. Int J Mol Med. 2008;22:529–539. [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci. 2013;17:525–544. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson TG, Mok SS, Henry A, Cappai R, Lander AD, Nurcombe V, Beyreuther K, Masters CL, Small DH. Secreted glypican binds to the amyloid precursor protein of Alzheimer’s disease (APP) and inhibits APP-induced neurite outgrowth. J Biol Chem. 1996;271:31215–31221. doi: 10.1074/jbc.271.49.31215. [DOI] [PubMed] [Google Scholar]
- Yeyeodu ST, Witherspoon SM, Gilyazova N, Ibeanu GC. A rapid, inexpensive high throughput screen method for neurite outgrowth. Curr Chem Genomics. 2010;4:74–83. doi: 10.2174/1875397301004010074. [DOI] [PMC free article] [PubMed] [Google Scholar]