Abstract
G protein-coupled receptors (GPCRs) comprise the largest family of receptors in humans. Traditional activation of GPCRs involves binding of a ligand to the receptor, activation of heterotrimeric G proteins and induction of subsequent signaling molecules. It is now known that GPCR signaling occurs through G protein-independent pathways including signaling through β-arrestin as well as transactivation of other receptor types. Generally, transactivation occurs when activation of one receptor leads to the activation of another receptor(s). GPCR-mediated transactivation is an essential component of GPCR signaling, as activation of other receptor types, such as receptor tyrosine kinases (RTKs), allows GPCRs to expand their signal transduction and affect various cellular responses. Several mechanisms have been identified for receptor transactivation downstream of GPCRs, one of which involves activation of extracellular proteases, such as A Disintegrin and Metalloprotease (ADAMs) and matrix metalloproteases (MMPs). These proteases cleave and release ligands that are then able to activate their respective receptors. ADAMs and MMPs can be activated via various mechanisms downstream of GPCR activation, including activation via second messenger, direct phosphorylation or direct G protein interaction. Additional understanding of the mechanisms involved in GPCR-mediated protease activation and subsequent receptor transactivation could lead to identification of new therapeutic targets.
Keywords: GPCRs, MMPs, ADAMs, Transactivation
Introduction
G protein-coupled receptors (GPCRs) represent the largest family of membrane proteins in the human genome with approximately 800 genes encoding functional GPCRs (1). These seven-transmembrane receptors bind a diverse range of ligands including proteins, small molecules, drugs, hormones, odorants and photons (2, 3). GPCRs are involved in many physiologic and pathologic processes and, therefore, are one of the main targets for researchers and pharmaceutical companies. In fact, nearly 50% of all drugs prescribed today target GPCRs (4).
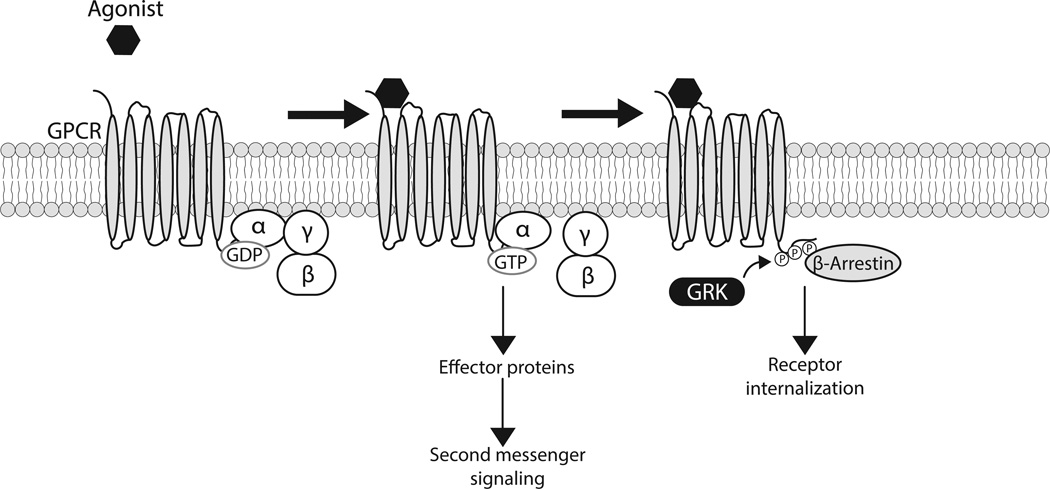
GPCRs lack intrinsic enzymatic activity and are coupled to heterotrimeric guanine nucleotide-binding proteins (G proteins), Gα, Gβ, and Gγ (4–6). Early understanding of GPCR signaling involved binding of a ligand to the receptor, stabilization of the receptor in an active conformation and subsequent activation of G proteins leading to an array of downstream signaling pathways through various effector proteins (5, 7). In an inactive receptor state, Gα is bound to the Gβγ dimer and GDP. After agonist activation, the receptor acts as a guanine nucleotide exchange factor to facilitate the exchange of GDP for GTP within the Gα subunit. The activated Gα protein dissociates from the Gβγ subunits, and the G proteins can then trigger effectors such as adenylyl cyclase, calcium, phospholipase C and various kinases, which further propagate the signal in the cell. After the receptor has been activated, downstream signaling is attenuated through receptor internalization, which is facilitated by phosphorylation of the receptor via G protein-coupled receptor kinases (GRKs) followed by binding of β-arrestin (Figure 1) (8, 9). While β-arrestin was first believed to play a specific role in signal termination and receptor internalization, it is now known that GPCR signaling also involves β-arrestin-dependent activation of divergent signaling pathways (10, 11). Various studies have been performed to delineate G protein and β-arrestin-mediated signaling, including genetic deletion of GRKs or β-arrestin, application of small molecule inhibitors of specific signaling molecules, and RNA silencing of G protein and β-arrestin pathway components (11–14).
Figure 1. GPCR signaling and desensitization.
Activation of GPCRs begins with binding of an agonist to the receptor. This leads to the exchange of GDP for GTP within the G protein α subunit followed by dissociation of the βγ subunit. These G proteins can then activate downstream effectors leading to various signaling pathways. GPCR signaling is halted via phosphorylation of the receptor by GRK, which then leads to internalization of the receptor through β-arrestin.
It is now understood that GPCRs are also able to exert effects through transactivation of other receptor types. Receptor transactivation allows for crosstalk between different signaling systems and plays a key role in coordination of extracellular stimuli and intracellular signaling (15–17). This crosstalk works to diversify signal transduction pathways that are involved in both physiological and pathological conditions (15). Several mechanisms have been identified by which GPCRs transactivate receptor tyrosine kinases (RTKs), as well as other receptor types, which are separated into ligand independent and ligand dependent mechanisms. In the ligand-independent mechanism, GPCR activation triggers second messengers, such as protein kinase C (PKC), non-receptor protein tyrosine kinases, β-arrestin, reactive oxygen species, and Ca2+ ions, which induce tyrosine phosphorylation and RTK activation. The ligand-dependent mechanism, more specifically known as triple-membrane-passing-signal (TMPS) mechanism, involves activation of extracellular proteases, namely, matrix metalloproteinases (MMPs) and A Disintegrin And Metalloproteases (ADAMs), which cleave and release agonists that are then free to activate their respective receptors (15).
This review focuses on the TMPS mechanism of receptor transactivation and the mechanisms of extracellular protease activation downstream of GPCRs, as this activation plays an essential role in propagation of GPCR signaling.
Receptor Transactivation
The process of transactivation was first defined by Berry et al. as “the process whereby ligand stimulation of one receptor leads to activation of another, distinct receptor” (18). Recently, a new definition of receptor transactivation has been proposed as “the agonist occupancy of its cognate GPCR complex which leads in a relatively short time and in the absence of “de novo” protein synthesis to the activation of and cytosolic generation of the immediate downstream product(s) of a second cell surface protein kinase receptor” (19). In 1996, Ullrich and colleagues discovered the GPCR-mediated transactivation of an RTK, the epidermal growth factor receptor (EGFR). RTKs are cell surface receptors that are activated by ligand binding to the extracellular domain, which induces dimerization of the receptor and autophosphorylation of tyrosine residues on the cytosolic domains. It was reported that GPCR agonists, endothelin-1 and thrombin, could transactivate the EGFR in Rat-1 fibroblasts (16). Stimulation of GPCRs led to an increase in ERK1/2 phosphorylation that could be mitigated by the EGFR inhibitor, indicating that ERK1/2 activation occurs downstream of EGFR activation (16). This is important because GPCRs are not capable of directly generating cell growth signals, but their ability to transactivate a growth factor receptor allows GPCR signaling to generate a cell growth response.
Since the first report of GPCR-mediated receptor transactivation, there have been nearly 200 studies involving RTK transactivation by GPCRs. GPCR-EGFR crosstalk has been identified in a variety of cell types such as vascular smooth muscle cells, PC12 cells, human keratinocytes, and a variety of cancer cells (15, 20–22). EGFR activation occurs downstream of several GPCRs and remains the paradigm of GPCR-mediated transactivation (17). The concept of receptor transactivation via GPCRs has also been expanded to include activation of other receptor types, such as receptor serine/threonine kinases and other GPCRs (19, 23–25). For example, transactivation of the TGFβ receptor appears to occur downstream of the lysophophatidic acid receptor and protease activated receptor (PAR)-1 in mice subjected to bleomycin-induced lung injury indicating a potential role for TGFβ receptor transactivation in acute lung injury and fibrosis (26, 27).
Triple-membrane-passing-signal (TMPS) mechanism of receptor transactivation
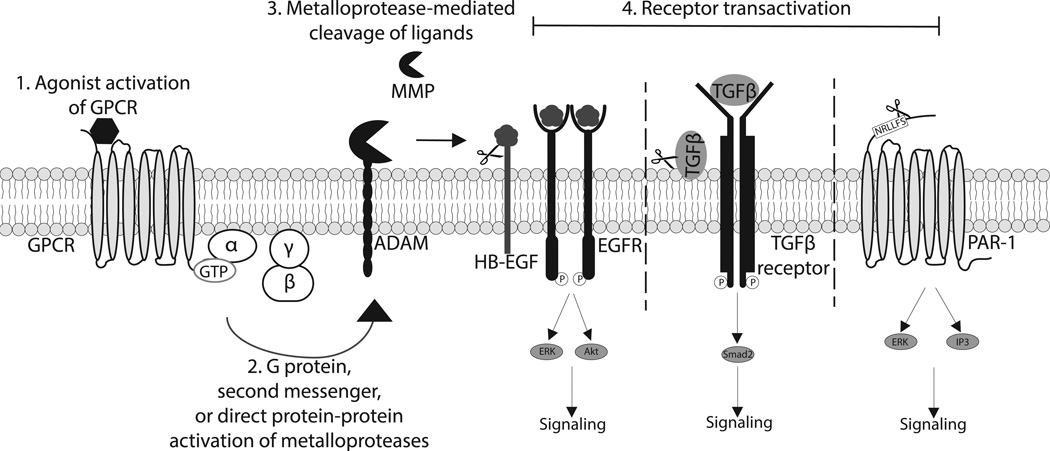
The involvement of extracellular proteases in receptor transactivation was first described in 1999 using a chimeric RTK that contained the EGFR ectodomain with the transmembrane and intracellular domains of the platelet-derived growth factor receptor (28). Treatment of fibroblasts containing the chimeric RTK with GPCR agonists led to transactivation of the artificial RTK but not endogenous platelet derived growth factor receptors, indicating that transactivation of the chimeric RTK did not involve intracellular signaling pathways, but was dependent on extracellular EGFR ligand-binding (28). The involvement of extracellular proteases in this receptor transactivation process was confirmed through inhibition of heparin-binding EGF (HB-EGF) function and metalloprotease activity, which completely abrogated GPCR-mediated transactivation of the EGFR (28). These studies led to the understanding that GPCR activation induces extracellular protease activation, which cleaves HB-EGF and allows the growth factor to bind to the EGFR and induce signaling (Figure 2).
Figure 2. Ligand-dependent mechanism of GPCR-mediated receptor transactivation.
1) Agonist binding of a GPCR leads to activation of heterotrimeric G proteins. 2) Downstream GPCR signaling can lead to activation of ADAMs or MMPs via direct G protein interaction, second messenger activation, or direct protein-protein interaction. 3) Activated ADAMs and MMPs can cleave and release various ligands leading to subsequent 4) receptor transactivation. Activation of EGFR by its ligands, such as HB-EGF, elicits multiple signaling pathways including ERK and Akt activation. Protease release of TGFβ and subsequent activation of the TGFβ receptor leads to canonical signaling through Smad2. Activation of PAR1 via MMP13 leads to ERK1/2 phosphorylation and generation of IP3. MMP13-mediated PAR1 transactivation appears to elicit biased signaling at this receptor, as IP3 generation is decreased compared to canonical agonist, thrombin, stimulation.
Since the original studies identifying the involvement of HB-EGF and proteases in RTK transactivation, several other ligands have been identified including amphiregulin (AR) and transforming growth factor-α, and the proteases involved were identified as members of the ADAM or MMP families of metalloproteases (29–31).
A Disintegrin and Metalloprotease (ADAMs)
ADAMs are membrane-bound members of the metzincin superfamily and generally consist of an NH2−terminal signal sequence, a pro-domain, a metalloprotease domain, a disintegrin domain, a cysteine-rich region, an EGF-like domain, a transmembrane domain, and a cytoplasmic domain (31). The metalloprotease domain allows for ectodomain shedding and cleaving of extracellular matrix (ECM) proteins, which can release active cytokines and growth factors. The disintegrin and cysteine-rich domains allow for adhesive activity. Due to their ectodomain shedding and adhesive properties, ADAMs can regulate various cellular processes such as growth, migration and adhesion of cells (31). Several ADAM family members are involved in GPCR-mediated receptor transactivation. Specific ADAM activation differs depending on the GPCR agonist and cell type. Various mechanisms of ADAM activation downstream of GPCRs have been identified, including G protein-mediated as well as protein-protein interaction (32).
In human breast cancer cells, ADAM17 is required for HB-EGF activation, which plays a role in cell migration (33). Treatment of these cells with pertussis toxin has been shown to inhibit EGFR transactivation via the GPCR agonists, lysophosphatidic acid and sphingosine-1-phosphate, suggesting that Gαi is involved in ADAM17 activation downstream of these receptors (34). Phospholipase C, which is mainly coupled to Gαq, is also required for ADAM17-dependent shedding of HB-EGF and subsequent EGFR transactivation by the angiotensin II (AngII) receptor, AT1. In fact, no HB-EGF shedding occurred with an AT1 receptor mutant that lacked Gαq coupling. Inhibition of Gαq also blocked HB-EGF shedding via the AT1 receptor, suggesting that Gαq and the second messenger phospholipase C, are required for ADAM17 activation (35). AngII and its receptor, AT1, play critical roles in cardiovascular diseases, hypertension and atherosclerosis, by inducing vascular remodeling, hypertrophy, and migration of vascular smooth muscle cells (VSMCs) (18). EGFR transactivation is probably one of the main mechanisms by which AngII induces several of its pathophysiologic functions. The second messenger PKC also plays a role in receptor activation through ADAMs. PKC-δ has been shown to mediate HB-EGF by activating ADAM9 in kidney cells (36). Likewise, PKC induces metalloprotease activity downstream of gonadotropin-releasing hormone receptors and AT1 receptors (37, 38). ADAM activity can also be regulated by direct or indirect protein interactions after GPCR activation. PACSIN3, a kinase, interacts with ADAM9, 10, 12 and 15. Specifically, PACSIN3 has been shown to associate with ADAM12 through the SH3 domain and is required for ADAM12-mediated shedding of HB-EGF after PMA or AngII stimulation in HT1080 cells (39). Other proteins have been identified that interact with ADAMs including growth factor receptor-bound protein-2, phosphatidyl inositol 3-kinase, Src, Fish and endophilin-1 (31, 40).
Matrix Metalloproteases (MMPs)
MMPs are zinc-dependent proteinases that are traditionally thought of as key players in maintenance and degradation of the ECM (41). To date, 23 MMPs have been identified in humans and are classified based on their preferential substrates. The structure of MMPs includes a prodomain, catalytic domain, hinge region and a hemopexin domain. MMPs are secreted from the cell as inactive zymogens (pro-MMPs) or anchored to the cell membrane (42). While MMPs are important in ECM protein cleavage, MMPs have also been shown to have several other functions, including release of growth factors from the cell membrane or ECM, activation of other MMPs, shedding of adhesion molecules and ECM-cell communication (42, 43).
Several MMPs are involved in GPCR-mediated transactivation of receptors, including RTKs, receptor serine/threonine kinases, and other GPCRs (15). Numerous studies have been performed indicating that MMPs are involved in ectodomain shedding of EGFR ligands and EGFR activation. In isolated ovarian follicles, MMP-2 and MMP-9 regulate EGFR ligand release in response to the pituitary luteinizing hormone (44). These same MMPs have a similar role in EGFR activation in gonadotropin-releasing hormone-stimulated gonadotropic cells (45). MMP-7 has been shown to shed HB-EGF and lead to EGFR transactivation in phenylephrine-stimulated arteries and plays a role in regulating vascular tone (46, 47). Additionally, stimulation of vascular smooth muscle cells (VSMCs) with phenylephrine or AngII leads to increased expression of MMP-2 and MMP-7 (46). In addition to their role in EGFR activation, MMPs are also important in transactivation of other cell surface membrane receptors. For example, studies in our lab have shown that MMP-13 activity increases after adrenergic receptor stimulation of cardiac cells, leading to transactivation of another GPCR, PAR-1 (25). In human proximal tubular epithelial cells, activation of PAR-2 induces MMP-mediated activation of EGFR as well as activation of the serine/threonine kinase TGFβ receptor (48).
Although the exact mechanism for the activation of many of the MMPs downstream of GPCRs has not fully been determined, one study suggests a direct activation of an MMP via the G proteins. Isolated membranes from adult rat cardiac myocytes and fibroblasts indicate that MMP-14 activity is increased after AngII, phenylephrine, GTP and GTPγS stimulation. Activation of MMP-14 is attenuated by treatment with pertussis toxin and a Gβγ inhibitor, gallein. Purified Gβγ subunits were also able to activate recombinant MMP-14, suggesting a direct role of G proteins in the activation of this MMP (49). Other studies have found that activation of MMPs downstream of GPCRs requires Src and β-arrestin (50–52). Specifically, one study suggests that activation of the β1-adrenergic receptor (β1AR) in HEK293 cells results in receptor phosphorylation via GRK5/6 and β-arrestin recruitment. β-arrestin then recruits Src, which leads to MMP activation and HB-EGF release and subsequent EGFR transactivation (50). Interestingly, MMP-mediated transactivation of the EGFR has also been shown to occur with classical antagonists of the β1AR, Alprenolol and Carvedilol. In fact, these β-blockers act as “biased agonists” at their receptor by activating only the β-arrestin signaling pathway downstream of the β1AR. Several aspects of biased agonism at particular GPCRs are now under investigation as possible therapeutic targets for cardiovascular disease.
Signal transduction downstream of extracellular protease-mediated receptor transactivation
Activation of GPCRs can lead to multiple downstream signaling pathways, but generally, GPCR agonists do not elicit robust cell growth signals. The ability of GPCRs to transactivate other receptors allows GPCR agonists to generate a cell growth response and ultimately expanding their signaling repertoire. For example, GPCRs can regulate several cellular functions such as hypertrophy, proliferation and migration through the transactivation of the EGFR (53, 54). Pathways downstream of EGFR include the RAS/RAF/MEK/ERK and PI3K/Akt pathways (17, 54, 55), which are likely mediated by metalloprotease activation of the receptor. It has been shown that pharmacologic inhibition of metalloproteases blocks EGFR transactivation and subsequent ERK activation after stimulation of cells with GPCR agonists, lysophophatidic acid, AngII and ET-1 (28, 56). Stimulation of SCC-9 cells with carbachol or lysophophatidic acid leads to ADAM17-mediated activation of EGFR and phosphorylation of Shc, ERK and Akt (57). In addition, metalloprotease activation of EGFR appears to elicit signaling through JNK and p38 in certain cell types. In TccSup cells, stimulation with LPA induces JNK and p38 activation that is blocked by a metalloprotease inhibitor (58). However, inhibition of metalloproteases blocks activation of p38, but not JNK, in response to AngII stimulation in VSMCs (56). Additionally, GPCR activation can lead to transactivation of the TGFβ receptor (Figure 2). Specifically, the GPCR agonists endothelin-1 and thrombin can induce phosphorylation of Smad2, which is directly downstream of the TGFβ receptor. This Smad2 phosphorylation is inhibited by treatment with endothelin receptor and PAR1 antagonists (24, 59, 60). Recently, our lab has reported that MMP-mediated receptor transactivation can also lead to biased agonism at the transactivated receptor, PAR1. Specifically, we have shown that MMP-13 is upregulated following adrenergic receptor stimulation (Figure 2). The canonical agonist of PAR1 is thrombin, which acts as an unbiased agonist and leads to signaling through Gαq, Gαi and Gα12/13. We found that MMP-13 is also capable of activating PAR1 and that this cleavage occurs at a unique site compared to that of thrombin. Thrombin-mediated activation of PAR1 leads to ERK1/2 phosphorylation as well as generation of inositol triphosphate (IP3). However, this novel MMP-13-mediated activation of PAR1 appears to cause unique, “biased” signaling through the Gαq pathway as evidenced by robust ERK1/2 phosphorylation but decreased IP3 generation compared to thrombin stimulation (25).
Conclusions
GPCRs respond to a diverse array of ligands and are essential for a cell’s ability to translate outside stimuli into an intracellular response. These receptors are also capable of activating a highly interconnected signaling network. While early knowledge of GPCR signaling mainly focused on a linear signaling pathway through activation of heterotrimeric G proteins, it is now understood that an important component of GPCR signaling involves transactivation of other receptors. GPCR-mediated transactivation can occur via several different mechanisms, including activation of ADAMs or MMPs. These proteases can cleave receptor agonists, leading to receptor activation and downstream signaling (Figure 2). This crosstalk between GPCRs and other receptors, such as RTKs and serine/threonine kinase receptors, provides GPCRs the ability to activate a wider array of signaling pathways. GPCR-mediated transactivation has been identified in various cells types including vascular, cardiac, and cancer cells, and has been shown to play a key role in migration and proliferation of these cells. ADAMs and MMPs have been implicated in a number of human diseases including cancer, cardiovascular diseases, inflammation, lung fibrosis and Alzheimer’s disease (26, 61–65). GPCR-induced EGFR transactivation likely plays a key role in the development of these diseases. The recent discovery of biased agonism and the role of β-arrestin in receptor transactivation may provide a unique tool to further understand the pathways downstream of GPCRs that lead to extracellular protease activation and receptor transactivation as well as the generation of new therapeutic targets. Biased agonism has been described for many different receptors and could lead to discovery of new therapeutic agents that block deleterious effects of some receptor signaling while allowing for protective effects of separate signaling pathways. This has been demonstrated in the biased signaling of β-blockers at the β1AR discussed above. The resulting β-arrestin-mediated MMP activation of EGFR was shown to be cardioprotective in an acute model of cardiac hypertrophy (50). Additionally, β-arrestin has been implicated in various physiologic and pathologic processes in numerous organ systems and may be a key player in receptor transactivation (66). While numerous studies have shown that proteases play a crucial role in receptor transactivation, little is known regarding the mechanism of protease activation downstream of the initial receptor activation. Targeting the mechanisms of protease activation with selective inhibitors of downstream second messengers would provide further understanding of the pathways leading to protease activation and receptor transactivation downstream of GPCRs, which could ultimately provide novel therapeutic targets, especially in proliferative diseases such as cancer and fibrosis.
Acknowledgments
Sources of funding:
This work was supported in part by: NIH 1R01HL129772-01, 1R01HL132551-01, NIH 1R01HL134321-01, NIH 1R01HL133695-01 (BCB) and T32HL125204 (AES).
List of abbreviations
- GPCR
G protein-coupled receptor
- ADAM
A Disintegrin And Metalloprotease
- MMP
Matrix metalloprotease
- TMPS
Triple-membrane-passing-signal mechanism
- GRK
G protein-coupled receptor kinase
- RTK
Receptor tyrosine kinase
- EGFR
Epidermal growth factor receptor
- PAR
Protease activated receptor
- AngII
Angiotensin II
- AR
Adrenergic receptor
- ECM
Extracellular matrix
- IP3
Inositol triphosphate
References
- 1.Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Molecular pharmacology. 2003 Jun;63(6):1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 2.Ji TH, Grossmann M, Ji I. G protein-coupled receptors. I. Diversity of receptor-ligand interactions. The Journal of biological chemistry. 1998 Jul 10;273(28):17299–17302. doi: 10.1074/jbc.273.28.17299. [DOI] [PubMed] [Google Scholar]
- 3.Kobilka BK. G protein coupled receptor structure and activation. Biochimica et biophysica acta. 2007 Apr;1768(4):794–807. doi: 10.1016/j.bbamem.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flower DR. Modelling G-protein-coupled receptors for drug design. Bba-Rev Biomembranes. 1999 Nov 16;1422(3):207–234. doi: 10.1016/s0304-4157(99)00006-4. [DOI] [PubMed] [Google Scholar]
- 5.Kobilka B. Adrenergic receptors as models for G protein-coupled receptors. Annu Rev Neurosci. 1992;15:87–114. doi: 10.1146/annurev.ne.15.030192.000511. [DOI] [PubMed] [Google Scholar]
- 6.Deupi X, Kobilka B. Activation of G protein-coupled receptors. Adv Protein Chem. 2007;74:137–166. doi: 10.1016/S0065-3233(07)74004-4. [DOI] [PubMed] [Google Scholar]
- 7.Gether U, Kobilka BK. G protein-coupled receptors. II. Mechanism of agonist activation. The Journal of biological chemistry. 1998 Jul 17;273(29):17979–17982. doi: 10.1074/jbc.273.29.17979. [DOI] [PubMed] [Google Scholar]
- 8.Kroeze WK, Sheffler DJ, Roth BL. G-protein-coupled receptors at a glance. Journal of Cell Science. 2003 Dec 15;116(24):4867–4869. doi: 10.1242/jcs.00902. [DOI] [PubMed] [Google Scholar]
- 9.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annual Review of Biochemistry. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 10.Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, Lefkowitz RJ. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proceedings of the National Academy of Sciences of the United States of America. 2001 Feb 27;98(5):2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nature reviews Drug discovery. 2010 May;9(5):373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ, Luttrell LM. beta-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. The Journal of biological chemistry. 2002 Mar 15;277(11):9429–9436. doi: 10.1074/jbc.M106457200. [DOI] [PubMed] [Google Scholar]
- 13.Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends in pharmacological sciences. 2007 Aug;28(8):416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Violin JD, Crombie AL, Soergel DG, Lark MW. Biased ligands at G-protein-coupled receptors: promise and progress. Trends in pharmacological sciences. 2014 Jul;35(7):308–316. doi: 10.1016/j.tips.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Cattaneo F, Guerra G, Parisi M, De Marinis M, Tafuri D, Cinelli M, Ammendola R. Cell-surface receptors transactivation mediated by g protein-coupled receptors. Int J Mol Sci. 2014;15(11):19700–19728. doi: 10.3390/ijms151119700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996 Feb 8;379(6565):557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 17.Gschwind A, Zwick E, Prenzel N, Leserer M, Ullrich A. Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene. 2001 Mar 26;20(13):1594–1600. doi: 10.1038/sj.onc.1204192. [DOI] [PubMed] [Google Scholar]
- 18.Berry C, Touyz R, Dominiczak AF, Webb RC, Johns DG. Angiotensin receptors: signaling, vascular pathophysiology, and interactions with ceramide. American journal of physiology Heart and circulatory physiology. 2001 Dec;281(6):H2337–H2365. doi: 10.1152/ajpheart.2001.281.6.H2337. [DOI] [PubMed] [Google Scholar]
- 19.Little PJ, Burch ML, Al-aryahi S, Zheng W. The paradigm of G protein receptor transactivation: a mechanistic definition and novel example. ScientificWorldJournal. 2011;11:709–714. doi: 10.1100/tsw.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zwick E, Daub H, Aoki N, Yamaguchi-Aoki Y, Tinhofer I, Maly K, Ullrich A. Critical role of calcium- dependent epidermal growth factor receptor transactivation in PC12 cell membrane depolarization and bradykinin signaling. The Journal of biological chemistry. 1997 Oct 3;272(40):24767–24770. doi: 10.1074/jbc.272.40.24767. [DOI] [PubMed] [Google Scholar]
- 21.Daub H, Wallasch C, Lankenau A, Herrlich A, Ullrich A. Signal characteristics of G protein-transactivated EGF receptor. EMBO J. 1997 Dec 1;16(23):7032–7044. doi: 10.1093/emboj/16.23.7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eguchi S, Iwasaki H, Hirata Y, Marumo F, Yamakawa T, Numaguchi K, Inagami T. Angiotensin II-induced growth-promoting signal requires EGF receptor transactivation. J Hypertens. 1998 Jun;16:S78-S. [Google Scholar]
- 23.Kamato D, Rostam MA, Bernard R, Piva TJ, Mantri N, Guidone D, Zheng W, Osman N, Little PJ. The expansion of GPCR transactivation-dependent signalling to include serine/threonine kinase receptors represents a new cell signalling frontier. Cell Mol Life Sci. 2015 Feb;72(4):799–808. doi: 10.1007/s00018-014-1775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burch ML, Osman N, Getachew R, Al-Aryahi S, Poronnik P, Zheng W, Hill MA, Little PJ. G protein coupled receptor transactivation: extending the paradigm to include serine/threonine kinase receptors. Int J Biochem Cell Biol. 2012 May;44(5):722–727. doi: 10.1016/j.biocel.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Jaffre F, Friedman AE, Hu Z, Mackman N, Blaxall BC. beta-adrenergic receptor stimulation transactivates protease-activated receptor 1 via matrix metalloproteinase 13 in cardiac cells. Circulation. 2012 Jun 19;125(24):2993–3003. doi: 10.1161/CIRCULATIONAHA.111.066787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu MY, Porte J, Knox AJ, Weinreb PH, Maher TM, Violette SM, McAnulty RJ, Sheppard D, Jenkins G. Lysophosphatidic acid induces alphavbeta6 integrin-mediated TGF-beta activation via the LPA2 receptor and the small G protein G alpha(q) The American journal of pathology. 2009 Apr;174(4):1264–1279. doi: 10.2353/ajpath.2009.080160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenkins RG, Su X, Su G, Scotton CJ, Camerer E, Laurent GJ, Davis GE, Chambers RC, Matthay MA, Sheppard D. Ligation of protease-activated receptor 1 enhances alpha(v)beta(6) integrin-dependent TGF-beta activation and promotes acute lung injury. Journal of Clinical Investigation. 2006 Jun;116(6):1606–1614. doi: 10.1172/JCI27183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999 Dec 23–30;402(6764):884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 29.Massague J, Pandiella A. Membrane-anchored growth factors. Annu Rev Biochem. 1993;62:515–541. doi: 10.1146/annurev.bi.62.070193.002503. [DOI] [PubMed] [Google Scholar]
- 30.Kasina S, Scherle PA, Hall CL, Macoska JA. ADAM-mediated amphiregulin shedding and EGFR transactivation. Cell Prolif. 2009 Dec;42(6):799–812. doi: 10.1111/j.1365-2184.2009.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes & development. 2003 Jan 1;17(1):7–30. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]
- 32.Ohtsu H, Dempsey PJ, Eguchi S. ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am J Physiol-Cell Ph. 2006 Jul;291(1):C1–C10. doi: 10.1152/ajpcell.00620.2005. [DOI] [PubMed] [Google Scholar]
- 33.Zheng X, Jiang F, Katakowski M, Zhang ZG, Lu QE, Chopp M. ADAM17 promotes breast cancer cell malignant phenotype through EGFR-PI3K-AKT activation. Cancer Biol Ther. 2009 Jun 1;8(11):1045–1054. doi: 10.4161/cbt.8.11.8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hart S, Fischer OM, Prenzel N, Zwick-Wallasch E, Schneider M, Hennighausen L, Ullrich A. GPCR-induced migration of breast carcinoma cells depends on both EGFR signal transactivation and EGFR-independent pathways. Biol Chem. 2005 Sep;386(9):845–855. doi: 10.1515/BC.2005.099. [DOI] [PubMed] [Google Scholar]
- 35.Mifune M, Ohtsu H, Suzuki H, Nakashima H, Brailoiu E, Dun NJ, Frank GD, Inagami T, Higashiyama S, Thomas WG, Eckhart AD, Dempsey PJ, Eguchi S. G protein coupling and second messenger generation are indispensable for metalloprotease-dependent, heparin-binding epidermal growth factor shedding through angiotensin II type-1 receptor. Journal of Biological Chemistry. 2005 Jul 15;280(28):26592–26599. doi: 10.1074/jbc.M502906200. [DOI] [PubMed] [Google Scholar]
- 36.Izumi Y, Hirata M, Hasuwa H, Iwamoto R, Umata T, Miyado K, Tamai Y, Kurisaki T, Sehara-Fujisawa A, Ohno S, Mekada E. A metalloprotease-disintegrin, MDC9/meltrin-gamma/ADAM9 and PKCdelta are involved in TPA-induced ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor. EMBO J. 1998 Dec 15;17(24):7260–7272. doi: 10.1093/emboj/17.24.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah BH, Farshori MP, Jambusaria A, Catt KJ. Roles of Src and epidermal growth factor receptor transactivation in transient and sustained ERK1/2 responses to gonadotropin-releasing hormone receptor activation. The Journal of biological chemistry. 2003 May 23;278(21):19118–19126. doi: 10.1074/jbc.M212932200. [DOI] [PubMed] [Google Scholar]
- 38.Shah BH, Yesilkaya A, Olivares-Reyes JA, Chen HD, Hunyady L, Catt KJ. Differential pathways of angiotensin II-induced extracellularly regulated kinase 1/2 phosphorylation in specific cell types: Role of heparin-binding epidermal growth factor. Mol Endocrinol. 2004 Aug;18(8):2035–2048. doi: 10.1210/me.2003-0476. [DOI] [PubMed] [Google Scholar]
- 39.Mori S, Tanaka M, Nanba D, Nishiwaki E, Ishiguro H, Higashiyama S, Matsuura N. PACSIN3 binds ADAM12/meltrin alpha and up-regulates ectodomain shedding of heparin-binding epidermal growth factor-like growth factor. The Journal of biological chemistry. 2003 Nov 14;278(46):46029–46034. doi: 10.1074/jbc.M306393200. [DOI] [PubMed] [Google Scholar]
- 40.Abram CL, Seals DF, Pass I, Salinsky D, Maurer L, Roth TM, Courtneidge SA. The adaptor protein fish associates with members of the ADAMs family and localizes to podosomes of Src-transformed cells. The Journal of biological chemistry. 2003 May 9;278(19):16844–16851. doi: 10.1074/jbc.M300267200. [DOI] [PubMed] [Google Scholar]
- 41.Marion MMHv. Matrix Metalloproteinases and Collagen Remodeling. 2006 [Google Scholar]
- 42.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovascular research. 2006 Feb 15;69(3):562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Valiente-Alandi I, Schafer AE, Blaxall BC. Extracellular matrix-mediated cellular communication in the heart. Journal of molecular and cellular cardiology. 2016 Feb;91:228–237. doi: 10.1016/j.yjmcc.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carbajal L, Biswas A, Niswander LM, Prizant H, Hammes SR. GPCR/EGFR Cross Talk Is Conserved in Gonadal and Adrenal Steroidogenesis but Is Uniquely Regulated by Matrix Metalloproteinases 2 and 9 in the Ovary. Mol Endocrinol. 2011 Jun;25(6):1055–1065. doi: 10.1210/me.2010-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roelle S, Grosse R, Aigner A, Krell HW, Czubayko F, Gudermann T. Matrix metalloproteinases 2 and 9 mediate epidermal growth factor receptor transactivation by gonadotropin-releasing hormone. The Journal of biological chemistry. 2003 Nov 21;278(47):47307–47318. doi: 10.1074/jbc.M304377200. [DOI] [PubMed] [Google Scholar]
- 46.Nagareddy PR, Chow FL, Hao L, Wang X, Nishimura T, MacLeod KM, McNeill JH, Fernandez-Patron C. Maintenance of adrenergic vascular tone by MMP transactivation of the EGFR requires PI3K and mitochondrial ATP synthesis. Cardiovascular research. 2009 Dec 1;84(3):368–377. doi: 10.1093/cvr/cvp230. [DOI] [PubMed] [Google Scholar]
- 47.Hao L, Du M, Lopez-Campistrous A, Fernandez-Patron C. Agonist-induced activation of matrix metalloproteinase-7 promotes vasoconstriction through the epidermal growth factor-receptor pathway. Circulation research. 2004 Jan 9;94(1):68–76. doi: 10.1161/01.RES.0000109413.57726.91. [DOI] [PubMed] [Google Scholar]
- 48.Chung H, Ramachandran R, Hollenberg MD, Muruve DA. Proteinase-activated receptor-2 transactivation of epidermal growth factor receptor and transforming growth factor-beta receptor signaling pathways contributes to renal fibrosis. The Journal of biological chemistry. 2013 Dec 27;288(52):37319–37331. doi: 10.1074/jbc.M113.492793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Overland AC, Insel PA. Heterotrimeric G proteins directly regulate MMP14/membrane type-1 matrix metalloprotease: a novel mechanism for GPCR-EGFR transactivation. The Journal of biological chemistry. 2015 Apr 17;290(16):9941–9947. doi: 10.1074/jbc.C115.647073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. The Journal of clinical investigation. 2007 Sep;117(9):2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pierce KL, Tohgo A, Ahn S, Field ME, Luttrell LM, Lefkowitz RJ. Epidermal growth factor (EGF) receptor-dependent ERK activation by G protein-coupled receptors: a co-culture system for identifying intermediates upstream and downstream of heparin-binding EGF shedding. The Journal of biological chemistry. 2001 Jun 22;276(25):23155–23160. doi: 10.1074/jbc.M101303200. [DOI] [PubMed] [Google Scholar]
- 52.Kim IM, Tilley DG, Chen J, Salazar NC, Whalen EJ, Violin JD, Rockman HA. Beta-blockers alprenolol and carvedilol stimulate beta-arrestin-mediated EGFR transactivation. Proceedings of the National Academy of Sciences of the United States of America. 2008 Sep 23;105(38):14555–14560. doi: 10.1073/pnas.0804745105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fischer OM, Hart S, Gschwind A, Ullrich A. EGFR signal transactivation in cancer cells. Biochem Soc Trans. 2003 Dec;31(Pt 6):1203–1208. doi: 10.1042/bst0311203. [DOI] [PubMed] [Google Scholar]
- 54.Eguchi S, Frank GD, Mifune M, Inagami T. Metalloprotease-dependent ErbB ligand shedding in mediating EGFR transactivation and vascular remodelling. Biochem Soc Trans. 2003 Dec;31(Pt 6):1198–1202. doi: 10.1042/bst0311198. [DOI] [PubMed] [Google Scholar]
- 55.Shah BH, Catt KJ. Matrix metalloproteinase-dependent EGF receptor activation in hypertension and left ventricular hypertrophy. Trends Endocrin Met. 2004 Aug;15(6):241–243. doi: 10.1016/j.tem.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 56.Eguchi S, Dempsey PJ, Frank GD, Motley ED, Inagami T. Activation of MAPKs by angiotensin II in vascular smooth muscle cells. Metalloprotease-dependent EGF receptor activation is required for activation of ERK and p38 MAPK but not for JNK. The Journal of biological chemistry. 2001 Mar 16;276(11):7957–7962. doi: 10.1074/jbc.M008570200. [DOI] [PubMed] [Google Scholar]
- 57.Gschwind A, Hart S, Fischer OM, Ullrich A. TACE cleavage of proamphiregulin regulates GPCR-induced proliferation and motility of cancer cells. EMBO J. 2003 May 15;22(10):2411–2421. doi: 10.1093/emboj/cdg231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schafer B, Gschwind A, Ullrich A. Multiple G-protein-coupled receptor signals converge on the epidermal growth factor receptor to promote migration and invasion. Oncogene. 2004 Jan 29;23(4):991–999. doi: 10.1038/sj.onc.1207278. [DOI] [PubMed] [Google Scholar]
- 59.Little PJ, Burch ML, Getachew R, Al-aryahi S, Osman N. Endothelin-1 stimulation of proteoglycan synthesis in vascular smooth muscle is mediated by endothelin receptor transactivation of the transforming growth factor-[beta] type I receptor. J Cardiovasc Pharmacol. 2010 Oct;56(4):360–368. doi: 10.1097/FJC.0b013e3181ee6811. [DOI] [PubMed] [Google Scholar]
- 60.Burch ML, Ballinger ML, Yang SN, Getachew R, Itman C, Loveland K, Osman N, Little PJ. Thrombin stimulation of proteoglycan synthesis in vascular smooth muscle is mediated by protease-activated receptor-1 transactivation of the transforming growth factor beta type I receptor. The Journal of biological chemistry. 2010 Aug 27;285(35):26798–26805. doi: 10.1074/jbc.M109.092767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amalinei C, Caruntu ID, Giusca SE, Balan RA. Matrix metalloproteinases involvement in pathologic conditions. Rom J Morphol Embryol. 2010;51(2):215–228. [PubMed] [Google Scholar]
- 62.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiological reviews. 2007 Oct;87(4):1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 63.Deuss M, Reiss K, Hartmann D. Part-time alpha-secretases: the functional biology of ADAM 9, 10 and 17. Curr Alzheimer Res. 2008 Apr;5(2):187–201. doi: 10.2174/156720508783954686. [DOI] [PubMed] [Google Scholar]
- 64.Duffy MJ, McKiernan E, O'Donovan N, McGowan PM. Role of ADAMs in cancer formation and progression. Clin Cancer Res. 2009 Feb 15;15(4):1140–1144. doi: 10.1158/1078-0432.CCR-08-1585. [DOI] [PubMed] [Google Scholar]
- 65.Mohammed FF, Smookler DS, Khokha R. Metalloproteinases, inflammation, and rheumatoid arthritis. Annals of the rheumatic diseases. 2003 Nov;62(Suppl 2):ii43–ii47. doi: 10.1136/ard.62.suppl_2.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends in molecular medicine. 2011 Mar;17(3):126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]