Abstract
Objective
The aim of this study is to evaluate the oncological outcomes of robotic total mesorectal excision (TME) at an NCI designated cancer center.
Summary Background Data
The effectiveness of laparoscopic TME could not be established, but the robotic assisted approach may hold some promise, with improved visualization and ergonomics for pelvic dissection. Oncological outcome data is presently lacking.
Methods
Patients who underwent total mesorectal excision or tumor specific mesorectal excision for rectal cancer between April 2009 and April 2016 via a robotic approach were identified from a prospective single-institution database. The circumferential resection margin (CRM), distal resection margin (DRM), and TME completeness rates were determined. Kaplan-Meier analysis of disease free survival (DFS) and overall survival (OS) was performed for all patients treated with curative intent.
Results
A total of 276 patients underwent robotic proctectomy during the study period. Robotic surgery was performed initially by 1 surgeon with 3 additional surgeons progressively transitioning from open to robotic during the study period with annual increase in the total number of cases performed robotically. Seven patients had involved circumferential resection margins (2.5%), and there were no positive distal or proximal resection margins. One hundred and eighty six patients had TME quality assessed, and only 1 patient (0.5%) had an incomplete TME. Eighty-three patients were followed up for a minimum of 3 years, with a local recurrence rate of 2.4%, and a distant recurrence rate of 16.9%. Five year DFS on Kaplan Meier analysis was 82%, and five year OS was 87%.
Conclusion
Robotic proctectomy for rectal cancer can be performed with good short and medium term oncological outcomes in selected patients.
Keywords: Robot, Rectal cancer, total mesorectal excision, minimally invasive surgery, laparoscopic surgery
Introduction
Oncological outcome after surgery for rectal cancer is highly dependent on surgical technique, with an established role for tumor specific mesorectal excision (TsME) for tumors in the upper rectum, total mesorectal excision (TME) for tumors in the low rectum, and dissection beyond the TME plane for locally advanced disease.1 While minimally invasive surgery for rectal cancer is now commonly performed in many institutions worldwide, the effectiveness of laparoscopic TME has not been established compared with traditional open surgery despite multiple randomized controlled trials specifically investigating this question.2-4 For patients requiring extended resections beyond the TME plane, the laparoscopic approach may be particularly challenging with reports of incomplete resection rates as high as 20– 30%.5, 6 Fundamental issues with the laparoscopic approach to proctectomy are poor visibility and difficult access in the low pelvis, which combine to make dissection and distal division challenging. This has led some surgeons to adopt alternative approaches which aim to improve the ease and quality of proctectomy while still retaining any potential benefits of minimally invasive incisions, with the two dominant techniques currently in use being robotic assisted dissection7 and trans-anal TME.8
The robotic assisted approach affords several technical advantages over standard laparoscopy, which are of particular importance in pelvic surgery, and can facilitate dissection.9 These include: wristed instruments, improved ergonomics and angle of approach, a magnified three dimensional view with immersive field of vision, and a stable camera platform with direct camera control by the operating surgeon. There is some evidence from a single, small, randomized trial and several case-control studies that these advantages may translate to improved TME quality and autonomic functional outcomes compared with laparoscopy, but larger randomized controlled trials are required to confirm those results.10-12 A preliminary presentation of the ROLARR randomized trial results, which compared laparoscopic versus robotic surgery, suggested that the robotic approach may have a lower overall conversion rate particularly when operating on obese male patients, but no other major benefits were shown.13 The final results of the ROLARR and COLRAR (NCT01423214) trials are expected to clarify these issues further, but they remain awaited.14
Most of the published data on robotic proctectomy addresses short term outcomes after surgery, with little discussion of survival and long term oncological outcomes.15 In addition, most large single center series originate from high volume experienced units in Asia,16 with little data on oncological outcomes of robotic resection of rectal cancer from individual centers in the United States.17 At our institution, robotic proctectomy has been performed for rectal cancer since 2009, and has become the procedure of choice in patients who are suitable for a minimally invasive approach.18 The aim of this study was to prospectively evaluate the perioperative and oncologic outcomes of robotic rectal cancer resection and evaluate the pathologic outcomes in the context of data from recently published randomized trials.
Methods
Patients
Consecutive patients who underwent robot-assisted rectal cancer surgery for biopsy-proven primary rectal adenocarcinoma at The University of Texas MD Anderson Cancer Center, Houston, Texas, between April 2009 and April 2016 were identified from a prospectively collected institutional colorectal robotic surgery database (DR08-0864). Patients who underwent pelvic dissection in the form of tumor specific mesorectal excision, total mesorectal excision, or extended resection via a robotic approach were included in the analysis. Selection of patients for robotic resection was based on surgeon experience with the technique and informed patient consent. The robotic approach was precluded in patients expected to have significant intra-abdominal adhesions limiting access to the distal colon, rectum, or root of the mesentery for lympho-vascular dissection. Patients requiring extended or multi-visceral resection, complex abdominal wall reconstruction, or synchronous liver resection with laparotomy were not offered robotic surgery.
Data collection
All demographic, operative, pathological and post-operative recovery data were obtained from the institutional colorectal robotic surgery database. Complications were stratified by the Clavien Dindo classification system for surgical complications, with no time limit placed on recording of surgical complications.19 Oncological outcomes such as local recurrence, distant recurrence, disease free survival and overall survival were recorded and subsequently confirmed by chart review to ensure up to date follow-up data.
Ethics
The study was approved by the MD Anderson Cancer Center institutional review board.
Staging and surgery
All patients underwent preoperative staging with computed tomography (CT) of the chest, abdomen, and pelvis. High-resolution pelvic magnetic resonance imaging (MRI) was performed with a surface coil and without the use of intravenous contrast. Diffusion weighted imaging was also performed. Images were obtained in the axial, sagittal and coronal planes and reviewed for T stage, relationship to the mesorectal fascia, N stage, relationship of tumor to the sphincter complex, vascular invasion and pelvic sidewall adenopathy. Mid and low rectal cancer were defined as tumors ≤ 12cm from the anal verge (as per the Z0651 trial), and low rectal cancer was defined as tumors located ≤ 5 cm from the anal verge.3 Surgery was performed at a median of 8 weeks after preoperative long-course chemoradiation therapy (if this was given). In cases with distant metastases, primary tumor resections were performed with curative or palliative intent, depending on the resectability of the metastases and this was documented at the outset. In some cases short course pelvic radiotherapy was performed for patients with distant metastases to minimize the duration of time without systemically active therapy. For all procedures, a four-arm da Vinci surgical system (Intuitive Surgical, Sunnyvale, CA, USA) was used, with a second generation system used earlier in the series and then a third or fourth generation system later in the series depending on availability. All primary and extended pelvic dissections, and vascular pedicle dissections were performed totally robotically. Patients subsequently underwent either robotic or laparoscopic mobilization of the splenic flexure after completion of the rectal dissection.18 Conversion to open surgery was defined as the use of an abdominal incision to continue the procedures under direct visualization before completion of the TME.
Pathological assessment
Specimens were taken fresh by the operating surgeon immediately after retrieval to the pathology laboratory so the specimen could be oriented, marked and assessed. The relevant macroscopic surgical margins including the vascular pedicle, the circumferential resection margin (CRM) and the distal resection margin (DRM) were reviewed jointly by the operating surgeon and the lead pathologist and documented. Microscopic margins were then assessed by the pathologist at the inked sites and reviewed. CRM and DRM involvement were defined as a resection margin of 1 mm or less and this information was recorded for all patients in the dataset. In the more recent subset of patients, completeness of TME was also assessed and documented. This was defined as complete, nearly complete, or incomplete according to the definitions employed during the ACOSOG Z6051 trial.3 “Complete TME” was defined as: intact mesorectum and covering mesorectal envelope to the level of rectal transection with no coning above the point of transection. The surface of the mesorectal covering should be smooth and shiny with no defects exposing the underlying fat. “Nearly complete TME” was defined as: presence of 1 or 2 areas of violation of the mesorectal envelope which were <5 mm and had no loss of fat and no coning. “Incomplete TME” was defined as a specimen that failed to meet the above criteria. Using the above data, a “successful resection” composite outcome was derived for the patients that had TME quality assessed using the same methodology as that used in the Z6051 trial.3 All 3 of the following parameters must have been achieved for the surgery to be considered a success: CRM > 1mm, DRM > 1mm, and TME complete / nearly complete.3
Statistical analysis
Data were analyzed using IBM SPSS Statistics for Windows, Version 22.0 (Armonk, NY: IBM Corp). Continuous variable parametricity was tested using the Kolmogorov–Smirnov test (K–S test), and results presented as mean (standard deviation) for parametric data and median (inter-quartile range) for non-parametric data. Kaplan-Meier analysis of disease free survival and overall survival was performed for all patients treated with curative intent. Median follow-up was estimated using Kaplan-Meier estimate of potential follow-up (KM-PF) method.
Results
A total of 1391 patients underwent proctectomy for rectal cancer during the study period, of which 322 patients underwent minimally invasive surgery: 46 via a laparoscopic-assisted approach, and 276 via a robotic approach. All robotic cases were included in the current analysis. Robotic surgery was performed initially by 1 surgeon with 3 additional surgeons progressively transitioning from open to robotic during the study period with annual increase in the total number of cases performed robotically. (Figure 1). Median follow-up for the robotic cases was 23.8 months, and baseline patient parameters are shown in Table 1. Most patients had tumors located in the mid and low rectum (82.6%), had T3/T4 disease (76.5%), and node positive disease (68.8%) on pre-treatment staging. Seventy-five percent of patients underwent neo-adjuvant treatment, of which the vast majority underwent long course chemo-radiotherapy.
Figure 1.
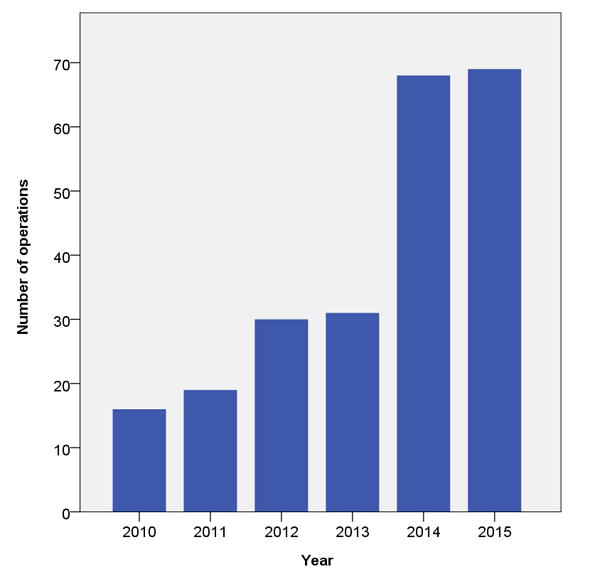
Number of robotic proctectomies performed each complete year during the study period (years 2009 and 2016 not shown as data only available for part of those years).
Table 1.
Baseline characteristics of patients with rectal cancer who underwent robotic proctectomy between April 2009 and April 2016 (N=276).*
| Characteristic | |
|---|---|
|
| |
| Mean age (SD), years | 54 (12) |
|
| |
| Sex | |
| Male | 168 (60.9) |
| Female | 108 (39.1) |
|
| |
| Ethnicity | |
| Caucasian | 236 (85.5) |
| African American | 15 (5.4) |
| Asian | 20 (7.2) |
| Other | 5 (1.8) |
|
| |
| Median BMI (IQR) | 27.0 (7.2) |
|
| |
| ASA score | |
| I | 0 |
| II | 63 (22.8) |
| III | 210 (76.1) |
| IV | 3 (1.1) |
|
| |
| Previous abdominal surgery | 63 (22.8) |
|
| |
| Pre-treatment AJCC staging | |
| I | 42 (15.2) |
| II | 34 (12.3) |
| III | 176 (63.8) |
| IV | 24 (8.7) |
|
| |
| Clinical T stage (cT) | |
| T0 / Tis | 9 (3.3) |
| T1 | 13 (5.7) |
| T2 | 43 (15.6) |
| T3 | 185 (67.0) |
| T4 | 26 (9.5) |
|
| |
| Clinical N stage (cN) | |
| N0 | 86 (31.2) |
| N1 | 137 (49.6) |
| N2 | 53 (19.2) |
|
| |
| Clinical M stage (cM) | |
| M0 | 252 (91.3) |
| M1 | 24 (8.7) |
|
| |
| Neo-adjuvant treatment | |
| Long course chemo-radiotherapy | 171 (61.9) |
| Short course radiotherapy | 1 (0.4) |
| Induction chemotherapy, followed by radiotherapy | 14 (5.1) |
| Chemotherapy only | 20 (7.2) |
| Did not receive neo-adjuvant treatment | 70 (25.4) |
|
| |
| Adjuvant treatment | |
| FOLFOX / CAPOX | 125 (45.3) |
| Fluorouracil / Capecitabine only | 76 (27.5) |
| FOLFOX + Bevacizumab | 12 (4.3) |
| FOLFIRI + Bevacizumab | 8 (2.9) |
| Other | 5 (1.8) |
| Did not receive chemotherapy | 50 (18.9) |
|
| |
| Median time to adjuvant treatment (IQR), days | 42 (21) |
Data are number of patients (%) unless otherwise indicated.
Abbreviations: SD, Standard Deviation; BMI, body mass index; IQR, Inter-quartile range; ASA, American Society of Anesthesiologists; AJCC, American Joint Committee on Cancer; FOLFOX, Folinic acid + Fluorouracil + Oxaliplatin; CAPOX: Capecitabine + Oxaliplatin; FOLFIRI: Folinic acid + Fluorouracil + Irinotecan.
Intra-operative and post-operative data
Peri-operative data is shown in Table 2. The majority of patients (80%) underwent sphincter preservation and reconstruction, despite a relatively high proportion of low tumors and locally advanced disease. Median total operating time was 345 min, and median console time was 122 min (docking time 4min). The overall conversion to open laparotomy rate was 2.2%. There were five instances of major intra-operative complication: distal stapler misfire requiring conversion to a hand sewn colo-anal anastomosis, rectal disruption during insertion of a stapler device requiring anastomosis revision, significant anastomotic bleeding after stapling requiring anastomosis revision, bleeding from the inferior mesenteric vein pedicle after using the vessel sealer requiring conversion, and bleeding from small bowel mesentery in a patient with portal hypertension requiring conversion. The anastomotic leak rate was 5.4%, the re-operation rate was 2.9%, and the median day stay was 4 days. There were two post-operative deaths, one due to an unexpected post-operative cardiac event that occurred after the patient was discharged from the hospital, and the other due to post-operative sepsis in a patient who had undergone a palliative resection and was readmitted to an external facility 3 weeks after the patient was discharged from the hospital. The source of the sepsis was not isolated as the patient elected to be treated with supportive care only.
Table 2.
Perioperative data of patients with rectal cancer who underwent robotic proctectomy between April 2009 and April 2016 (N=276).*
| Characteristic | |
|---|---|
|
| |
| Technique | |
| Complete robotic | 87 (31.5) |
| Reverse hybrid (laparoscopic flexure mobilization) | 175 (66.3) |
| Converted to open | 6 (2.2) |
|
| |
| Intent | |
| Curative | 267 (96.7) |
| Palliative | 9 (3.3) |
|
| |
| Procedure | |
| Low anterior resection (Tumor Specific TME) | 48 (17.4) |
| Ultralow anterior resection (stapled) | 125 (45.3) |
| Ultralow anterior resection (hand-sewn coloanal) | 49 (17.8) |
| Abdominoperineal Resection | 51 (18.5) |
| Total Pelvic Exenteration | 2 (0.7) |
| Total Proctocolectomy | 1 (0.4) |
|
| |
| Extended / Contiguous resections | 52 (18.8) |
| Extended lymph node dissections (aortic, iliac, or obturator) | 24 (8.7) |
| Hysterectomy / oophorectomy | 18 (6.5) |
| Hepatectomy (synchronous) | 7 (2.5) |
| Total Cystectomy | 2 (0.7) |
| Prostatectomy (bladder preserving) | 1 (0.4) |
|
| |
| Additional procedures | |
| VRAM perineal reconstruction | 5 (1.8) |
| Intra-operative radiotherapy | 2 (0.7) |
|
| |
| Stoma | |
| Loop Ileostomy | 188 (68.1) |
| End Colostomy | 53 (19.2) |
| None | 35 (12.7) |
|
| |
| Median blood loss (IQR), mL | 100 (150) |
|
| |
| Major intra-op complication | 5 (1.8) |
|
| |
| Median hospital stay (IQR), days | 4 (3) |
|
| |
| Post-operative complications (Clavien Dindo, per event) | |
| Grade I | 36 (13) |
| Grade II | 22 (8.0) |
| Grade III | 34 (12.3) |
| Grade IV | 2 (0.7) |
| Grade V | 2 (0.7) |
Data are number of patients (%) unless otherwise indicated.
TME: Total Mesorectal Excision, IQR: Inter-quartile range, VRAM: Vertical Rectus Abdominis Myocutaneous flap
Pathology
Histological data is shown in Table 3. Seven patients (2.5%) had CRM involvement, including one patient (0.4%) with a grossly involved margin due to tumor perforation, and six patients (2.2%) with microscopically positive margins. There were no positive distal or proximal resection margins. One-hundred and eighty-six patients had TME quality assessed, and of these 141 patients (75.8%) were graded as complete, 44 (23.7%) graded nearly complete, and only 1 patient (0.5%) graded as an incomplete TME. Of the patients who had TME quality assessed, 97.3% met the Z6051 trial criteria for the composite outcome of successful resection.
Table 3.
Pathology data of patients with rectal cancer who underwent robotic proctectomy between April 2009 and April 2016 (N=276).*
| Characteristic | |
|---|---|
|
| |
| Circumferential Resection Margin (CRM) | |
| > 1mm | 269 (97.5) |
| ≤ 1 mm | 7 (2.5) |
|
| |
| Distal Resection Margin (DRM) | |
| > 1mm | 276 (100) |
| ≤ 1 mm | 0 |
|
| |
| Completeness of TME (n = 186) | |
| Complete TME | 141 (75.8) |
| Nearly Complete | 44 (23.7) |
| Incomplete | 1 (0.5) |
|
| |
| Successful resection (n = 186)† | |
| Achieved | 5 (2.7) |
| Not achieved | 181 (97.3) |
|
| |
| Pathological T stage (pT) | |
| T0 / Tis | 36 (13.0) |
| T1 | 43 (15.6) |
| T2 | 83 (30.1) |
| T3 | 102 (37.0) |
| T4 | 12 (4.3) |
|
| |
| Pathological N stage (pN) | |
| N0 | 158 (57.2) |
| N1 | 88 (31.9) |
| N2 | 30 (10.9) |
|
| |
| Median lymph nodes (IQR) | |
| Total nodes | 22 (11) |
| Positive nodes | 0 (1) |
Data are number of patients (%) unless otherwise indicated.
CRM > 1mm, DRM > 1mm, and TME complete / nearly complete
TME: Total Mesorectal Excision, IQR: Inter-quartile range
Recurrence and Survival
Of the patients treated with curative intent (n = 267 patients), 7 patients developed loco-regional recurrence during the follow-up period, of which 3 had local pelvic recurrences (2 anastomotic), and 4 had regional nodal recurrences. None of the patients with a local recurrence, and only one of the patients with a regional nodal recurrence, had a positive margin at the initial surgery. There were 22 patients who developed distant recurrences, of which 8 had widespread multi-visceral recurrence, 9 had isolated recurrence in the lung, 2 had recurrence in the liver, and 3 had distant nodal recurrence. The median time to recurrence (local and / or distant) was 14.8 months (IQR 6.7). A total of 83 patients were followed up for a minimum of 3 years, and in those patients the local recurrence rate was 2.4%, and the distant recurrence rate was 16.9%. Kaplan Meier analysis of five-year disease free survival (DFS) and overall survival (OS) for all patients treated with curative intent (N=267) is shown in Figures 1 and 2. Five year estimated DFS was 82%, and five year estimated OS was 87%.
Figure 2.
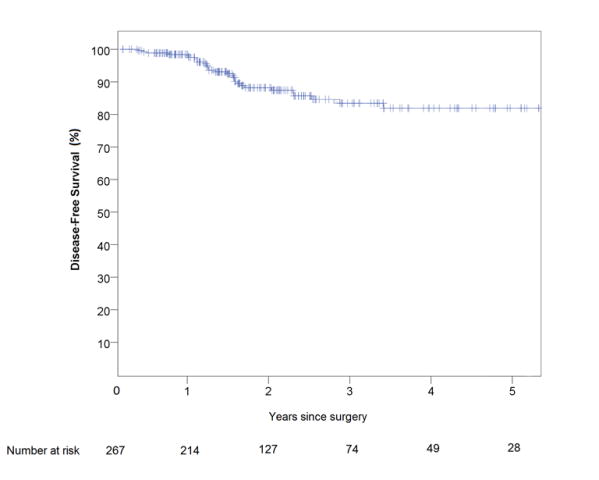
Kaplan-Meier estimate of disease-free survival of patients with rectal cancer who underwent robotic proctectomy with curative intent between April 2009 and April 2016 (N=267)
Discussion
We have conducted an analysis of short and medium term oncological outcomes after robotic proctectomy for rectal cancer using a prospectively maintained oncological database from a single large NCI designated cancer center in the US. The results demonstrate that robotic rectal surgery could be performed with a very low rate of CRM involvement, high rate TME completeness, and good recurrence free survival. Moreover there was a low risk of conversion to open surgery despite a relatively advanced stage at presentation.
Our data compare favorably with published prospective data on open and laparoscopic proctectomy. The results of two large randomized controlled trials comparing laparoscopic and open TME have recently been published.3, 4 In this current study, we used very similar definitions for margin positivity and TME completeness to the randomized trials and the results serve as a good reference point for the outcomes of modern rectal cancer surgery in selected patients and centers. In the ALaCaRT study, the CRM positive rate was 7% in the laparoscopic arm and 3% in the open arm (the DRM positive rate was 1% in both arms). In the Z6051 trial, which included 35 robotic cases in the minimally invasive arm, the CRM positive rate was 12% in the minimally invasive arm and 8% in the open arm (the DRM positive rate was 2% in both arms). The overall rate of incomplete TME was 2% in the ALaCaRT study, and 6% in the Z6051 study (with a non-significant trend in favor of open surgery in both trials). As a result of these findings, the effectiveness of laparoscopic surgery could not be established. When compared with the above results, the outcomes in the current series appear quite favorable with a CRM positive rate of 2.6% overall. Moreover the composite outcome of “successful resection” also compares favorably: 97.3% in the current study versus 84.2% in the Z6051 trial and 82% in AlaCaRT (although we did have some missing data for TME quality which limits this comparison somewhat). While it should be recognized that there are also limitations when comparing single institutional data to that from randomized trials, it should be noted that the pre-operative stage was more advanced in this series than in both the trials with 73% staged as AJCC III/IV versus approximately 46% in AlaCaRT and 55% in in Z6051 trial. Moreover our perioperative assessment criteria was modeled after the approach used in Z6051.This is consistent with the fact that both trials excluded all patients with clinical T4 tumors and that the AlaCaRT trial also excluded patients with T3 tumors with a radiographically involved CRM. In addition, the percentage of male patients, the proportion of tumors that were in the low rectum, and the median BMI were similar in all three data sets.
To the authors' knowledge, this is the largest series of robotic rectal cancer surgery to be published from a Western center.20-22 The only larger series identified in the literature was published by Baik et al from South Korea and included 370 patients undergoing tumor-specific TME. They reported a CRM positive rate of 5.7%, and 3-year cumulative local recurrence rate of 3.6%.16 While it is notable that in their series patients had comparatively earlier stage disease, a lower BMI (23.3kg/m2), and were much less likely to undergo neo-adjuvant chemoradiation (only 21%), perhaps reflecting differences between Eastern and Western practice, the reported oncological outcomes are comparable to our results, and lend support to the oncological safety of the robotic approach.
The rate of conversion to open surgery was comparable to the lowest rates from prior international single institutional reports at 2.2%.20 We attribute this largely to preoperative patient evaluation and selection. Patients with disease unsuitable for a minimally invasive approach based on pre-operative cross sectional imaging, or those with prior history of extensive intra-abdominal surgery were recommended to undergo open resection up front. In addition, we frequently utilized the reverse hybrid technique with laparoscopic dissection to mobilize the splenic flexure or omental flap after vascular ligation and TME was completed, particularly in the earlier cohort of patients where a second or third generation robot (rather than the current fourth generation) was used.18 There is also evidence that the robotic approach may have a lower overall conversion rate than a purely laparoscopic one, in particular when operating on obese male patients.22, 23 In a preliminary presentation of the ROLARR trial results, which compared laparoscopic versus robotic surgery, this finding was supported, but it was noted that a major cause of conversions in both study arms was failure to progress in the pelvis.13 We have adapted additional techniques to facilitate rectal retraction to reduce the risk for failure to progress in the pelvis, which may have contributed to our low rate of conversion. Indeed the rate of conversion in the current series is lower than reported in ROLARR but consistent with other reports from experienced centers that undertake high volume robotic surgery, suggesting that the learning curve with respect to reducing conversion risk during robotic surgery may be longer than previously considered.16
Currently, the single biggest drawback of the robotic assisted approach is the higher cost associated with the equipment and reported longer operative time compared with laparoscopic and open surgery.22, 24, 25 It is expected that if current trends continue, both of these cost drivers may reduce owing to economies of scale, improved instrument design, and increased experience of the operating team.26 Furthermore advances in competing technologies have the potential to impact cost as well. We did note that in our series the total console time was relatively short at 122 min and our median docking time was only 4 minutes. Console time included vascular dissection, colonic mobilization, and TME. The remainder of the operating time was for splenic flexure mobilization (in hybrid cases), colon division, rectal division, anastomosis (including coloanal in a significant number of cases), evaluation of anastomosis by flexible endoscopy, ileostomy formation, pathology review, and wound closure. Therefore non-console operating time was actually longer than time spent operating on the robot. In addition, the technology and surgical technique itself continue to be refined, and while this may not necessarily lower the cost of the procedure, incremental patient and provider benefits may improve the effectiveness side of the equation.27 Finally, there may be cost-savings associated with reduced hospitalization in patients who might have otherwise required an open approach. The 4-day median length of stay in this study was achieved with most patients treated prior to standardization of enhanced recovery after surgery pathways. Thus further gains in perioperative care and reduced length of stay may be possible. Nevertheless, cost remains an important barrier to utilization and must be addressed in a climate of increasing pressure on healthcare resources if the technique is to be widely adopted internationally.28
This study has several limitations. Firstly, the study cohort reflects a single institutional experience which may be subject to bias as patients were selected for robotic surgery at the discretion of the operating surgeon based on the general principles as outlined. The nature of the practice in our center predisposes to the selective referral of complex and re-operative rectal cancer cases, many of which require synchronous multi-organ resection, exenterative procedures, complex abdominal wall reconstruction, or extended aorto-iliac lymphadenectomy. Most of these cases were not suitable for a minimally invasive approach, and it is notable that the majority of proctectomies done over the study period were actually performed open (77%). Nevertheless, the current robotic dataset still includes a relatively high number of male patients with low rectal cancers, as well as some selected extended resections for locally advanced disease.7 Another possible limitation is that the reverse-hybrid technique was used in many cases (laparoscopic flexure mobilization), particularly earlier in the series, and so not all cases had a totally robotic technique,18 although all pelvic dissections were completed robotically in the cases that were not converted to laparotomy. Assessment of the quality of TME was incompletely reported during the early study period, with almost one third of patients having missing data for this parameter, limiting comparisons with ALaCart and Z6051 trials. Photo-documentation was not performed as per published criteria but the information was collected prospectively and at the time of resection. Finally, our median follow-up was relatively short at 24 months; however it was sufficient for actuarial estimates of disease free survival and covers the period of time when the majority of recurrences are identified.
Conclusion
Robotic proctectomy for rectal cancer can be performed with good short and medium-term oncological outcomes in selected patients. Long-term oncological results, and outcomes of randomized controlled trials are awaited.
Figure 3.
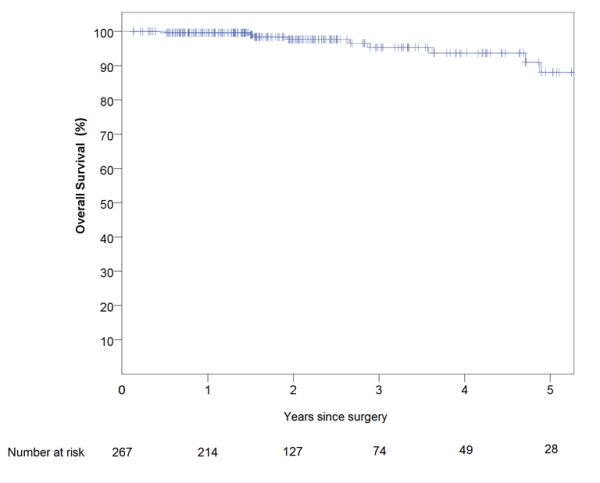
Kaplan-Meier estimate of overall survival of patients with rectal cancer who underwent robotic proctectomy with curative intent between April 2009 and April 2016 (N=267)
Acknowledgments
The authors would like to acknowledge Sa T Nguyen, Research Programmer, Department of Surgical Oncology for her assistance with database management and Jill Delsigne-Russell, Associate Scientific Editor, Department of Scientific Publications for her assistance with manuscript editing.
Financial Support: This study is supported in part by the Aman Trust for Colorectal Cancer Research and Education (GJC) and National Institutes of Health/National Cancer Institute grant CA016672 (The University of Texas MD Anderson Cancer Center Support Grant). The NIH had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
We declare that we have no relevant conflicts of interest.
References
- 1.The Beyond TME Collaborative. Consensus statement on the multidisciplinary management of patients with recurrent and primary rectal cancer beyond total mesorectal excision planes. Br J Surg. 2013;100(8):1009–14. doi: 10.1002/bjs.9192. [DOI] [PubMed] [Google Scholar]
- 2.Bonjer HJ, Deijen CL, Abis GA, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372(14):1324–32. doi: 10.1056/NEJMoa1414882. [DOI] [PubMed] [Google Scholar]
- 3.Fleshman J, Branda M, Sargent DJ, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA. 2015;314(13):1346–55. doi: 10.1001/jama.2015.10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevenson AR, Solomon MJ, Lumley JW, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA. 2015;314(13):1356–63. doi: 10.1001/jama.2015.12009. [DOI] [PubMed] [Google Scholar]
- 5.Bretagnol F, Dedieu A, Zappa M, et al. T4 colorectal cancer: is laparoscopic resection contraindicated? Colorectal Dis. 2011;13(2):138–43. doi: 10.1111/j.1463-1318.2010.02380.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim KY, Hwang DW, Park YK, et al. A single surgeon's experience with 54 consecutive cases of multivisceral resection for locally advanced primary colorectal cancer: can the laparoscopic approach be performed safely? Surg Endosc. 2012;26(2):493–500. doi: 10.1007/s00464-011-1907-7. [DOI] [PubMed] [Google Scholar]
- 7.Shin US, Nancy You Y, Nguyen AT, et al. Oncologic Outcomes of Extended Robotic Resection for Rectal Cancer. Ann Surg Oncol. 2016 doi: 10.1245/s10434-016-5117-3. [DOI] [PubMed] [Google Scholar]
- 8.Fleshman JW, Boughey JC, You YN. Rectal cancer resection: Laparoscopic or open--which way forward? Bull Am Coll Surg. 2016;101(1):64–6. [PubMed] [Google Scholar]
- 9.Baek SJ, Kim CH, Cho MS, et al. Robotic surgery for rectal cancer can overcome difficulties associated with pelvic anatomy. Surg Endosc. 2015;29(6):1419–24. doi: 10.1007/s00464-014-3818-x. [DOI] [PubMed] [Google Scholar]
- 10.Allemann P, Duvoisin C, Di Mare L, et al. Robotic-Assisted Surgery Improves the Quality of Total Mesorectal Excision for Rectal Cancer Compared to Laparoscopy: Results of a Case-Controlled Analysis. World J Surg. 2016;40(4):1010–6. doi: 10.1007/s00268-015-3303-2. [DOI] [PubMed] [Google Scholar]
- 11.Kang J, Yoon KJ, Min BS, et al. The impact of robotic surgery for mid and low rectal cancer: a case-matched analysis of a 3-arm comparison--open, laparoscopic, and robotic surgery. Ann Surg. 2013;257(1):95–101. doi: 10.1097/SLA.0b013e3182686bbd. [DOI] [PubMed] [Google Scholar]
- 12.Baik SH, Ko YT, Kang CM, et al. Robotic tumor-specific mesorectal excision of rectal cancer: short-term outcome of a pilot randomized trial. Surg Endosc. 2008;22(7):1601–8. doi: 10.1007/s00464-008-9752-z. [DOI] [PubMed] [Google Scholar]
- 13.Pigazzi A. Results of robotic vs laparoscopic resection for rectal cancer: ROLARR Study. Verbal presentation: American Society of Colon and Rectal Surgeons Annual Scientific Meeting; 2015. [Google Scholar]
- 14.Collinson FJ, Jayne DG, Pigazzi A, et al. An international, multicentre, prospective, randomised, controlled, unblinded, parallel-group trial of robotic-assisted versus standard laparoscopic surgery for the curative treatment of rectal cancer. Int J Colorectal Dis. 2012;27(2):233–41. doi: 10.1007/s00384-011-1313-6. [DOI] [PubMed] [Google Scholar]
- 15.Speicher PJ, Englum BR, Ganapathi AM, et al. Robotic Low Anterior Resection for Rectal Cancer: A National Perspective on Short-term Oncologic Outcomes. Ann Surg. 2015;262(6):1040–5. doi: 10.1097/SLA.0000000000001017. [DOI] [PubMed] [Google Scholar]
- 16.Baik SH, Kim NK, Lim DR, et al. Oncologic outcomes and perioperative clinicopathologic results after robot-assisted tumor-specific mesorectal excision for rectal cancer. Ann Surg Oncol. 2013;20(8):2625–32. doi: 10.1245/s10434-013-2895-8. [DOI] [PubMed] [Google Scholar]
- 17.Sun Z, Kim J, Adam MA, et al. Minimally Invasive Versus Open Low Anterior Resection: Equivalent Survival in a National Analysis of 14,033 Patients With Rectal Cancer. Ann Surg. 2016;263(6):1152–8. doi: 10.1097/SLA.0000000000001388. [DOI] [PubMed] [Google Scholar]
- 18.Park IJ, You YN, Schlette E, et al. Reverse-hybrid robotic mesorectal excision for rectal cancer. Dis Colon Rectum. 2012;55(2):228–33. doi: 10.1097/DCR.0b013e31823c0bd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 20.Kim CW, Kim CH, Baik SH. Outcomes of robotic-assisted colorectal surgery compared with laparoscopic and open surgery: a systematic review. J Gastrointest Surg. 2014;18(4):816–30. doi: 10.1007/s11605-014-2469-5. [DOI] [PubMed] [Google Scholar]
- 21.Guerra F, Pesi B, Bonapasta SA, et al. Does robotics improve minimally invasive rectal surgery? Functional and oncological implications Journal of Digestive Diseases. 2016;17:88–94. doi: 10.1111/1751-2980.12312. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Wei Z, Bie M, et al. Robot-assisted versus laparoscopic-assisted surgery for colorectal cancer: a meta-analysis. Surg Endosc. 2016 doi: 10.1007/s00464-016-4892-z. [DOI] [PubMed] [Google Scholar]
- 23.Feinberg AE, Elnahas A, Bashir S, et al. Comparison of robotic and laparoscopic colorectal resections with respect to 30-day perioperative morbidity. Can J Surg. 2016;59(4):16615. doi: 10.1503/cjs.016615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moghadamyeghaneh Z, Phelan M, Smith BR, et al. Outcomes of Open, Laparoscopic, and Robotic Abdominoperineal Resections in Patients With Rectal Cancer. Dis Colon Rectum. 2015;58(12):1123–9. doi: 10.1097/DCR.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 25.Baek SJ, Kim SH, Cho JS, et al. Robotic versus conventional laparoscopic surgery for rectal cancer: a cost analysis from a single institute in Korea. World J Surg. 2012;36(11):2722–9. doi: 10.1007/s00268-012-1728-4. [DOI] [PubMed] [Google Scholar]
- 26.Young M, Pigazzi A. Total mesorectal excision: open, laparoscopic or robotic. Recent Results Cancer Res. 2014;203:47–55. doi: 10.1007/978-3-319-08060-4_6. [DOI] [PubMed] [Google Scholar]
- 27.Atallah S, Nassif G, Polavarapu H, et al. Robotic-assisted transanal surgery for total mesorectal excision (RATS-TME): a description of a novel surgical approach with video demonstration. Tech Coloproctol. 2013;17(4):441–7. doi: 10.1007/s10151-013-1039-2. [DOI] [PubMed] [Google Scholar]
- 28.Sammour T, Macleod A, Chittleborough TJ, et al. Total caseload of a colorectal surgical unit: baseline measurement and identification of areas for efficiency gains. Int J Colorectal Dis. 2016;31(6):1141–8. doi: 10.1007/s00384-016-2556-z. [DOI] [PubMed] [Google Scholar]


