Abstract
Goal
To examine screening adenoma detection rates (ADR) and serrated detection rates (SDR) among smokers and obese adults in the New Hampshire Colonoscopy Registry.
Background
ADR, a quality measure for screening colonoscopies, is associated with protection from interval colorectal cancer (CRC). Currently, only sex-specific ADR benchmarks are reported. However, obesity and smoking ≥ 20 pack-years are strong predictors for colorectal neoplasia, as highlighted by the 2009 American College of Gastroenterology CRC Screening Guidelines. Data comparing ADR in smokers and obese adults to those without these risks are limited.
Study
We calculated ADR, SDR and 95% confidence intervals for screening colonoscopies in participants ≥ 50 years. Sex and sex-age specific rates were compared by smoking exposure (never versus < 20 pack-years versus ≥ 20 pack-years) and body mass index (BMI < 30 versus BMI ≥ 30).
Results
21,539 screening colonoscopies were performed by 77 endoscopists at 20 facilities (4/2009 -9/2013). The difference in ADR between non-smokers and smokers with ≥ 20 pack-years was 8.8% (p < 0.0001) and between obesity groups 5.0% (p<0.0001). Significant sex specific and sex-age specific increases in ADR and SDR were found among smokers and obese participants.
Conclusions
ADR and SDR for smokers and obese adults were significantly higher than their counterparts without those risks. Endoscopists should consider the prevalence of these risks within their screening population when comparing their rates to established benchmarks. Calculating sex or sex-age specific ADR and SDR based on smoking and obesity may provide optimal protection for populations with a particularly high prevalence of smokers and obese adults.
Keywords: Adenoma detection, serrated polyp, colonoscopy
Introduction
The adenoma detection rate (ADR), introduced as a method for measuring the quality of screening colonoscopies1,2, has been shown to be inversely associated with interval cancer rates3,4. The ADR is defined as the proportion of screening colonoscopies in which one or more adenomas or colorectal cancers are detected. Recent recommendations suggest that endoscopists achieve an overall ADR of 25%5.
The recent joint American College of Gastroenterology (ACG) and American Society for Gastrointestinal Endoscopy (ASGE) paper on quality indicators for colonoscopy5 states that the principal factor that influences ADR in average-risk adults is sex, as supported by other studies6. Accordingly, the primary consideration in setting ADR benchmarks for screening exams in adults 50 years or older is sex, with recommendations that endoscopists achieve a mean ADR of 30% for men and 20% for women. Guideline recommendations do not take into account other factors that influence adenoma prevalence, such as smoking or obesity5, two factors which were highlighted in the recent ACG CRC screening guidelines7. Consideration was given to recommend that CRC screening should begin at age 45 years for both groups but the ACG guidelines concluded that additional study was warranted. Both factors have also been shown to be associated with an increased risk for adenomas8-12, with one meta-analysis finding an almost two-fold increase in adenoma risk among smokers13.
Data examining the ADR in smokers and obese adults are limited. Our goal was to use data from the New Hampshire Colonoscopy Registry (NHCR) to compare the ADR in screening colonoscopies conducted in obese adults and smokers to those in individuals without these risk factors. Given the association between the serrated pathway and a large proportion of CRC14 as well as interest in using the detection of serrated polyps as a potential quality measure for colonoscopy15,16, we also examined the serrated polyp detection rates (SDR) in these risk groups.
Methods
The NHCR17-20 is a population-based, statewide registry collecting data from endoscopy sites throughout New Hampshire (NH). Prior to colonoscopy, consenting patients complete a self-administered patient questionnaire. Participants are queried about their smoking history21, including questions about current smoking status (never, past or current), number of years smoked, and the number of cigarettes per day if applicable. Body mass index (BMI) is calculated from the participant’s self-report of height and weight.
The NHCR Procedure Form is completed during or immediately following colonoscopy by endoscopists or endoscopy nurses. Data collected include screening (with three detailed options: no symptoms and no family history of polyps or CRC, family history of polyp(s), or family history of CRC with specification of first degree relative), surveillance or diagnostic indication for the colonoscopy, findings (location, size and specific treatment of polyps, cancer, or other findings), type and quality of bowel preparation, sedation medication, anatomical location reached during the procedure, withdrawal time, follow-up recommendations, and immediate complications.
The NHCR requests pathology reports for all colonoscopies with findings directly from the pathology laboratory used by each participating endoscopy facility. Trained NHCR staff abstract and enter these pathology reports, including location, size, and histology of each polyp, into the NHCR database, linking individual polyp level data to information from the Procedure Form.19 All data collection and study procedures were approved by the Committee for the Protection of Human Subjects at Dartmouth College (study # 00015834), as well as by other relevant human subjects reviewing bodies at participating sites.
Cohort
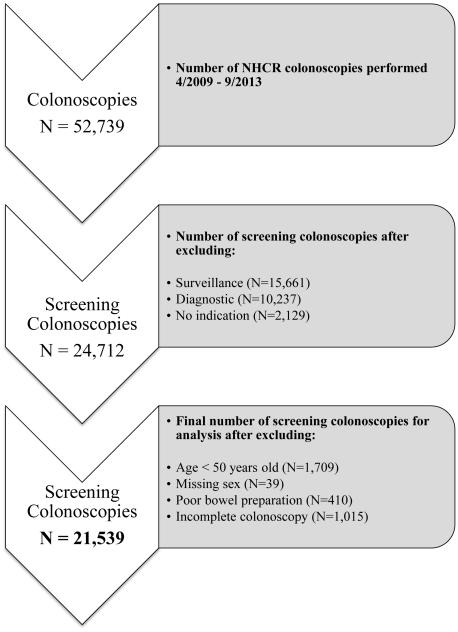
Our analysis involved selecting screening colonoscopies from a total of 52,739 colonoscopies performed from 4/6/2009 to 9/13/2013 at 20 endoscopy facilities, including hospitals, ambulatory surgery centers, and community practices across NH. Since our intent was to examine the ADR for screening colonoscopies, surveillance colonoscopies (N = 15,661), diagnostic colonoscopies (N = 10,237) and colonoscopies with no indication (N = 2,129) were not included in this analysis22. A surveillance indication was specified for a colonoscopy performed on patients with a personal history of CRC and/or personal history of polyps and on patients with familial polyposis or hereditary non-polyposis colon cancer or inflammatory bowel disease (IBD). An indication of diagnostic included evaluation of gastrointestinal bleeding, ruling out IBD, biopsy of suspected cancer, follow-up of a positive fecal occult blood test, abnormal virtual colonoscopy or barium enema test, polypectomy of known polyp and iron deficiency anemia. As shown in Figure 1, there were 24,712 screening colonoscopies.
Figure 1.
Flow diagram for study analysis of the New Hampshire Colonoscopy Registry
Since average-risk CRC screening begins at age 50 and the guideline benchmarks apply to individuals aged 50 years and older,5,23 we excluded colonoscopies among patients less than 50 years of age at the time of colonoscopy (N = 1,709). We also excluded colonoscopies for which sex of the participant was missing (N = 39), colonoscopies with a poor colonic preparation (N = 410) and incomplete colonoscopies (N = 1,015). After excluding 3,173 colonoscopies, 21,539 screening colonoscopies performed by 77 endoscopists comprised our study cohort.
Risk Factors
The variables of interest were sex, age at screening colonoscopy, and the patient’s self-reported risk factors of smoking and body mass index (BMI). Consistent with the ACG CRC Screening Guidelines7, we defined smokers into three groups: ≥ 20 pack years, < 20 pack years, or never smoked, and classified patients as obese (BMI ≥ 30) or not obese (BMI < 30). Age at the time of screening colonoscopy was categorized into two groups (50-59 and ≥ 60).
Outcome Measures
We calculated the ADR (the number of colonoscopies with at least one adenoma or adenocarcinoma detected, divided by the total number of colonoscopies), and 95% confidence interval (95% CI), for screening colonoscopies. In calculating the ADR, the numerator contained all colonoscopies in which at least one lesion with adenomatous tissue was detected, including tubular, tubulovillous or villous adenomas, and adenomatous polyps with high grade dysplasia, intra-mucosal carcinoma or invasive adenocarcinoma.
Our calculation for SDR was similar to that for ADR. The numerator for our calculation was the number of screening colonoscopies with at least one serrated lesion. Based on recent studies15,16 as well as the expert panel guidelines24, we defined a serrated lesion for the purpose of calculating SDR as any sessile serrated polyp or adenoma (SSP/A), traditional serrated adenoma (TSA), or hyperplastic polyp, proximal to the sigmoid. The denominator, as in the ADR calculation, was the total number of screening colonoscopies.
Analysis
We report overall, sex specific and sex-age specific ADR, SDR and 95% confidence intervals (95% CI), by smoking status and BMI. The chi-square test and two-sample tests of proportions were performed to assess differences in ADR and SDR for smoking and BMI groups. A p-value <0.05 was considered statistically significant. The analyses were conducted in SAS (SAS Institute Inc. 2015. SAS® 9.4 System Options: Reference, Fourth Edition. Cary, NC: SAS Institute Inc.) and STATA (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP).
Results
The study cohort had a mean (±SD) age of 58.2 (±7.7) years, and was 56% female. Forty-seven percent of the participants were smokers and 33.8% were obese (BMI ≥ 30). Less than 4% of patients did not complete the patient questionnaire, and information on the number missing relevant covariate data (smoking status and BMI) is provided in the footnotes to Tables 1 - 3.
Table 1.
Overall and sex specific adenoma detection rates and 95% confidence intervals for patient risk factors among NHCR screening colonoscopies (4/6/2009 -9/13/2013)
| Risk Factors1 | All Colonoscopies N=21,539 |
Male N=9,491 (44%) |
Female N=12,048 (56%) |
||
|---|---|---|---|---|---|
| Adenoma N |
Rate, % (95% CI) |
Rate, % (95% CI) |
Rate, % (95% CI) |
||
| All Patients | 5,083 | 23.6 (23.0 - 24.2) |
30.2 (29.3 - 31.2) |
18.4 (17.7 - 19.1) |
|
| Smoking | Never (N=10,921) |
2,294 | 21.0 (20.2 - 21.8) |
27.5 (26.2 - 28.8) |
16.0 (15.1 - 17.0) |
| < 20 Pack Years (N=4,254) |
940 | 22.1 (20.9 - 23.4) |
29.2 (27.1 - 31.4) |
17.3 (15.8 - 18.8) |
|
| ≥ 20 Pack Years (N=5,423) |
1,614 | 29.8 (28.5 - 31.0) |
35.8 (34.0 - 37.7) |
24.2 (22.7 - 25.9) |
|
| BMI | < 30 (N=13,778) |
3,002 | 21.8 (21.1 - 22.5) |
28.1 (27.0 - 29.3) |
17.1 (16.2 - 17.9) |
| ≥ 30 (Obese) (N=7,036) |
1,887 | 26.8 (25.8 - 27.9) |
33.7 (32.1 - 35.3) |
20.7 (19.4 - 22.0) |
|
Missing (N): Smoking (941); BMI (725).
Table 3.
Sex-age specific adenoma and serrated detection rates and 95% confidence intervals for patient risk factors among NHCR screening colonoscopies (4/6/2009 -9/13/2013)
| Risk Factors1 | Male N = 9,491 (44%) | Female N = 12,048 (56%) | |||
|---|---|---|---|---|---|
| 50-59 years N = 5,936 (62%) |
≥ 60 years N = 3,555 (38%) |
50-59 years N = 7,457 (62%) |
≥ 60 years N = 4,591 (38%) |
||
| Rate, % (95% CI) | Rate, % (95% CI) | Rate, % (95% CI) | Rate, % (95% CI) | ||
| All Patients |
ADR SDR |
28.7 (27.5 - 29.8) 11.4 (10.6 - 12.3) |
32.8 (31.3 - 34.4) 10.3 (9.3 - 11.4) |
17.1 (16.3 - 18.0) 8.6 (8.0 - 9.3) |
20.4 (19.3 - 21.6) 8.2 (7.5 - 9.1) |
| Smoking | Never ADR SDR |
25.7 (24.2 - 27.2) 10.0 (9.0 - 11.1) |
31.6 (29.2 - 34.0) 9.0 (7.6 - 10.6) |
14.4 (13.3 - 15.6) 7.1 (6.3 - 7.9) |
18.8 (17.2 - 20.5) 7.5 (6.4 - 8.6) |
| < 20 Pack Years ADR SDR |
28.4 (25.6 - 31.4) 10.5 (8.6 - 12.6) |
30.2 (26.9 - 33.6) 11.5 (9.4 - 14.0) |
16.2 (14.4 - 18.1) 9.6 (8.2 - 11.2) |
19.2 (16.7 - 21.9) 7.3 (5.7 - 9.1) |
|
| ≥ 20 Pack Years ADR SDR |
35.4 (32.9 - 37.9) 10.7 (9.0 - 12.7) |
36.3 (33.5 - 39.2) 15.2 (13.4 - 17.2) |
24.0 (21.9 - 26.1) 11.1 (9.6 - 12.7) |
24.6 (22.2 - 27.2) 10.7 (9.0 - 12.6) |
|
| BMI | < 30 ADR SDR |
26.4 (24.9 - 27.8) 10.8 (9.8 - 11.9) |
30.8 (29.0 - 32.7) 9.1 (8.0 - 10.3) |
15.8 (14.8 - 16.9) 8.3 (7.5 - 9.1) |
19.1 (17.7 - 20.5) 7.2 (6.3 - 8.1) |
| ≥ 30 (Obese) ADR SDR |
32.0 (30.1 - 34.0) 12.3 (11.0 - 13.8) |
37.1 (34.2 - 40.0) 13.1 (11.1 - 15.2) |
19.3 (17.7 - 20.9) 9.3 (8.2 - 10.6) |
23.1 (20.9 - 25.5) 10.4 (8.9 - 12.2) |
|
Missing (N): Smoking (941); BMI (725).
Shown in Table 1, the overall screening ADR (95% CI) was 23.6% (23.0% - 24.2%). A small proportion (0.1%) of the overall screening ADR was attributable to CRC (n=27). The sex specific ADR was 30.2% (29.3% - 31.2%) for males and lower among females at 18.4% (17.7% - 19.1%). The ADR for screening colonoscopies among smokers with more than 20 pack years (29.8% (28.5% - 31.0%)), was significantly higher than for screening colonoscopies among those who smoked less than 20 pack years (22.1% (20.9% - 23.4%)) or who never smoked (21.0% (20.2% - 21.8%)), a pattern that held true within patient sex groups. The difference in ADR between patients who never smoked and smokers with a 20 pack year exposure was 8.8% (p <0.0001). The highest rate for smokers with 20 or more pack years was among males (35.8% (34.0% - 37.7%). The ADR for screening colonoscopies performed in obese participants (26.8% (25.8% - 27.9%)) was significantly higher than that for screening colonoscopies performed in non-obese adults (21.8% (21.1% - 22.5%); p <0.0001), with similar results found for sex specific rates.
The overall screening SDR (95% CI) was 9.6% (9.2% - 10.0%; Table 2). The SDR was higher among males (11.0% (10.4% - 11.7%)) than among females (8.5% (8.0% - 9.0%); p <0.0001). Similar to the ADR results, significant increases in overall (p<0.0001) and sex specific SDR were found among smokers (males: p<0.0001; females: p<0.0001) and obese participants (p<0.0001).
Table 2.
Overall and sex specific serrated detection rates and 95% confidence intervals for patient risk factors among NHCR screening colonoscopies (4/6/2009 -9/13/2013)
| Risk Factors1 | All Colonoscopies N=21,539 |
Male N=9,491 (44%) |
Female N=12,048 (56%) |
||
|---|---|---|---|---|---|
| Serrated N |
Rate, % (95% CI) |
Rate, % (95% CI) |
Rate, % (95% CI) |
||
| All Patients | 2,071 | 9.6 (9.2 - 10.0) |
11.0 (10.4 - 11.7) |
8.5 (8.0 - 9.0) |
|
| Smoking | Never (N=10,921) |
905 | 8.2 (7.8 - 8.8) |
9.7 (8.9 - 10.6) |
7.2 (6.6 - 7.9) |
| < 20 Pack Years (N=4,254) |
410 | 9.6 (8.8 - 10.6) |
11.0 (9.5 - 12.5) |
8.7 (7.7 - 9.9) |
|
| ≥ 20 Pack Years (N=5,423) |
652 | 12.0 (11.2 - 12.9) |
13.2 (12.0 - 14.6) |
10.9 (9.8 - 12.1) |
|
| BMI | < 30 (N=13,778) |
1,214 | 8.8 (8.3 - 9.3) |
10.1 (9.4 - 10.9) |
7.8 (7.3 - 8.4) |
| ≥ 30 (Obese) (N=7,036) |
779 | 11.1 (10.3 - 11.8) |
12.6 (11.5 - 13.7) |
9.7 (8.8 - 10.7) |
|
Missing (N): Smoking (941); BMI (725).
Table 3 presents the results for sex-age specific ADR (95% CI) and SDR (95% CI) for each patient risk group. ADR for males 60 years or older (32.8 % (31.3% - 34.4%)) was elevated as compared to that in younger males (28.7% (27.5% - 29.8%)). Older females (60+ years) also had a higher ADR (20.4% (19.3% - 21.6%) than younger females (17.1% (16.3% - 18.0%)). The highest ADRs (95% CI) were observed in older men with 20 pack years exposure (36.3% (33.5% - 39.2%)) and older obese men (37.1% (34.2% - 40.0%)) The highest SDRs (95% CI) were also found among males aged 60 years or older who smoked more than 20 pack years (15.2% (13.4% - 17.2%)) and older obese males (13.1% (11.1% - 15.2%)).
Discussion
In our study, NHCR participants who had a 20-pack year or greater history of smoking had a significantly higher ADR as compared to those individuals with lower smoking exposure or those adults who never smoked. We observed a higher ADR for obese participants as compared to non-obese adults. Of note, we found the ADR difference between the high smoking exposure group and the adults who never smoked was 8.8%. ADR differences among men and women non-smokers and smokers were even more pronounced and reached nearly 10% among the younger age groups (50-59 years old); a result which is similar to the difference found in our study for the overall ADR between men and women5. Additionally, we found ADRs for some of the subgroups were notably higher than the current recommended benchmarks (ACG/ASGE recommendations5). For example, the ADR for obese males older than 60 years of age was 37.1%. However, despite having a significant proportion (47%) of current or former smokers, our registry’s overall ADR was 23.6%, which is consistent with known screening population benchmarks. One interesting finding is that the ADR increased by 5.9% for the men in the 50-59 year age group to the ≥ 60 year group, but it increased by less than 1% for heavy smokers. Similar findings were observed in women, an increase of 4.4% for the younger group and 0.6% for the older group. These findings might suggest that perhaps smoking accelerates production of adenomas so that smokers develop a peak adenoma level at a younger age.
A recent joint statement from the ACG/ASGE recommends that endoscopists achieve an overall ADR of 25% in screening colonoscopies, 20% for women and 30% for men5. It is unclear whether and how the ADR should be adjusted for the presence of risk factors other than sex. Studies have examined the association between ADR and family history of colorectal neoplasia25, sex,6 and exam related factors.26-29 One study found a small change in ADR when adjusting for demographics such as patient race30. However, few studies have investigated ADRs in adults with other risk factors that are associated with an increased risk of CRC. Based on many studies9,10,12,31, recent ACG CRC Screening Guidelines identify adults who are obese or have smoked more than 20 pack years as being at a higher risk for colorectal neoplasia than average-risk individuals without these risk factors7. In addition, smoking and obesity were also highlighted by the recent ACG/ASGE joint paper5 on quality indicators for colonoscopy as factors that influence adenoma prevalence. However, there are no current recommendations to adjust ADR benchmarks for these risk factors5.
To the best of our knowledge, our study is the first analysis to present adenoma and serrated polyp detection rates for screening colonoscopies in individuals stratified by additional risk factors of smoking and obesity. As highlighted by the fact that almost 50% of people within the statewide NHCR database have a smoking history, this distinction may have wide applicability. ADR is the primary colonoscopy quality measure and higher detection rates have been shown to be inversely correlated with interval cancer risk3. Recent data suggest that an ADR higher than the currently recommended 25% may be required to ensure maximal protection from interval cancer4. Setting appropriate ADR benchmarks is critical to optimizing quality of colonoscopy. Therefore, whether and/or when to adjust ADR benchmarks for higher risk populations merits investigation.
Our study findings suggest that endoscopists should be cognizant of the prevalence of these risk factors in their screening populations. The increasing use of electronic medical records facilitates the assessment of smoking and obesity within the population of patients for a given endoscopist or a given group practice. For example, the software that is used for Clinical Outcomes Research Initiative (CORI) captures smoking history as well as height and weight32. This information would allow stratification of ADR benchmarks within the large group of screening patients, informing optimal care. As with sex, endoscopists could stratify their ADR into smokers versus non-smokers and obese versus non-obese adults. Based on our data, the recommended ADR benchmark goal may more appropriately be at least 35% for male heavy smokers. A screening study of over 3000 veterans observed an adenoma detection rate of 37.5%33. In this cohort of male veterans, nearly three quarters of all men were smokers and nearly half had a 25 pack-year of more smoking exposure34. Thus, an endoscopist in a Veterans Affairs hospital, where many patients are male and have a heavy smoking history, might need to achieve a higher benchmark than the current recommendation of 30%, in order to ensure patients adequate protection from CRC34.
However, it should be noted that despite the high ADR in smokers with a 20 pack year exposure as well as the high prevalence of all smokers in our sample, the overall ADR was 23.6%, which is not notably different from established benchmarks for a typical screening population. Furthermore, the 50% prevalence of adults in the NHCR who had a smoking exposure, past or current, has also been observed in other similar screening populations11. Therefore, while adjustment for smoking or obesity may be needed in a population with a particularly high prevalence of these risk factors, especially if many individuals have a 20 pack year or greater exposure, stratification may not be necessary in typical screening populations.
Given the association between the serrated pathway and CRC as well as the variation in endoscopist serrated polyp detection rates, SDR is another metric that has been proposed as a potential colonoscopy quality measure15,16,26. In our study, we found a significant difference in SDR between smokers with a 20 pack year history and those individuals who never smoked (12.0% versus 8.2%), which is similar to the ADR findings among the smoking groups. These results are not surprising given previous studies demonstrating a link between obesity, smoking and serrated histology35,36. As with ADR, our data suggest that once SDR benchmarks are clearly established, in order to optimize quality, those benchmarks may require further investigation to determine whether adjustment for obesity and smoking is needed. It is noteworthy that unlike ADR, SDR for each sex was not higher among older individuals. This finding supports previously published data demonstrating that while risk for conventional adenomas increases dramatically with age, the risk for serrated lesions is not increased in older adults24,34.
A limitation of this analysis is the presence of minimal racial diversity in NH. While this may limit generalizability, the racial homogeneity of the population may have reduced the possibility of confounding variables related to race, thus allowing us to study the specific exposure variables for a defined population. Further studies will be needed to clarify the impact of smoking and obesity on ADR and SDR in other populations. We also acknowledge that BMI was self-reported in our sample. With regards to the feasibility of measuring ADR for these high risk adults in practice, we acknowledge that not all electronic health records (EHRs) or endoscopy software capture smoking exposure and thus it may not be feasible to know the prevalence of heavy smokers in all practices. However, clinical practice would suggest that smoking may be more frequently assessed in health centers serving populations where there is a high prevalence of this important risk factor.
There are several strengths in our analysis. Our main exposure variables, obesity and smoking, have been identified in several models as predicting the presence of advanced adenomas in large populations37,38. In addition, our findings were consistent when the results were stratified by two other powerful adenoma predictors, age and sex. The large database of the NHCR, with nearly 22,000 screening colonoscopies included in this analysis, provided excellent power to examine the impact of obesity and smoking. The screening colonoscopies were performed by 77 endoscopists, practicing at 20 diverse facilities including community and academic practices, both urban and rural, thereby increasing the generalizability of the results. Furthermore, the detailed exam and specimen level data of the NHCR allowed this analysis to exclude incomplete exams and those with poor bowel preparation. Excluded exams within the screening group (i.e., poor prep and incomplete colonoscopies) represented only a small fraction of that group, limiting the potential for bias in the determination of a screening ADR in that population. Finally, comprehensive pathology capture with matching at the individual polyp level19 enabled accurate calculation of ADR and SDR.
In summary, our investigation demonstrates that ADRs in male and female smokers and obese males are higher than non-smokers and non-obese individuals in our population, and also higher than the recommended benchmark ADRs for screening populations without these risk factors. Given the importance of achieving adequate ADR in ensuring protection from interval cancers4, adjustment in ADR benchmarks to appropriately account for these factors may be indicated in populations with particularly high prevalence of these risk factors. For example, a population in which 50% of the screening population has a 20 pack year or greater smoking exposure, such as those observed in VA facilities, might require a higher ADR to ensure adequate protection from interval cancer (35% for men and 25% for women). While 47% of our sample had any smoking exposure, only half of that group (25% of the screening population) had a significant exposure of ≥ 20 pack years. The overall ADR for the entire sample was not higher than that of a typical screening population. Therefore, for most typical screening populations, no adjustment may be necessary since ADR outcomes fall within the range of current benchmarks. However, endoscopists should be aware of the prevalence of these risk factors, especially of a high prevalence of heavy smokers in their screening practice. Studies in other populations are also needed to support optimal benchmark goals.
Acknowledgments
Financial support: The project described was supported by Grant # R01CA131141 and contract # HHSN261201400595P from the National Cancer Institute, as well as by the Norris Cotton Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
The contents of this work do not represent the views of the Department of Veterans Affairs or the United States Government
Conflict of interests: The authors have no conflicts of interest to declare.
Guarantors of the article: Joseph C. Anderson, M.D.; Julia E. Weiss, M.S.; Christina M. Robinson, M.S.; Lynn Butterly, M.D.
Specific Author Contributions:
Conception and design: Anderson, Joseph C.; Weiss, Julia E.; Robinson, Christina M.; Butterly, Lynn
Analysis and interpretation of the data: Anderson, Joseph C.; Weiss, Julia E.; Robinson, Christina M.; Butterly, Lynn
Drafting of article: Anderson, Joseph C.; Weiss, Julia E.; Robinson, Christina M.; Butterly, Lynn
Critical revision of the article for important intellectual content: Anderson, Joseph C.; Weiss, Julia E.; Robinson, Christina M.; Butterly, Lynn
Final approval of the article: Anderson, Joseph C.; Weiss, Julia E.; Robinson, Christina M.; Butterly, Lynn
None of the authors have any conflicts of interest to declare
References
- 1.Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. The American journal of gastroenterology. 2002;97:1296–308. doi: 10.1111/j.1572-0241.2002.05812.x. [DOI] [PubMed] [Google Scholar]
- 2.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101:873–85. doi: 10.1111/j.1572-0241.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 3.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 4.Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. The New England journal of medicine. 2014;370:1298–306. doi: 10.1056/NEJMoa1309086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. The American journal of gastroenterology. 2015;110:72–90. doi: 10.1038/ajg.2014.385. [DOI] [PubMed] [Google Scholar]
- 6.Coe SG, Wallace MB. Assessment of adenoma detection rate benchmarks in women versus men. Gastrointestinal endoscopy. 2013;77:631–5. doi: 10.1016/j.gie.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] The American journal of gastroenterology. 2009;104:739–50. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 8.Anderson JC, Attam R, Alpern Z, et al. Prevalence of colorectal neoplasia in smokers. The American journal of gastroenterology. 2003;98:2777–83. doi: 10.1111/j.1572-0241.2003.08671.x. [DOI] [PubMed] [Google Scholar]
- 9.Anderson JC, Latreille M, Messina C, et al. Smokers as a high-risk group: data from a screening population. Journal of clinical gastroenterology. 2009;43:747–52. doi: 10.1097/MCG.0b013e3181956f33. [DOI] [PubMed] [Google Scholar]
- 10.Anderson JC, Messina CR, Dakhllalah F, et al. Body mass index: a marker for significant colorectal neoplasia in a screening population. Journal of clinical gastroenterology. 2007;41:285–90. doi: 10.1097/01.mcg.0000247988.96838.60. [DOI] [PubMed] [Google Scholar]
- 11.Anderson JC, Moezardalan K, Messina CR, Latreille M, Shaw RD. Smoking and the association of advanced colorectal neoplasia in an asymptomatic average risk population: analysis of exposure and anatomical location in men and women. Dig Dis Sci. 2011;56:3616–23. doi: 10.1007/s10620-011-1814-8. [DOI] [PubMed] [Google Scholar]
- 12.Stein B, Anderson JC, Rajapakse R, Alpern ZA, Messina CR, Walker G. Body mass index as a predictor of colorectal neoplasia in ethnically diverse screening population. Digestive diseases and sciences. 2010;55:2945–52. doi: 10.1007/s10620-009-1113-9. [DOI] [PubMed] [Google Scholar]
- 13.Botteri E, Iodice S, Raimondi S, Maisonneuve P, Lowenfels AB. Cigarette smoking and adenomatous polyps: a meta-analysis. Gastroenterology. 2008;134:388–95. doi: 10.1053/j.gastro.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Anderson JC. Pathogenesis and management of serrated polyps: current status and future directions. Gut and liver. 2014;8:582–9. doi: 10.5009/gnl14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahi CJ, Hewett DG, Norton DL, Eckert GJ, Rex DK. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9:42–6. doi: 10.1016/j.cgh.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Kahi CJ, Li X, Eckert GJ, Rex DK. High colonoscopic prevalence of proximal colon serrated polyps in average-risk men and women. Gastrointestinal endoscopy. 2012;75:515–20. doi: 10.1016/j.gie.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Weaver DL, Rosenberg RD, Barlow WE, et al. Pathologic findings from the Breast Cancer Surveillance Consortium: population-based outcomes in women undergoing biopsy after screening mammography. Cancer. 2006;106:732–42. doi: 10.1002/cncr.21652. [DOI] [PubMed] [Google Scholar]
- 18.Butterly LF, Goodrich M, Onega T, et al. Improving the quality of colorectal cancer screening: assessment of familial risk. Dig Dis Sci. 2010;55:754–60. doi: 10.1007/s10620-009-1058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene MA, Butterly LF, Goodrich M, et al. Matching colonoscopy and pathology data in population-based registries: development of a novel algorithm and the initial experience of the New Hampshire Colonoscopy Registry. Gastrointestinal endoscopy. 2011;74:334–40. doi: 10.1016/j.gie.2011.03.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carney P, Butterly L, Goodrich M, Weiss JE, Dietrich A. Design and Development of a Population-based Colonoscopy Registry. Journal of Registry Management. 2006;33:91–9. [Google Scholar]
- 21.Onega T, Goodrich M, Dietrich A, Butterly L. The influence of smoking, gender, and family history on colorectal adenomas. J Cancer Epidemiol. 2010;2010:509347. doi: 10.1155/2010/509347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcondes FO, Dean KM, Schoen RE, et al. The impact of exclusion criteria on a physician's adenoma detection rate. Gastrointestinal endoscopy. 2015;82:668–75. doi: 10.1016/j.gie.2014.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine. 2008;149:627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 24.Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. The American journal of gastroenterology. 2012;107:1315–29. doi: 10.1038/ajg.2012.161. quiz 4, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanaka MR, Rai T, Navaneethan U, et al. Adenoma detection rate in high-risk patients differs from that in average-risk patients. Gastrointestinal endoscopy. 2015 doi: 10.1016/j.gie.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 26.Anderson JC, Butterly LF, Goodrich M, Robinson CM, Weiss JE. Differences in detection rates of adenomas and serrated polyps in screening versus surveillance colonoscopies, based on the new hampshire colonoscopy registry. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11:1308–12. doi: 10.1016/j.cgh.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson JC, Butterly LF, Robinson CM, Goodrich M, Weiss JE. Impact of fair bowel preparation quality on adenoma and serrated polyp detection: data from the New Hampshire colonoscopy registry by using a standardized preparation-quality rating. Gastrointestinal endoscopy. 2014;80:463–70. doi: 10.1016/j.gie.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butterly L, Robinson CM, Anderson JC, et al. Serrated and adenomatous polyp detection increases with longer withdrawal time: results from the New Hampshire Colonoscopy Registry. The American journal of gastroenterology. 2014;109:417–26. doi: 10.1038/ajg.2013.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jover R, Zapater P, Polania E, et al. Modifiable endoscopic factors that influence the adenoma detection rate in colorectal cancer screening colonoscopies. Gastrointestinal endoscopy. 2013;77:381–9. doi: 10.1016/j.gie.2012.09.027. e1. [DOI] [PubMed] [Google Scholar]
- 30.Jensen CD, Doubeni CA, Quinn VP, et al. Adjusting for patient demographics has minimal effects on rates of adenoma detection in a large, community-based setting. Clin Gastroenterol Hepatol. 2015;13:739–46. doi: 10.1016/j.cgh.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson JC, Attam R, Alpern Z, et al. Prevalence of colorectal neoplasia in smokers. The American journal of gastroenterology. 2003;98:2777–83. doi: 10.1111/j.1572-0241.2003.08671.x. [DOI] [PubMed] [Google Scholar]
- 32.Lieberman DA, Holub J, Eisen G, Kraemer D, Morris CD. Utilization of colonoscopy in the United States: results from a national consortium. Gastrointestinal endoscopy. 2005;62:875–83. doi: 10.1016/j.gie.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 33.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162–8. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 34.Lieberman DA, Prindiville S, Weiss DG, Willett W. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. Jama. 2003;290:2959–67. doi: 10.1001/jama.290.22.2959. [DOI] [PubMed] [Google Scholar]
- 35.Anderson JC, Pleau DC, Rajan TV, et al. Increased frequency of serrated aberrant crypt foci among smokers. The American journal of gastroenterology. 2010;105:1648–54. doi: 10.1038/ajg.2010.109. [DOI] [PubMed] [Google Scholar]
- 36.Anderson JC, Rangasamy P, Rustagi T, et al. Risk factors for sessile serrated adenomas. Journal of clinical gastroenterology. 2011;45:694–9. doi: 10.1097/MCG.0b013e318207f3cf. [DOI] [PubMed] [Google Scholar]
- 37.Kaminski MF, Polkowski M, Kraszewska E, Rupinski M, Butruk E, Regula J. A score to estimate the likelihood of detecting advanced colorectal neoplasia at colonoscopy. Gut. 2014;63:1112–9. doi: 10.1136/gutjnl-2013-304965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bortniker E, Anderson JC. Do recent epidemiologic observations impact who and how we should screen for CRC? Digestive diseases and sciences. 2015;60:781–94. doi: 10.1007/s10620-014-3467-x. [DOI] [PubMed] [Google Scholar]