Abstract
Background
Internet-based cancer risk assessment tools might be one strategy for translating epidemiological risk prediction research into public health practice. Understanding how such tools might affect key social-cognitive precursors of behavior change is crucial for leveraging their potential into effective interventions.
Purpose
To test the effects of a publicly-available Internet-based breast cancer risk assessment tool on social-cognitive precursors of physical activity.
Methods
Women (N=132) aged 40–78 with no personal cancer history indicated their perceived risk of breast cancer and were randomly assigned to receive personalized (www.yourdiseaserisk.wustl.edu) or non-personalized breast cancer risk information. Immediately thereafter, breast cancer risk perceptions and physical activity-related behavioral intentions, self-efficacy, and response-efficacy were assessed.
Results
Personalized information elicited higher intentions, self-efficacy, and response efficacy than non-personalized information, ps<.05. Self-efficacy and response efficacy mediated the effect of personalizing information on intentions. Women who received personalized information corrected their inaccurate risk perceptions to some extent, ps<.05, but few fully accepted the information.
Conclusion
Internet-based risk assessment tools can produce beneficial effects on important social-cognitive precursors of behavior change, but lingering skepticism, possibly due to defensive processing, needs to be addressed before the effects can be maximized.
Keywords: Health communication, risk prediction, health cognitions, risk perception, cancer
Introduction
Over 230,000 women in the U.S. developed breast cancer in 2015 (1). Several risk prediction algorithms can calculate a woman’s likelihood of developing breast cancer (e.g., 2, 3), and the results can identify women who may be eligible for intensive risk reduction and/or detection efforts (4, 5), help women gain a more accurate understanding of their risk of developing cancer (6), and facilitate informed decisions about cancer screening (7, 8). However, the potential for breast cancer risk assessment tools to motivate other risk reduction behaviors in the general population deserves more attention. For example, an Internet-based tool that produces a modest improvement in physical activity behavior (3, 9) could yield profound impacts on public health due to its wide reach into the population.
Risk Assessment Tools and Health Cognitions
Using risk assessment tools to provide personalized disease risk information can improve the accuracy of risk perceptions (10–12), but providing information in a way that is useful and meaningful to the public is challenging. Numerical percentage estimates are easily misunderstood and may not be necessary in all situations (13, 14). Furthermore, people may be less likely to understand and incorporate personalized risk information into their personal perceptions of risk when the information is provided in terms of absolute risk rather than in terms of how their risk compares to that of similar others (11, 15). These misunderstandings may limit the effectiveness of personalized risk communication interventions (16–20).
Another factor that may limit the effectiveness of risk assessment tools is a tendency to focus on only one of several social cognitions that are critical precursors of health behavior (16–23). The Health Action Process Approach (HAPA) asserts that interventions that target motivation or intentions to engage in a healthy behavior should incorporate components that not only increase participants’ perceived risk of disease, but also increase their confidence in their ability to perform the behavior (i.e., self-efficacy) and their confidence that engaging in the behavior will reduce risk (i.e., response efficacy) (23). Meta-analyses support these assertions (24–27). However, few personalized risk communication interventions have intervened on all of these key constructs. This could be because little is known about how to incorporate brief self-efficacy and response efficacy intervention components (e.g., 28) into personalized risk assessment tools in general (10, 11, 16–18, 29) and breast cancer risk assessment tools in particular (7). We addressed this gap by evaluating a publically-available Internet-based risk assessment tool that uses risk communication strategies that are easy for the public to understand and also incorporates efforts to increase response efficacy and self-efficacy (11, 30, 31).
Your Disease Risk
Dozens of cancer risk assessment tools are available online, and the majority provide feedback about the risk of developing breast cancer (19). However, few use evidence-based risk communication strategies or target key social-cognitive precursors of behavior change. In contrast, Your Disease Risk was developed by a transdisciplinary team that included experts in health behavior change and risk perception research (30, 32, 33). In addition to personalized information about the risk of developing breast cancer and other diseases, it attempts to increase response efficacy by informing participants of the extent to which behavior change could reduce their risk. It targets self-efficacy by suggesting specific strategies for changing behavior. Your Disease Risk has received extensive publicity and has, on average, attracted over 1000 visitors per day since inception (34). Yet, like many such tools, little is known about how it affects social-cognitive precursors of behavior change. Understanding these effects will provide insights into how to use personalized risk assessment tools to influence key social-cognitive precursors of behavior change, inform the development of future risk assessment tools, offer advice about whether and how such tools should be incorporated into comprehensive multi-component behavior change interventions, and guide ongoing development of the Your Disease Risk tool.
Physical Activity
We examined the effects of Your Disease Risk in the context of physical activity for several reasons. First, it is one of the few modifiable behavioral risk factors for breast cancer that applies broadly across the population, is unrelated to reproductive factors (3, 9), and does not rely on medical technology or services. Second, less than 37% of U.S. women realize that insufficient physical activity is a risk factor for breast cancer (35). Third, only half of U.S. women obtain the recommended amount of physical activity (36). Lastly, despite the sizeable potential audience, no published data describe an intervention that uses a breast cancer risk assessment tool to encourage interest in physical activity.
Objectives and Hypotheses
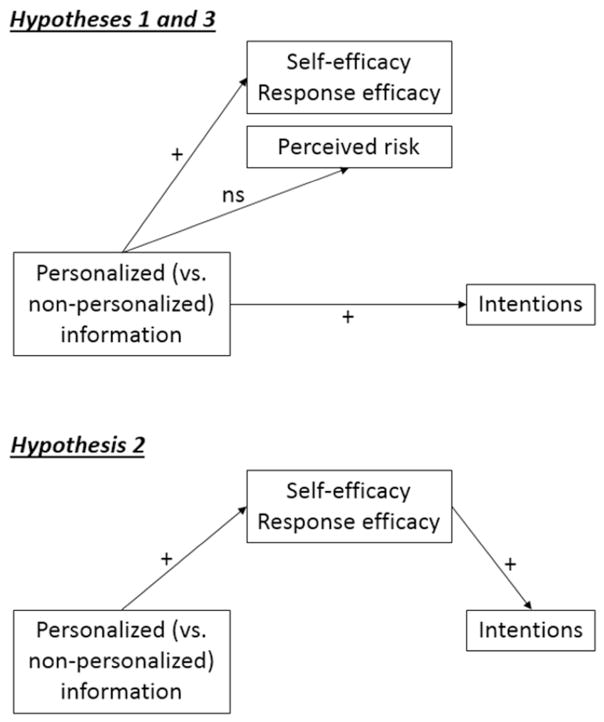
Our primary objective was to test the effects of an evidence-based, publically-available, online breast cancer risk assessment tool on key social-cognitive precursors of physical activity: behavioral intentions, self-efficacy, response efficacy, and perceived risk. The hypotheses were in accordance with HAPA and empirical research results demonstrating that higher self-efficacy, response efficacy, and perceived risk were associated with higher intentions to change behavior (21, 23, 24, 37, 38). We hypothesized that personalized information from the Your Disease Risk website (vs. non-personalized website information) would elicit higher levels of physical activity-related behavioral intentions, self-efficacy, and response efficacy (H1, see top of Figure 1). Also consistent with HAPA predictions, we hypothesized that any effect of personalized (versus non-personalized) information on intentions would be mediated by self-efficacy and response efficacy (H2; see bottom of Figure 1).
Figure 1.
Hypotheses 1–3.
Hypotheses regarding the effect of the tool on risk perceptions, on the other hand, were nuanced because the effect of personalizing information is contingent upon individuals’ objectively calculated risk estimate. First, we expected no main effect of study condition (personalized vs. non-personalized) on post-intervention risk perceptions (H3, see top of Figure 1); women who have many risk-increasing factors would be told they are at above average risk, which would elicit high risk perceptions. In contrast, women who have many risk-reducing factors would be told they are at below average risk, which would produce low risk perceptions. Comparing the risk perceptions of one group of people who are not given any personal risk information to another group comprised of both people who are told their personal risk is above average and people whose personal risk is below average should produce a null effect.
Second, based on research described above (10–12), we expected changes in risk perceptions within the personalized condition such that providing personalized information would increase the accuracy of women’s risk perceptions from pre- to post-intervention. Thus, among women in the personalized condition who initially underestimated their risk, risk perceptions were expected to increase pre- to post-intervention. Among women in the personalized condition who initially overestimated their risk, risk perceptions would decrease (H4).
Our secondary objective was to obtain preliminary data for a future randomized controlled trial. Specifically, we explored the potential for the tool to affect self-reported physical activity in the absence of other health behavior intervention components such as those that increase self-regulatory capabilities (23, 27) or address socio-contextual barriers (39). Due to the exploratory nature of this objective, no hypotheses were developed.
Methods
Participants
Individuals were eligible to participate if they were White or African American women aged 18–80 years who spoke and read English. Exclusion criteria were: a prior cancer diagnosis, having ever had a biopsy, and having ever visited the Your Disease Risk or the Susan G. Komen websites. Pilot testing revealed that limited computer literacy might prevent some participants from completing the study. Therefore, we excluded individuals who did not use the internet at least three days per week.
Participants were recruited from the St. Louis Metropolitan area from January 13, 2013 through April 28, 2014. Recruitment strategies included posting flyers in local small businesses such as restaurants and clothing stores, advertising in local newspapers, and contacting members of two research participant registries. One registry was managed by the Washington University Institute for Clinical and Translational Sciences. Participants were recruited to this registry via extensive community outreach activities, including mass media advertising and face-to-face recruitment in community locations. The Women’s Health Repository registry included women recruited by clinical staff immediately after undergoing annual mammographic screening.
Of the 373 women screened from all three recruitment strategies combined, 165 enrolled (44.1%). Of those, 132 (80.0%) completed the baseline survey and 105 (80.8%) completed the 4-week follow-up survey (Supplemental Table 1). Although the individuals who completed the eligibility screening differed by recruitment modality in terms of age, education, race/ethnicity, and reasons for eligibility (Supplemental Table 2), due to the properties of randomization these differences should be approximately equally distributed between study conditions.
Procedure
Participants completed the Time 1 study materials online at a location of their choice. After consenting, participants completed the Pre-Intervention Questionnaire. Participants were then randomly assigned to receive either personalized information about their risk of developing breast cancer via the Your Disease Risk website or a static list of risk factors that was listed on the Susan G. Komen website. Both websites addressed the same risk factors. Randomization was implemented automatically when participants clicked on the link to the study. Immediately after viewing the risk information, participants completed the Post-Intervention Questionnaire. Approximately 4 weeks later, at Time 2, each participant received a follow-up survey by mail. If participants did not respond, they received a reminder two weeks later. Time 1 took approximately 20 minutes to complete and Time 2 took approximately 5 minutes. Participants received $10 to complete the Time 1 survey and $5 for the Time 2 survey.
Study Conditions
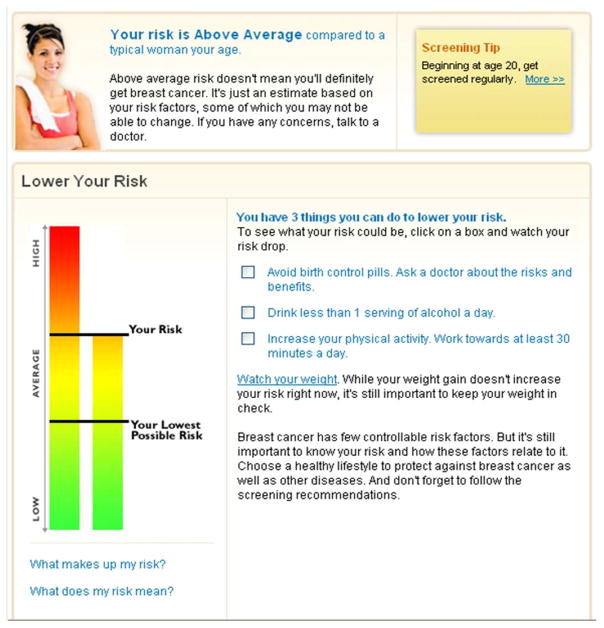
The personalized risk condition exposed participants to the breast cancer module of a version of the Your Disease Risk tool that had been formatted for use by university employees (30). The employee version differs from the current “live” version of Your Disease Risk only in the coloring of the background and in the addition of a picture of a young woman. All content and risk communication strategies remain the same. The tool assessed a variety of demographic, medical history, and behavioral risk factors (40). Then participants were provided with a vertical bar chart that depicted their risk visually along with text that indicated their risk was: very much below average, much below average, below average, average, above average, much above average, or very much above average (Figure 2). The bar chart and text were intended to target users’ risk perceptions. Information intended to affect response efficacy was also provided via text and by a visual depiction. Specifically, the risk output identified which of the participant’s current characteristics and behaviors increased and decreased her risk. Clicking on a box located beside a given health-protective behavior would lower the bar chart depicting their risk and alter the text as appropriate (e.g., “your risk is below average”). To increase self-efficacy, a given recommendation could be clicked to obtain information about risk reduction strategies. The interactive features were only available for modifiable risk factors such as physical activity, and it was not possible to determine whether a participant clicked on a given risk factor.
Figure 2.
Personalized risk results.
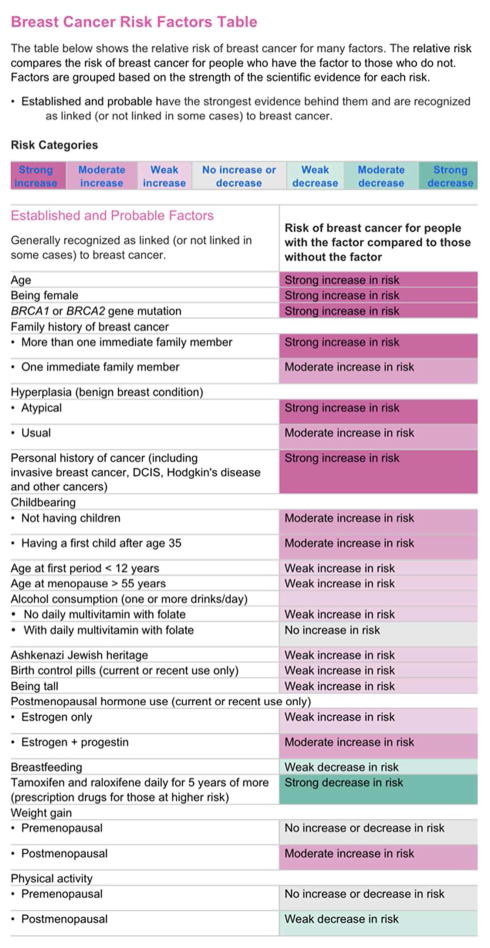
The non-personalized control condition exposed participants to a static list of the demographic, medical, and behavioral risk factors that were identical to those used to calculate the risk estimates for participants in the personalized condition. The static list used words and colors to indicate whether the factor conferred a strong, moderate, or weak increase or decrease in risk (Figure 3). The current list is located in the Risk Factors section of the Susan G. Komen website http://ww5.komen.org/BreastCancer/BreastCancerRiskFactorsTable.html.
Figure 3.
Non-personalized risk factor table.
Measures
All measures were obtained from prior sources and, where necessary, adapted based on feedback from 10 cognitive interviews (41). To minimize burden, we used many single-item measures that were obtained from existing literature or national surveys (22). Time 1 measures included the Pre- and Post- Intervention Questionnaire; Time 2 measures included the follow-up survey. The full questionnaire can be obtained from the senior author.
Pre-Intervention Questionnaire (Time 1a)
Demographics included age, race, ethnicity, educational attainment, and family history of breast cancer. Pre-intervention perceived risk was adapted from (11): “Compared to the average woman your age and race, do you think your risk of getting breast cancer is: 1=very much below average; 2=much below average; 3=below average; 4=average; 5=above average; 6=much above average; or 7=very much above average; 8=Don’t know?” Don’t know responses were treated as missing (n=6). The questionnaire also included an item assessing the possibility that responses might vary according to current physical activity motivation and behavior (42): “Exercising at “moderate” intensity involves activities like walking fast, bicycling, swimming, and vacuuming. Which of the following statements do you agree with most about exercising at moderate intensity for at least 30 minutes 4 or more days of the week? 1=I have never heard about doing this; 2=I already do this; 3=I’ve never thought about doing this; 4=I try to do this but I can’t always; 5=I’m not sure about doing this; 6=I don’t want to do this; 7=I do want to do this but I have not started yet.
Post-Intervention Questionnaire (Time 1b)
Following the intervention (described above) the main outcome measures were assessed: intentions to engage in physical activity, self-efficacy of engaging in physical activity, response efficacy, and perceived risk. The intentions item read (43): “I plan to exercise at moderate intensity for at least 30 minutes 4 or more days of the week, starting within the next 4 weeks. 1=definitely not; 2=probably not; 3=maybe; 4=probably yes; 5=definitely yes.” The wording for self-efficacy was (44): “Overall, how confident are you that you will exercise at moderate intensity for at least 30 minutes 4 or more days of the week, starting in the next 4 weeks? 1=not at all confident; 2=a little confident; 3=somewhat confident; 4=very confident; 5=extremely confident.” The response efficacy item read (45): “If you exercised at moderate intensity for at least 30 minutes 4 or more days of the week, would it reduce your chance of getting breast cancer? 1=definitely not; 2=probably not; 3=maybe; 4=probably yes; 5=definitely yes.” Consistent with prior research related to personalized risk communication (11), post-intervention perceived risk was adapted to accommodate the possibility that participants did not accept the information provided: “You may or may not agree with what the website said about breast cancer risk and risk factors. We are interested in what you believe. Compared to the average woman your age and race, what is your risk of developing breast cancer?” The response scale was identical to the scale for pre-intervention perceived risk. Breast cancer worry was also assessed but, due to a programming error, cannot be presented here.
Women in the personalized risk condition (but not the non-personalized condition) were asked to recall the personalized risk information they were provided. Recalled risk was assessed with the item (11): “According to the information on the website, is your risk of getting breast cancer…1=very much below average; 2=much below average; 3=below average; 4=average; 5=above average; 6=much above average; or 7=very much above average; 8=Don’t know.” Recalled risk was not asked of participants in the non-personalized condition because they did not receive a risk personalized risk estimate.
Follow-Up Questionnaire (Time 2)
Physical activity behavior was adapted from (22): “Within the past week, have you exercised at moderate intensity for at least 30 minutes 4 or more days of the week? (Yes/No).” Breast cancer worry, self-efficacy, response efficacy, and recall of risk information were assessed but not reported here.
Analysis Plan
Sample size
Sample size calculations were based on achieving a moderate effect (Cohen’s d of 0.5) of study condition on our primary outcome of intentions (24). This required 128 participants. Calculations were based on intentions because it is the cognition that is most proximal to behavior (21, 43), and HAPA and other stage theories of health behavior identify it as the most appropriate outcome for an intervention that targets the motivational phase of behavior change (23, 42). Logistical constraints required us to revise our initial effect size goal from d=0.4 (N=212). No data were analyzed prior to revising the effect size and recruitment goals.
Preliminary Analyses
Chi-square and t-tests were used to verify that participant demographic characteristics, pre-intervention risk perceptions, and physical activity stage (i.e., “I already [engage in the recommended amount of activity]” vs. all others) were distributed equally between study conditions, ps>.05. Pearson correlations were used to explore interrelationships among Time 1 social-cognitive variables.
Main Analyses
ANCOVAs were conducted to separately test the main effect of study condition on the social-cognitive variables of intentions, self-efficacy, response efficacy, and perceived risk. The 6 “don’t know” responses to the perceived risk item were treated as missing. Small cell sizes required using Fisher’s exact tests to determine whether and how women adjusted their risk perceptions in response to personalized risk information. Logistic regressions were used to examine the effect of study condition on self-reported engagement of physical activity at follow-up. All analyses included the following covariates: educational attainment (Less than Bachelor’s degree vs. Bachelor’s degree or higher), race/ethnicity (Non-Hispanic White vs. all others), family history of breast cancer (yes vs. no), and stage of physical activity (i.e., “I already [engage in the recommended amount of activity]” vs. all others).
Mediation of the effect of study condition on intentions was tested using the %INDIRECT macro for SAS. %INDIRECT calculates the bias-corrected bootstrapped 95% confidence intervals of the indirect effect of an independent variable on a dependent variable through the mediator while controlling for covariates (46, 47). Prior to running %INDIRECT, intentions, self-efficacy, and response efficacy were standardized using PROC STANDARD.
Results
Participants were randomized to the personalized (n=66) or non-personalized (n=66) study condition. Most participants were Non-Hispanic White (74.5%). Only 29% of participants had a Bachelor’s degree or higher, which is comparable to that of the U.S. adult population (Table 1). Most women in the personalized condition (56.8%) were at above average risk of breast cancer. Due to randomization, we expect that participants in the non-personalized condition were also at above average risk. Intercorrelations among the social cognitive variables were in the direction predicted by many theories of health behavior: Higher self-efficacy and response efficacy were associated with higher intentions to engage in physical activity, ps<.01 (Supplemental Table 3). Pre- and post-intervention risk perceptions were moderately correlated (r=.60, p<.01), as were the objective risk estimates and post-intervention risk perceptions in the personalized condition (r=.54, p<.01).
Table 1.
Participant characteristics (N=132)
| Participant characteristic | n | % |
|---|---|---|
| Educational attainment | ||
| Less than high school | 1 | 0.8% |
| High school degree | 41 | 31.1% |
| Some college | 38 | 28.8% |
| Associate's or technical degree | 13 | 9.8% |
| Bachelor's degree | 17 | 12.9% |
| Master's or doctorate degree | 21 | 15.9% |
| Missing | 1 | 0.8% |
| Race | ||
| Non-Hispanic White | 98 | 74.2% |
| Non-White | 34 | 25.8% |
| Objective risk (personalized condition only)a | ||
| Very much below average | 0 | 0.0% |
| Much below average | 1 | 2.3% |
| Below average | 16 | 27.3% |
| Average | 7 | 13.6% |
| Above average | 33 | 47.7% |
| Much above average | 5 | 9.1% |
| Very much above average | 0 | 0.0% |
|
|
||
| M | SD | |
|
|
||
| Objective risk (personalized condition only)a | 4.4 | 1.0 |
| Age | 55.8 | 9.1 |
Of the 66 participants randomized to the personalized risk condition, 4 did not complete the items required to calculate their objective risk estimates.
Primary Objective
Between-Group Effects of Study Condition on Social-Cognitive Constructs at Time 1 (H1)
As predicted, participants who received personalized risk information reported statistically significantly higher physical activity intentions, self-efficacy, and response efficacy compared to participants who received non-personalized risk information, ps<.05 (Table 2).1
Table 2.
Effect of personalized breast cancer risk information on Time 1 social-cognitive variablesa
| Outcome | F | p | η2partial | Non-personalized | Personalized | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean | SE | Mean | SE | ||||
| Hypothesis 1 (entire sample) | |||||||
| Intentions | 11.5 | 0.001 | 0.09 | 4.0 | 0.1 | 4.5 | 0.1 |
| Self-efficacy | 5.2 | 0.02 | 0.04 | 3.4 | 0.1 | 3.8 | 0.1 |
| Response efficacy | 7.2 | 0.01 | 0.06 | 3.5 | 0.1 | 3.9 | 0.1 |
|
| |||||||
| Hypothesis 3 (entire sample) | |||||||
| Post-intervention perceived risk | 0.0 | 0.99 | 0.00 | 4.1 | 0.2 | 4.1 | 0.2 |
|
| |||||||
| Hypothesis 4 (personalized only) | |||||||
| Change in perceived riskb | 15.2 | .001 | 0.35 | - | - | - | - |
| Initial underestimators | 4.4c | .001 | 0.78d | - | - | 0.7 | 0.2 |
| Initial overestimators | −2.6c | .02 | 0.69d | - | - | −1.1 | 0.4 |
| Initial accurate | −2.2c | .05 | 0.58d | - | - | −.5 | 0.2 |
All analyses controlled for the following covariates: educational attainment, race/ethnicity, family history of breast cancer, and stage of engagement in physical activity.
Change in perceived risk from pre- to post-intervention among women who received personalized risk information only, according to whether they overestimated, underestimated, or accurately underestimated their risk prior to engaging with the risk assessment tool, n=66. Mean values are difference scores.
Numbers are t-values, not F-values.
Numbers are Cohen’s D effect sizes, not partial η2 effect sizes.
Between-Group Effects of Study Condition on Post-Intervention Perceived Risk at Time 1 (H3)
Personalized and non-personalized risk information elicited similar levels of perceived risk, p=.99 (Table 2).
Mediators of Between-Group Effects of Study Condition on Intentions at Time 1 (H2)
A multiple mediator model confirmed that personalized information elicited higher intentions to engage in physical activity than non-personalized information by eliciting higher levels of self-efficacy and response efficacy. Study condition was a significant predictor of self-efficacy (β=0.3, SE=0.1, p=.02) and response efficacy (β=0.5, SE=0.2, p=.01). Higher self-efficacy was associated with higher intentions (β=0.8, SE=0.1, p<.0001), and higher response efficacy was associated with higher intentions (β=0.1, SE=0.1, p=.03). Based on 5000 bootstrapped resamples with bias-corrected confidence intervals, the indirect effect of study condition on intentions through self-efficacy was statistically significant (β=0.25, 95% CI=0.05–0.50). The indirect effect through response efficacy was also significant (β=0.07, 95% CI=0.01–0.16). The relative strength of self-efficacy and response efficacy on intentions were approximately equivalent (β=−0.19, 95% CI=−0.45–0.03). Perceived risk could not be tested as a mediator because doing so would have required stratifying analyses by risk status, which was unavailable for the non-personalized group.
Within-Group Pre-Post Intervention Changes in Accuracy of Perceived Risk at Time 1 (H4).2
To examine change in accuracy, we compared women’s objective risk estimates provided by Your Disease Risk to their pre-intervention and post-intervention risk perceptions. We began by identifying women who overestimated, accurately estimated, or underestimated their risk prior to receiving personalized information. Of the 59 women in the personalized condition who provided complete data on the risk assessment and perception variables, 31 (52.5%) initially underestimated their risk prior to receiving personalized risk information (i.e., their pre-intervention risk perceptions were lower than their objective risk), 14 (23.7%) initially accurately estimated their risk, and 14 (23.7%) initially overestimated their risk.
Next, we examined whether women adjusted their risk perceptions to become more consistent with the objective personalized risk estimates they were provided. The mean change in perceived risk from pre- to post- intervention was significantly different among women who initially underestimated, accurately estimated, or overestimated their risk, F(2, 56)=15.2, p<.001, η2=.35. Follow up one-sample t-tests showed that personalized information increased perceptions of risk among women who initially underestimated their risk and decreased perceptions of risk among women who initially overestimated their risk, ps<.05, as predicted (Table 2). It also marginally decreased risk perceptions among women who initially estimated their risk accurately, p=.05.
Third, we examined the proportion of women who fully adjusted their post-intervention risk perceptions to the exact same level as the objective risk estimate provided by the risk assessment tool (i.e., exact accuracy). Of the 31 women who initially underestimated their risk prior to receiving personalized risk information, 24 (77.4%) continued to underestimate their risk after receiving personalized information. Of the 14 women who initially overestimated their risk, 7 (50.0%) continued to overestimate, Fisher’s exact test, Cramer’s V=0.56, p<.001.
In sum, although providing risk personalized risk information prompted women to adjust their risk perceptions to be more consistent with their objective risk, persistent misestimation of risk was widespread (i.e., few women’s risk perceptions were equal to the estimate they were provided).
Secondary Objective
Between-Group Effects on Self-Reported Physical Activity at Follow-Up (Time 2)
Participants who received personalized and non-personalized risk information had equal odds of reporting engaging in the recommended amount of physical activity, OR=0.9, 95% CI 0.4–0.9, p=.64.
Exploratory Supplemental Analyses
We sought to explore possible factors that were associated with persistent misestimation of risk. Because only 7 women engaged in persistent overestimation, we examined only persistent underestimation.
We began by considering whether persistent underestimation was due to misremembering the risk estimates provided. However, correct recall was similar between women who did and who did not underestimate their risk, Fisher’s exact test p=0.69. Specifically, 18 of the 24 women (75%) who continued underestimating their risk recalled the information correctly, as did 24 of the 35 women (68.6%) who did not continue underestimating their risk (i.e., those who had accurate pre-intervention perceptions, who fully corrected their perceptions, and who continued to overestimate their risk). This suggests that persistent underestimation of risk was not due to misremembering.
We next explored whether women purposefully rejected the risk information by comparing women’s post-intervention risk perceptions to their recalled risk estimates (11). Of the 24 women who continued underestimating their risk (i.e., both pre- and post-intervention risk perceptions were lower than their objective risk), 18 (75%) believed that their risk was lower than they recalled being told. Only 2 of the 35 (5.7%) women who did not continue underestimating their risk believed their risk was lower than they recalled being told (i.e., those who had accurate pre-intervention perceptions, who fully corrected their perceptions, and who continued to overestimate their risk), Fisher’s exact test p<.001. This suggests that persistent underestimation of risk may be due to skepticism about the validity of the provided estimate.
Discussion
This study makes three key contributions. First, it shows that a tool that provides laypeople with the results of a risk prediction model using theoretically and empirically-supported strategies can have beneficial effects on crucial social-cognitive precursors of behavior change (H1) and that the mediational pathways are consistent with those predicted by many health behavior theories (H2)(21). Second, it is the first study to identify, in a sample of women recruited from the general population, possible defensive processing of real breast cancer risk information provided by a highly-publicized Internet-based risk assessment tool. Third, it demonstrates that a publically-available, widely used breast cancer risk assessment tool is associated with higher accuracy of risk perceptions in a way consistent with risk assessment tools used in clinical settings (48), thereby demonstrating a potentially significant impact at the population level. Our research also expands on prior research by assessing several key mediators of behavior change in a single model. Consequently, we were able to improve understanding of the “active ingredients” through which Your Disease Risk increased intentions to engage in physical activity. Critically, our findings were consistent with prior research reporting that self-efficacy and response efficacy are important mediators of effective interventions (24).
The absence of any effect on self-reported physical activity at follow-up is consistent with prior interventions that seek to modify physical activity and other health behaviors by providing personalized risk information (16–18, 49). Volitional behavior change often involves moving through several stages (23, 42). The goal of the first phase is to increase motivation/intentions to change by increasing perceptions of risk, self-efficacy, and response-efficacy. Our study achieved this important milestone. However, to help motivated people actually enact and maintain behavior change often requires building self-regulatory skills (23, 50). We plan to develop such an intervention component and incorporate it into Your Disease Risk.
Effects on Perceived Risk
Although risk perceptions among women who received personalized information became more consistent with their objective risk estimates (H4), many women continued to underestimate their risk. This finding, in addition to the discrepancy between high recall of personalized information and limited full acceptance of that same information, is consistent with prior reports of skepticism about the validity of the information derived from risk assessment tools (11, 51, 52). Some of the skepticism might be due to the tendency for people to view their health status as embedded within a broader context that includes personal and family “lived experiences,” trust in physicians, and emotions (20, 51, 52). For these individuals, probabilistic information is only one piece of information to consider when evaluating their risk status.
The data also offer very preliminary evidence that people might engage in defensive processing when receiving personalized breast cancer risk results. Although most women who received personalized risk information were at above average objective risk of developing breast cancer (57%), most women also began the study believing that they were at average or below average risk (79%). Such “unrealistic optimism” is a type of motivated reasoning in which people draw conclusions about their risk status to reduce negative affect and to protect their sense of self in the face of threatening information (53, 54). That many participants continued to underestimate their risk despite accurately recalling the information provided is consistent with other research reporting that participants rejected threatening health information when they expected good news (55).
Another possible indication of defensive processing is that women who continued to underestimate their risk were at disproportionately higher objective risk than were women who accepted the information. This is also consistent with research reporting reluctance to accept threatening health feedback (56). However, determining the causal link between information rejection and defensive processing was not the primary objective of this research, so we cannot say with certainty that our participants displayed defensive processing. It is also possible that participants continued to underestimate their risk because they were uncertain about whether the health history data they entered into YourDiseaseRisk was correct, not because they were concerned about the accuracy of the algorithm or that they were threatened by the findings. Therefore, these results should be viewed as hypothesis-generating and other possible explanations, such as limited health literacy or numeracy, should be ruled out.
Limitations and Future Research
These results should be interpreted in light of the following considerations. Most importantly, the non-personalized condition differed from the personalized condition in the way the information was organized. This could have led to differences in readability and interpretability that were not accounted for by randomization and are not due to personalization. That said, it is difficult to see how a non-personalized list of risk factors could be formatted as simply as the vertical bar chart. Importantly, the Komen site’s use of colors to indicate the strength and direction of the risk factor effect provides a strong visual cue that viewers can use to draw conclusions that are not provided by lists of risk factors that are not shaded (57). This could have mitigated some of the negative effect of the list format on readability and comprehensibility, but it is not possible to rule out the possibility that our effects were due to factors other than personalization of information.
An important caveat to the findings about changes in risk perceptions from pre- to post-intervention is that, because objective risk estimates could not be calculated for participants in the control condition, the Hypothesis 4 and exploratory analyses could not take advantage of the benefits of randomization and are therefore vulnerable to the limitations of a pre-post design (58). Although our findings could be due to regression to the mean or demand effects, it is unlikely that our findings are due to an unknown event occurring in the very short time period (5 minutes) separating the pre- and post-tests. In addition, other recently-published research also reported that personalizing risk information can improve accuracy in some circumstances.(48) Nevertheless, we cannot draw causal inferences about changes in risk perception accuracy or defensive processing from our data. Future research should explore these issues using a true experimental design.
Several other potential limitations should be considered. Participant characteristics differed by recruitment modality, but we could not control for or stratify by this variable. Individuals recruited in different ways may respond differently to the intervention. We also do not know which participants clicked the link to a page explaining how they could get more physical activity, nor do we know how much time they spent exploring the contents of the website. The nature of the comparison group and timing of measures do not allow us to determine whether our findings are due to the specific results of the tool or due to deeper information processing as a result of actively engaging with the tool. Future research could examine how people engage with the tool using eye tracking technologies. More fine-grained assessments of physical activity are also needed to examine incremental changes in activity. It is also important to examine how people’s responses to tools that estimate the risk of dying from cancer differ from tools like Your Disease Risk that estimate the risk of developing cancer. Furthermore, single-item measures may not be as stable as multiple item measures when measuring psychosocial constructs. Finally, many of our sub-analyses are based on relatively small sample sizes and should be replicated.
Another important area of future research is examining how different sources of information influence responses to online risk assessment tools. For example, would participants react more favorably to such a tool if it were “prescribed” by their primary care provider than if they simply found it online while searching for health information? It is also important to examine factors that might inhibit and facilitate implementing risk assessment tools in different types of healthcare settings. Future research should also investigate how best to incorporate risk assessment tools into more comprehensive interventions, including those that help participants develop self-regulatory strategies such as making action plans to overcoming barriers to behavior change.
Conclusions
Well-designed risk assessment tools can target constructs most likely to motivate behavior change. This may be particularly promising if used as a facilitation tool for doctor-patient communication or in contexts and settings where access to traditional modes of risk information delivery are limited, such as in underserved urban and rural communities.
Furthermore, because online risk assessment tools may provide greater reach to potential audience members than non-Internet-based interventions, an online tool that increases motivation to change behavior may have a considerable positive public health impact.
Supplementary Material
Acknowledgments
Funding: This research was supported by funding from the Barnes Jewish Hospital Foundation, NIH grant R01CA190391 (PI: Waters), the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH), and the National Cancer Institute’s Cancer Prevention Fellowship Program. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Footnotes
Because different communication strategies sometimes work differently for people from different socio-demographic backgrounds, we explored whether education, race, and health literacy moderated between-group effects on cognitions. A statistically significant interaction suggested that family history of breast cancer may moderate the effect of study condition on intentions to engage in physical activity, p=.03. Follow-up t-tests showed that, among participants with a breast cancer family history, personalized information elicited higher physical activity intentions than did non-personalized information, p=.02. However, among participants with no family history, personalized and non-personalized information elicited similar intentions, p=.12. No other statistically significant interactions emerged, ps>.05.
Only women in the personalized condition completed a risk assessment. Therefore, we could only examine whether providing personalized risk increased the accuracy of risk perceptions within that group.
Conflict of Interest: The authors declare that they have no conflicts of interest.
References
- 1.American Cancer Society. Cancer Facts & Figures 2015. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 2.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–86. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 3.Colditz GA, Hankinson SE. The Nurses' Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5(5):388–96. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 4.Freedman AN, Graubard BI, Rao SR, McCaskill-Stevens W, Ballard-Barbash R, Gail MH. How many US women are eligible to use tamoxifen for breast cancer chemoprevention? How many women would benefit? The American Journal of Oncology Review. 2004;3(1):47. [Google Scholar]
- 5.Graubard BI, Freedman AN, Gail MH. Five-year and lifetime risk of breast cancer among U.S. subpopulations: implications for magnetic resonance imaging screening. Cancer Epidemiol Biomarkers Prev. 2010;19(10):2430–6. doi: 10.1158/1055-9965.EPI-10-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helmes AW, Culver JO, Bowen DJ. Results of a randomized study of telephone versus in-person breast cancer risk counseling. Patient Educ Couns. 2006;64(1–3):96–103. doi: 10.1016/j.pec.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Edwards AG, Naik G, Ahmed H, Elwyn GJ, Pickles T, Hood K, et al. Personalised risk communication for informed decision making about taking screening tests. The Cochrane database of systematic reviews. 2013;2:CD001865. doi: 10.1002/14651858.CD001865.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards A, Unigwe S, Elwyn G, Hood K. Effects of communicating individual risks in screening programmes: Cochrane systematic review. BMJ. 2003;327:703. doi: 10.1136/bmj.327.7417.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA internal medicine. 2016;176(6):816–25. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreuter MW, Strecher VJ. Changing inaccurate perceptions of health risk: Results from a clinical trial. Health Psychol. 1995;14(1):56–63. doi: 10.1037//0278-6133.14.1.56. [DOI] [PubMed] [Google Scholar]
- 11.Weinstein ND, Atwood K, Puleo E, Fletcher R, Colditz G, Emmons KM. Colon cancer: Risk perceptions and risk communication. Journal of Health Communication. 2004;9:53–65. doi: 10.1080/10810730490271647. [DOI] [PubMed] [Google Scholar]
- 12.Croyle RT, Lerman C. Risk communication in genetic testing for cancer susceptibility. J Natl Cancer Inst Monogr. 1999;25:59–66. doi: 10.1093/oxfordjournals.jncimonographs.a024210. [DOI] [PubMed] [Google Scholar]
- 13.Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21(1):37–44. doi: 10.1177/0272989X0102100105. [DOI] [PubMed] [Google Scholar]
- 14.Zikmund-Fisher BJ. The right tool is what they need, not what we have: a taxonomy of appropriate levels of precision in patient risk communication. Med Care Res Rev. 2013;70(1 Suppl):37S– 49S. doi: 10.1177/1077558712458541. [DOI] [PubMed] [Google Scholar]
- 15.Windschitl PD, Martin R, Flugstad AR. Context and the interpretation of likelihood information: the role of intergroup comparisons on perceived vulnerability. J Pers Soc Psychol. 2002;82(5):742–55. doi: 10.1037//0022-3514.82.5.742. [DOI] [PubMed] [Google Scholar]
- 16.Drieling RL, Ma J, Thiyagarajan S, Stafford RS. An Internet-based osteoporotic fracture risk program, effect on knowledge, attitudes, and behaviors. Journal of Women’s Health. 2011;20(12):1895– 907. doi: 10.1089/jwh.2010.2515. [DOI] [PubMed] [Google Scholar]
- 17.Price HC, Griffin SJ, Holman RR. Impact of personalized cardiovascular disease risk estimates on physical activity-a randomized controlled trial. Diabet Med. 2011;28(3):363–72. doi: 10.1111/j.1464-5491.2010.03212.x. [DOI] [PubMed] [Google Scholar]
- 18.Welschen LM, Bot SD, Kostense PJ, Dekker JM, Timmermans DR, van der Weijden T, et al. Effects of cardiovascular disease risk communication for patients with type 2 diabetes on risk perception in a randomized controlled trial: the @RISK study. Diabetes Care. 2012;35(12):2485–92. doi: 10.2337/dc11-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waters EA, Sullivan HW, Nelson W, Hesse BW. What is my cancer risk? Identifying how Internetbased cancer risk calculators convey individualized risk estimates to the public. J Med Internet Res. 2009;11(3):e33. doi: 10.2196/jmir.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmberg C, Waters EA, Whitehouse K, Daly M, McCaskill-Stevens W. My Lived Experiences Are More Important Than Your Probabilities: The Role of Individualized Risk Estimates for Decision Making About Participation in the Study of Tamoxifen and Raloxifene (STAR) Med Decis Making. 2015 doi: 10.1177/0272989X15594382. [DOI] [PMC free article] [PubMed]
- 21.Conner M, Norman P, editors. Predicting Health Behaviour. Buckingham/Philadelphia: Open University Press; 1995. [Google Scholar]
- 22.National Cancer Institute. Health Information National Trends Survey (HINTS) items for years 2003–2007. Bethesda, MD: National Cancer Institute; 2009. [Available from: http://hints.cancer.gov/questions/all-questions1.jsp. [Google Scholar]
- 23.Schwarzer R. Social-cognitive factors in changing health-related behavior. Curr Dir Psychol Sci. 2001;10(2):47–51. [Google Scholar]
- 24.Sheeran P, Harris PR, Epton T. Does Heightening Risk Appraisals Change People's Intentions and Behavior? A Meta-Analysis of Experimental Studies. Psychol Bull. 2013 doi: 10.1037/a0033065. [DOI] [PubMed] [Google Scholar]
- 25.Sheeran P, Maki A, Montanaro E, Avishai-Yitshak A, Bryan A, Klein WM, et al. The Impact of Changing Attitudes, Norms, and Self-Efficacy on Health-Related Intentions and Behavior: A Meta-Analysis. Health Psychol. 2016 doi: 10.1037/hea0000387. [DOI] [PubMed] [Google Scholar]
- 26.Harkin B, Webb TL, Chang BP, Prestwich A, Conner M, Kellar I, et al. Does monitoring goal progress promote goal attainment? A meta-analysis of the experimental evidence. Psychol Bull. 2016;142(2):198–229. doi: 10.1037/bul0000025. [DOI] [PubMed] [Google Scholar]
- 27.Gollwitzer PM, Sheeran P. Implementation intentions and goal achievement: A meta-analysis of effects and processes. Adv Exp Soc Psychol. 2006;38:69–119. [Google Scholar]
- 28.Jackson KM, Aiken LS. Evaluation of a multicomponent appearance-based sun-protective intervention for young women: uncovering the mechanisms of program efficacy. Health Psychol. 2006;25(1):34–46. doi: 10.1037/0278-6133.25.1.34. [DOI] [PubMed] [Google Scholar]
- 29.Kypri K, McAnally HM. Randomized controlled trial of a web-based primary care intervention for multiple health risk behaviors. Prev Med. 2005;41(3–4):761–6. doi: 10.1016/j.ypmed.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Colditz GA. Your Disease Risk. 2004 www.yourdiseaserisk.wustl.edu. [Available from: www.yourdiseaserisk.wustl.edu.
- 31.Emmons KM, Koch-Weser S, Atwood K, Conboy L, Rudd R, Colditz G. A qualitative evaluation of the Harvard Cancer Risk Index. Journal of health communication. 1999;4:181–93. doi: 10.1080/108107399126904. [DOI] [PubMed] [Google Scholar]
- 32.Emmons KM, Wong M, Puleo E, Weinstein N, Fletcher R, Colditz G. Tailored computer-based cancer risk communication: Correcting colorectal cancer risk perception. Journal of health communication. 2004;9:127–41. doi: 10.1080/10810730490425295. [DOI] [PubMed] [Google Scholar]
- 33.Colditz GA. Your Cancer Risk [replaced by Your Disease Risk] 2000 [Available from: www.yourdiseaserisk.wustl.edu.
- 34.Waters EA, Colditz GA, Dart H. Your Disease Risk: The culmination of 17 years of transdisciplinary research. National Cancer Institute; Mar 22, 2012. [Google Scholar]
- 35.Wang C, Miller SM, Egleston BL, Hay JL, Weinberg DS. Beliefs about the causes of breast and colorectal cancer among women in the general population. Cancer Causes Control. 2010;21(1):99–107. doi: 10.1007/s10552-009-9439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control & Prevention. Adult participation in aerobic and musclestrengthening physical activities--United States, 2011. MMWR Morb Mortal Wkly Rep. 2013;62(17):326– 30. [PMC free article] [PubMed] [Google Scholar]
- 37.Webb TL, Sheeran P. Does changing behavioral intentions engender behavior change? A metaanalysis of the experimental evidence. Psychol Bull. 2006;132(2):249–68. doi: 10.1037/0033-2909.132.2.249. [DOI] [PubMed] [Google Scholar]
- 38.Milne S, Sheeran P, Orbell S. Prediction and intervention in health-related behavior: A metaanalytic review of protection motivation theory. J Appl Soc Psychol. 2000;30(1):106–43. [Google Scholar]
- 39.Stokols D. Social ecology and behavioral medicine: implications for training, practice and policy. Behav Med. 2000;26:129–38. doi: 10.1080/08964280009595760. [DOI] [PubMed] [Google Scholar]
- 40.Colditz GA, Rosner B. Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses' Health Study. Am J Epidemiol. 2000;152(10):950–64. doi: 10.1093/aje/152.10.950. [DOI] [PubMed] [Google Scholar]
- 41.Willis GB. Cognitive Interviewing: A Tool for Improving Questionnaire Design. Thousand Oaks, CA: Sage Publications; 2004. p. 352. [Google Scholar]
- 42.Weinstein ND, Sandman PM. The precaution adoption process model. In: Glanz K, Rimer BK, Lewis FM, editors. Health behavior and health education: Theory, research, and practice. 3. San Francisco, CA: John Wiley and Sons; 2002. pp. 121–43. [Google Scholar]
- 43.Conner M, Sparks P. The theory of planned behaviour and health behaviours. In: Conner M, Norman P, editors. Predicting Health Behaviour. Philadelphia, PA: Open University Press; 1995. pp. 121–62. [Google Scholar]
- 44.Lorig K, Stewart A, Ritter P, González V, Laurent D, Lynch J. Outcome measures for health education and other health care interventions. Thousand Oaks, CA: Sage; 1996. [Google Scholar]
- 45.Schuz B, Sniehotta FF, Wiedemann A, Seemann R. Adherence to a daily flossing regimen in university students: effects of planning when, where, how and what to do in the face of barriers. J Clin Periodontol. 2006;33(9):612–9. doi: 10.1111/j.1600-051X.2006.00967.x. [DOI] [PubMed] [Google Scholar]
- 46.Hayes AF. SPSS, SAS, and Mplus macros and code. [Available from: http://www.afhayes.com/spss-sas-and-mplus-macros-and-code.html.
- 47.Hayes AF, Scharkow M. The relative trustworthiness of inferential tests of the indirect effect in statistical mediation analysis: does method really matter? Psychol Sci. 2013;24(10):1918–27. doi: 10.1177/0956797613480187. [DOI] [PubMed] [Google Scholar]
- 48.Haas JS, Baer HJ, Eibensteiner K, Klinger EV, St Hubert S, Getty G, et al. A Cluster Randomized Trial of a Personalized Multi-Condition Risk Assessment in Primary Care. Am J Prev Med. 2016 doi: 10.1016/j.amepre.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harle CA, Downs JS, Padman R. Effectiveness of personalized and interactive health risk calculators: a randomized trial. Med Decis Making. 2012;32(4):594–605. doi: 10.1177/0272989X11431736. [DOI] [PubMed] [Google Scholar]
- 50.de Bruin M, Sheeran P, Kok G, Hiemstra A, Prins JM, Hospers HJ, et al. Self-regulatory processes mediate the intention-behavior relation for adherence and exercise behaviors. Health Psychol. 2012;31(6):695–703. doi: 10.1037/a0027425. [DOI] [PubMed] [Google Scholar]
- 51.Han PK, Hootsmans N, Neilson M, Roy B, Kungel T, Gutheil C, et al. The value of personalised risk information: a qualitative study of the perceptions of patients with prostate cancer. BMJ open. 2013;3(9):e003226. doi: 10.1136/bmjopen-2013-003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scherer LD, Ubel PA, McClure J, Greene SM, Alford SH, Holtzman L, et al. Belief in numbers: When and why women disbelieve tailored breast cancer risk statistics. Patient Educ Couns. 2013;92(2):253–9. doi: 10.1016/j.pec.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shepperd JA, Klein WM, Waters EA, Weinstein ND. Taking stock of unrealistic optimism. Perspect Psychol Sci. 2013;8(4):395–411. doi: 10.1177/1745691613485247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kunda Z. The case for motivated reasoning. Psychol Bull. 1990;108(3):480–98. doi: 10.1037/0033-2909.108.3.480. [DOI] [PubMed] [Google Scholar]
- 55.Shepperd JA, Novell CA, O'Neill SC, Docherty SL, Sanderson SC, McBride CM, et al. Contemplating genetic feedback regarding lung cancer susceptibility. Ann Behav Med. 2014;47(3):395– 403. doi: 10.1007/s12160-013-9561-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Croyle RT, Sun Y-C, Louie DH. Psychological minimization of cholesterol test results: Moderators of appraisal in college students and community residents. Health Psychol. 1993;12(6):503–7. doi: 10.1037//0278-6133.12.6.503. [DOI] [PubMed] [Google Scholar]
- 57.Lipkus IM, Hollands JG. The visual communication of risk. J Natl Cancer Inst Monogr. 1999;25(1):149–63. doi: 10.1093/oxfordjournals.jncimonographs.a024191. [DOI] [PubMed] [Google Scholar]
- 58.Cook TD, Campbell DT. Quasi-Experimentation: Design & Analysis Issues for Field Settings. Boston, MA: Houghton Mifflin Company; 1979. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.