Abstract
Introduction
Failure to change risk behaviors following myocardial infarction (MI) increases the likelihood of recurrent MI and death. Lower-socioeconomic status (SES) patients are more likely to engage in high-risk behaviors prior to MI. Less well known is whether propensity to change risk behaviors after MI also varies inversely with SES.
Methods
We performed a systematized literature review addressing changes in risk behaviors following MI as a function of SES.
Results
2160 abstracts were reviewed and 44 met eligibility criteria. Behaviors included smoking cessation, cardiac rehabilitation (CR), medication adherence, diet, and physical activity (PA). For each behavior, lower-SES patients were less likely to change after MI. Overall, lower-SES patients were 2 to 4 times less likely to make needed behavior changes (OR's 0.25–0.56).
Conclusion
Lower-SES populations are less successful at changing risk behaviors post-MI. Increasing their participation in CR/secondary prevention programs, which address multiple risk behaviors, including increasing PA and exercise, should be a priority of healthy lifestyle medicine (HLM).
Keywords: myocardial infarction, socioeconomic status, risk factors, secondary prevention, health behaviors
Introduction
Coronary heart disease is the leading cause of death in the United States, accounting for roughly 14% of deaths annually. An estimated 750,000 Americans experience a myocardial infarction (MI) each year (1). Rehospitalizations after an initial cardiovascular (CV) disease (CVD) event are also a major concern, as there are more than 200,000 recurrent MI's each year (1). In one study 30% of those hospitalized for an MI were readmitted within 90 days (2).
Behavior change following an MI is critical for addressing the risk of future morbidity and mortality. Risk of reinfarction, as well as other sources of morbidity and mortality, can be significantly reduced through adherence to secondary prevention guidelines that include the modification of lifestyle behaviors such as smoking cessation (3), improving diet (4, 5), increasing moderate physical activity (PA) (6), adhering to prescribed medications (7), and attending cardiac rehabilitation (CR) (8). Attendance at CR is particularly important as at CR patients receive counseling and support to alter a host of behavioral risk factors (9).
Certain vulnerable populations are at a particularly increased risk for developing CVD. One such at-risk population is patients with lower-socioeconomic status (SES), which has a long history of strong associations with CV health (10) and continues to be a robust predictor of MI incidence (11, 12) even at the level of countries (13, 14).
SES is also a strong predictor of morbidity and mortality following an MI. Multiple studies have found that morbidity and mortality rates following MI are higher among those with lower SES (15–19), which is also predictive of progression of CVD, severity of MI presentation, and mortality (20–21). Associations between SES and mortality following MI persist even in countries with universal healthcare (18). Similarly, within the Medicare population (where you would assume equity of medical care) lower-SES patients have higher rates of mortality than higher-SES patients (22–23).
This association between lower SES and poor post-MI outcomes is not well explained by access to healthcare and is thought to be more related to behavioral risk-factor patterns (24). Lower-SES patients have behavioral profiles consistent with a higher risk of incident and recurrent MI, as lower-SES patients are more likely to smoke, engage in low levels of PA, have poor blood pressure, type 2 diabetes mellitus, or lipid control, have higher body mass indices, and consume more fat and fewer fruits and vegetables than their higher-SES counterparts (17, 25–28). It is estimated that controlling these potentially modifiable risk factors could address up 80–90% of the risk for initial MI (29, 30). These risky behaviors not only predict development of CVD but also subsequent morbidity and mortality after its onset. Indeed, studies that have examined these relationships have demonstrated that most of the discrepancy in cardiovascular morbidity and mortality between lower- and higher-SES populations can be explained by the higher risk profiles of lower-SES patients (15–17, 27, 28, 31). For example, morbidity risk drops from a hazard ratio of 2.68 to 1.52 after adjusting for behavioral risk-factors (17) while mortality rates of 5.1% vs. 1.9% are no longer significantly different after a similar adjustment (31). In essence, it is not SES that is responsible for morbidity and mortality disparities, it is the behavioral risk factors associated with SES that drive health outcome disparities.
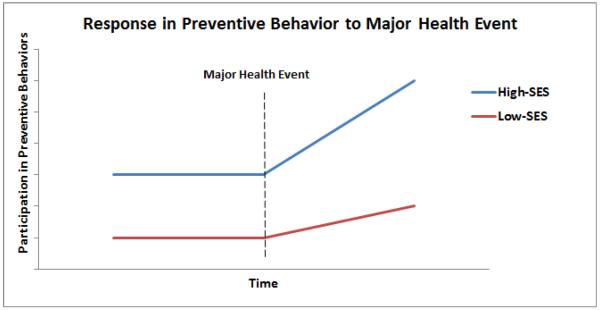
Assuming that lower-SES patients are higher risk for CVD given their relatively high rates of risk behaviors (smoking, sedentary lifestyles, etc.), an additional important determinant of future morbidity and mortality risk is how lower-SES patients respond to the necessity of making behavioral changes after experiencing an MI. Other researchers have demonstrated that, in general, lower-SES populations are “vulnerable” to life event challenges and may respond differently to such events (e.g. 32). If lower-SES populations are less likely to change their behavior in response to major health challenges than higher-SES populations, we would expect to see that health disparities by SES would actually increase over time (Figure 1). Indeed, there is some evidence to support this view as studies modeling disparities over time show that the discrepancy in health between lower- and higher-SES populations increase as the population ages and accumulates more health challenges (e.g. 33). If indeed lower-SES cardiac patients are struggling to change risk behaviors following a major health event, like an MI, a preventive approach such as healthy lifestyle medicine (HLM) would suggest increased efforts to engage these patients in secondary prevention programs, such as CR (34).
Figure 1.
Hypothetical trajectories of participation in preventive health behaviors over time in which reduced response to a major helth event in lower-SES populations would predict an increasing divergence in health disparities.
Given that risk behaviors account for much of morbidity and mortality following a CVD event, changing behavioral risk factors to align with secondary prevention guidelines should significantly attenuate future risk. However, if lower-SES patients are less likely to make the needed behavior changes following an MI, this could help explain the sustained and diverging disparities in health outcomes seen by SES. The goal of this review is to help inform HLM by examining the extent to which behavior change following an MI, as measured by changes to risk behaviors, varies as a function of SES.
Methods
A systemized literature review was conducted using PubMed, PsychInfo, and Web of Science to identify articles potentially relevant to the relationship between SES and post-MI behavior change. SES is defined in a variety of ways in the literature and thus search terms were included to encompass a diversity of definitions such as income, insurance type, and educational attainment. The following search string was used: (((myocardial infarction) AND (deprivation OR SES OR poverty OR neighborhood OR high school OR Medicaid OR socioeconomic status OR education OR income)) AND (behavior OR smoking OR cardiac rehabilitation OR fitness change OR physical activity OR medication adherence OR diet change))). Articles had to be published by July 1, 2016. Abstracts were reviewed independently by two of the authors (DEG, RJE). To be included, articles had to: (a) Report data regarding changes in secondary prevention behavior following an MI; (b) Report outcomes by SES level; (c) Include a sample of at least 100 patients to protect against biased results due to small sample sizes; (d) Be available in English; (e) Include a sample where MI made up >70% of eligible diagnoses. Where SES was measured several ways within a study, individual-level characteristics were reported preferentially over group-level variables (e.g. individual educational attainment vs. neighborhood average income). Within individual-level characteristics, reporting preferences was given in the following order: educational attainment, insurance type (e.g. Medicaid or other income-based insurance), income, and then job-type. In instances where effects of SES were reported as significant at a univariate but not multivariate level, the effect was considered non-significant.
Results
We identified 2160 abstracts for review in the initial search. After removing duplicates, 1552 unique articles were identified and reviewed for potential inclusion with 47 articles meeting inclusion criteria. Three additional articles were excluded due to overlap with an earlier report by the same investigators. A final sample of 44 articles represented 50 data points for this review, as some articles reported on multiple behaviors. Articles included for each behavior are summarized in Tables 1–4. Most articles identified examined one of three behaviors: smoking cessation (16), attendance at CR (14), or medication adherence (14). Changes in PA and diet were represented by only three articles each. Data were from 19 countries and encompassed results from over 400,000 patients. Articles used a variety of SES markers, most commonly educational attainment, income, or a composite measure that estimated deprivation level on a neighborhood level, taking into account variables such as local income and employment levels, and car and home ownership.
Table 1.
Effect of Socioeconomic Status on Change in Smoking Status Following Myocardial Infarction
| Author | Year | Country | n | Definition of SES | Effect Direction | Effect Size |
|---|---|---|---|---|---|---|
| Altenhoener et al. (66) | 2012 | Germany | 543 | Composite measure | −* | OR of continued smoking lower in medium and higher SES groups (ORs 0.27, 0.31) |
| Gerber et al. (a) (67) | 2011 | Israel | 768 | Education | −* | OR of continued smoking 0.80 for each additional 4 years of education |
| Greenwood et al. (36) | 1995 | UK | 532 | Composite measure | −* | OR for stopping smoking 0.80 for lower social classes |
| Ockene et al. (68) | 1985 | USA | 200 | Education | −* | Discriminate function coefficient of −0.397 in a model differentiating current and ex-smokers |
| Smith et al. (37) | 2009 | Canada | 248 | Education | −* | Higher education predicts higher cessation at 12 months (OR 2.34) |
| Wray et al. (45) | 1998 | USA | 2,391 | Education | −* | Each additional year of education beyond HS increased odds of quitting by 44% |
| Attebring et al. (69) | 2004 | Sweden | 1,320 | Job type | − | 44% of those with manual labor jobs quit vs. 61% with non-manual jobs |
| Conroy et al. (70) | 1986 | USA | 299 | Job type | − | Probability of continued abstinence correlated with job type (Tau=−0.22, p=0.003). |
| Tofler et al. (71) | 1993 | USA | 816 | Education | − | Those with less than HS education less likely to be quit at 6 months (38% vs. 49%) |
| Chan et al. (72) | 2008 | Canada | 1,801 | Income | =* | Smoking cessation did not differ by educational attainment |
| Dawood et al. (3) | 2008 | USA | 834 | Education | =* | No differences in abstinence at 6 months by SES |
| Quist-Paulsen et al. (73) | 2005 | Norway | 218 | Education | =* | Education did not predict cessation at 12 months |
| Hajeck et al. (74) | 2002 | UK | 540 | Education | =* | Education was a univariate but not multivariate predictor of cessation at 12 months |
| Dornelas et al. (75) | 2000 | USA | 100 | Education | = | No differences in abstinence at 6 months by SES |
| Rallidis et al. (76) | 2005 | Greece | 607 | Education | = | Education did not predict cessation at 30 months |
| Shapiro et al. (77) | 1970 | USA | 564 | Job type | + | Those with a blue collar job more likely to quit (35% vs. 27%) |
Note: Significance is defined as the original author's determination of statistical significance. A criterion of p < .05 was used across all studies. A + denotes low-SES is correlated with behavior change, an = denotes no significant relationship and a − denotes low-SES is negatively correlated with behavior change. An * denotes studies using positively multivariate analyses that accounted for other common predictors of behavior change such as age, gender, race and history and severity of disease.
Table 4.
Effect of Socioeconomic Status on Change in Physical Activity and Diet Following Myocardial Infarction
| Author | Year | Country | n | Behavior | Definition of SES | Effect Direction | Effect Size |
|---|---|---|---|---|---|---|---|
| Salisbury et al. (95) | 2011 | USA | 2,481 | Diet | Education | −* | Patients without a college degree more likely to keep eating fast food after MI (RR 1.27) |
| Conroy et al. (70) | 1986 | USA | 299 | Diet | Job type | − | Those of a lower social class were less likely to achieve a healthy BMI by 1 year (Tau = −0.21) |
| Chan et al. (72) | 2008 | Canada | 1,801 | Diet | Income | =* | Education was not predictive of dietary change in a multi-variate model |
| Gerber et al. (b) (96) | 2011 | Israel | 1,410 | Physical Activity | Education | −* | Lower educational attainment is associated with decreasing physical activity after MI (AOR 0.87) |
| Shapiro et al., (77) | 1970 | USA | 564 | Physical Activity | Job type | − | Having a blue collar job is associated with decreasing physical activity after MI (decrease of 31% vs. 22%) |
| Conroy et al. (70) | 1986 | USA | 299 | Physical Activity | Job type | − | Those of a lower social class were less likely to increase their physical activity by 1 year (Tau = −0.21) |
Note: Significance is defined as the original author's determination of statistical significance. A criterion of p < .05 was used across all studies. A + denotes low-SES is correlated with behavior change, an = denotes no significant relationship and a − denotes low-SES is negatively correlated with behavior change. An * denotes studies using positively multivariate analyses that accounted for other common predictors of behavior change such as age, gender, race and history and severity of disease.
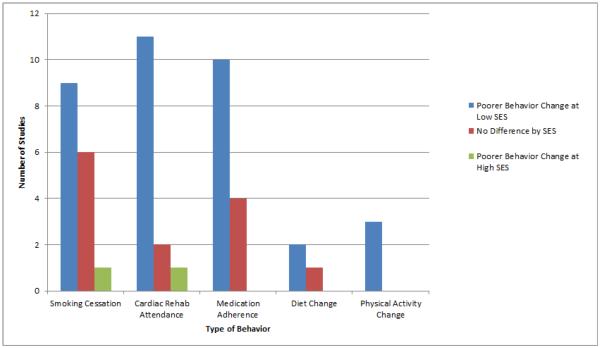
Figure 2 summarizes the effects of SES on successful engagement in secondary prevention for the six behaviors examined. Overall, the majority of articles reported that lower SES predicted a lower likelihood of success. For smoking cessation, 56% (9/16) of studies reported less cessation in lower-SES patients. For attendance at CR, 79% (11/14) of studies reported poorer attendance by lower-SES patients. For medication adherence, 71% (10/14) of studies reported lower adherence among lower-SES patients. Relatively few articles examined changes in PA and diet after MI by SES, however, within the articles identified, 100% (3/3) reported less improvement in PA among lower-SES patients and 67% (2/3) reported less improvement in diet among lower-SES patients. Overall, within the risk behaviors examined, lower-SES consistently predicted less success with behavior change. Summarizing across comparisons examined, 70% (35/50) reported that lower SES predicted less positive behavior change following MI, 26% (13/50) reported no difference by SES, and only 4% (2/50) reported an effect in the opposite direction. While reporting of effect sizes varied considerably in this sample, examining a subset of studies within the three most commonly reported behaviors can begin to provide an estimate. Looking within studies demonstrating a negative impact of lower SES using multivariate modeling and SES as a dichotomous variable (lower vs. higher), odds ratios ranged from 0.27 to 0.80 (median 0.43) for smoking cessation (35–37), 0.12 to 0.40 (median 0.25) for CR attendance (8, 38–40), and 0.30 to 0.79 (median 0.56) for medication adherence (41–43).
Figure 2.
Summary showing numbers of comparisions reporting poorer changes in behavior at lower-SES, higher-SES, or no difference by SES.
Discussion
Our results indicate that lower-SES patients are less likely to make needed behavior changes following an MI than their better educated and more affluent counterparts. Given that lower-SES patients have higher-risk cardiac behavioral profiles prior to their clinical event (15, 17, 44), and that much of the risk of morbidity and mortality associated with CVD is accounted for by behavioral risk factors (15–17, 27, 28, 31), this subsequent divergence in adherence to secondary prevention guidelines only serves to further widen already established SES-based health disparities (Figure 1). As the field of medicine contemplates a shift from reactionary care towards preventive care, such as in HLM, increasing engagement among lower-SES patients in preventive care programs after MI should be a priority.
SES clearly has large effects on changing behavioral risk factors after MI. For example, with smoking cessation, one study reported that each year of education a patient had beyond high school increased the chance of quitting smoking after an MI by 44% (45). Similarly, in a nationwide study of CR, being lower SES decreased the chances of attending the program by more than half (40). Engagement in CR/secondary prevention programs is particularly important as patients are counseled and guided towards adherence with multiple behavioral risk factors such as physical activity, smoking cessation, healthy eating and medication adherence (9, 34).
The consequences of not adhering to these behaviors following an MI are powerful. Those who continue smoking have twice the mortality rate of those who quit (46). Similarly, attendance at CR results in a 26% reduction in cardiac mortality and a 31% reduction in one-year hospital readmissions (47). One of the seemingly most straightforward behaviors is taking recommended medications such as statins. However, adherence is far from optimal and suboptimal adherence to medications can increase rehospitalization rates by 50% (48). One study estimated that 28.9% of post-MI deaths could be avoided if all patients received all the guideline-recommended interventions including attending CR, taking statins, and receiving guidance on smoking cessation and diet change (49). Again, improvements in attendance at CR could potentially impact multiple behaviors as patients are carefully monitored for symptom changes that could reveal lack of medication adherence and are counseled on other areas of cardiac health such as improving diet and smoking cessation (50).
Given the serious consequences of not adhering to secondary prevention guidelines following MI improving risky behaviors should be a priority. Indeed, even in the general population, participation in secondary prevention behaviors can be very low. For example, in one study of a population that had enrolled in a CR/secondary prevention program, adherence to some of the secondary prevention guidelines was as low as 15% (PA) (51). However, given existing health disparities, improving adherence in lower-SES populations should be a priority. As poor outcomes are likely related to post-MI behavior and given that lower-SES patients who adopt appropriate changes benefit similarly to their higher-SES counterparts (52–54), much of the disparities seen in outcomes following MI could be addressed by improving behavioral interventions for lower-SES patients following MI.
Improving behavioral risk factors post-MI in lower-SES populations could be challenging. One approach would be to use interventions that have proven successful in improving high-risk behaviors in the CAD population generally and adapting them for or targeting them to those with lower SES. For example, success at promoting smoking cessation has been demonstrated with intensive programs initiated in the hospital combined with medication therapy and regular follow up by health professionals (55). Better attendance at CR has been seen by automating CR referral during the hospital discharge process and by providing a liaison during the hospital discharge transition (56, 57). Adjusting CR programs to make them more accessible, for example by providing expanded hours for scheduling, may also be useful (34, 58). Additionally, reducing co-pays may help with improving adherence to prescribed medications (59). However, it is unclear which interventions would be successful specifically within the lower-SES population and more research in this area is needed. Very little investigation has been done thus far aimed at improving adherence with secondary prevention behaviors specifically in lower-SES populations. However, one promising approach that is currently being tested is the use of incentives, where objectively measured outcomes of behavior change, such as attendance at CR, are reinforced with gift cards, vouchers, or other financial incentives (60).
One interesting aspect of the studies reviewed was the significant variation in how SES was measured. SES was measured by income, educational attainment, insurance status, or by composite measures that were used to construct a “deprivation index.” SES was also reported either on an individual level, or on a neighborhood level. Some studies also included multiple measures of SES. While having any SES measure will go far towards helping characterize a population, multiple measures may provide a more nuanced picture of associations between SES and various outcome measures. Some studies have demonstrated, for example, that income and education are both independently associated with health outcomes (17, 19, 61). However, if using only a single measure, the patient characteristic that may be most useful at teasing apart SES differences in health behaviors and outcomes appears to be educational attainment. In one study examining risk for MI across 52 countries, educational attainment was consistently the most predictive SES measure (13).
However SES is measured, lower-SES patients are a high-risk group in need of additional support post-MI to make healthy behavior change. Not only does this population have higher rates of morbidity and mortality following MI they are also more likely to fail to regain their previous quality of life. Lower-SES patients are more likely to develop frailty (61), lose their independence (62), have limited functional recovery (63), and are less likely to successfully return to work following their MI (64), all negative outcomes that are ameliorated with participation in CR (9, 65). Overall these findings paint a picture of a high-risk population with myriad challenges in need of intensive intervention post-MI. It seems clear that the period of time following an MI is a particularly vulnerable time for lower-SES patients but also a time where important behavior changes may occur. Engagement in appropriate CR/secondary prevention programs could reduce the likelihood of future events and improve the likelihood of regaining functional capacity and remaining independent, all very important outcomes from a HLM standpoint.
Conclusion
Lower-SES patients are less likely to change risk behaviors and adhere to secondary prevention guidelines following an MI than their more affluent and better educated counterparts. This differential response likely leads to increasing health disparities over time. Vulnerable, high-risk patients may need intensive interventions to make significant changes and reduce risk for future morbidity and mortality. Encouraging participation by lower-SES patients in CR/secondary prevention programs where multiple risk behaviors are addressed, including PA and exercise, should be a high priority of HLM.
Table 2.
Effect of Socioeconomic Status on Cardiac Rehabilitation Participation and Adherence Following Myocardial Infarction
| Author | Year | Country | n | Definition of SES | Effect Direction | Effect Size |
|---|---|---|---|---|---|---|
| Dunlay et al. (38) | 2009 | USA | 179 | Education | −* | Those with post-secondary education more likely to attend CR (OR 3.32) |
| Jin et al. (39) | 2014 | China | 328 | Education | −* | Those with lower education are less likely to attend CR (OR 8.13) |
| Melville et al. (78) | 1999 | UK | 464 | Composite measure | −* | Each unit increase in deprivation score reduces odds of attending CR by 8–15% (ORs 0.92, 0.85) |
| Nielsen et al. (8) | 2008 | Denmark | 200 | Income | −* | Low-income patients less likely to attend CR (OR 0.20) |
| Oldridge (79) | 1984 | Canada | 733 | Job type | −* | Dropout from CR higher for those in blue collar jobs (adjusted RR 1.71) |
| Pell et al. (80) | 1996 | Scotland | 316 | Composite measure | −* | Each unit increase in deprivation score reduces odds of completing CR (OR 0.96) |
| Suaya et al (40) | 2007 | USA | 267,427 | Insurance type | −* | Those with Medicaid insurance are less likely to attend CR (adjusted OR 0.44) |
| Alter et al. (81) | 2013 | Canada | 1,368 | Income | − | 37% of low-income patients attended CR vs 60% of higher-income |
| Ramm et al. (82) | 2001 | New Zealand | 324 | Education | − | Those with secondary school or less were less likely to attend CR (p<0.05) |
| Stern & Cleary (83) | 1981 | USA | 784 | Composite measure | − | Those dropping out of CR were more likely to be lower-social class (46% of dropouts vs. 34% of completers) |
| Young et al., (84) | 1990 | USA | 246 | Education | − | Patients with lower educational attainment were less likely to enroll in CR (RR 0.40) |
| Lane et al. (85) | 2001 | UK | 263 | Education | =* | Education was not a significant predictor in multivariate models |
| Winberg & Fridlund (86) | 2002 | Sweden | 200 | Education | = | Educational attainment did not predict CR attendance |
| Altenhoener et al. (66) | 2005 | Germany | 536 | Composite measure | + | 90% of low- and middle-income patients went to CR compared to 78% of high-income patients |
Note: Significance is defined as the original author's determination of statistical significance. A criterion of p < .05 was used across all studies. A + denotes low-SES is correlated with behavior change, an = denotes no significant relationship and a − denotes low-SES is negatively correlated with behavior change. An * denotes studies using positively multivariate analyses that accounted for other common predictors of behavior change such as age, gender, race and history and severity of disease.
Table 3.
Effect of Socioeconomic Status on Medication Adherence Following Myocardial Infarction
| Author | Year | Country | n | Definition of SES | Effect Direction | Effect Size |
|---|---|---|---|---|---|---|
| Carey et al. (87) | 2012 | UK | 6,673 | Composite measure | −* | RR of continued adherence to statins 0.92 in more deprived population |
| Danchin et al. (88) | 2011 | France | 4,939 | Insurance Type | −* | Continuing secondary prevention medications adjusted RR was 0.82 in those with subsidized insurance |
| Gonarkar et al. (41) | 2016 | India | 101 | Education | −* | High educational level (graduate and above) predicts higher adherence to multiple medications (OR = 3.3) |
| Ohlsson et al. (42) | 2010 | Sweden | 1,346 | Income | −* | High income patients had higher adherence (ORs lipid: 1.29; and ACE-inhibitor therapy: 1.22) |
| Spertus et al. (43) | 2006 | USA | 500 | Education | −* | Less than high school associated with discontinuation of thienopyridine therapy (OR 1.79) |
| Akincigil et al. (89) | 2008 | USA | 1,025 | Income | − | HR of continued adherence to beta-blockers 0.72 in lower income population |
| Alter et al. (81) | 2013 | Canada | 1,368 | Income | − | High-income patients more likely to be taking medications at 1 year for each of 4 preventive medicines |
| Castellano et al. (90) | 2014 | Argentina, Brazil, Paraguay, Italy, Spain | 2,118 | Education | − | Those with primary education or less were less adherent (42% vs. 47%) |
| Shapiro et al. (77) | 1970 | USA | 564 | Job type | − | Those with a blue collar job were less likely to be taking their medications at 6 months (25% vs. 41%) |
| Shimony et al., (91) | 2009 | Israel | 1,397 | Composite measure | − | Low-SES patients had fewer continuous days of treatment with asprin (453 vs. 585) and clopigorel (94 vs 301) |
| Rasmussen et al. (a) (92) | 2007 | Denmark | 30,078 | Education | =* | Education does not consistently predict discontinuation of statin or beta-blocker treatment |
| Rasmussen et al. (b) (93) | 2007 | Canada | 31,455 | Income | =* | In multivariate analyses income does not consistently predict discontinuation 3 preventive medications |
| Wei et al. (7) | 2004 | Scotland | 865 | Composite measure | =* | Deprivation level was not a predictor of high adherence to beta-blockers |
| Matthews et al. (94) | 2015 | USA | 7,425 | Education | =* | Education a univariate but not multivariate predictor of poor adherence |
Note: Significance is defined as the original author's determination of statistical significance. A criterion of p < .05 was used across all studies. A + denotes low-SES is correlated with behavior change, an = denotes no significant relationship and a − denotes low-SES is negatively correlated with behavior change. An * denotes studies using positively multivariate analyses that accounted for other common predictors of behavior change such as age, gender, race and history and severity of disease.
Acknowledgements
This research was supported in part by Center of Biomedical Research Excellence award P20GM103644 from the National Institute of General Medical Sciences.
Abbreviations
- CR
Cardiac Rehabilitation
- CV
Cardiovascular
- CVD
Cardiovascular Disease
- HLM
Healthy Lifestyle Medicine
- MI
Myocardial infarction
- PA
Physical Activity
- SES
Socioeconomic Status
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e1–e323. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Chen HY, Tisminetzky M, Yarzebski J, et al. Decade-long trends in the frequency of 90-Day rehospitalizations after hospital discharge for acute myocardial infarction. Am J Cardiol. 2016;117:743–748. doi: 10.1016/j.amjcard.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.*Dawood N, Vaccarino V, Reid KJ, et al. Predictors of smoking cessation after a myocardial infarction: the role of institutional smoking cessation programs in improving success. Arch Intern Med. 2008;168:1961–1967. doi: 10.1001/archinte.168.18.1961. [DOI] [PubMed] [Google Scholar]
- 4.Liu S, Manson JE, Lee IM, et al. Fruit and vegetable intake and risk of cardiovascular disease: the Women's health Study. Am J Clin Nutr. 2001;72:922–928. doi: 10.1093/ajcn/72.4.922. [DOI] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Fried LP, Burke GL, et al. Lifestyles of older adults: can we influence cardiovascular risk in older adults? Am J Geriatr Cardiol. 2004;13:153–160. doi: 10.1111/j.1076-7460.2004.02122.x. [DOI] [PubMed] [Google Scholar]
- 6.Mons U, Hahmann H, Brenner H. A reverse J-shaped association of leisure time physical activity with prognosis in patients with stable coronary heart disease: evidence from a large cohort with repeated measurements. Heart. 2014;100:1043–1049. doi: 10.1136/heartjnl-2013-305242. [DOI] [PubMed] [Google Scholar]
- 7.*Wei L, Flynn R, Murray GD, et al. Use and adherence to beta-blockers for secondary prevention of myocardial infarction: who is not getting the treatment? Pharmacoepidemiol Drug Saf. 2004;13:761–766. doi: 10.1002/pds.963. [DOI] [PubMed] [Google Scholar]
- 8.*Nielsen KM, Faergeman O, Foldspang A, et al. Cardiac rehabilitation: health characteristics and socio-economic status among those who do not attend. The European Journal of Public Health. 2008;18:479–483. doi: 10.1093/eurpub/ckn060. [DOI] [PubMed] [Google Scholar]
- 9.Ades PA. Cardiac rehabilitation and secondary prevention of coronary heart disease. N Engl J Med. 2001;345:892–902. doi: 10.1056/NEJMra001529. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993;88:1973–1998. doi: 10.1161/01.cir.88.4.1973. [DOI] [PubMed] [Google Scholar]
- 11.Salomaa V, Niemelä M, Miettinen H, et al. Relationship of socioeconomic status to the incidence and prehospital, 28-day, and 1-year mortality rates of acute coronary events in the FINMONICA myocardial infarction register study. Circulation. 2000;101:1913–1918. doi: 10.1161/01.cir.101.16.1913. [DOI] [PubMed] [Google Scholar]
- 12.Van Lenthe FJ, Gevers E, Joung IM, et al. Material and behavioral factors in the explanation of educational differences in incidence of acute myocardial infarction: the Globe study. AEP. 2002;12:535–542. doi: 10.1016/s1047-2797(01)00279-4. [DOI] [PubMed] [Google Scholar]
- 13.Rosengren A, Subramanian SV, Islam S, et al. Education and risk for acute myocardial infarction in 52 high, middle and low-income countries: INTERHEART case-control study. Heart. 2009;95:2014–2022. doi: 10.1136/hrt.2009.182436. [DOI] [PubMed] [Google Scholar]
- 14.Grace SL, Turk-Adawi KI, Contractor A, et al. Cardiac rehabilitation delivery model for low-resource settings: an international council of cardiovascular prevention and rehabilitation consensus statement. Prog Cardiovasc Dis. 2016;59:303–322. doi: 10.1016/j.pcad.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Alter DA, Chong A, Austin PC, et al. Socioeconomic status and mortality after acute myocardial infarction. Ann Intern Med. 2006;144:82–93. doi: 10.7326/0003-4819-144-2-200601170-00005. [DOI] [PubMed] [Google Scholar]
- 16.Bernheim SM, Spertus JA, Reid KJ, et al. Socioeconomic disparities in outcomes after acute myocardial infarction. Am Heart J. 2007;153:313–319. doi: 10.1016/j.ahj.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 17.Gerber Y, Goldbourt U, Drory Y, et al. Interaction between income and education in predicting long-term survival after acute myocardial infarction. Eur J Cardiovasc Prev Rehabil. 2008;15:526–532. doi: 10.1097/HJR.0b013e328304feac. [DOI] [PubMed] [Google Scholar]
- 18.Wang JY, Wang CY, Juang SY, et al. Low socioeconomic status increases short-term mortality of acute myocardial infarction despite universal health coverage. Int J Cardiol. 2014;172:82–87. doi: 10.1016/j.ijcard.2013.12.082. [DOI] [PubMed] [Google Scholar]
- 19.Rasmussen JN, Rasmussen S, Gislason GH, et al. Mortality after acute myocardial infarction according to income and education. J Epidemiol Community Health. 2006;60:351–356. doi: 10.1136/jech.200X.040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang WC, Kaul P, Westerhout CM, et al. Effects of socioeconomic status on mortality after acute myocardial infarction. Am J Med. 2007;120:33–39. doi: 10.1016/j.amjmed.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 21.Latour-Pérez J, Gutiérrez-Vicén T, Lópezcamps V, et al. Socioeconomic status and severity of illness on admission in acute myocardial infarction patients. Soc Sci Med. 1996;43:1025–1029. doi: 10.1016/0277-9536(96)00006-8. [DOI] [PubMed] [Google Scholar]
- 22.Rao SV, Schulman KA, Curtis LH, et al. Socioeconomic status and outcome following acute myocardial infarction in elderly patients. Arch Intern Med. 2004;164:1128–1133. doi: 10.1001/archinte.164.10.1128. [DOI] [PubMed] [Google Scholar]
- 23.Doll JA, Hellkamp AS, Goyal A, et al. Treatment, outcomes, and adherence to medication regimens among dual medicare-medicaid–eligible adults with myocardial infarction. JAMA Cardiology. 2016;1:787–794. doi: 10.1001/jamacardio.2016.2724. [DOI] [PubMed] [Google Scholar]
- 24.Van Lenthe FJ, Schrijvers CT, Droomers M, et al. Investigating explanations of socio-economic inequalities in health. Eur J Pub Health. 2004;14:63–70. doi: 10.1093/eurpub/14.1.63. [DOI] [PubMed] [Google Scholar]
- 25.Erkkila AT, Sarkkinen ES, Lehto S, et al. Diet in relation to socioeconomic status in patients with coronary heart disease. Eur J Clin Nutr. 1999;53:662–668. doi: 10.1038/sj.ejcn.1600829. [DOI] [PubMed] [Google Scholar]
- 26.Papadopoulou SK, Papadopoulou SD, Zerva A, et al. Health status and socioeconomic factors as determinants of physical activity level in the elderly. Med Sci Monit. 2003;9:CR79–CR83. [PubMed] [Google Scholar]
- 27.Strand BH, Tverdal A. Can cardiovascular risk factors and lifestyle explain the educational inequalities in mortality from ischaemic heart disease and from other heart diseases? 26 year follow up of 50 000 Norwegian men and women. J Epidemiol Community Health. 2004;58:705–709. doi: 10.1136/jech.2003.014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yarnell J, Yu S, McCrum E, et al. Education, socioeconomic and lifestyle factors, and risk of coronary heart disease: the PRIME Study. Int J Epidemiol. 2005;34:268–275. doi: 10.1093/ije/dyh267. [DOI] [PubMed] [Google Scholar]
- 29.Stampfer MJ, Hu FB, Manson JE, et al. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- 30.Yusuf S, Hawken S, Ounpuu S, et al. Investigators IS. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 31.Alter DA, Iron K, Austin PC, et al. Socioeconomic status, service patterns, and perceptions of care among survivors of acute myocardial infarction in Canada. JAMA. 2004;291:1100–1107. doi: 10.1001/jama.291.9.1100. [DOI] [PubMed] [Google Scholar]
- 32.McLeod JD, Kessler RC. Socioeconomic status differences in vulnerability to undesirable life events. J Health Soc Behav. 1990:162–172. [PubMed] [Google Scholar]
- 33.Prus SG. Age, SES, and health: a population level analysis of health inequalities over the lifecourse. Sociol Health Illn. 2007;29:275–296. doi: 10.1111/j.1467-9566.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 34.Ades PA, Keteyian SJ, Wright JS, et al. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc. doi: 10.1016/j.mayocp.2016.10.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.*Altenhöner T, Baczkiewicz C, Weishaar H, et al. Inequalities in therapeutic treatment during cardiac inpatient rehabilitation in Germany. Int J Public Health. 2012;57:175–184. doi: 10.1007/s00038-011-0298-9. [DOI] [PubMed] [Google Scholar]
- 36.*Greenwood DC, Muir KR, Packham CJ, et al. Stress, social support, and stopping smoking after myocardial infarction in England. J Epidemiol Community Health. 1995;49:583–587. doi: 10.1136/jech.49.6.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.*Smith PM, Burgess E. Smoking cessation initiated during hospital stay for patients with coronary artery disease: a randomized controlled trial. CMAJ. 2009;180:1297–1303. doi: 10.1503/cmaj.080862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.*Dunlay SM, Witt BJ, Allison TG, et al. Barriers to participation in cardiac rehabilitation. Am Heart J. 2009;158:852–859. doi: 10.1016/j.ahj.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.*Jin H, Wei Q, Chen L, et al. Obstacles and alternative options for cardiac rehabilitation in Nanjing, China: an exploratory study. BMC Cardiovasc Disord. 2014;14:1. doi: 10.1186/1471-2261-14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.*Suaya JA, Shepard DS, Normand SLT, et al. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116:1653–1662. doi: 10.1161/CIRCULATIONAHA.107.701466. [DOI] [PubMed] [Google Scholar]
- 41.*Gonarkar SB, Dhande PP. Medication adherence and its determinants in myocardial infarction patients: An Indian scenario. J Clin Prev Cardiol. 2016;5:2. [Google Scholar]
- 42.*Ohlsson H, Rosvall M, Hansen O, et al. Socioeconomic position and secondary preventive therapy after an AMI. Pharmacoepidemiol Drug Saf. 2010;19:358–366. doi: 10.1002/pds.1917. [DOI] [PubMed] [Google Scholar]
- 43.*Spertus JA, Kettelkamp R, Vance C, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement results from the PREMIER registry. Circulation. 2006;113:2803–2809. doi: 10.1161/CIRCULATIONAHA.106.618066. [DOI] [PubMed] [Google Scholar]
- 44.Igland J, Vollset SE, Nygård OK, et al. Educational inequalities in 28day and 1-year mortality after hospitalisation for incident acute myocardial infarction - a nationwide cohort study. Int J Cardiol. 2014;177:874–880. doi: 10.1016/j.ijcard.2014.10.045. [DOI] [PubMed] [Google Scholar]
- 45.*Wray LA, Herzog AR, Willis RJ, et al. The impact of education and heart attack on smoking cessation among middle-aged adults. J Health Soc Behav. 1998;39:271–294. [PubMed] [Google Scholar]
- 46.Arefalk G, Hambraeus K, Lind L, et al. Discontinuation of smokeless tobacco and mortality risk after myocardial infarction. Circulation. 2014;130:325–332. doi: 10.1161/CIRCULATIONAHA.113.007252. [DOI] [PubMed] [Google Scholar]
- 47.Heran BS, Chen JM, Ebrahim S, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;7 doi: 10.1002/14651858.CD001800.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rahimi AR, Spertus JA, Reid KJ, et al. Financial barriers to health care and outcomes after acute myocardial infarction. JAMA. 2007;297:1063–1072. doi: 10.1001/jama.297.10.1063. [DOI] [PubMed] [Google Scholar]
- 49.Dondo TB, Hall M, Timmis AD, et al. Excess mortality and guideline-indicated care following non-ST-elevation myocardial infarction. Eur Heart J: Acute Cardiovascular Care. 2016 doi: 10.1177/2048872616647705. DOI: 10.1177/2048872616647705. [DOI] [PubMed] [Google Scholar]
- 50.Griffo R, Ambrosetti M, Tramarin R, et al. Effective secondary prevention through cardiac rehabilitation after coronary revascularization and predictors of poor adherence to lifestyle modification and medication. Results of the ICAROS Survey. Int J Cardiol. 2013;167:1390–1395. doi: 10.1016/j.ijcard.2012.04.069. [DOI] [PubMed] [Google Scholar]
- 51.Turk-Adawi KI, Oldridge NB, Vitcenda MJ, et al. Secondary Prevention Recommendation Attainment with Cardiac Rehabilitation: Is There a Gender Disparity? WHI. 2016;26:278–287. doi: 10.1016/j.whi.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Mayer-Berger W, Simic D, Mahmoodzad J, et al. Efficacy of a long-term secondary prevention programme following inpatient cardiovascular rehabilitation on risk and health-related quality of life in a low-education cohort: a randomized controlled study. Eur J Prev Cardiol. 2014;21:145–152. doi: 10.1177/2047487312465526. [DOI] [PubMed] [Google Scholar]
- 53.Nielsen KM, Meillier LK, Larsen ML. Extended cardiac rehabilitation for socially vulnerable patients improves attendance and outcome. Dan Med J. 2013;60:A4591–A4591. [PubMed] [Google Scholar]
- 54.Szalewska D, Zielinski P, Tomaszewski J, et al. Effects of outpatient followed by home-based telemonitored cardiac rehabilitation in patients with coronary artery disease. Kardiol Pol. 2015;73:1101–1107. doi: 10.5603/KP.a2015.0095. [DOI] [PubMed] [Google Scholar]
- 55.Rigotti NA, Clair C, Munafò MR, et al. Interventions for smoking cessation in hospitalised patients. The Cochrane Library. 2012 doi: 10.1002/14651858.CD001837.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grace SL, Russell KL, Reid RD, et al. Effect of cardiac rehabilitation referral strategies on utilization rates: a prospective, controlled study. Arch Intern Med. 2011;171:235–241. doi: 10.1001/archinternmed.2010.501. [DOI] [PubMed] [Google Scholar]
- 57.Gravely-Witte S, Leung YW, Nariani R, et al. Effects of cardiac rehabilitation referral strategies on referral and enrollment rates. Nat Rev Cardiol. 2010;7:87–96. doi: 10.1038/nrcardio.2009.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bjarnason PD, Benesch L, Bischoff KO, et al. Die Effektivität einer ambulanten kardiologischen Rehabilitation der Phase II. Herz. 2003;28:404–412. doi: 10.1007/s00059-003-2433-8. [DOI] [PubMed] [Google Scholar]
- 59.Ito K, Avorn J, Shrank WH, et al. Long-term cost-effectiveness of providing full coverage for preventive medications after myocardial infarction. Circ Cardiovasc Qual Outcomes. 2015;8:252–259. doi: 10.1161/CIRCOUTCOMES.114.001330. [DOI] [PubMed] [Google Scholar]
- 60.Gaalema DE, Savage PD, Rengo JL, et al. Financial incentives to promote cardiac rehabilitation participation and adherence among Medicaid patients. Prev Med. 2016;92:47–50. doi: 10.1016/j.ypmed.2015.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Myers V, Drory Y, Goldbourt U, et al. Multilevel socioeconomic status and incidence of frailty post myocardial infarction. Int J Cardiol. 2014;170:338–343. doi: 10.1016/j.ijcard.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 62.Dodson JA, Arnold SV, Reid KJ, et al. Physical function and independence 1 year after myocardial infarction: observations from the translational research investigating underlying disparities in recovery from acute myocardial infarction: patients' health status registry. Am Heart J. 2012;163:790–796. doi: 10.1016/j.ahj.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ickovics JR, Viscoli CM, Horwitz RI. Functional recovery after myocardial infarction in men: the independent effects of social class. Ann Intern Med. 1997;127:518–525. doi: 10.7326/0003-4819-127-7-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 64.Babić Z, Pavlov M, Oštrić M, et al. Re-initiating professional working activity after myocardial infarction in primary percutaneous coronary intervention networks era. Int J Occup Med Environ Health. 2015;28:999–1010. doi: 10.13075/ijomeh.1896.00478. [DOI] [PubMed] [Google Scholar]
- 65.Valencia HE, Savage PD, Ades PA. Cardiac rehabilitation participation in underserved populations. JCRP. 2011;31:203–210. doi: 10.1097/HCR.0b013e318220a7da. [DOI] [PubMed] [Google Scholar]
- 66.*Altenhoener T, Leppin A, Grande G, et al. Social inequality in patients' physical and psychological state and participation in rehabilitation after myocardial infarction in Germany. Int J Rehabil Res. 2005;28:251–257. doi: 10.1097/00004356-200509000-00008. [DOI] [PubMed] [Google Scholar]
- 67.*Gerber Y, Koren-Morag N, Myers V, et al. Long-term predictors of smoking cessation in a cohort of myocardial infarction survivors: a longitudinal study. Eur J Cardiovasc Prev Rehabil. 2011a;18:533–541. doi: 10.1177/1741826710389371. [DOI] [PubMed] [Google Scholar]
- 68.*Ockene JK, Hosmer D, Rippe J, et al. Factors affecting cigarette smoking status in patients with ischemic heart disease. J Chronic Dis. 1985;38:985–994. doi: 10.1016/0021-9681(85)90096-7. [DOI] [PubMed] [Google Scholar]
- 69.*Attebring MF, Hartford M, Hjalmarson A, et al. Smoking habits and predictors of continued smoking in patients with acute coronary syndromes. J Adv Nurs. 2004;46:614–623. doi: 10.1111/j.1365-2648.2004.03052.x. [DOI] [PubMed] [Google Scholar]
- 70.*Conroy RM, Cahill S, Mulcahy R, et al. The relation of social class to risk factors, rehabilitation, compliance and mortality in survivors of acute coronary heart disease. Scand J Public Health. 1986;14:51–56. doi: 10.1177/140349488601400202. [DOI] [PubMed] [Google Scholar]
- 71.*Tofler GH, Muller JE, Stone PH, et al. Comparison of long-term outcome after acute myocardial infarction in patients never graduated from high school with that in more educated patients. Am J Cardiol. 1993;71:1031–1035. doi: 10.1016/0002-9149(93)90568-w. [DOI] [PubMed] [Google Scholar]
- 72.*Chan RH, Gordon NF, Chong A, et al. Influence of socioeconomic status on lifestyle behavior modifications among survivors of acute myocardial infarction. Am J Cardiol. 2008;102:1583–1588. doi: 10.1016/j.amjcard.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 73.*Quist-Paulsen P, Bakke PS, Gallefoss F. Predictors of smoking cessation in patients admitted for acute coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2005;12:472–477. doi: 10.1097/01.hjr.0000183914.90236.01. [DOI] [PubMed] [Google Scholar]
- 74.*Hajek P, Taylor TZ, Mills P. Brief intervention during hospital admission to help patients to give up smoking after myocardial infarction and bypass surgery: randomised controlled trial. BMJ. 2002;324:87–89. doi: 10.1136/bmj.324.7329.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.*Dornelas EA, Sampson RA, Gray JF, et al. A randomized controlled trial of smoking cessation counseling after myocardial infarction. Prev Med. 2000;30:261–268. doi: 10.1006/pmed.2000.0644. [DOI] [PubMed] [Google Scholar]
- 76.*Rallidis LS, Hamodraka ES, Foulidis VO, et al. Persistent smokers after myocardial infarction: a group that requires special attention. Int J Cardiol. 2005;100:241–245. doi: 10.1016/j.ijcard.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 77.*Shapiro S, Weinblatt E, Frank CW, et al. Social factors in the prognosis of men following first myocardial infarction. Milbank Q. 1970;48:37–50. [PubMed] [Google Scholar]
- 78.*Melville MR, Packham C, Brown N, et al. Cardiac rehabilitation: socially deprived patients are less likely to attend but patients ineligible for thrombolysis are less likely to be invited. Heart. 1999;82:373–377. doi: 10.1136/hrt.82.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.*Oldridge NB. Compliance and dropout in cardiac exercise rehabilitation. J Cardiac Rehabil. 1984;4:166–177. [Google Scholar]
- 80.*Pell J, Pell A, Morrison C, et al. Retrospective study of influence of deprivation on uptake of cardiac rehabilitation. BMJ. 1996;313:267–268. doi: 10.1136/bmj.313.7052.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.*Alter DA, Franklin B, Ko DT, et al. Socioeconomic status, functional recovery, and long-term mortality among patients surviving acute myocardial infarction. PLoS One. 2013;8:e65130. doi: 10.1371/journal.pone.0065130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.*Ramm C, Robinson S, Sharpe N. Factors determining non-attendance at a cardiac rehabilitation programme following myocardial infarction. N Z Med J. 2001;114:227–229. [PubMed] [Google Scholar]
- 83.*Stern MJ, Cleary P. National Exercise and Heart Disease Project: Psychosocial changes observed during a low-level exercise program. Arch Intern Med. 1981;141:1463–1467. [PubMed] [Google Scholar]
- 84.*Young RF, Waller JB, Jr, Kahana E. Racial and socioeconomic aspects of myocardial infarction recovery: studying confounds. Am J Prev Med. 1990;7:438–444. [PubMed] [Google Scholar]
- 85.*Lane D, Carroll D, Ring C, et al. Predictors of attendance at cardiac rehabilitation after myocardial infarction. J Psychosom Res. 2001;51:497–501. doi: 10.1016/s0022-3999(01)00225-2. [DOI] [PubMed] [Google Scholar]
- 86.*Winberg B, Fridlund B. Self-reported behavioural and medical changes in women after their first myocardial infarction: a 4-year comparison between participation and non-participation in a cardiac rehabilitation programme. Eur J Cardiovasc Nurs. 2002;1:101–107. doi: 10.1016/s1474-5151(02)00012-9. [DOI] [PubMed] [Google Scholar]
- 87.*Carey IM, DeWilde S, Shah SM, et al. Statin use after first myocardial infarction in UK men and women from 1997 to 2006: who started and who continued treatment? Nutr Metab Cardiovasc Dis. 2012;22:400–408. doi: 10.1016/j.numecd.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 88.*Danchin N, Neumann A, Tuppin P, et al. Impact of free universal medical coverage on medical care and outcomes in low-income patients hospitalized for acute myocardial infarction an analysis from the French National Health Insurance System. Circ Cardiovasc Qual Outcomes. 2011;4:619–625. doi: 10.1161/CIRCOUTCOMES.111.961193. [DOI] [PubMed] [Google Scholar]
- 89.*Akincigil A, Bowblis JR, Levin C, et al. Long-term adherence to evidence based secondary prevention therapies after acute myocardial infarction. J Gen Intern Med. 2008;23:115–121. doi: 10.1007/s11606-007-0351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.*Castellano JM, Sanz G, Peñalvo JL, et al. A polypill strategy to improve adherence: results from the FOCUS project. JACC. 2014;64:2071–2082. doi: 10.1016/j.jacc.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 91.*Shimony A, Zahger D, Ilia R, et al. Impact of the community's socioeconomic status on characteristics and outcomes of patients undergoing percutaneous coronary intervention. Int J Cardiol. 2010;144:379–382. doi: 10.1016/j.ijcard.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 92.*Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297:177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 93.*Rasmussen JN, Gislason GH, Rasmussen S, et al. Use of statins and beta-blockers after acute myocardial infarction according to income and education. J Epidemiol Community Health. 2007;61:1091–1097. doi: 10.1136/jech.2006.055525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.*Mathews R, Peterson ED, Honeycutt E, et al. Early medication nonadherence after acute myocardial infarction insights into actionable opportunities from the treatment with ADP receptor iNhibitorS: longitudinal assessment of treatment patterns and events after acute coronary syndrome (TRANSLATE-ACS) study. Circ Cardiovasc Qual Outcomes. 2015;8:347–356. doi: 10.1161/CIRCOUTCOMES.114.001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.*Salisbury AC, Chan PS, Gosch KL, et al. Patterns and predictors of fast food consumption after acute myocardial infarction. Am J Cardiol. 2011;107:1105–1110. doi: 10.1016/j.amjcard.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.*Gerber Y, Myers V, Goldbourt U, et al. Neighborhood socioeconomic status and leisure-time physical activity after myocardial infarction: a longitudinal study. Am J Prev Med. 2011(b);41:266–273. doi: 10.1016/j.amepre.2011.05.016. [DOI] [PubMed] [Google Scholar]