Abstract
There has been increasing use of novel synthetic hallucinogenic compounds, 25B-NBOMe, 25C-NBOMe, 25I-NBOMe, and 5-MeO-DALT, which have been associated with severe toxicities. These four compounds were tested for discriminative stimulus effects similar to a prototypical hallucinogen (−)-2,5-dimethoxy-4-methylamphetamine (DOM) and to the entactogen (±)-3,4-methylenedioxymethamphetamine (MDMA). Locomotor activity in mice was tested to obtain dose range and time course information. 25B-NBOMe, 25C-NBOMe and 25I-NBOMe decreased locomotor activity. 5-MeO-DALT dose-dependently increased locomotor activity with a peak at 10 mg/kg. A higher dose (25 mg/kg) suppressed activity. 25B-NBOMe fully substituted (≥80%) in both DOM- and MDMA-trained rats at 0.5 mg/kg. However, higher doses produced much lower levels of drug-appropriate responding in both DOM- and MDMA-trained rats. 25C-NBOMe fully substituted in DOM-trained rats, but produced only 67% drug-appropriate responding in MDMA-trained rats at doses that suppressed responding. 25I-NBOMe produced 74 to 78% drug-appropriate responding in DOM- and MDMA-trained rats at doses that suppressed responding. 5-MeO-DALT fully substituted for DOM, but produced little few or no MDMA-like effects. All of the compounds but except 25I-NBOMe produced fully substituted for DOM, whereas only 25B-NBOMe fully substituted for MDMA. However, the failure of 25I-NBOMe to fully substitute for either MDMA or DOM was more likely due to its substantial rate rate-depressant effects than to weak discriminative stimulus effects. All of the compounds are likely to attract recreational users for their hallucinogenic properties, but likely probably much less interest as substitutes for MDMA. Although no acute adverse effects were observed at the doses tested, the substantial toxicities reported in humans, coupled with the high likelihood for illicit use, suggests that these compounds have the same potential for abuse as other, currently scheduled compounds.
Keywords: Novel hallucinogens, DOM, MDMA, drug discrimination, locomotor activity, mouse, rat
Introduction
Use of “N-Bomb”, a street drug primarily comprised of any of three phenethylamine hallucinogens, 2-(4-bromo-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine hydrochloride (25B-NBOMe), 2-(4-chloro-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine hydrochloride (25C-NBOMe), and 2-(4-iodo-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine hydrochloride (25I-NBOMe), has drawn increasing attention due to their ease of synthesis (Halberstadt & Geyer; 2014; Kyriakou et al., 2015), toxic effects (Kyriakou et al, 2015; Weaver et al., 2015) and increasing use (Lawn et al., 2014; Weaver et al., 2015). All three compounds have been placed in temporary scheduling as controlled substances by the United States Drug Enforcement Agency (DEA, 2016). N,N-diallyl-5-methoxy tryptamine (5-MeO-DALT) is currently not scheduled by the DEA, but recently increased trafficking (Odoardi et al., 2016) and severe toxicities, including lethality (Corkery et al., 2012; Jovel et al., 2014; Kalasho & Nielsen, 2016), have increased interest in its pharmacological testing.
Review articles have summarized case reports from emergency rooms and poison control centers for the NBOMe compounds, and consistently found that high levels of agitation, tachycardia, hypertension and convulsions were observed in users (Forrester, 2014; Suzuki et al., 2015; Wood et al., 2015). A disturbing number of fatalities were reported, up to 15% of NBOMe-related cases, particularly with 25I-NBOMe (Suzuki et al., 2015). Studies of 25B-NBOMe reported hallucinations, violent agitation, cardiac events and/or serotonin syndrome, and convulsions (Tang et al., 2014; Laskowski et al., 2015; Gee et al., 2016). 25C-NBOMe overdose has been associated with serotonin syndrome (Andreasen et al., 2015) and convulsions (Laskowski et al., 2015).
The NBOMe compounds have high affinity for the 5-HT2A and 5-HT2C receptors (Juncosa et al., 2013; Rickli et al., 2015), with much lower potencies observed for other receptors (e.g., 3000–7100 fold more selective for 5-HT2A over 5-HT1A). Lower-affinity binding was observed for α1 adrenergic and H1 histaminergic receptors, and very low levels of binding were seen to monoamine transporters or dopamine receptors in these studies.
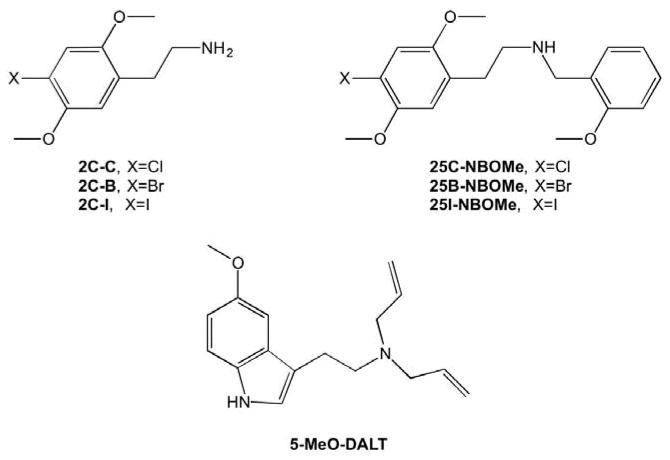
These compounds (Figure 1) are chemically related to the phenethylamine compounds, 2C-B (2,5-dimethoxy-4-bromophenethylamine), 2C-C (2,5-dimethoxy-4-chlorophenethylamine), and 2C-I (2,5-dimethoxy-4-iodophenethylamine), which are high potency and efficacy agonists at 5-HT2A and 5-HT2C receptors (Eshleman et al., 2014; Rickli et al., 2015). 2C-C and 2C-I produce DOM and/or LSD-like discriminative stimulus effects (Eshleman et al., 2014), and also produce adverse effects, such as convulsions and serotonin syndrome, in mice (Eshleman et al., 2014) and in human users (Bosak et al. 2013). Adding the N-benzylmethoxy group decreases activity at 5-HT1A receptors, and increases activity at adrenergic receptors and monoamine transporters (Rickli et al., 2015). How this translates to differences in behavioral effects is not clear, but one study noted that 25I-NBOMe was about 14-fold more potent at inducing 5-HT2A-mediated head twitch than 2C-I (Halberstadt & Geyer, 2014).
Figure 1. Structures of test compounds.
The illustration on at the top left shows the basic structure of the 2C phenethylamines. On At the top right is the structure for the NBOMe compounds tested in this study (25B-NBOMe, 25C-NBOMe, and 25I-NBOMe), which are structurally similar to the 2C phenethylamines, with the addition of the N-benzylmethoxy group. On At the bottom is shown the structure for 5-MeO-DALT.
There has been increasing use and trafficking of the substituted tryptamine 5-MeO-DALT over the past few years (Odoardi et al., 2016). 5-MeO-DALT produces a range of adverse effects similar to those of the NBOMe compounds including tachycardia, agitation and combativeness (Jovel et al., 2014). In addition, diaphoresis was observed (Jovel et al., 2014), which may indicate cholinergic effects in addition to serotonin syndrome.
5-MeO-DALT binds 5-HT1A, 2A, 2B and 2C receptors with high affinity, acting as a full agonist at these receptors (Nonaka et al., 2007; Arunotayanum et al., 2013; Cozzi & Daley, 2016). It also binds a range of other receptors, including adrenergic, D3, H1, H2, and sigma 1 and 2 receptors (Arunotayanum et al., 2013; Cozzi & Daley, 2016). However, 5-MeO-DALT showed little or no effects on monoamine release or uptake (Nagai et al., 2007; Cozzi & Daley, 2016).
Hallucinogens produce little or no self-administration or conditioned place preference (CPP) in animal models, and much of their recreational or therapeutic effects are based on their subjective effects (e.g., Carbonaro & Gatch, 2016; Winter, 2009; Nichols, 2016), which leaves drug discrimination as the best model for testing abuse liability of these compounds. Drug discrimination has been a useful animal model of the subjective effects of drugs, and correlates well with likelihood of use in humans (Young 2009; Horton et al. 2013). In the present study, 25B-NBOMe, 25C-NBOMe, 25I-NBOMe, and 5-MeO-DALT were first tested in the mouse locomotor assay to obtain dose ranges and time courses for their psychoactive effects. The mice were not habituated to the apparatus before testing, so that both locomotor depressant and stimulant effects could be detected. The compounds were then tested in rats trained to discriminate the classic serotonergic hallucinogen (−)-2,5-dimethoxy-4-methylamphetamine (DOM), and in rats trained to discriminate the entactogenic compound (±)-3,4-methylenedioxymethamphetamine (MDMA).
Methods
Subjects
Male Swiss–Webster mice were obtained from Harlan (Indianapolis, IN) at approximately 8 weeks of age and tested at approximately 10 weeks of age. Mice were group housed in cages on a 12:12-h light/dark cycle and were allowed free access to food and water. Male Sprague-Dawley rats were obtained from Harlan. All rats were housed individually and were maintained on a 12:12 light/dark cycle (lights on at 7:00 AM). Body weights were maintained at 320–350 g by limiting food to 15 g/day which included the food received during operant sessions. Water was readily available. All housing and procedures were in accordance with Guidelines for the Care and Use of Laboratory Animals (National Research Council, 2011) and were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
Locomotor Activity
The study was conducted using 40 Digiscan (model RXYZCM, Omnitech Electronics, Columbus, OH) locomotor activity testing chambers (40.5 X 40.5 X 30.5 cm) housed within sound-attenuating chambers in sets of two. A panel of infrared beams (16 beams) and corresponding photodetectors were located in the horizontal direction along the sides of each activity chamber. A 7.5-W incandescent light above each chamber provided dim illumination and fans provided an 80-dB ambient noise level within the chamber.
Separate groups of 8 mice were injected with either vehicle (0.9% saline), 25B-NBOMe, 25C-NBOMe, 25I-NBOMe, or 5-MeO-DALT immediately prior to locomotor activity testing. In all studies, horizontal activity (interruption of photocell beams) was measured for 8 hours within 10-min periods, beginning at 0800 hrs (1 hr after lights on). When testing 25B-NBOMe, an unusually low vehicle control value was observed, so the experiment was replicated in a separate group of mice and the data combined (n=16).
Discrimination Procedures
Standard behavior-testing chambers (Coulbourn Instruments, Allentown, PA) were connected to IBM-PC compatible computers via LVB interfaces (Med Associates, East Fairfield, VT). The computers were programmed in Med-PC for Windows, version IV (Med Associates, East Fairfield, VT) for the operation of the chambers and collection of data.
Using a two-lever choice methodology, a pool of rats previously trained to discriminate either MDMA (1.5 mg/kg) or DOM (0.5 mg/kg) from saline, as previously described (Gatch et al., 2009), were tested. Rats received an injection of either saline or drug and were subsequently placed in the behavior-testing chambers, where food (45 mg food pellets; Bio-Serve, Frenchtown, NJ) was available as a reinforcer for every ten responses on a designated injection-appropriate lever. The pretreatment time was 15 min for MDMA, and 30 min for DOM. Each training session lasted a maximum of 10 min, and the rats could earn up to 20 food pellets. The rats received approximately 60 of these sessions before they were used in tests for substitution of the experimental compounds. Rats were used in testing once they had achieved 9 of 10 sessions at 85% injection-appropriate responding for both the first reinforcer and total session. The training sessions occurred on separate days in a double alternating fashion (drug-drug-saline-saline-drug; etc.) until the training phase was complete, after which substitution tests were introduced into the training schedule, such that at least one saline and one drug session occurred between each test (drug-saline-test-saline-drug-test-drug; etc.). The substitution tests occurred only if the rats had achieved 85% injection-appropriate responding on the two prior training sessions.
Test sessions lasted for a maximum of 20 min. In contrast with training sessions, both levers were active, such that 10 consecutive responses on either lever led to reinforcement. Data were collected until the first reinforcer was obtained, or for a maximum of 20 min. Each compound was tested in groups of six rats. The dose effect of each compound was tested from no effect to full effect, or rate suppression (<20% of vehicle control) or adverse effects. Starting doses and pretreatment times were inferred from the locomotor activity testing. Doses were tested in no particular order. For dose-effect experiments, intraperitoneal (i.p.) injections (1 ml/kg) of vehicle or test compound were administered with a pretreatment of 15 min (25C-NBOMe and 25I-NBOMe), 40 min (25B-NBOMe), or 50 min (5-MeO-DALT). A repeated-measures design was used, such that each rat was tested at all doses of a given test compound. Preliminary data from 25B-NBOMe suggested that it might have locomotor stimulant effects at 40 min, so drug discrimination was tested using a 40-min pretreatment time. The other compounds were tested at the time of peak locomotor depressant effects.
Drugs
(±)-3,4-Methylenedioxymethamphetamine hydrochloride (MDMA), (−)-2,5-dimethoxy-4-methylamphetamine hydrochloride (DOM), 2-(4-bromo-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine hydrochloride (25B-NBOMe), 2-(4-chloro-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine hydrochloride (25C-NBOMe), 2-(4-iodo-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine hydrochloride (25I-NBOMe), and N,N-di allyl-5-methoxy tryptamine free base (5-MeO-DALT) were provided by the National Institute on Drug Abuse Drug Supply Program. DOM and MDMA were dissolved in 0.9% saline, whereas 25B-NBOMe, 25C-NBOMe, and 25I-NBOMe were dissolved in deionized water. 5-MeO-DALT was suspended in 2% methylcellulose. All compounds were administered intraperitoneally in a volume of 1 ml/kg in rats and 10 ml/kg in mice.
Data Analysis
Locomotor activity data were expressed as the mean number of photocell counts in the horizontal plane (ambulation counts) during each 10-min period of testing. A 30-min period, beginning when maximal stimulation of locomotor activity first appeared, as a function of dose, was used for analysis of dose-response data. A two-way repeated measures analysis of variance was conducted on horizontal activity counts/10 min interval. A one-way analysis of variance was conducted on horizontal activity counts for the 30-min period of maximal effect, and planned comparisons were conducted for each dose against saline control using single degree-of-freedom F tests.
Drug discrimination data are expressed as the mean percentage of drug-appropriate responses occurring in each test period. Rates of responding were expressed as a function of the number of responses made divided by the total session time. Graphs for percent drug-appropriate responding and response rate were plotted as a function of dose of test compound (log scale). Percent drug-appropriate responding was shown only if at least 3 rats completed the first fixed ratio. Full substitution was defined as ≥80% drug-appropriate responding and not statistically different from the training drug. Response rate data was analyzed by one-way repeated measures analysis of variance. Effects of individual doses were compared to the vehicle control value using a priori contrasts.
Results
Locomotor Activity
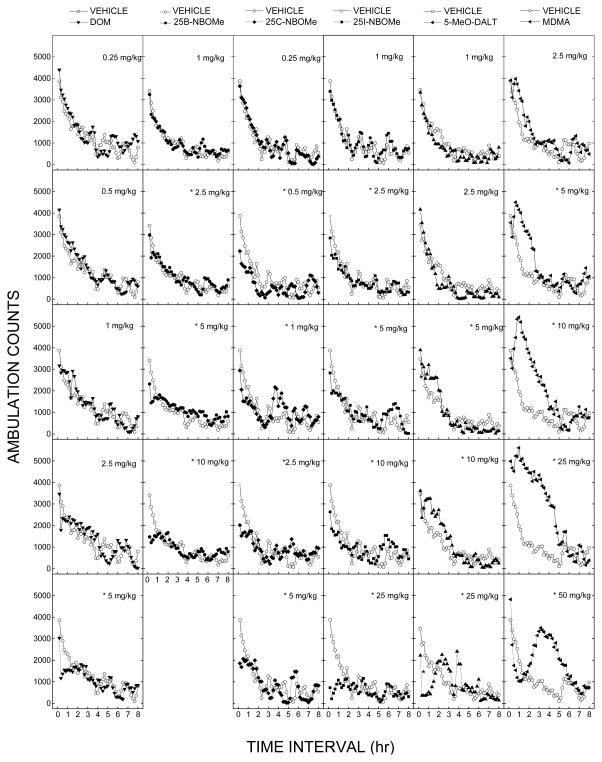
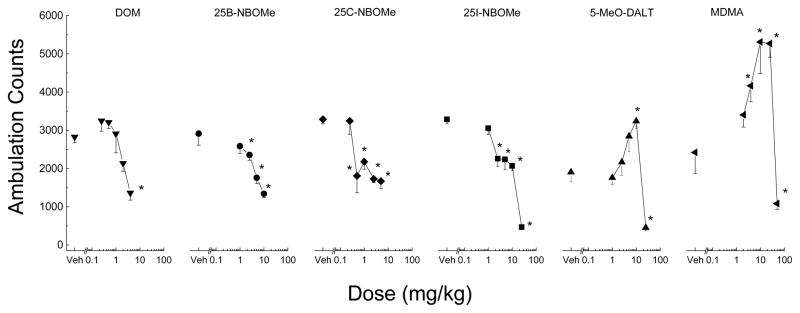
DOM decreased locomotor activity following 5 mg/kg (fig 2). A two-way analysis of variance failed to indicate a significant effect of Treatment [F(5,42)=0.645, p=.667NS [, but did indicate significant effects of 10-Minute Periods F(47,1974)=37.14915, p<.001, and the the interaction of Periods and × Treatment interaction F(235,1974)=1.582, p<.001. Locomotor depressant effects occurred within 20 minutes following injection and lasted 70 minutes. The dose-effect curve at the time of peak effects (10–40 min) showed a significant locomotor depressant effect at 5 mg/kg [F(5,42)=7.123, p<.001], as shown in figure 3.
Figure 2. Time course of locomotor activity.
Mean horizontal activity (Ambulation counts± SEM) as a function of time (10-min bins) and dose for each test compound (left to right). Each of the compounds produced time- and dose-dependent decreases in ambulation counts. 5-MeO-DALT also produced increases in ambulation counts at and 10 mg/kg. Data are from independent groups of 8 mice per dose, except 25B-NBOMe with 16 mice per dose. * indicates stimulant or depressant effects (p < 0.05) against vehicle control.
Figure 3. Dose effect of locomotor activity.
Average horizontal activity counts/10 min (± SEM) during the 30 min of peak effect as a function of dose for each of the test compounds. All of the compounds decreased ambulation. 5-MeO-DALT showed an inverted U-shaped dose response with the peak stimulant dose producing ambulation counts greater than vehicle control, and the highest dose producing ambulation counts less than vehicle control. n=8 for each dose, except 25B-NBOMe with 16 mice per dose. Veh indicates vehicle control. * indicates (p < 0.05) against vehicle control.
Treatment with 25B-NBOMe resulted in time- and dose-dependent depression of locomotor activity (fig 2). Depressant effects occurred within 10 minutes following injection and lasted 30–60 minutes. A two-way analysis of variance conducted on horizontal activity counts/10 min failed to indicate a significant effect of Treatment F(4,75)=0.42, p=.794NS, but did indicate significant effects of 10-Minute Periods F(47,3525)=31.05, p<.001 and the interaction of Periods and × Treatment interaction F(188,3525)=1.66, p<.001. The dose-effect curve at the time of peak effects (0–30 min) showed a significant locomotor depressant effect for 2.5, 5, and 10 mg/kg [F(4,75)=11.35, p<.001], as shown in figure 3.
Treatment with 25C-NBOMe resulted in time- and dose-dependent depression of locomotor activity (fig 2). Depressant effects occurred within 10 minutes following injection and lasted 30–60 minutes. A two-way analysis of variance conducted on horizontal activity counts/10 min failed to indicate a significant effect of Treatment F(5,42)=1.71972, p=.151NS, but did indicate significant effects of 10-Minute Periods F(47,1974)=20.25, p<.001 and the interaction of Periods and × Treatment interaction F(235,1974)=1.822, p<.001. The dose-effect curve at the time of peak effects (0–30 min) showed a significant locomotor depressant effect for 0.5, 1, 2.5, and 5 mg/kg [F(5,42)=7.33734, p<.001].
25I-NBOMe produced time- and dose-dependent depression of locomotor activity. A two-way analysis of variance failed to indicate a significant effect of Treatment F(5,42)=1.52, p=.206NS, but did indicate significant effects of 10-Minute Periods F(47,1974)=19.07, p<.001, and the interaction of Periods and × Treatment interaction F(235,1974)=1.70, p=.<.001. Locomotor depressant effects occurred within 10 minutes following injection and lasted 30–90 minutes. The dose-effect curve at the time of peak effects (0–30 min) showed a significant locomotor depressant effect at 2.5, 5, 10, and 25 mg/kg [F(5,42)=65.17, p<.001].
Treatment with 5-MeO-DALT resulted in time-dependent stimulation of locomotor activity following 10 mg/kg. A two-way analysis of variance conducted on horizontal activity counts/10 min failed to indicate a significant effect of Treatment F(5,42)=2.15, p=.078NS, but did indicate a significant effects of 10-Min Periods F(47,1974)=40.41, p<.001, and the interaction of Periods and × Treatment interaction F(235,1974)=3.20, p<.001. Locomotor stimulant effects of 10 mg/kg 5-MeO-DALT occurred within 40 min following injection and lasted 180 min. Depressant effects of 25 mg/kg suppressed activity within 20 min and lasted 90 min. The dose-effect curve at the time of peak effects (40–70 minutes following injection) showed a locomotor stimulant effect at 10 mg/kg and a depressant effect following 25 mg/kg [F(5,42)=25.86, p<.001].
MDMA produced time- and dose-dependent stimulation of locomotor activity (fig 2). A two-way analysis of variance conducted on horizontal activity counts/10 min indicated a significant effects of Treatment F(5,42)=7.27928, p<.001, 10-Minute Periods F(47,1974)=42.98699, p<.001 and the interaction of Periods and × Treatment interaction F(235,1974)=4.28729, p<.001. The dose-effect curve at the time of peak effects (40–70 min) showed a significant locomotor depressant effect for 5, 10 and 25 mg/kg [F(5,42)=11.702, p<.001], as shown in figure 3. Locomotor stimulant effects of 5 to 25 mg/kg occurred within 40 min following administration and lasted 3 to 5 h. In contrast, 50 mg/kg depressed locomotor activity from 30 to 60 min, and stimulant effects of 50 mg/kg were observed from 2 to 6 h after administration.
Drug Discrimination
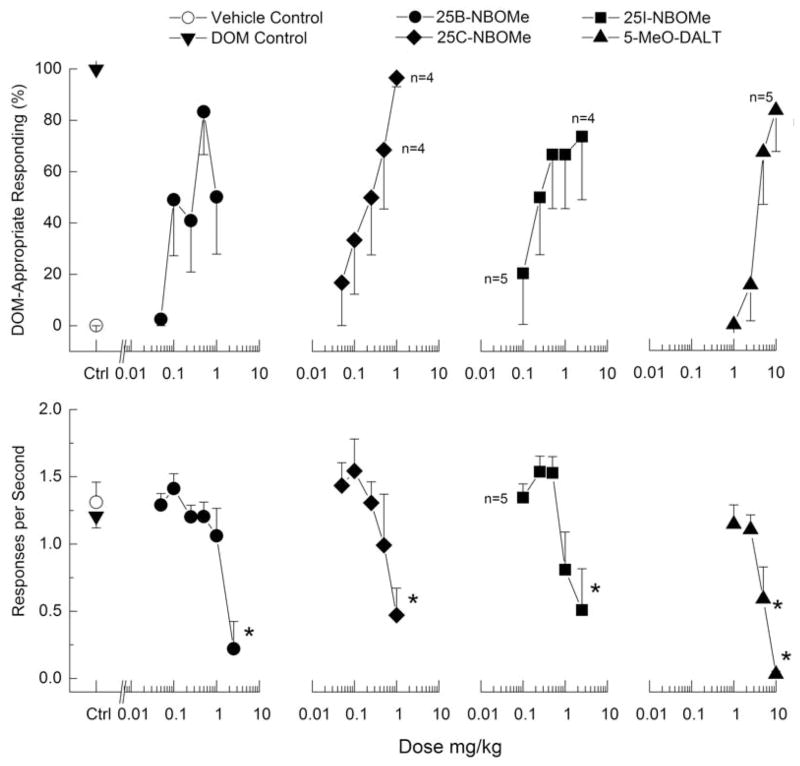
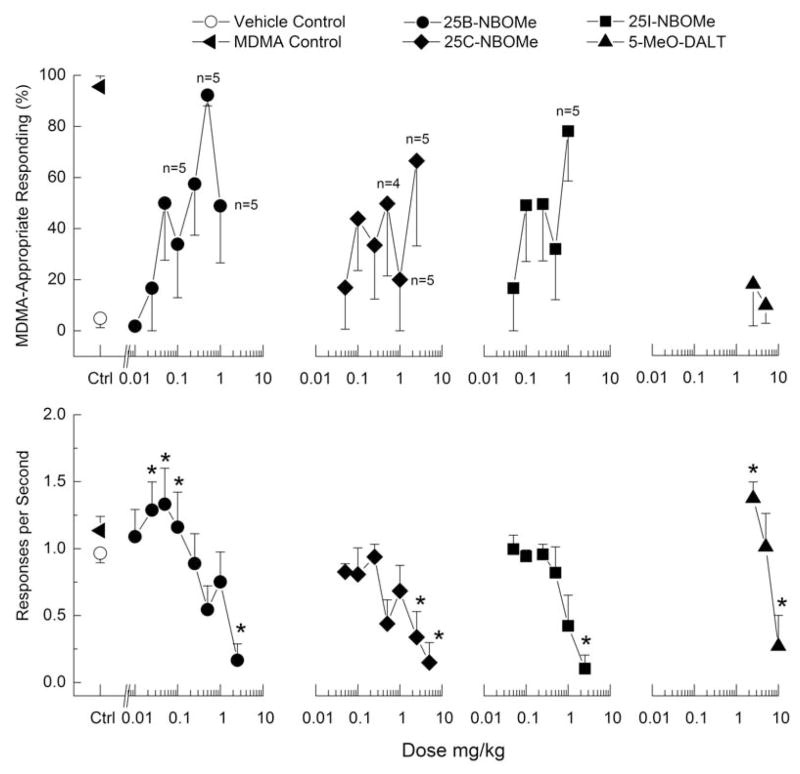
Discriminative stimulus effects of 25B-NBOMe in DOM-trained rats are shown in Figure 4, and for MDMA-trained rats in Figure 5. 25B-NBOMe produced dose-dependent increases in drug-appropriate responding in both DOM-(83%) and MDMA-trained rats (92%) at a dose of 0.5 mg/kg. However, 1 mg/kg produced substantially less drug-appropriate responding (49–50%). Higher doses suppressed responding. In DOM-trained rats, response rate was decreased to 18% of vehicle control following 2.5 mg/kg 25B-NBOMe [F(6,30)=10.25, p≤<0.001], whereas in MDMA-trained rats, response rate was increased in the dose range of 0.01 to 0.05 mg/kg 25B-NBOMe following 0.05 mg/kg, and decreased to 17% of vehicle control following 2.5 mg/kg of 25B-NBOMe [F(8,40)=7.79, p<.001].
Figure 4. Substitution for the discriminative stimulus effects of DOM.
Top Upper panels show percentage of total responses made on the drug-appropriate lever. Bottom Lower panels show rate of responding in responses per second (r/s). n=7 for 5-MeO-DALT; all others n=6 except where shown. Ctrl indicates vehicle and DOM control values. * indicates response rate different from vehicle control (p < 0.05).
Figure 5. Substitution for the discriminative stimulus effects of MDMA.
Top Upper panels show percentage of total responses made on the drug-appropriate lever. Bottom Lower panels show rate of responding in responses per second (r/s). n=6 except where shown. Ctrl indicates vehicle and MDMA control values. * indicates response rate different from vehicle control (p < 0.05).
25C-NBOMe produced dose-dependent increases in drug-appropriate responding to a maximum of 97% DOM-appropriate responding at 1 mg/kg, which also produced a decrease in response rate [F(5,25)=4.00, p=.<.0108]. In contrast, 25C-NBOMe produced substantial fluctuations in MDMA-appropriate responding with a peak effect of only 67%. 25C-NBOMe did produce dose-dependent decreases in rate of responding to 16% of vehicle control following 2.5 mg/kg [F(7,35)=3.72, p=.<.004005].
25C-NBOMe produced dose-dependent increases in drug-appropriate responding to a maximum of 97% DOM-appropriate responding at 1 mg/kg, which also produced a decrease in response rate [F(5,25)=4.00, p=.<.0108]. In contrast, 25C-NBOMe produced substantial fluctuations in MDMA-appropriate responding with a peak effect of only 67%. 25C-NBOMe did produce dose-dependent decreases in rate of responding to 16% of vehicle control following 2.5 mg/kg [F(7,35)=3.72, p=.<.0054].
25I-NBOMe dose-dependently increased DOM-appropriate responding to a peak effect of 74% at a dose of 2.5 mg/kg (fig 4), which also decreased rate of responding [F(5,20)=6.63, p=.<.001]. In MDMA-trained rats, 25I-NBOMe produced a peak of 78% drug-appropriate responding at 1 mg/kg (fig 5). A higher dose decreased rate of responding to 12% of vehicle control [F(6,30)=7.49, p<.001].
5-MeO-DALT produced a maximum of 84% DOM-appropriate responding (fig 4). Response rate was decreased following 5 and 10 mg/kg [F(4,24)=22.30, p<.001]. In contrast, 5-MeO-DALT failed to substitute in MDMA-trained rats, with a maximum of 18% drug-appropriate responding (fig 5). Response rate was increased to 122% of vehicle control following 2.5 mg/kg and decreased to 24% of vehicle control following 10 mg/kg of 5-MeO-DALT [F(3,15)=15.79, p<.001].
Discussion
In the present study, the hallucinogenic compounds 25B-NBOMe, 25C-NBOMe, 25I-NBOMe and 5-MeO-DALT were tested to determine the similarity of their behavioral effects to known abused and controlled substances. Hallucinogens produce little or no self-administration or CPP, so drug discrimination was the most appropriate model for testing abuse liability. Locomotor activity was tested to determine active doses and time courses. All of the compounds were locomotor depressants; however, 5-MeO-DALT also acted as a locomotor stimulant at lower doses.
All of the NBOMe compounds and DOM dose-dependently suppressed locomotor activity, similar to other phenethylamine hallucinogens such as 2C-C, 2C-D, 2C-E, 2C-I, 2C-T-2, and DOC (Eshleman et al., 2014). In contrast, 5-MeO-DALT increased locomotor activity at intermediate doses (5–10 mg/kg), and the highest dose tested (25 mg/kg) produced a sharp decrease in locomotor activity. MDMA also produced increased locomotor activity at intermediate doses (5–25 mg/kg), although they were much larger and longer lasting, and the highest dose (50 m/kg) also decreased activity levels to below vehicle control levels. A delayed stimulant effect was observed following 50 mg/kg of MDMA, which was not observed following 5-MeO-DALT. Hallucinogenic tryptamines like 5-MeO-dimethyltryptamine and 5-MeO-α methyltryptamine were locomotor depressants (Halberstadt et al., 2008; Gatch et al., 2011), but 5-MeO-diethyltryptamine produced locomotor stimulant effects at intermediate doses and locomotor depressant effects at the highest dose (Gatch et al., 2011), similar to 5-MeO-DALT in the present study. 5-MeO-isopropylmethyltryptamine also produced mixed stimulant/depressant effects on locomotor activity (Goodwin et., in submission2017).
25B-NBOMe, 25C-NBOMe and 5-MeO-DALT all produced full substitution (≥80% drug-appropriate responding) for DOM; however, 25B-NBOMe produced an inverted U-shaped dose-effect curve and 25I-NBOMe failed to produce 80% drug-appropriate responding in DOM-trained rats. In MDMA-trained rats, only 25B-NBOMe produced full substitution, but again produced an inverted U-shaped dose-effect curve. Both 25C-NBOMe and 25I-NBOMe produced substantial fluctuations in drug-appropriate responding with maximum effects of approaching 80% drug-appropriate responding, but higher doses tested produced substantial suppression of responding. Given that the effects of 25I-NBOMe were more linear than those of 25C-NBOMe, and because both of the rats that did respond at the 2.5 mg/kg dose selected the drug lever (data for points with less than 3 subjects were censored from the figures), it is possible that the failure of 25I-NBOMe to fully substitute was due to the substantial rate effects. In contrast, 5-MeO-DALT produced little few or no MDMA-like effects.
In previous testing, phenethylamines have produced robust hallucinogen-like discriminative stimulus effects. Several phenethylamines, including 2C-C, 2C-D, 2C-E, 2C-T-7, DOC, and LSD have fully substituted for the discriminative stimulus effects of DOM (Eshleman et al., 2014; Li et al., 2010). Most of these compounds also fully substituted for LSD or DMT, but 2C-C only produced a maximum of 75% drug-appropriate responding in DMT- or LSD-trained rats (Eshleman et al., 2014). In contrast, only three phenethylamines have fully substituted for MDMA (2C-C, 2C-E, 2C-T-7) and others produced significant, but submaximal levels of MDMA-appropriate responding (51 to 65%), including 2C-D, 2C-I, DOC and DOM (Eshleman et al., 2014; Murnane et al., 2009).
2C-C and 2C-I, which are chemical analogues of 25C-NBOMe and 25I-NBOMe, but missing the NBOMe moiety, produced discriminative stimulus effects similar to, but not completely overlapping their NBOMe-containing analogues. 2C-C and 25C-NBOMe both fully substituted for DOM, whereas 2C-C fully substituted for MDMA, but 25C-NBOMe produced a peak of only 67% MDMA-appropriate responding. This difference is not surprising as less fewer than half of the phenethylamines tested have fully substituted for MDMA and the rest produced partial substitution.
2C-I has not been tested in DOM-trained rats, but fully substituted for LSD and DMT (Eshleman et al., 2014). In contrast, 25I-NBOMe produced only 74% DOM-appropriate responding in the present study. Both compounds produced intermediate levels of MDMA-appropriate responding: 65% for 2C-I (Eshleman et al., 2014), and 78% for 25I-NBOMe. Taken together, these findings indicate that the NBOMe substitution may cause a slightly reduced efficacy to produce hallucinogenic-like discriminative stimulus effects without altering entactogen-like discriminative stimulus effects. One possible explanation for the reduced efficacy of the NBOMe compounds coupled with the increased reduction in rate is a result of the limited efficacy of these compounds at 5HT2A/2C receptors relative to the 2C series (Rickli et al., 2015). Another possibility is the finding that adding the N-benzylmethoxy group decreased activity at 5-HT1A receptors, and increased activity at adrenergic receptors and monoamine transporters (Rickli et al., 2015). 5-HT1A receptors are known to contribute at least in part to hallucinogenic-like effects and it is possible that increased activity at adrenergic receptors could result in an increased sensitivity to rate-decreasing effects.
Other tryptamine hallucinogens also fully substituted for the discriminative stimulus effects of DOM, including DMT, DPT, N,N-DiPT, 4-OH-DiPT, and 5-MeO-MIPT), although 5-MeO-DET produced little or no DOM-like discriminative stimulus effects (Gatch et al., 2009; 2011; Goodwin et al., in submission; Li et al., 2010). However, the tryptamine hallucinogens do not appear to be as efficacious at producing discriminative stimulus effects in LSD- or DMT-trained rats as the phenethylamine hallucinogens. Psilocybin and 4-OH-DiPT fully substituted for LSD (Goodwin et al., in submission; Jarbe 1980), whereas DMT, DPT, NN-DiPT, 5-MeO-DiPT, and 5-MeO-MIPT produced maximal effects of 56% to 75% drug-appropriate responding and 5-MeO-DET produced no LSD-like effects at all (Fantegrossi et al., 2006; 2008; Gatch et al., 2009, 2011). Similarly, only N,N-DiPT and 5-MeO-DET fully substituted for DMT, and 4-OH-DiPT and 5-MeO-MIPT produced only 60–67% drug appropriate responding (Gatch et al., 2011; Goodwin et al., in submission2017).
Similarly, of eight tryptamine hallucinogens tested in MDMA-trained rats, only one (DPT) produced full substitution (Fantegrossi et al., 2008), others produced 45 to 64% MDMA-appropriate responding (DMT, psilocybin, 4-OH-DiPT, 5-MeO-DET and 5-MeO-MIPT), and one (N,N-DiPT) failed to produce any MDMA-appropriate responding (Gatch et al., 2009; 2011; Goodwin et al., in submission; Winter et al., 2007).
Taken together, these findings suggest that many of the tryptamines tested may have less efficacy at producing hallucinogenic and/or entactogenic discriminative stimulus effects. Alternatively, their discriminative stimulus effects may be more DOM-like than LSD-, DMT-, or MDMA-like. This hypothesis is weakened by findings that DOM and LSD fully cross-substitute for each other’s discriminative stimulus effects; however, although LSD and DOM fully substitute in DMT-trained rats and DMT substitutes in DOM-trained rats, DMT only partially substitutes in LSD-trained rats (see review in Gatch, et al., 2009).
The reasons for the differential substitution patterns and changes in locomotor activity among the various phenethylamine and tryptamine hallucinogens have not been readily apparent from the in vitro literature. There have been no consistent differences in the potency and efficacy of these compounds at the various monoaminergic receptors or transporters (Cozzi & Daley, 2016; Gatch et al., 2011; Goodwin et al., in submission; Nagai et al., 2007) that might account for the differing patterns of substitution for various training drugs or for their effects on locomotor activity. Similarly, there are no differences in potency or selectivity for 5-HT2A/C receptors among the compounds tested in the present study (Juncosa et al., 2013; Rickli et al., 2015). Furthermore, the potencies for off-target receptors (D1, alpha-2, etc.) are roughly 1000-fold lower for each compound than for 5HT2A/C, so non-serotonergic effects seem unlikely (Juncosa et al., 2013; Rickli et al., 2015).
It may seem somewhat unusual that 5-MeO-DALT produced stimulant effects in the locomotor assay, but did not substitute at all for the discriminative stimulus effects of MDMA, given that MDMA at least partially substitutes for psychostimulants such as cocaine and methamphetamine (see review in Gatch et al., 2009). Testing took place at comparable times, as the stimulant effects were seen in the locomotor assay at 40–70 min, and the pretreatment for 5-MeO-DALT in the discrimination studies was 50 min, so the difference was not due to parametric factors. However, other hallucinogenic tryptamines generally produced no more than partial substitution for MDMA. For example, the two other synthetic tryptamines which also produced increases in locomotor activity (5-MeO-DET and 5-MeO-MIPT), produced only 59 to 64% MDMA-appropriate responding (Gatch et al., 2011; Goodwin et al., in submission2017). Further, it has been previously noted that the effects of hallucinogenic tryptamines on locomotor activity are not predictive of discriminative stimulus effects (Gatch et al., 2011). Unfortunately, there have been no consistent differences in the potency and efficacy of these tryptamine compounds at the various monoaminergic receptors or transporters (Cozzi & Daley, 2016; Gatch et al., 2011; Goodwin et al., in submission; Nagai et al., 2007) that might account for the differing patterns of substitution for various training drugs or for their effects on locomotor activity.
In summary, all of the compounds but 25I-NBOMe fully substituted for DOM. The failure of 25I-NBOMe to fully substitute was more likely due to its rate-decreasing effects preventing the testing of higher doses rather than to weak stimulus effects. 25B-NBOMe also fully substituted for MDMA and the other two NBOMe compounds produced greater than 50% drug-appropriate responding, but less than full substitution, whereas 5-MeO-DALT produced no MDMA-like effects. These findings suggest that the four test compounds are most likely to be used as recreational hallucinogens, rather than as Ecstasy substitutes, although 25B-NBOMe has the potential to be used as both a hallucinogen and an entactogen. However, the inverted U-shaped dose effect curve of 25B-NBOMe suggests that it may have a narrow range of effects, which may decrease its liking among recreational users. Data on patterns of human use (i.e., hallucinogen vs. entactogen) is are necessary to determine whether the animal model data is are useful for predicting the settings in which people use these compounds.
Acknowledgments
Support: Supported by NIH N01DA-13-8908.
References
- Andreasen MF, Telving R, Rosendal I, Eg MB, Hasselstrøm JB, Andersen LV. A fatal poisoning involving 25C-NBOMe. Forensic Sci Int. 2015;251:e1–8. doi: 10.1016/j.forsciint.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Arunotayanun W, Dalley JW, Huang XP, Setola V, Treble R, Iversen L, Roth BL, Gibbons S. An analysis of the synthetic tryptamines AMT and 5-MeO-DALT: Emerging ‘Novel Psychoactive Drugs’. Bioorg Med Chem Lett. 2013;23:3411–3415. doi: 10.1016/j.bmcl.2013.03.066. [DOI] [PubMed] [Google Scholar]
- Bosak A, LoVecchio F, Levine M. Recurrent seizures and serotonin syndrome following “2C-I” ingestion. J Med Toxicol. 2013;9:196–8. doi: 10.1007/s13181-013-0287-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonaro TM, Gatch MB. Neuropharmacology of N,N-dimethyltryptamine. Brain Res Bull. 2016;126:74–88. doi: 10.1016/j.brainresbull.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkery JM, Durkin E, Elliot S, Schifano F, Ghodse AH. The recreational tryptamine 5-MeO-DALT (N,N-diallyl-5-methoxytryptamine): A brief review. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:259–62. doi: 10.1016/j.pnpbp.2012.05.022. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration. Schedules of Controlled Substances: Extension of Temporary Placement of Three Synthetic Phenethylamines in Schedule I. Final order. Federal Register. 2015;80:70657–70659. [PubMed] [Google Scholar]
- Eshleman AJ, Forster MJ, Wolfrum KM, Johnson RA, Janowsky A, Gatch MB. Behavioral and neurochemical pharmacology of six psychoactive substituted phenethylamines: Mouse locomotion, rat drug discrimination and in vitro receptor and transporter binding and function. Psychopharmacology. 2014;231:875–888. doi: 10.1007/s00213-013-3303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Murnane KS, Reissig CJ. mThe behavioral pharmacology of hallucinogens. Biochem Pharmacol. 2008;75:17–33. doi: 10.1016/j.bcp.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Kiessel CL, et al. Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats. Pharmacol Biochem Behav. 2006;83:122–9. doi: 10.1016/j.pbb.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Forrester MB. NBOMe designer drug exposures reported to Texas poison centers. (2014) J Addict Dis. 33:196–201. doi: 10.1080/10550887.2014.950027. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ, Janowsky A, Eshleman AJ. Abuse liability profile of three substituted tryptamines. J Pharmacol Exp Ther. 2011;338:280–9. doi: 10.1124/jpet.111.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Rutledge M, Carbonaro T, Forster MJ. Comparison of the discriminative stimulus effects of dimethyltryptamine with different classes of psychoactive compounds in rats. Psychopharmacology. 2009;204:715–724. doi: 10.1007/s00213-009-1501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee P, Schep LJ, Jensen BP, Moore G, Barrington S. Case series: toxicity from 25B-NBOMe--a cluster of N-bomb cases. Clin Toxicol (Phila) 2016;54:141–6. doi: 10.3109/15563650.2015.1115056. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Gatch MB, Eshleman AJ, Forster MJ, Janowsky A, Ator N. The locomotor, discriminative, reinforcing, and neurochemical effects of two substituted tryptamines: 5-MeO-MIPT and 4-OH-DIPT. Drug Alcohol Depend (in submission 2017) [Google Scholar]
- Halberstadt AL, Buell MR, Masten VL, Risbrough VB, Geyer MA. Modification of the effects of 5-methoxy-N,N-dimethyltryptamine on exploratory behavior in rats by monoamine oxidase inhibitors. Psychopharmacology (Berl) 2008;201:55–66. doi: 10.1007/s00213-008-1247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton DB, Potter DM, Mead AN. A translational pharmacology approach to understanding the predictive value of abuse potential assessments. Behav Pharmacol. 2013;24:10–36. doi: 10.1097/FBP.0b013e3283644d2e. [DOI] [PubMed] [Google Scholar]
- Jarbe TUC. LSD-25 as a discriminative stimulus for response selection by pigeons. Pharmacol Biochem Behav. 1980;13:549–554. doi: 10.1016/0091-3057(80)90279-8. [DOI] [PubMed] [Google Scholar]
- Jovel A, Felthous A, Bhattacharyya A. Delirium due to intoxication from the novel synthetic tryptamine 5-MeO-DALT. J Forensic Sci. 2014;59:844–6. doi: 10.1111/1556-4029.12367. [DOI] [PubMed] [Google Scholar]
- Juncosa JI, Jr, Hansen M, Bonner LA, Cueva JP, Maglathlin R, McCorvy JD, Marona-Lewicka D, Lill MA, Nichols DE. Extensive rigid analogue design maps the binding conformation of potent N-benzylphenethylamine 5-HT2A serotonin receptor agonist ligands. ACS Chem Neurosci. 2013;16:96–109. doi: 10.1021/cn3000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalasho A, Nielsen SV. 5-MeO-DALT; a novel designer drug on the market causing acute delirium and rhabdomyolysis. Acta Anaesthesiol Scand. 2016;60:1332–6. doi: 10.1111/aas.12765. [DOI] [PubMed] [Google Scholar]
- Kyriakou C, Marinelli E, Frati P, Santurro A, Afxentiou M, Zaami S, Busardo FP. NBOMe: new potent hallucinogens--pharmacology, analytical methods, toxicities, fatalities: a review. Eur Rev Med Pharmacol Sci. 2015;19:3270–81. [PubMed] [Google Scholar]
- Lawn W, Barratt M, Williams M, Horne A, Winstock A. The NBOMe hallucinogenic drug series: Patterns of use, characteristics of users and self-reported effects in a large international sample. J Psychopharmacol. 2014;28:780–8. doi: 10.1177/0269881114523866. [DOI] [PubMed] [Google Scholar]
- Laskowski LK, Elbakoush F, Calvo J, Exantus-Bernard G, Fong J, Poklis JL, Poklis A, Nelson LS. Evolution of the NBOMes: 25C- and 25B- Sold as 25I-NBOMe. J Med Toxicol. 2015;11:237–41. doi: 10.1007/s13181-014-0445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Koek W, Rice KC, France CP. Differential effects of serotonin 5-HT1A receptor agonists on the discriminative stimulus effects of the 5-HT2A receptor agonist 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane in rats and rhesus monkeys. J Pharmacol Exp Ther. 2010;333:244–52. doi: 10.1124/jpet.109.163451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Murai N, Howell LL, Fantegrossi WE. Discriminative stimulus effects of psychostimulants and hallucinogens in S(+)-3,4-methylenedioxymethamphetamine (MDMA) and R(−)-MDMA trained mice. J Pharmacol Exp Ther. 2009;331:717–23. doi: 10.1124/jpet.109.156174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai F, Nonaka R, Satoh Hisashi Kamimura K. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur J Pharmacol. 2007;559:132–137. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. The National Academies Press; Washington, D.C: 2011. [Google Scholar]
- Nichols DE. Psychedelics. Pharmacol Rev. 2016;68:264–355. doi: 10.1124/pr.115.011478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka R, Nagai F, Ogata A, Satoh K. In Vitro Screening of Psychoactive Drugs by [35S]GTPγS Binding in Rat Brain Membranes. Biol Pharm Bull. 2007;30:2328–2333. doi: 10.1248/bpb.30.2328. [DOI] [PubMed] [Google Scholar]
- Odoardi S, Romolo FS, Strano-Rossi S. A snapshot on NPS in Italy: Distribution of drugs in seized materials analysed in an Italian forensic laboratory in the period 2013–2015. Forensic Sci Int. 2016;265:116–120. doi: 10.1016/j.forsciint.2016.01.037. [DOI] [PubMed] [Google Scholar]
- Rickli A, Luethi D, Reinisch J, Buchy D, Hoener MC, Liechti ME. Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs) Neuropharmacology. 2015;99:546–53. doi: 10.1016/j.neuropharm.2015.08.034. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Dekker MA, Valenti ES, Arbelo Cruz FA, Correa AM, Poklis JL, Poklis A. Toxicities associated with NBOMe ingestion-a novel class of potent hallucinogens: a review of the literature. Psychosomatics. 2015;56:129–39. doi: 10.1016/j.psym.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang MH, Ching CK, Tsui MS, Chu FK, Mak TW. Two cases of severe intoxication associated with analytically confirmed use of the novel psychoactive substances 25B-NBOMe and 25C-NBOMe. Clin Toxicol (Phila) 2014;52:561–5. doi: 10.3109/15563650.2014.909932. [DOI] [PubMed] [Google Scholar]
- Weaver MF, Hopper JA, Gunderson EW. Designer drugs 2015: assessment and management. Addict Sci Clin Pract. 2015;25(10):8. doi: 10.1186/s13722-015-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter JC. Hallucinogens as discriminative stimuli in animals: LSD, phenethylamines, and tryptamines. Psychopharmacology. 2009;203:251–263. doi: 10.1007/s00213-008-1356-8. [DOI] [PubMed] [Google Scholar]
- Winter JC, Rice KC, Amorosi DJ, Rabin RA. Psilocybin-induced stimulus control in the rat. Pharmacol Biochem Behav. 2007;87:472–480. doi: 10.1016/j.pbb.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DM, Sedefov R, Cunningham A, Dargan PI. Prevalence of use and acute toxicity associated with the use of NBOMe drugs. Clin Toxicol (Phila) 2015;53:85–92. doi: 10.3109/15563650.2015.1004179. [DOI] [PubMed] [Google Scholar]
- Young R. Drug Discrimination. In: Buccafuso Jerry J., editor. Methods of behavior analysis in neuroscience. 2. CRC Press, Taylor & Francis Group LLC; Boca Raton: 2009. ( http://www.ncbi.nlm.nih.gov/books/NBK5228/) [Google Scholar]