Abstract
Objective
Paroxysmal sympathetic hyperactivity (PSH) is characterized by episodic, hyperadrenergic alterations in vital signs after traumatic brain injury (TBI). We sought to apply an objective scale to the vital sign alterations of PSH in order to determine if one element might be predictive of developing PSH.
Setting/Participants/Design
We conducted an observational study of consecutive TBI patients (GCS ≤12), and monitored the cohort for clinical evidence of PSH. PSH was defined as a paroxysm of 3 or more of the following characteristics: (1) tachycardia, (2) tachypnea, (3) hypertension, (4) fever, and (5) dystonia (rigidity or decerebrate posturing) and (6) diaphoresis, with no other obvious causation (i.e. alcohol withdrawal, sepsis).
Main Measures
The “Modified Clinical Feature Severity Scale” (mCFSS) was applied to each participant once daily for the first 5 days of hospitalization.
Results
Nineteen (11%) of the 167 patients met criteria for PSH. Patients with PSH had a higher 5-day cumulative mCFSS than those without PSH (36 [29–42] v. 29 [22–35], P=0.01). Of the four components of the mCFSS, elevated temperature appeared to be most predictive of the development of PSH, especially during the first 24 hours (OR=1.95, 95%CI [1.12, 3.40]).
Conclusion
Early fever after TBI may signal impending autonomic dysfunction.
Keywords: Traumatic Brain Injury, Paroxysmal Sympathetic Hyperactivity, Sympathetic Storms, Autonomic Instability, Fever
Introduction
Despite advances in both prevention and treatment, traumatic brain injury (TBI) remains one of the most burdensome diseases; 2% of the US population currently lives with disabilities resulting from TBI 1. Patients with severe TBI may have lengthy intensive care unit (ICU) stays driven by complications from their underlying brain injury such as autonomic dysfunction. Autonomic dysfunction may clinically manifest as episodic alterations in vital signs, such as tachycardia and hypertension. Fulminant autonomic dysfunction is known as paroxysmal sympathetic hyperactivity (PSH), has just recently been formally defined2. PSH occurs in about 10% (8–33%) of moderate to severe TBI patients3. Patients with both TBI and PSH have worse Glasgow Outcome Scales-Extended (GOSE), Functional Independence Measures (FIM), longer hospital courses, and higher healthcare costs than their counterparts without PSH.4 A major barrier to the mechanistic understanding and impact of PSH is the lack of objective measures for its diagnosis and severity5. Additionally, as PSH is a diagnosis of exclusion, there may be a delay in diagnosis, even if the index of suspicion is high.
To address this pressing need, we conducted an observational cohort study of TBI patients and applied a recently developed severity score for the episodes of PSH, the Clinical Features Severity Score (CFSS)2. Our goals were to use an objective scale to quantify the elements of PSH and to determine if one of the vital sign derangements might predict the development of PSH more than the others. Determination of the elements of the CFSS has been described elsewhere2, but in short, an international working group applied the Delphi method6 to build a consensus definition of PSH and a scale to describe its severity, the CFSS. The CFSS attempts to rate the severity of the sympathetic and motor clinical features of the condition. The severity of the clinical features are scored from 0 (i.e., within normal parameters, for example, temperature < 37°C or heart rate < 100 beats per minute) up to a score of 3 for the highest tier of severity. Thus, the CFSS became an 18-point scale (from 0 to 18). (Figure 1) In the same manuscript, the group also developed a score to quantify the likelihood that the symptoms observed were consistent with a diagnosis of PSH (“Diagnosis Likelihood Tool” Figure 2).
Figure 1.
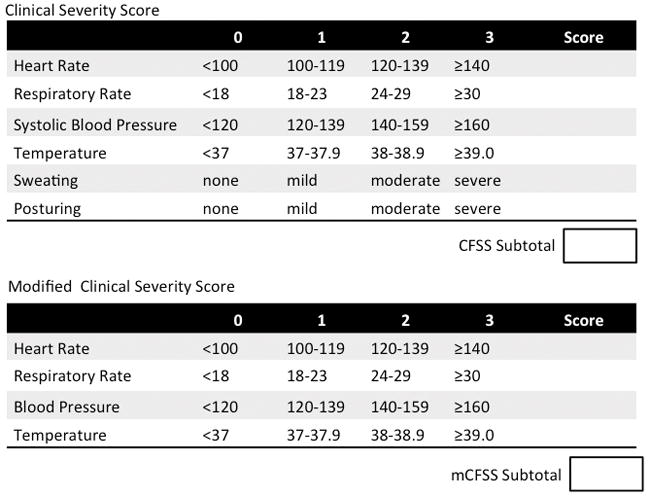
Clinical Severity Scores
Figure 2.
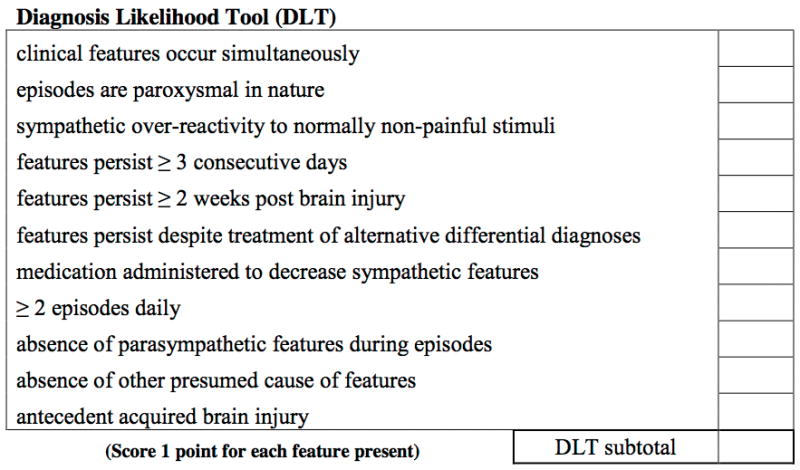
Methods
Setting/Participants/Design
IRB approval was obtained prior to enrolling subjects. We identified consecutive adult head trauma patients presenting to the Trauma Bay of the Emergency Department (ED) with a GCS ≤12 and subsequently admitted to the Trauma Intensive Care Unit (ICU). Patients were excluded if the depressed GCS was attributable to a cause other than brain injury such as sedation, intoxication, or hemorrhagic shock. Patients were excluded if there were discharged from the ED or admitted to the non-ICU ward. Admission characteristics of the cohort were recorded from the electronic medical record.
PSH was identified by the clinical team, and defined as at least two serial events of paroxysms of 3 or more of the following simultaneous characteristics: (1) tachycardia, (2) tachypnea, (3) hypertension, (4) fever, and (5) dystonia (rigidity or decerebrate posturing) and (6) diaphoresis, with no other obvious causation (i.e. alcohol withdrawal, sepsis). Subjects were monitored for the entirety of their hospitalization for PSH. Patients were monitored on telemetry with hourly heart rate, respiratory rate, and systolic blood pressures recorded. In an effort to confirm the clinical suspicion of PSH, we retrospectively reviewed the charts of all patients identified as having PSH, and calculated a Diagnosis Likelihood Tool score for each case. Per ICU protocol, temperatures were recorded at least every 4 hours (and as frequently as hourly) for the first 48 hours after admission. Temperatures were measured with a bladder catheter thermistor in the majority of measurements. Rarely, oral or axillary temperatures were used if there was no foley catheter. If the fever threshold (38.4°C) was met or exceeded, cultures of blood and urine, as well as sputum for intubated patients, were routinely obtained, unless the patient was immediately post-operative (<24 hours). Additionally, febrile patients received acetaminophen, followed by external cooling measures such as ice packs or cooling blankets if medication alone was ineffective after the first hour. Rarely, an adhesive cooling device (Arctic Sun™) was applied for refractory fever. Infection was determined by the presence of a culture drawn that grew a pathogenic organism not felt to be a contaminant.
Main Measures: Clinical Feature Severity Scale (CFSS)
The “Clinical Feature Severity Scale” (CFSS)2 was determined retrospectively on each participant, regardless of PSH status, once daily for the first 5 days of admission. The CFSS was calculated from the most deranged set of vitals recorded in that 24-hour period in the medical record, e.g. the highest scoring set of vitals assessed by the CFSS. The CFSS is calculated by summing points attributed for each vital sign derangement for a total score. (Figure 1)
Statistical Analysis
Chi squared test for binary data, a student’s t test for normally distributed continuous data, and Wilcoxon rank sum test for ordinal and non-normally distributed continuous data were applied for intragroup comparisons. Logistic regression was employed to determine if elements of the CFSS predicted the development of PSH for each day of the first 5 days of ICU stay. Each day was analyzed separately with all vital sign derangement indicators included in the same model to control for any potential confounding. Only patients with complete data were included in the regression analyses; patients that died or were discharged before 5 days were excluded from the analysis. Data is reported as Median [Interquartile Range] or Mean [± Standard Deviation].
Results
Patient Characteristics and Hospital Course
Three hundred and forty-eight patients were screened in the emergency department. Of those 348, 181 were excluded from enrollment, mostly as a result of having a GCS of 13 or greater. (Figure 3) One hundred and sixty-seven patients met enrollment criteria, and were followed through their hospitalization. (Figure 3) Nineteen (11%) of the 167 patients were deemed to meet criteria for PSH by the treating team as outlined in the Methods. (Table 1) The median Diagnosis Likelihood Tool (DLT) score was 7 [5–9] in the identified cases. There were a similar proportion of men in both groups. Patients exhibiting PSH were significantly younger than those without PSH (31 [±15] v. 47 [±21] years, P<0.01). There was a trend toward lower admission GCS scores in the PSH group, but this did not meet significance (3 [3–3] v. 3 [3–6], P=0.07). Subjects with PSH had higher Injury Severity Scores (ISS) (48 [34–57] v. 27 [24–35], P<0.01) and higher Head Abbreviated Injury Scale scores than those who did not display the syndrome (5 [5–5] v. 5[4–5], P=0.05).
Figure 3.
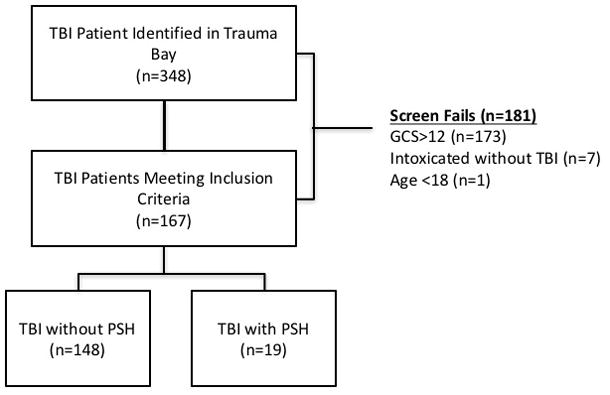
Screening of trauma patients for inclusion into the study. TBI= Traumatic Brain Injury, GCS=Glasgow Coma Score
Table 1.
Admission Characteristics and Hospital Course of TBI cohort.
| PSH(n=19) | Non-PSH(n=148) | p | |
|---|---|---|---|
| Admission Characteristics | |||
| Mean Age [±STD] | 31 [±15] | 47 [±21] | <0.01 |
| Male Gender | 68% | 78% | 0.39 |
| Median Admit GCS [IQR] | 3 [3–3] | 3 [3–6] | 0.07 |
| Median Head AIS [IQR] | 5 [5–5] | 5 [4–5] | 0.05 |
| Median ISS [IQR] | 48 [34–57] | 27 [24–35] | <0.01 |
| Hospital Course | |||
| Median ICU LOS [IQR] days | 17 [13–22] | 6[3–11] | <0.001 |
| Median Hospital LOS [IQR] days | 30 [24–53] | 10 [5–23] | <0.001 |
| Median GCS at ICU discharge [IQR] | 5 [4–6] | 4 [3–7] | 0.08 |
Patients exhibiting PSH had longer stays in the ICU (17 [13–22] v. 6 [3–11] days, P<0.001) and in the hospital (30 [24–53] v. 10 [5–23] days, P<0.001) than those without PSH. (Table 1)
Modification of the CFSS
CFSS was calculated retrospectively from the electronic medical record. Reliable documentation of “sweating” and “posturing” were not always recorded. Thus these two items were removed from the CFSS, collapsing the scale to a 0–12 point measurement termed the “modified CFSS” (Figure 1). Once the CFSS was modified, CFSS was compared between patients with and without PSH. Only patients with scores for all 5 days were included in the analysis (n=134). Patients with PSH had a higher 5-day cumulative score than those without PSH (36 [29–42] v. 29 [22–35], P=0.01).
PSH Prediction
Each element of the CFSS total was interrogated in a logistic regression model with the designated outcome of developing PSH. (Table 2) Respiratory rate and heart rate scores were not associated with the development of PSH on days 1–5. Higher temperature scores were associated with the development of PSH on days 1 and 3, but not on days 4 or 5. There was a trend toward association on day 2, but it did not meet statistical significance. For each level of increased temperature on the mCFSS on day 1, there was an increased likelihood of development of PSH of 1.95 (95% CI: 1.12 to 3.40; p=0.018). For each level of increased temperature on the mCFSS on day 3, there was an increase of in odds of development of PSH of 2.10 (95% CI: 1.11–3.99; p=0.023). Low blood pressure on day 1 was associated with the development of PSH (p=0.043), but this association was not present on days 2–5. (Table 2)
Table 2.
Prediction of PSH by Elements of the mCFSS
| Day l | Day 2 | Day 3 | Day 4 | Day 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR(95% CI) | P | OR(95% CI) | P | OR(95% CI) | P | OR(95% CI) | P | OR(95% CI) | P | |
| Heart Rate | 1.33(0.79–2.25) | 0.282 | 1.38(0.79–2.41) | 0.25 | 1.12(0.62–2.02) | 0.714 | 0.89(0.49–1.64) | 0.717 | 1.53(0.87–2.70) | 0.143 |
| Respiratory Rate | 1.52(0.78–2.96) | 0.221 | 1.59(0.81–3.14) | 0.182 | 1.20(0.66–2.16) | 0.549 | 1.37(0.75–2.50) | 0.307 | 1.26(0.66–2.39) | 0.486 |
| Blood Pressure | 0.56 (0.32–0.98) | 0.043 | 0.76(0.41–1.39) | 0.369 | 0.65(0.37–1.13) | 0.124 | 0.78(0.43–1.43) | 0.425 | 0.75(0.42–1.30) | 0.306 |
| Temperature | 1.95(1.12–3.40) | 0.018 | 2.01(0.96–4.49) | 0.062 | 2.10(1.11–3.99) | 0.023 | 1.74(0.92–3.31) | 0.089 | 1.47(0.80–2.71) | 0.215 |
Given the relationship between early elevations in temperature and development of PSH, an exploratory analysis was conducted to examine the relationship between fever in the first 24 and 48 hours, and PSH. Fifty-three percent of patients with PSH had a temperature of 38.0°C or higher (scores of 2 or 3 on the mCFSS) during the first 24 hours of hospitalization (P=0.12). Conversely, there was a significant association between temperatures of 38.0°C or higher during the first 24 hours of hospitalization and the development of PSH (P=0.015). That relationship persisted for the first 48 hours as well (P=0.05). Only 2 (2%) of the 86 patients exhibiting temperatures of 38.0°C or greater on day 1 had positive cultures from the first 24 hours, both of which were obtained from sputum. Only 16 (19%) of the 86 had one or more positive cultures from the first 5 days of hospitalization.
Discussion
We observed a statistically significant association between the presence of PSH and severity of injury (higher ISS and HAIS), longer hospital courses, and higher temperature scores on the mCFSS in patients with TBI. Our results echo previous work indicating patients exhibiting PSH are more severely injured and have longer ICU stays when compared to their counterparts without PSH7,8. Our work adds to the existing base of knowledge by suggesting that early fever might be an early indication of autonomic dysfunction. Investigators have established a firm link between elevated temperature and poor outcome after a variety of neurologic injuries9. Early fever (occurring in the first 24–72 hours after injury) appears to be especially associated with mortality, reinforcing the significance of acute temperature elevation10,11. Whether the fever is a marker of severity of the injury, produces ongoing injury, or both remains to be determined. The association of lower blood pressure with PSH on day 1 might reflect the need for greater sedation for more severely injured patients resulting in hypotension, but this hypothesis would need to be tested directly.
Our work has several important limitations. It is possible that some patients were incorrectly categorized as having PSH, or conversely, some patients may have been missed, as diagnosis was at the discretion of the clinical team. Since the conception of this study, formal diagnostic criteria for PSH2 have been published, which did not inform the diagnosis in our cohort. To address this limitation, we retrospectively applied the DLT scores to our cohort, and found the median score to be 7 (out of a maximal score of 11 points), which is suggestive that the label of PSH was correctly applied. Additionally, the rate of PSH in out cohort (11%) is in line with the reported rates in the literature3. Our sample size was small, which may limit the generalizability of our findings. Modified CFSS scores were recorded without regard to therapy; thus scores may have been influenced by scheduled symptomatic treatments such as anti-pyretics. Future studies will carefully collect mCFSS scored before and after therapeutic intervention. We do not have long-term outcome data on our cohort. Additionally, not all infections may yield positive culture data thus our reported rate of infection for early fever might be artificially low. We cannot definitely exclude infection as a confound in our cohort. Future investigations will carefully record the timing of empiric antibiotic administration and response to antibiotics with PSH events. Finally, our exploratory analysis will need to be validated in other cohorts of PSH patients.
The CFSS was difficult to apply in its original form, as it requires special training of personnel to document sweating and posturing in a uniform fashion. Future studies should incorporate training on the instrument before utilizing the CFSS. In contrast, the mCFSS is practical and easy to apply retrospectively, as it relies only on standard vital sign measurements. The mCFSS is not intended to make the diagnosis of PSH and should not substitute for clinician judgment in the correct clinical scenario. Instead, applying the mCFSS may aid clinician in quantifying the severity of PSH and grading the response of the syndrome to therapeutic interventions. It remains to be shown if reduction of mCFSS scores might translate to decreased LOS or improved outcomes. However, we hope that application of the mCFSS might provide an objective framework to make this determination. Additionally, the links between early fever and autonomic dysfunction should be investigated, as early fever may herald the arrival of PSH.
Acknowledgments
The project described was supported in part by Award Number 5K12HL108974-03 from the National Heart, Lung, and Blood Institute. This publication was supported by Oregon Clinical and Translational Research Institute (OCTRI), grant number (UL1TR000128) from the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. The authors wish to acknowledge Brittney Brown and Maya Vanderbark for their work in data collection.
Footnotes
Declaration of Interest
The authors report no conflicts of interest.
References
- 1.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Baguley IJ, Perkes IE, Fernandez-Ortega JF, Rabinstein A, Dolce G, Hendricks H. Paroxysmal sympathetic hyperactivity after acquired brain injury: consensus on conceptual definition, nomenclature, and diagnostic criteria. J Neurotrauma. doi: 10.1089/neu.2013.3301. Epub 2014 Apr 14. [DOI] [PubMed] [Google Scholar]
- 3.Baguley IJ, Slewa-Younan S, Heriseanu RE, Nott MT, Mudaliar Y, Nayyar V. The incidence of dysautonomia and its relationship with autonomic arousal following traumatic brain injury. Brain Inj BI. 2007;21:1175–1181. doi: 10.1080/02699050701687375. [DOI] [PubMed] [Google Scholar]
- 4.Baguley IJ. Autonomic complications following central nervous system injury. Semin Neurol. 2008;28:716–725. doi: 10.1055/s-0028-1105971. [DOI] [PubMed] [Google Scholar]
- 5.Perkes IE, Menon DK, Nott MT, Baguley IJ. Paroxysmal sympathetic hyperactivity after acquired brain injury: A review of diagnostic criteria. Brain Inj BI. 2011;25:925–932. doi: 10.3109/02699052.2011.589797. [DOI] [PubMed] [Google Scholar]
- 6.Milholland A, Wheeler S, Heieck J. Medical assessment by a Delphi group opinion technic. N Engl J Med. 1973;288:1272–1275. doi: 10.1056/NEJM197306142882405. [DOI] [PubMed] [Google Scholar]
- 7.Lv L-Q, Hou L-J, Yu M-K, et al. Prognostic influence and magnetic resonance imaging findings in paroxysmal sympathetic hyperactivity after severe traumatic brain injury. J Neurotrauma. 2010;27:1945–1950. doi: 10.1089/neu.2010.1391. [DOI] [PubMed] [Google Scholar]
- 8.Lv L-Q, Hou L-J, Yu M-K, et al. Risk Factors Related to Dysautonomia After Severe Traumatic Brain Injury. [Accessed August 24, 2011];J Trauma. doi: 10.1097/TA.0b013e31820ebee1. [online serial]. Epub 2011 Mar 21. Accessed at: http://www.ncbi.nlm.nih.gov/pubmed/21427610. [DOI] [PubMed]
- 9.Greer DM, Funk SE, Reaven NL, Ouzounelli M, Uman GC. Impact of fever on outcome in patients with stroke and neurologic injury: a comprehensive meta-analysis. Stroke J Cereb Circ. 2008;39:3029–3035. doi: 10.1161/STROKEAHA.108.521583. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Jiang J. Chinese Head Trauma Data Bank: effect of hyperthermia on the outcome of acute head trauma patients. J Neurotrauma. 2012;29:96–100. doi: 10.1089/neu.2011.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saxena M, Young P, Pilcher D, et al. Early temperature and mortality in critically ill patients with acute neurological diseases: trauma and stroke differ from infection. Intensive Care Med. 2015;41:823–832. doi: 10.1007/s00134-015-3676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


