Abstract
High-fat (HF) diets result in weight gain, hyperphagia, and reduced dopamine D2 signaling; however, these findings have been shown only under free-feeding conditions. This study tested the extent to which HF diet affects effort-dependent food procurement and the extent to which dopamine signaling is involved. Male Sprague-Dawley rats consumed either a HF (n = 20) or standard-chow (n = 20) diet. We assessed sensitivity to effort-based reinforcement in ten rats from each group by measuring consumption across a series of fixed-ratio schedules (FR 5 – FR 300) under a closed economy, and quantified performance using the exponential demand equation. For each FR, acute injections of 0 or 0.1 mg/kg of haloperidol, a D2 antagonist, were administered to assess dopamine-related changes in consumption. Rats fed a HF diet consumed more calories and weighed significantly more than rats fed standard chow. Food consumption decreased in both groups in an effort-dependent manner, but there were no group differences. Haloperidol reduced responding in a FR-dependent manner for both groups. Animals exposed to a HF diet showed an altered sensitivity to haloperidol relative to rats fed a standard diet, suggesting that HF diet alters sensitivity to DA signaling underlying effort-based food procurement.
Keywords: behavioral economics, demand, diet-induced obesity, effort, food availability, haloperidol, rat
Introduction
Diet-induced obesity (DIO) is a laboratory model of obesity in which nonhuman animals — usually rodents — are given chronic access to a HF diet, which results in hyperphagia, excess body weight, and changes in neurobiology related to food intake, relative to controls fed standard-chow diets (Rolls et al., 1980; Geiger et al., 2009; Johnson & Kenny, 2010; Vucetic et al., 2012). DIO-related hyperphagia is traditionally tested in environments that employ unlimited access to food, in which large amounts of food are readily available with little response-cost required (Rolls et al., 1980). Therefore, these economic conditions likely favor heightened food consumption (See Rasmussen et al., 2016).
Studies from the field of behavioral economics (Hursh, 1980, 1984), show that effort plays an integral part of consumption of food, drugs, and other reinforcers relevant to human behavior (see Bickel & Vuchinich, 2000; Bickel et al., 2014). Demand refers to the extent to which behavior for a commodity changes as a function of its price. In nonhuman animal studies, price is manipulated by fixed-ratio (FR) schedules of reinforcement, where a set number of responses result in a reinforcer (e.g. a single food pellet). To assess demand, the number of reinforcers earned at a range of response requirements is plotted as a function of FR (log scaled) to produce a demand curve (Hursh & Silberberg, 2008). Demand curves typically show that consumption decreases as the FR increases in a positively accelerating fashion, with little change in consumption at lower prices (inelasticity) and higher reductions in consumption at higher prices (elasticity; Hursh & Winger, 1995).
One way to quantify sensitivity to effort is the exponential-demand equation, which quantifies the essential value of a reinforcer (Hursh & Silberberg, 2008):
| (1) |
where Q0 is the number of reinforcers consumed at the lowest price (e.g., akin to a free-feeding environment), k is the range of reinforcers earned expressed in log units, and α (i.e. “essential value”) is a free parameter that estimates changes in consumption as a function of price (P; Hursh & Silberberg, 2008). Lower values of α indicate lower elasticity (i.e. consumption is relatively insensitive to price increases) and therefore is associated with a commodity with higher reinforcer efficacy, whereas higher values of α indicate higher elasticity and lower reinforcer efficacy. Behavioral-economic analyses offer a fuller characterization of the reinforcing efficacy of food by measuring consumption across a wide range of prices (i.e. response requirements) rather than simply observing consumption at one price, such as in free-feeding environments.
Previous studies have utilized demand procedures to characterize effort-related food consumption in the genetically obese Zucker rat (fa/fa)—an animal model of obesity that repeatedly has been shown to engage in hyperphagia (Rasmussen et al., 2010; Rasmussen et al., 2012). In these studies, genetically obese Zucker rats consumed significantly more sucrose pellets than lean Zucker rats, but only when food was delivered contingent on lower response requirements. When higher response requirements were tested, there were no group differences. Obese Zucker rats also had higher Q0 estimates compared to lean Zucker rats, supporting that the differences were related to low effort. No group differences in elasticity (α values) were evident. It is important to note that the Rasmussen et al. studies assessed demand in an open economy, in which additional food was available outside of the experimental session. Research suggests that demand in open economies is more elastic than demand in closed economies, in which all daily food is earned (Hursh, 1980, 1984; Collier et al., 1992). Therefore, because the Rasmussen et al. studies were conducted in open economies, this may have limited the ability for differences in elasticity to be observed between lean and obese Zucker rats. Regardless, these studies support the finding that differences between lean and obese rats observed in previous studies using animal models of obesity (i.e. Rolls et al., 1980) may be inflated by ready access to food.
One likely neurobiological mechanism underlying difference in food-reinforced behavior in models of obesity is disturbed dopamine neurotransmission. DIO reduces dopamine D2 signaling in brain areas that underlie choice and motivation, which may lead to alterations in sensitivity to reinforcement (Geiger et al., 2009; Johnson & Kenny, 2010; Vucetic et al., 2012). For example, Johnson and Kenny (2010) showed that rats exposed to 40 days of a HF, cafeteria-style diet showed a 75% reduction of striatal D2-receptor availability that was associated with hyperphagia. Whether hyperphagia following DIO was maintained across a variety of response requirements is unknown, as food consumption was measured in a free-feed (low-effort) environment.
Research suggests that dopamine D2 signaling underlies effort-based food procurement. Salamone and colleagues have demonstrated that depleting dopamine (DA) in the nucleus accumbens via neurotoxic lesions reduces responding for food under fixed ratio (FR) schedules of reinforcement, and does so in a ratio-dependent manner; that is, higher FRs reduce food consumption more strongly than lower FRs. However, these same DA-depleting lesions do not reduce the amount of food consumed in a free-feeding situation (Cousins & Salamone, 1994; Cousins et al., 1996; Salamone et al., 2001). A similar pattern of results is observed when haloperidol, a D2 antagonist, rather than neurotoxic lesions, is used to reduce DA activity, such that following a 0.1 mg/kg injection of haloperidol, rats tend to consume more food that is less-preferred, but freely available in the experimental chamber relative to a more-preferred food that is available contingent on a FR 5 schedule (Salamone et al., 1991). Therefore, accumbens-based D2 appears to be involved in effort-based food procurement.
Another study by Soto et al. (2011) supports D2 involvement in effort-based food consumption. Here, genetic variations in D2 receptor densities affected demand for food in three different mouse strains: a D2 knockout lean mouse (KO), which lacked D2 receptors, a heterozygous (HET) mouse and a wildtype (WT) mouse: the latter two had typical levels of D2 receptors and served as controls. Across a range of FRs from 5 to 160, HET and WT mice showed similar rates of demand, reaching elasticity around 80 lever presses. However, demand curves for D2 receptor KO mice were much steeper, reaching elasticity around FR 20. The estimated α values for D2 receptor KO mice were higher than α values for WT and HET mice, indicating that the food had lower essential value for the lean D2 receptor KO mice. Thus, DA activity — especially D2 signaling — is implicated in effort-based food procurement.
Given that reduced D2 signaling has been shown to reduce the amount of effort rodents will produce to gain access to food, it is possible that DIO-induced DA reductions also modulate effort-based food responding. Behavioral pharmacological procedures offer one alternative to test the role of DA in effort-based feeding by comparing behavior of HF-diet exposed rats to controls following an injection of a DA antagonist and measuring the extent to which demand changes as a function of diet condition (i.e. HF vs. standard) and drug administration. Haloperidol has been shown to unmask differences in delay discounting between rats fed a HF diet and rats fed standard chow (Boomhower & Rasmussen, 2014), though it is not clear whether effort-based responding is also relevant. Acute administration of haloperidol may allow general diet-induced changes in DA levels, which may be too subtle to detect under baseline conditions, to be unmasked.
The current study had two primary aims. First, we examined the extent to which the essential value of food changed as a result of exposure to a HF diet in rats, using a demand procedure in a closed economy. Rats responded under a variety of FR schedules and the number of reinforcers earned and responses made were analyzed using the exponential demand equation. Second, we examined the extent to which HF diet would result in dopaminergic alterations that may manifest in sensitivity to haloperidol. As such, behavior following an acute injection of haloperidol was compared between animals fed a standard diet versus a HF diet.
Methods
Subjects and Diets
Male Sprague-Dawley rats (n = 40) were obtained from a commercial breeder (Harlan, Livermore, CA) at three weeks of age and individually housed in plexiglass, shoebox cages with unlimited access to water and food. Rats were housed in a temperature- and humidity-controlled colony room and maintained on a 12-h light/dark cycle (lights on at 0700 h). Dietary exposure was based on that described in Huang, et al. (2006). Upon arrival, rats were randomly assigned to a HF diet (n = 20; 5.49 kcal/g, 36% from fat; Bioserv, Frenchtown, NJ) or a standard-chow diet (n = 20; 3.0 kcal/g, 17% from fat) and remained on these diets for 15 weeks. Following this dietary exposure period, the ten rats with the highest body mass (g) from the HF-diet group and the ten rats with the lowest body mass (g) from the standard-chow diet group were selected for operant testing (Huang et al., 2006; Boomhower & Rasmussen, 2014). This standard practice (e.g. Levin & Keesey, 1998; Huang et al., 2003; Huang et al., 2006; Johnson & Kenny, 2010; Boomhower & Rasmussen) is conducted to maximize the differences in weights and D2 in the striatum between groups (Wang et al, 2001; Johnson & Kenny, 2010) and to ensure that the animals were a model of obesity, and not simply an evaluation of the effects of a HF diet, per se. It is common to capitalize on individual differences in this manner to establish or begin to establish animal models of various phenomena, including obesity and alcohol use disorders (see Rasmussen et al, 2016; Bell et al, 2016). During operant testing, rats earned all of their food during the experimental session (i.e. closed economy). The Idaho State University Institutional Animal Care and Use Committee approved all procedures.
Apparatus
Seven Coulbourn®Habitest (Coulbourn Instruments, Whitehall, PA, USA) standard experimental chambers for rats were used for data collection. Each chamber was equipped with two levers and stimulus lights situated on the right side of the chamber and 5 cm above the grid floor. Only the right stimulus light was illuminated and the right lever was active during each session. When a response contingency was met, a 45-mg grain-based Precision pellet (Bioserv, Frenchtown, NJ; 3.35 kcals/g) was dropped into a food receptacle situated between the two response levers. White noise was generated via a speaker situated on the top-right corner of the left wall. The chamber was ventilated via a 5 cm × 5 cm fan on the top-left corner of the left wall. Each chamber was placed in a sound-attenuating cubicle. Experimental events were controlled and data were collected with a 0.01-s resolution using GraphicState® software (Coulbourn Instruments, Whitehall, PA, USA) on a Windows-based computer.
Behavioral Testing
Training
After the 15-week diet exposure concluded, lever-press training commenced. Lever-press training consisted of placing each rat in an operant chamber in which a FR 1 schedule of reinforcement was in effect for the right lever. After a single lever press, the house light was illuminated for 3-s and a 45-mg pellet was delivered. Once 70 responses were made, lever pressing was considered trained. Rats that did not meet the criterion in three days were trained to lever press by hand shaping (i.e. reinforcing successive approximations) until 70 responses were made independently.
Behavioral Testing
Once rats were trained to lever press, demand curves were determined. In this phase, rats earned all of their daily food during 6-h experimental sessions (i.e. closed economies) that were conducted five days per week (Sundays, Mondays, Tuesdays, Thursdays, and Fridays). On two days per week (Wednesdays and Saturdays), rats were allowed access to their originally assigned diet condition (standard-chow vs. HF) for 10h. Water was freely available during behavioral testing session. During experimental sessions, rats earned food pellets under FR schedules that increased systematically. For the first three weeks, an FR 5 was implemented, such that for every five lever presses, a single food pellet was delivered. In subsequent three-week periods, the FR values increased to 15, 30, 50, and 90. Only one week of testing was used for the FR 150 and 300 contingencies, as body masses dropped dramatically. Free-feeding body weights were calculated on the basis of weights at the end of the 15-week diet exposure. Rats that demonstrated weight losses to below 85% of their free-feeding weights were not exposed to subsequently higher FRs, as this compromised health of the rats. This practice was also in compliance with institutional welfare guidelines.
Drug
Haloperidol (Sigma-Aldrich, USA) was dissolved in a 1:1:18 vehicle solution of lactic acid (Sigma-Aldrich, USA), buffering agent, and saline (1 mL/kg). Both drug and vehicle solutions (which included the lactic acid, buffering agent, and saline) were held at a pH of 7. Acute i.p. injections were delivered 30 min prior to the experimental session to ensure that the drug was behaviorally active. Rats received one dose of 0.1 mg/kg, as well as one injection of vehicle for each FR schedule tested, as this dose has been shown to be behaviorally active, though not toxic, using high and low-effort operant conditions (e.g., Salamone et al, 1991; Rasmussen & Newland, 2001). Seven days elapsed between drug injections to minimize potential carry-over effects.
Statistical Analysis
Statistical analyses were performed using IBM SPSS Version 23.0 (IBM SPSS Statistics for Macintosh, Version 23.0, Armonk, NY: IBM Corp.). Weight gain during diet-exposure was analyzed using non-linear regression. For baseline data, reinforcers and responses for each rat under each FR were determined by averaging data from the last three sessions of each FR and compared using a two-way ANOVA with repeated measures, with FR as a within-subjects variable and dietary exposure as a between-subjects variable. Aspects of demand were quantified by fitting the exponential demand equation to the reinforcer data (expressed as number of food pellets earned and number of kcals consumed). Differences in free-feeding food consumption (g) and estimated parameters from the demand equation were compared using independent t-tests. Drug effects between groups were compared using a three-way repeated-measures ANOVA, with dose and FR as within-subject variables and diet as a between-subject variable. In some instances (e.g. higher FRs), when rats' behavior was placed under higher FR schedules, but no reinforcers were earned, a 1 was added to the values (including 0s) in that rat's data (e.g. if the rat earned 15 reinforcers under vehicle and 0 under haloperidol, we recorded it as 16 reinforcers under vehicle and 1 under haloperidol). This allowed us to not only conduct the analyses, as 0s in the denominator yields undefined values, but to keep the relative values in the data set consistent. This method has been used with other studies that employ demand procedures (Hursh, Association for Behavior Analysis International 2016 Convention).
Results
Weight gain and Food Intake
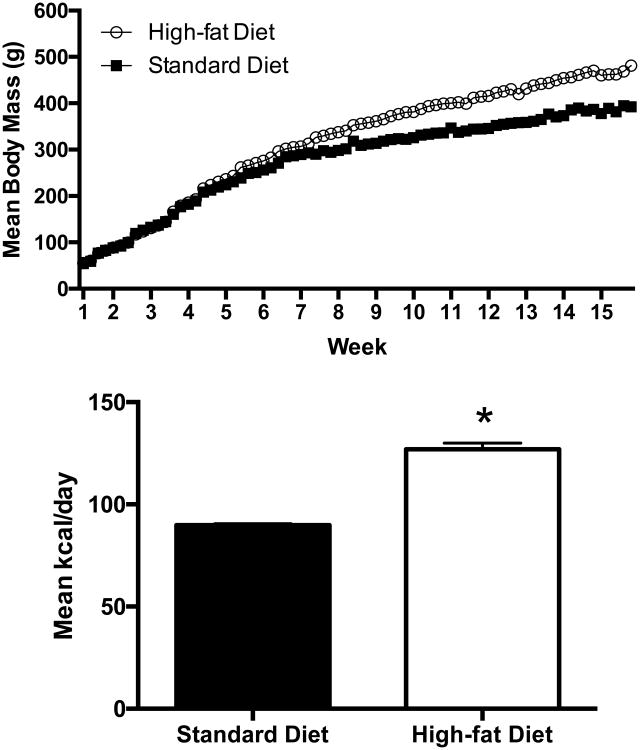
Weight gain was analyzed for the 20 rats that moved on to behavioral testing (n = 10 standard diet, n = 10 HF diet). Weight increased with age for both diet groups [HF diet: F (3, 70) = 8557.00, p < .001, R2 = 0.99; standard-chow diet: F (3, 70) = 5415.31, p < 0.001, R2 = 0.99] across the 15-week exposure period (Fig 1, top). By the final day of exposure, rats exposed to the HF diet demonstrated significantly higher weights than those in the standard diet conditions, t (38) = -2.69, p < 0.01, d = 0.87. The rats used in the operant portion of the study that were exposed to the HF diet also consumed significantly more calories than the rats exposed to the standard diet, t (9.978) = -3.85, p < 0.01, d = -1.72 (Fig. 1, bottom).
Figure 1.
Weight gain for rats that continued to behavioral testing (n = 20; top panel) and their caloric consumption (n = 20; lower panel) for rats given standard-chow vs. a HF diet. The lower panel shows mean caloric intake during the last three days of exposure as a function of diet. Error bars represent 1 SEM.
Baseline Demand
Demand curves (reinforcer data as a function of FR) are summarized as means for each group in Figure 2. Graphs depicting three representative individual animals from each group are also included. Generally, as FR increased, the number of reinforcers decreased, F(2.10, 37.88) = 106.36, p < 0.001,η2P = 0.85; however, there was no significant main effect of diet (p = 0.69) or interaction. Data from individual animals show similar patterns. Note that S55 exhibited a shallow demand curve with little elasticity. Free parameter values were estimated using the exponential demand analysis (Table 1). A significant between-groups difference in R2 was found but there were no differences for other estimated parameters. An exponential demand analysis was also applied to the reinforcer data, expressed as the number of kcals consumed at each FR (Table 1); there were no significant group differences in these data.
Figure 2.
The top panel show mean number of reinforcers earned as a function of diet (left panel). Error bars represent 1 SEM. Lower panels show representative data for individual demand curves of rats exposed to standard chow (left panels) or a HF diet (right panels).
Table 1.
Mean (SEM) free parameters of the exponential demand equation fit to reinforcer and kcals earned during operant testing.
| Reinforcers | ||||
|---|---|---|---|---|
|
| ||||
| Standard | HF | t(df) | p-value | |
| Q0 | 526.06 (8.15) | 474.52 (8.97) | -1.34(8) | Ns |
| α | 8.50 × 10 -6 (6.57 × 10 -7) | 5.02 × 10 -6 (1.99 × 10 -7) | -1.60(10.63) | Ns |
| k | 2.74 | 2.74 | ||
| R2 | 0.75 (0.01) | 0.89 (0.005) | 3.57(18) | .002 |
| n | 10 | 10 | ||
| kcals | ||||
|
| ||||
| Standard | HF | t(df) | p-value | |
|
| ||||
| Q0 | 82.90 (1.13) | 83.88 (1.95) | 0.137(18) | Ns |
| α | 6.72 × 10-5 (5.51 × 10 -6) | 5.15 × 10-5 (3.21×10-6) | -0.78(18) | Ns |
| k | 1.95 | 1.95 | ||
| R2 | 0.72 (0.02) | 0.84 (0.01) | 1.79(14.8) | Ns |
| n | 10 | 10 | ||
Response output curves are shown as a function of FR and summarized as means for each group; data from representative animals from each group are also shown (Fig. 3). There was a significant main effect of FR, F(1.72, 30.98) = 22.97, p < 0.001, η2P = 0.56, as responses increased and then decreased as FR increased, but no significant main effect of diet or interaction (p's > 0.76).
Figure 3.
The top panel shows mean number of responses emitted for each FR for rats exposed to standard chow (closed circles) or a HF diet (diamonds). Error bars represent 1 SEM. The lower panels show representative data for individual animals exposed to standard chow (left panels) or a HF diet (right panels).
Acute drug challenge
The top of Figure 4 shows mean reinforcers earned across all FRs for standard and HF groups for both vehicle (left) and 0.1 mg/kg (right) haloperidol conditions. The dotted horizontal line refers to the baseline half-Q0 value, or the value at which half of the maximal reinforcers were earned (this is a value based on the mean 0.5(Q0) values across group in Table 1). This line assists in observing deviations from baseline. For reinforcers, there was a significant main effect of FR, F(6, 96) = 51.04, p < 0.001, η2P = 0.76, such that reinforcers decreased as FR increased, but there was no significant main effect of drug or group. However, there was a significiant FR × Diet interaction, F(6, 96) = 2.45, p < 0.05, η2P = 0.13, such that the lowest number of reinforcers was earned by rats exposed to the HF diet at the highest FRs. There was also a significant FR × Diet × Drug interaction, F(6, 96) = 2.46, p < 0.05, η2P = 0.13, reflecting the observation that the lowest number of reinforcers was earned at the highest FRs following an injection of haloperidol in rats exposed to a HF diet. The interaction is best observed by comparing the number of bars above and below the half-Q0 line for vehicle (left) vs. 0.1 mg/kg (right) haloperidol and also attending to the differences between the standard and HF diet conditions across FRs.
Figure 4.
The upper panels show reinforcers as a function of FR for each group (standard diet = black bar; HF diet = white bars). Dotted horizontal lines show baseline ½-Q0 values (241) to allow for between-dose comparisons. The lower panels show responses; the dotted horizontal line represents ½ omax baseline values. The vehicle is represented in the left panels and the 0.1 mg/kg dose of haloperidol in the right panels. Note: Means in the FR 150 condition include only 7 and 9 rats from standard chow and HF diet, respectively; the means in FR 300 include 3 and 5 rats, respectively. Error bars = 1 SEM.
The lower panel of Figure 4 shows responses. There was a significant main effect of FR, F(6, 96) = 11.19, p < 0.001, η2P = 0.41. While there were no significant main effects of group or dose, there were marginally significant FR × Diet, F(3.42, 54.73) = 2.30, p < 0.08 and FR × Dose × Diet interactions, F(3.42, 54.73) = 2.36, p< 0.07, such that responses were lowest under the lowest and highest FRs under haloperidol and in animals exposed to the HF diet. These effects can best be observed by comparing the number of bars above and below the half-Omax lines and also examining differences between the standard and HF diet conditions across FRs. Omax values were calculated using the linear-elasticity demand equation (Hursh & Winger, 1995; Hursh, 2000) and represent the maximum responding during baseline for rats fed a HF diet (M = 21842.02, SEM = 575.30) and rats fed a standard diet (M = 26068.17, SEM = 1793.41). Because it is difficult to assess between-group differences in drug sensitivity from the data shown in Figure 4, the data were converted to percent change from vehicle. These data are showed in Figure 5. A note about analysis is warranted. Given that we used repeated-measures ANOVAs to analyze these data, only animals that completed all FR schedules under haloperidol (FR 5 – FR 90) can be included in the analysis. Data from FR 150 – FR 300 are shown in the right panels and were excluded from the repeated measures analysis, but are addressed independently.
Figure 5.
The upper left panel shows the mean number of reinforcers earned expressed as percent of vehicle (Haloperidol/Vehicle) for FR 5 – 90 (standard diet = black bar; HF diet = white bars). The upper right panel shows individual data for the number of reinforcers earned expressed as percent vehicle (Haloperidol/Vehicle) for FR 150 – 300 (standard diet = black circles; HF diet = white circles; median = -) The lower panels shows the mean number of responses emitted expressed as percent vehicle. In all panels, the dotted line shows the point at which responding under vehicle and haloperidol was equal. Bars that fall below the line indicate reductions in responding as a function of haloperidol administration and bars that fall above the line show increases as a function of haloperidol administration.
As the upper left panel of Figure 5 shows, data below the 100% line indicate a haloperidol-induced reduction in reinforcers and any above the line represent an increase. Rats in the standard diet condition exhibited haloperidol-induced reductions in reinforcement across all FRs. Rats exposed to a HF diet displayed lower haloperidol-induced suppression than rats in the standard diet group under FR 5 - 50. In some cases, a haloperidol-induced increase from vehicle was observed in the HF diet rats. In other words, for the HF diet group, sensitivity and behavioral change to haloperidol depended highly on FR. A repeated measures analysis supported a significant FR × Diet interaction, F(4, 36) = 2.53, p = 0.057, η2P = 0.22. There was no significant main effect of FR or diet.
The lower left panel of Figure 5 shows percent change from vehicle for responses. Consistent with the reinforcer analysis, only FR 5 – FR 90 were included. A repeated-measures ANOVA revealed a significant main effect of FR, F(4, 40) = 2.60, p = 0.053, η2P = 0.22. In addition, rats exposed to HF diet showed lower haloperidol-induced suppression than standard diet rats, but it depended on FR. A FR × Diet interaction trended toward significance, F(4, 40) = 2.36, p = 0.07, η2P = 0.21.
The right panels of Figure 5 also shows individual data (each datum = one rat) for FR 150 and FR 300, the schedules under which all rats did not complete the FRs. As previously mentioned, these schedule values were assessed separately due to the number of animals that were not able to complete FR 150 and FR 300. For FR 150, 5 standard diet rats 9 of the HF diet rats completed the schedule. A χ2 analysis confirmed a significant group difference, χ2 (1) = 3.81, p < 0.05. In addition, the medians and range of the data within each group indicate robust differences in the distributions of the groups. The majority of the data (8 of 9 rats) from the HF diet group demonstrated increases in both reinforcers and responses as a result of haloperidol, while 4 of 5 rats in the standard diet group showed reductions in reinforcers and responses (one rat showed a 500-fold increase). Indeed, the median score for the HF diet group is 10-fold higher than the mean of the standard diet group. A statistical analysis (i.e., t-test) on these data would be difficult to interpret, however, given the unequal sample sizes and large differences in variance, so none are reported here. For FR 300, only 3 and 7 standard diet and HF diet rats completed the schedule, respectively, and there was a trend toward a significant difference, χ2 (1) = 3.20, p = 0.074. The medians were similar but as with the FR 150 data, a statistical analysis was not useful due to unequal n's and unequal variances.
Because the haloperidol-induced reductions for reinforcers and responses were scaled more linearly (i.e., not logarithmically), an exponential demand analysis is not reported.
Discussion
Chronic exposure to a HF diet resulted in greater caloric intake and body mass than exposure to a standard diet
Rats given free access to achronic HF diet vs. standard chow showed differential patterns of food consumption and weight gain. Rats given the HF diet consumed significantly more calories and demonstrated significantly higher body mass than rats exposed to a standard diet. This is consistent with previous literature (e.g., Rolls,et al., 1980; Johnson & Kenny, 2010).
Baseline food consumption decreased as a function of effort requirement, though there were no diet-related differences
We examined the extent to which chronic exposure to a HF diet altered demand for food in a closed economy, across FR values ranging from 5 to 300. At all FR values, rats in both groups emitted a similar number of responses and earned a similar number of food reinforcers (i.e., there were no group differences). The exponential demand analysis did not yield significant group differences, but rats exposed to a HF diet showed a significantly higher R2 than rats fed a standard diet. This finding is consistent with Rasmussen et al., (2010) who found that obese Zucker rats showed a significantly better fit of the exponential demand equation than controls. Regardless of the differences in R2 the fits of the exponential demand model generally described the pattern of data for both groups well for both exponential demand analyses (average R2 = 0.82 for reinforcer analysis and R2 = 0.86 for kcal analysis), but did not reveal in any differences in behavior.
Previous literature suggests that rats will engage in hyperphagia when exposed to a chronic HF diet (Johnson & Kenny, 2010). The current study demonstrated that when a response requirement is added to food consumption, rats with different dietary histories show similar sensitivities to effort-based reinforcement. It is important to point out that under free-feeding conditions, rats in the HF diet group consumed 40% more calories daily than the standard chow group (130 kcal vs. 90 kcal, respectively). However, under the closed economy in which all food was response-dependent, both groups consumed a maximum of about 90 kcals per day. One interpretation of this observation is that 90 kcals per day is an average number of kcals that a rat will defend. This interpretation is further supported by the exponential demand analysis applied to the reinforcer data expressed as kcals earned, in that the Q0 values are 83.26 and 87.08 for the rats fed the standard diet and HF diet, respectively (bottom of Table 1). Other conditions that make food more readily available (i.e., the free-feed environment) or palatable (i.e., HF diet) may increase the amount consumed and promote excessive weight gain. Higher response requirements (e.g., greater than 90 lever presses) may challenge that threshold, resulting in reductions in food consumption and weight loss.
These findings replicate aspects of Rasmussen et al. (2010, 2012), which examined demand for sucrose in genetically lean and obese Zucker rats. In these studies, obese Zuckers earned more reinforcers at lower FRs than lean Zuckers, but as the FR increased, both lean and obese Zucker rats defended consumption at similar rates. The finding that the Zucker and DIO models of obesity show similar patterns of demand suggests that effort required for food procurement may be an important factor in obesity. The current study also expands these papers by using a closed economy (see Hursh, 1980; Collier et al., 1992) and grain-based pellets (instead of sucrose). Further, these findings support human correlational and experimental studies that show proximity to food results in greater consumption and a higher likelihood of obesity (Wansink et al., 2006; Epstein et al., 2007; Hanks et al., 2012).
Chronic exposure to a HF diet interacts with haloperidol-induced reductions in effort
An established behaviorally active, but non-toxic dose of haloperidol was used to assess the extent to which diet-induced alterations in D2 manifested as changes to effort-based food consumption. Figure 4 showed a number of findings. First, the 0.1 mg/kg dose of haloperidol appeared to generally decrease the number of reinforcers and responses in an FR-dependent manner. For vehicle, the vast majority of reinforcers in the lower FRs (1-50) were higher than the half-Q0 values. Under the 0.1 mg/kg dose, however, there were fewer means above this line. Also, across the higher FRs in which the vehicle values are below the half-Q0 line, the values for the 0.1 mg/kg dose are even lower. These haloperidol findings replicated Salamone et al (1991) and also extends this work by including FRs greater than those in the original report (FR ranged from 1 to 5), as the current study included FR values up to 300.
The most notable findings, however, were found in the interactions of dietary history, response requirement, and haloperidol. Specifically, the lowest number of reinforcers appeared to be earned at the highest FRs in rats exposed to the HF diet condition. In addition, diet interacted with haloperidol and FR, such that the lowest number of reinforcers earned tended to be in the highest-effort conditions following an injection of haloperidol in rats with a history of a HF diet. Haloperidol also influenced responses emitted. In the vehicle condition, the peak responses were in the medium FR range (50 – 90), which replicates other behavioral economic studies (Rasmussen et al., 2010; Soto et al., 2011; Rasmussen et al., 2012). For most FRs, especially those in that range, an injection of haloperidol reduced the number of responses emitted. Responses did not exceed half-max values in the 0.1 mg/kg conditions for any FR. However, these haloperidol induced reductions resulted in only marginally statistically significant interactions, possibly because lower responses emitted at very low (e.g., FR 5) and very high (e.g. FR 300) response requirements created a floor effect.
Chronic exposure to a HF diet changed sensitivity to effort following an injection of haloperidol compared to rats fed a standard diet
While the raw data suggest that haloperidol reduced reinforcers in a manner that was FR and diet-dependent, it is difficult to determine how diet influenced sensitivity to haloperidol by an examination of Figure 4. As such, drug data were analyzed as percent of vehicle (Fig 5). Assessing these effects statistically with repeated-measures analysis was difficult because the number of rats in each group completing higher FRs (150 and 300) under haloperidol was reduced, making a repeated-measures analysis across all FRs challenging. We approached this problem by analyzing the data for FR 5 – 90 using a repeated-measures analysis and examining behavior under FR 150 and 300 separately. The analyses revealed a number of findings. First, consistent with Figure 4, the 0.1 mg/kg dose of haloperidol generally decreased the number of reinforcers and responses for rats fed a standard diet. The single exception to this finding is that rats fed a standard diet showed a higher level of responding, but not reinforcers, at FR 15 under haloperidol. These haloperidol findings from the standard chow group replicate previous literature that has documented decreases in dopamine D2 by genetic (Soto et al., 2011), pharmacological (Salamone et al., 1991), and neurotoxic lesion (Cousins & Salamone, 1994; Cousins et al., 1996; Salamone et al., 2001) in standard rat models results in a reduction in effort-dependent food consumption.
Rats exposed to a HF diet, however, showed an altered sensitivity to haloperidol compared to the rats fed standard chow. At most FRs (FR 5 – 50, but not 90), rats exposed to the HF diet showed a lower haloperidol-induced reduction in reinforcers than rats fed a standard diet and in a number of cases haloperidol actually increased the number of reinforcers earned in this group relative to vehicle. This pattern also suggests the possibility that the effects of haloperidol on effortful responding documented in other studies (i.e. Salamone et al., 1991) depend highly on diet and FR value.
Data from the haloperidol sessions from FR 150 and FR 300 schedules add to these findings. First, significantly more rats from the HF diet condition completed the FR 150 schedule than the rats fed a standard diet and this trend was observed for FR 300. Second, for four of the five rats that completed FR 150, haloperidol decreased responding and reinforcers earned at these schedule values for rats fed a standard diet; however, haloperidol tended to increase responding for eight of the nine rats fed a HF diet. These differences support the repeated-measures analysis and offer some additional evidence that rats exposed to HF diets show a lowered sensitivity to haloperidol (in terms of its reducing effects) that interact with effort.
The altered sensitivity to haloperidol in rats exposed to a HF diet (compared to controls) extends the literature that chronic exposure to a HF diet leads to a reduction in D2 and changes in food consumption in free-food intake, often interpreted as a “blunting” of food reward processes (Geiger et al., 2009; Johnson & Kenny, 2010; Vucetic et al., 2012). This interpretation would support the conclusion that HF diet alters the D2 receptor in a manner that changes the rewarding properties of food; however, the findings in this study suggest that a reduction of D2 activity may enhance the reinforcing properties of food under some effort-based conditions and reduce them in others, though they are reduced to a lesser extent than controls.
Despite the strengths of this study, there were also some limitations. First, a closed economy with the same food type was necessary to examine a range of possible differences in elasticity. The inherent limitation with this methodological set-up is that the earlier diet exposure effects could be “washed out” during operant testing. To accommodate this problem, we continued some exposure to diet by allowing rats to come into contact with their respective diets twice per week. While it would be ideal to allow the animals to earn their daily diets in operant sessions across the entire study the differences in macronutrient content and palatability of these pellets would confound interpretation of the results. Future research could investigate this question by manipulating dietary exposure duration between demand curve sessions or by using open economies, in which rats are given outside access daily to their respective diets. This is complicated, however, because caloric intake must be controlled and the use of an open economy reduces elasticity for food. A second limitation is that we used only a single dose of haloperidol to test differences in sensitivity. While this dose of haloperidol has been used as an effective dose in a number of studies (Salamone et al., 1991; Salamone et al., 1994; Randall et al., 2012) and allowed a diet-related interaction to be observed, a fuller characterization using smaller and larger doses would provide more dose-response information and may improve the ability to detect altered sensitivity to dopaminergic function.
Conclusion
Despite these limitations, the data in the present study have implications for public health. Food environments often have high-fat foods, such as fast food or highly processed food, available at low effort and monetary costs (Drewnowski & Darmon, 2005). These data suggest that rats exposed to a HF diet will defend consumption of food at similar rates as rats exposed to standard chow, but that HF diet can alter sensitivity to food reward when unmasked with a D2 compound at response requirements that vary. Nonetheless, one way to reduce overeating and promote healthy food choices may be to make HF foods less convenient and more expensive than healthier alternatives, in order to promote healthy food choices (Wansink et al., 2006; Epstein et al., 2007; Hanks et al., 2012).
Acknowledgments
Sources of Funding: This research project was supported by the INBRE Program, NIH Grant No. P20 GM103408 (National Institute of General Medical Sciences) and the Idaho State University Faculty Research Committee as well as the College of Arts and Letters.
Footnotes
Conflicts of interest: None declared.
References
- Baladi M, Daws L, France C. You are what you eat: Influence of type and amount of food consumed on central dopamine systems and the behavioral effects of direct- and indirect-acting dopamine receptor agonists. Neuropharmacology. 2012;63:76–86. doi: 10.1016/j.neuropharm.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R, Rod Z, Lumeng L, Murphy J, McBride W. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–88. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Bickel W, Johnson M, Koffarnus M, MacKillop J, Murphy J. The behavioral economics of substance use disorders: Reinforcement pathologies and their repair. Annu Rev Clin Psychol. 2014;10:641–77. doi: 10.1146/annurev-clinpsy-032813-153724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomhower S, Rasmussen E. Haloperidol and rimonabant increase delay discounting in rats fed high-fat and standard-chow diets. Behav Pharmacol. 2014;25:705–16. doi: 10.1097/FBP.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier G, Johnson D, Morgan C. The magnitude-of-reinforcement function in closed and open economies. J Exp Anal Beh. 1992;57:81–9. doi: 10.1901/jeab.1992.57-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins M, Salamone J. Nucleus accumbens dopamine depletions in rats affect relative response allocation in a novel cost/benefit procedure. Pharmacol, Biochem Behav. 1994;49:85–91. doi: 10.1016/0091-3057(94)90460-x. [DOI] [PubMed] [Google Scholar]
- Cousins M, Atherton A, Turner L, Salamone J. Nucleus accumbens dopamine depletions alter relative response allocation in a T-maze cost/benefit task. Behav Brain Res. 1996;74:189–97. doi: 10.1016/0166-4328(95)00151-4. [DOI] [PubMed] [Google Scholar]
- Dews PB. Studies on behavior. I. Differential sensitiviy to pentobarbital of pecking performance in pigeons depending on the schedule of reward. J Pharm Exp Ther. 1955;113:393–401. [PubMed] [Google Scholar]
- Epstein L, Dearing K, Paluch R, Roemmich J, Cho D. Price and maternal obesity influence purchasing of low-and-high energy-dense foods. Am J Clin Nutr. 2007;86:914–22. doi: 10.1093/ajcn/86.4.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Haburcak M, Avena M, Moyer C, Hoebel B, Pathos E. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience. 2009;159:1193–99. doi: 10.1016/j.neuroscience.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Han M, Storlien L. The level of NPY receptor mRNA expression in diet-induced obese and resistant mice. Mol Brain Res. 2003;115:21–8. doi: 10.1016/s0169-328x(03)00174-8. [DOI] [PubMed] [Google Scholar]
- Huang X, Zavitsanou K, Huang X, Yu Y, Wang H, Chen F, Lawrence A, Deng C. Dopamine transporter and D2 receptor binding densities in mice prone or resistant to chronic high fat diet-induced obesity. Behav Brain Res. 2006;175:415–9. doi: 10.1016/j.bbr.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Hursh S. Economic concepts for the analysis of behavior. J Exp Anal Beh. 1980;34:219–238. doi: 10.1901/jeab.1980.34-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh S. Behavioral economics. J Exp Anal Beh. 1984;42:435–52. doi: 10.1901/jeab.1984.42-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh S. Behavioral economic concepts and methods for studying health behavior. In: Bickel WK, Vuchinch RE, editors. Reframing health behavior change with behavioral economics. Mahwah, NJ: Lawrence Erlbaum Associates; 2000. pp. 27–60. [Google Scholar]
- Hursh S, Silberberg Economic demand and essential value. Psychol Rev. 2008;115:186–98. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Hursh S, Winger G. Normalized demand for drugs and other reinforcers. J Exp Anal Beh. 1995;64:373–84. doi: 10.1901/jeab.1995.64-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh S, Raslear T, Shurtleff D, Bauman R, Simmons L. A cost-benefit analysis of demand for food. J Exp Anal Beh. 1988;50:419–40. doi: 10.1901/jeab.1988.50-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P, Kenny P. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature Neurosci. 2010;13:635–41. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin B, Keesy R. Defense of differing body weight set points in diet-induced obese and resistant rats. Am J Physiol. 1998;274:R412–19. doi: 10.1152/ajpregu.1998.274.2.R412. [DOI] [PubMed] [Google Scholar]
- Randall P, Pardo M, Nunes E, Lopez Cruz L, Vemuri V, Makriyannis A, Baqi Y, et al. Dopaminergic modulation of effort-related choice behavior as assessed by a progressive ratio chow feeding choice task: Pharmacological studies and the role of individual differences. PLoS One. 2012;7:e47934. doi: 10.1371/journal.pone.0047934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen E, Newland M. Developmental exposure to methylmercury alters behavioral sensitivity to d-amphetamine and pentobarbital in adult rats. Neurotoxicology and Teratology. 2001;23:45–55. doi: 10.1016/s0892-0362(00)00112-4. [DOI] [PubMed] [Google Scholar]
- Rasmussen E, Reilly W, Hillman Demand for sucrose in the genetically obese Zucker (fa/fa) rat. Behav Process. 2010;85:191–7. doi: 10.1016/j.beproc.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Rasmussen E, Reilly W, Buckley J, Boomhower S. Rimonabant reduces the essential value of food in the genetically obese Zucker rat: An exponential demand analysis. Physiol Behav. 2012;105:734–41. doi: 10.1016/j.physbeh.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Rasmussen E, Robertson S, Rodriguez L. The utility of behavioral economics in expanding the free-feed model of obesity. Behav Proc. 2016;127:25–34. doi: 10.1016/j.beproc.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls B, Rowe E, Turner R. Persistent obesity in rats following a period of consumption of a mixed, high-energy diet. J Physiol. 1980;298:415–27. doi: 10.1113/jphysiol.1980.sp013091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone J, Steinpreis R, McCullough L, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology. 1991;104:515–21. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- Salamone J, Cousins M, Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental reponses selection in a T-maze cost/benefit procedure. Behav Brain Res. 1994;65:221–9. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Salamone J, Wisniecki A, Carlson B, Correa M. Nucleus accumbens dopamine depletions make animals highly sensitive to high fixed ratio requirements but do not impair primary food reinforcement. Neuroscience. 2001;105:863–70. doi: 10.1016/s0306-4522(01)00249-4. [DOI] [PubMed] [Google Scholar]
- Soto P, Grandy D, Hursh S, Katz J. Behavioral economics of food reinforcement and the effects of prefeeding, extinction, eticlopride in dopamine D2 receptor mutant mice. Psychopharmacology. 2011;215:775–84. doi: 10.1007/s00213-011-2173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucetic Z, Carlin J, Totoki K, Reyes T. Epigenetic dysregulation of the dopamine system in diet-induced obesity. J Neurochem. 2012;120:891–8. doi: 10.1111/j.1471-4159.2012.07649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Volkow N, Logan J, Pappas N, Wong C, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357:354–7. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Wang X, Zavitsanou K, Huang X, Yu Y, Wang K, Chen F, Lawrence A, et al. Dopamine transporter and D2 receptor binding densities in mice prone or resistant to chronic high fat diet-induced obesity. Behav Brain Res. 2006;175:415–9. doi: 10.1016/j.bbr.2006.08.034. [DOI] [PubMed] [Google Scholar]