Abstract
A 16 year old young man was diagnosed with Ewing sarcoma of the ribcage with pulmonary metastases. Six months after completion of scheduled therapy he was found to have a new intracardiac mass, presumed recurrent Ewing sarcoma. EWSR1 fusion was not detected via droplet digital PCR from blood plasma. After no improvement with salvage chemotherapy, he underwent surgical resection which identified a low-grade spindle cell sarcoma. Despite the near synchronous presentation of two unrelated sarcomas, extensive genomic analyses did not reveal any unifying somatic or germline mutations nor any apparent cancer predisposition. This case also highlights the potential role of utilizing plasma cell-free DNA for diagnosing tumors in locations where biopsy confers high morbidity.
Keywords: Ewing sarcoma, Cardiac sarcoma, Low grade sarcoma, Cell-free DNA, Genomics
Introduction
Ewing sarcoma (ES) is an aggressive embryonal malignancy which presents in soft tissue or bone. 1 Metastases occur in 20-25% of patients, the majority of which are located in the lungs, bone, or bone marrow. 2 Here, we present an adolescent male with ES of the left posterior rib with multiple pulmonary metastases. He was found to have a new FDG-avid intracardiac mass 6 months following completion of planned initial therapy on routine radiographic surveillance. While an atypical location for an ES relapse, intracardiac ES cases have been reported both at diagnosis and relapse. 3–7 Intracardiac masses give rise to diagnostic, therapeutic, and management challenges. This case illustrates how new molecular technologies may provide some solutions to these challenges.
Case Report
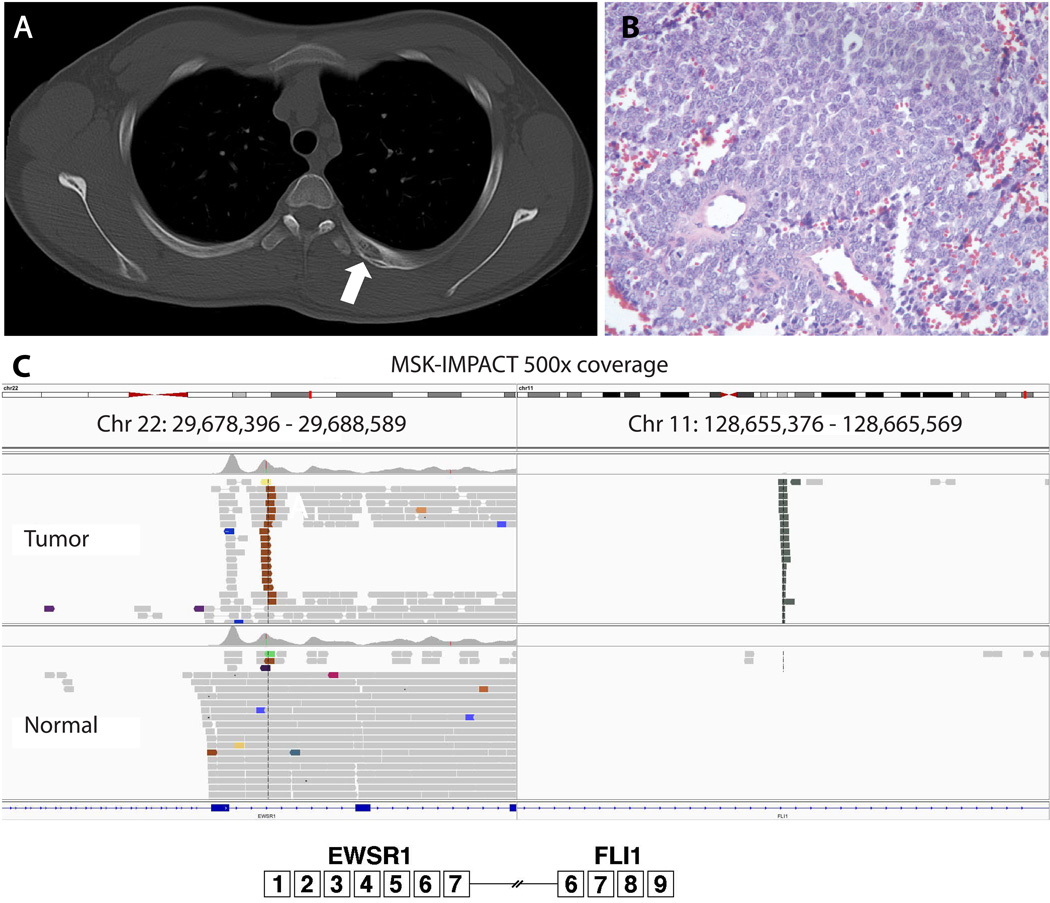
Our Caucasian male patient presented at age 16 to his pediatrician with a painless upper back mass which was biopsied and identified as a small round blue cell tumor (Figure 1). Cytogenetic evaluation identified an EWSR1 rearrangement and confirmed a diagnosis of ES. Staging demonstrated bilateral sub-centimeter pulmonary metastases but no evidence of extrapulmonary disease, which notably comprised multiple cross sectional modalities with fields including his heart. He was treated on a Memorial Sloan Kettering Cancer Center trial for metastatic ES (NCT01864109), which includes 4 cycles of cyclophosphamide (4.2 mg/m2/cycle), doxorubicin (75 mg/m2/cycle), and vincristine (2 mg/m2/cycle), 3 cycles of ifosphamide (14,000 mg/m2/cycle) and etoposide (500 mg/m2/cycle), as well as 10 cycles of irinotecan (200 mg/m2/cycle) and temozolomide (500 mg/m2/cycle).
Figure 1. Key imaging and pathology results from the Ewing sarcoma.
A: Axial CT scan revealed an expansile lytic lesion with periosteal reaction involving the left posterior fourth rib (arrow); B: H&E stain demonstrates a small round blue cell tumor; C: MSK-IMPACT sequencing reveals the precise coordinates of our patient’s EWSR1-FLI1 rearrangement. Note that this sequencing result had 500x coverage with no additional mutations identified.
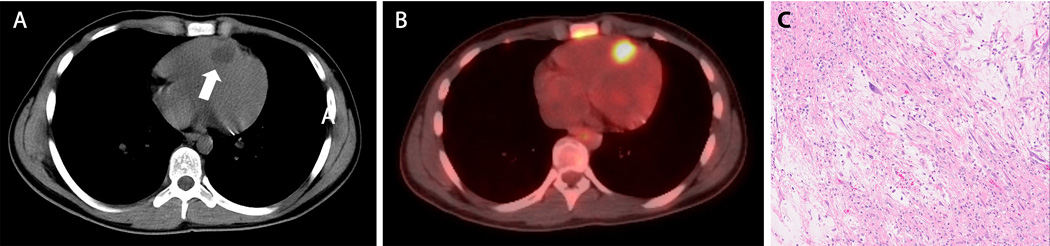
Six months after the completion of his scheduled therapy, a new FDG-avid nodule within the right ventricle of his heart was identified on a surveillance PET scan (Figure 2 A,B). Although rare, intracardiac metastases have been described in ES. Given the significant surgical risks associated with a biopsy or resection in this location, a decision was made to administer salvage chemotherapy with cyclophosphamide and topotecan for presumed relapsed disease. 8 Following two cycles of salvage therapy, no significant change in size or FDG-avidity was demonstrated. Given the lack of response, the patient then underwent surgical resection of the intracardiac mass, which identified a 3 cm low-grade spindle cell sarcoma with negative margins (Figure 2C). EWSR1 fusion testing was negative by both FISH and anchored multiplex PCR (Archer FusionPlex). Testing was negative for MDM2 and PDGFRA amplification indicating it was not an intimal sarcoma. 9 Finally, we profiled both his original ES biopsy specimen and low-grade intracardiac tumor on MSK-IMPACT, a 410 gene hybrid-capture based targeted next generation sequencing platform. 10 An EWSR1-FLI1 fusion was identified on the original ES tumor biopsy, while the intracardiac tumor revealed two silent somatic mutations, an intronic mutation of MRE11A and a synonymous mutation of AMER1.
Figure 2. Key imaging and pathology findings from the low-grade intracardiac sarcoma.
A: Axial noncontrast enhanced CT of the chest revealed a hypodense nodule at the apex of the right ventricle abutting the intraventricular septum (arrow); B: This lesion was FDG-avid on PET scan; C: Low-grade myxoid spindle cell neoplasm composed of atypical spindle cells with focal nuclear pleomorphism in a myxoid background is apparent on H&E stain.
After his first three cycles of chemotherapy, the patient was enrolled on an ongoing study evaluating the utility of identifying genomic EWSR1 fusions from plasma-derived DNA as a potential biomarker for subclinical disease. Multiple plasma samples were collected after the identification of the intracardiac mass, all of which were negative for the identification of an EWSR1 fusion by droplet digital PCR.
Although ES has not been associated with classic tumor susceptibility syndromes, recent literature demonstrates that germline predispositions are more common than previously believed. 11, 12 A targeted germline evaluation of 76 genes with known associations with cancer development did not reveal any constitutional mutations. Furthermore, an additional extensive interrogation of germline TP53 mutations was also negative. This included the bidirectional sequencing of splice junctions and corresponding coding regions (exons 2-11) along with deletion and duplication analysis to identify larger rearrangements. Given the limited differential for a cardiac tumor, notably, no mutations or copy number alterations in the TSC1 or TSC2 genes were detected and the young man did not exhibit any stigmata of tuberous sclerosis.
As of this submission date, the patient is 15 months post completion of therapy for his ES, excluding his two salvage cycles of chemotherapy. He does have a right bundle branch block, which was an expected result of the tumor location, but otherwise no impaired cardiac function.
Discussion
We report a case of a young Caucasian man with ES of the ribcage who was found to have a low-grade intracardiac sarcoma 6 months after completion of therapy. Despite extensive testing of somatic and germline mutations, we did not identify any consistent mutations which might indicate a common etiology of these two sarcomas. The fact that they both revealed distinct somatic mutations provides further evidence that the tumors are unlikely to be related. However, occult constitutional mutations or mosaicism cannot be excluded. Ultimately, despite a negative targeted evaluation of germline variants with known associations with cancer, it is possible that an uninterrogated gene aberration is responsible for the development of multiple malignancies in this patient.
At this point, there are no known underlying genetic predispositions which are specifically associated with ES. 11 However, the incidence of ES is nine times greater in individuals of Caucasian ancestry compared with African Americans, suggesting that there is likely a heritable factor which predisposes Caucasian patients but has yet to be defined. 13 Recent evidence has implicated GGAA-microsatellite polymorphisms in NR0B1 as a possible etiology for this racial disparity. 14, 15
It is possible but unlikely that this subsequent intracardiac sarcoma was treatment related. The patient received cardiac sparing radiation, and thus the dose to the heart was estimated to be quite low, approximately 10 Gy spread over 25 fractions. In a study of 266 survivors of ES, no secondary sarcomas developed in patients who had received less than 48 Gy and the shortest latency from the end of therapy to a second malignancy was 3.5 years with a median of 7.6 years, much longer than the latency in our patient. 16
The detection of genomic fusion products by blood plasma digital droplet PCR is a promising biomarker in ES but is not yet clinically actionable. 17–18 This case highlights the potential utility of circulating tumor DNA as a blood based biomarker for ES, particularly in situations where biopsy confers significant morbidity. Our group has recently demonstrated that EWSR1 fusions can be detected via blood droplet digital PCR in 11/11 ES patients, 7 of which were able to be detected at relapse. (Shukla N, personal communication) Ongoing studies are underway to ascertain the clinical utility of cell-free DNA in ES.
Acknowledgments
This work was supported by the Kristen Ann Carr Fund (M.O.), Marie Josee and Henry R. Kravis Center for Molecular Oncology, and the MSK Cancer Center Support Grant/Core Grant P30 CA008748.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest and have all made significant contributions to the manuscript. This project is approved by all authors, has not been published previously, and is not under consideration for publication elsewhere.
References
- 1.Karski EE, McIlvaine E, Segal MR, et al. Identification of Discrete Prognostic Groups in Ewing Sarcoma. Pediatric Blood & Cancer. 2016;63(1):47–53. doi: 10.1002/pbc.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Group TEESNW. Bone sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2014 Sep 1;25(suppl 3):iii113–iii123. doi: 10.1093/annonc/mdu256. [DOI] [PubMed] [Google Scholar]
- 3.Saremi F, Ho SY, Cabrera JA, et al. Right ventricular outflow tract imaging with CT and MRI: Part 2, Function. American Journal of Roentgenology. 2013 Jan;200(1):W51–W61. doi: 10.2214/AJR.12.9334. [DOI] [PubMed] [Google Scholar]
- 4.Coccia P, Ruggiero A, Rufini V, et al. Cardiac metastases of Ewing sarcoma detected by 18F-FDG PET/CT. Journal of Pediatric Hematology/Oncology. 2012 Apr;34(3):236–238. doi: 10.1097/MPH.0b013e318242754d. [DOI] [PubMed] [Google Scholar]
- 5.Wilson KS, Nyssen J, Alexander S. Ewing sarcoma: phalangeal primary with fatal cardiac metastases. Medical and Pediatric Oncology. 1979;7(4):361–364. doi: 10.1002/mpo.2950070411. [DOI] [PubMed] [Google Scholar]
- 6.Flinn RM, Foyle A, Montague TJ. Extraskeletal Ewing's sarcoma with fatal cardiac metastasis. Canadian Medical Association Journal. 1985;133(10):1017–1018. [PMC free article] [PubMed] [Google Scholar]
- 7.Raafat J, Brown JA, Oster MW. Metastatic Ewing sarcoma to the heart simulating adriamycin cardiotoxicity. Medical and Pediatric Oncology. 1978;5(1):51–54. doi: 10.1002/mpo.2950050107. [DOI] [PubMed] [Google Scholar]
- 8.Hunold A, Weddeling N, Paulussen M, et al. Topotecan and cyclophosphamide in patients with refractory or relapsed Ewing tumors. Pediatric Blood & Cancer. 2006 Nov;47(6):795–800. doi: 10.1002/pbc.20719. [DOI] [PubMed] [Google Scholar]
- 9.Neuville A, Collin F, Bruneval P, et al. Intimal sarcoma is the most frequent primary cardiac sarcoma: clinicopathologic and molecular retrospective analysis of 100 primary cardiac sarcomas. The American Journal of Surgical Pathology. 2014 Apr;38(4):461–469. doi: 10.1097/PAS.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 10.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. The Journal of Molecular Diagnostics. 2015 May;17(3):251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Randall RL, Lessnick SL, Jones KB, et al. Is There a Predisposition Gene for Ewing's Sarcoma? Journal of Oncology. 2010;2010:6. doi: 10.1155/2010/397632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Walsh MF, Wu G, et al. Germline Mutations in Predisposition Genes in Pediatric Cancer. The New England Journal of Medicine. 2015 Dec 10;373(24):2336–2346. doi: 10.1056/NEJMoa1508054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jawad MU, Cheung MC, Min ES, et al. Ewing sarcoma demonstrates racial disparities in incidence-related and sex-related differences in outcome: an analysis of 1631 cases from the SEER database, 1973-2005. Cancer. 2009 Aug 1;115(15):3526–3536. doi: 10.1002/cncr.24388. [DOI] [PubMed] [Google Scholar]
- 14.Beck R, Monument MJ, Watkins WS, et al. EWS/FLI-responsive GGAA microsatellites exhibit polymorphic differences between European and African populations. Cancer Genetics. 2012 Jun;205(6):304–312. doi: 10.1016/j.cancergen.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monument MJ, Johnson KM, McIlvaine E, et al. Clinical and biochemical function of polymorphic NR0B1 GGAA-microsatellites in Ewing sarcoma: a report from the Children's Oncology Group. PloS One. 2014;9(8):e104378. doi: 10.1371/journal.pone.0104378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuttesch JF, Wexler LH, Marcus RB, et al. Second malignancies after Ewing's sarcoma: radiation dose-dependency of secondary sarcomas. Journal of Clinical Oncology. 1996 Oct 1;14(10):2818–2825. doi: 10.1200/JCO.1996.14.10.2818. [DOI] [PubMed] [Google Scholar]
- 17.Krumbholz M, Hellberg J, Steif B, et al. Genomic EWSR1 Fusion Sequence as Highly Sensitive and Dynamic Plasma Tumor Marker in Ewing Sarcoma. Clinical Cancer Research. 2016 Sep 1;22(17):4356–4365. doi: 10.1158/1078-0432.CCR-15-3028. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi M, Chu D, Meyer CF, et al. Highly personalized detection of minimal Ewing sarcoma disease burden from plasma tumor DNA. Cancer. 2016 Oct;122(19):3015–3023. doi: 10.1002/cncr.30144. [DOI] [PMC free article] [PubMed] [Google Scholar]