Abstract
Many species of animal live in groups, and the group represents the organizational level within which ecological and evolutionary processes occur. Understanding these processes, therefore, relies on knowledge of the mechanisms that permit or constrain group formation. We suggest that physiological capacities and differences in physiology between individuals modify fission–fusion dynamics. Differences between individuals in locomotor capacity and metabolism may lead to fission of groups and sorting of individuals into groups with similar physiological phenotypes. Environmental impacts such as hypoxia can influence maximum group sizes and structure in fish schools by altering access to oxygenated water. The nutritional environment determines group cohesion, and the increase in information collected by the group means that individuals should rely more on social information and form more cohesive groups in uncertain environments. Changing environmental contexts require rapid responses by individuals to maintain group coordination, which are mediated by neuroendocrine signalling systems such as nonapeptides and steroid hormones. Brain processing capacity may constrain social complexity by limiting information processing. Failure to evaluate socially relevant information correctly limits social interactions, which is seen, for example, in autism. Hence, functioning of a group relies to a large extent on the perception and appropriate processing of signals from conspecifics. Many if not all physiological systems are mechanistically linked, and therefore have synergistic effects on social behaviour. A challenge for the future lies in understanding these interactive effects, which will improve understanding of group dynamics, particularly in changing environments.
This article is part of the themed issue ‘Physiological determinants of social behaviour in animals’.
Keywords: fission–fusion, glucocorticoids, locomotion, metabolism, neuroendocrine systems, sociality
1. Introduction
Many species of animal occur in groups, and fundamental processes such as reproduction, foraging, dispersal and migration occur within this social context [1]. The dynamics of the group, therefore, influence ecology and evolution of populations and species [2–4]. According to Tinbergen, the multi-dimensional nature of biological processes is encapsulated by four dimensions: phylogeny, development, proximate mechanisms and adaptive consequences [5–8]. Selection will lead to the evolution of adaptive behaviour within the constraints of the physiology of individuals [6]. Physiology is important as a proximate mechanism because it enables movement of individuals and interactions between individuals. Social behaviour is contingent on inter-individual differences [9], and physiology may act as a constraint if physiological capacities differ markedly between individuals, thereby decreasing cohesion of the group [10,11] or even preventing grouping behaviour of subsets within populations.
Physiology can influence cohesion of groups at several levels. Cohesion requires that individuals possess similar capacities for movement, and incur similar energetic (metabolic) costs of locomotion [12,13]. Additionally, animals navigate within a group by processing information transmitted by their environment, including between individuals. Collective behaviour of individuals within groups thereby follows particular interaction rules or decisions that reflect how information gathered about the environment is translated into motor responses and movement [11,14,15]. Information processing relies on the neurophysiological characteristics of individuals [16,17]. Physiological characteristics of individuals can, therefore, influence cohesion of the group, and segregation (fission) and association (fusion) patterns [18]. Determining the link between the physiology of individuals and higher-level interactions is significant, because it may explain dynamics at all group sizes: from groups of few individuals to populations that must respond to environmental variability such as climate change. In this introductory review, we will summarize physiological processes that may interact with grouping behaviour, and these themes are developed in detail in the articles that make up this Theme Issue.
Groups of animals do not associate randomly, and the behaviour of an individual can determine patterns of association with other individuals [19,20]. The membership of a group can change with environmental context, so that animals leave the group (fission) or new animals join the group (fusion) [18]. The organizational properties of fission–fusion societies, such as the frequency of fission–fusion events, provide insights into how interactions among individuals drive social relationships, and ultimately influence dispersal and population processes [20–22]. Temporal patterns of social organization can form the basis of large-scale effects such as rates of gene flow and speciation [3,4,18,23].
Theoretically, animals segregate based on their activity patterns. According to the activity synchronization theory [10], a group can stay cohesive only if all members of the group are at the same place at the same time. For this to occur, individuals have to synchronize their activity, such as foraging and migration, for the benefits of staying in the group. The benefits of living in a group, such as resource acquisition, defence against predators, access to information and mating success, are substantial [1,24], so that the need for activity synchronization is a general feature of social groups [25]. There may be a trade-off, however, if increasing or decreasing activity or movement speed comes at a cost to the individual. If activity patterns of two individuals are different, one or both may experience a cost if they are obliged to synchronize their behaviour [10]. For example, Pacific bluefin tuna (Thunnus orientalis) adopt intermittent locomotion, where active swimming episodes are interspersed with gliding, resulting in overall lower energetic costs of locomotion compared with continuous active swimming [26]. The speed of gliding, however, depends on animal size, and smaller animals glide more slowly than larger animals. Hence, equal proportions of gliding during locomotion of different-sized animals would lead to fission of the group. Instead, smaller animals show greater proportions of active propulsion than larger animals, thereby incurring a greater energetic cost [26]. Physiological differences between individuals may represent a similar proximate mechanism to size differences in causing trade-offs, and groups may segregate on the basis of their physiological capacities, thereby reducing the benefits of group membership. Alternatively, physiologically diverse individuals may remain within the same group, but individuals will incur differential costs by operating at suboptimal physiological levels, for example, an energetic cost resulting from moving at a suboptimal speed [27–29].
Currently, it is thought that individuals segregate because differences in size, sex or age are accompanied by different activity patterns, which can make animals of different sex, for example, incompatible for group living [10]. Here, we suggest that inter-individual differences in physiological capacities can have similar consequences for fission and fusion dynamics of animals groups (figure 1), which is discussed in detail in this issue [30]. Differences in physiology may be the result of genetic differences in underlying traits, or because individuals respond differently to environmental conditions [4,9]. A good example presented in this issue is different species of equid (horses, asses and their relatives), which possess relatively subtle differences in physiology that translate into different social behaviours under different environmental conditions [31].
Figure 1.
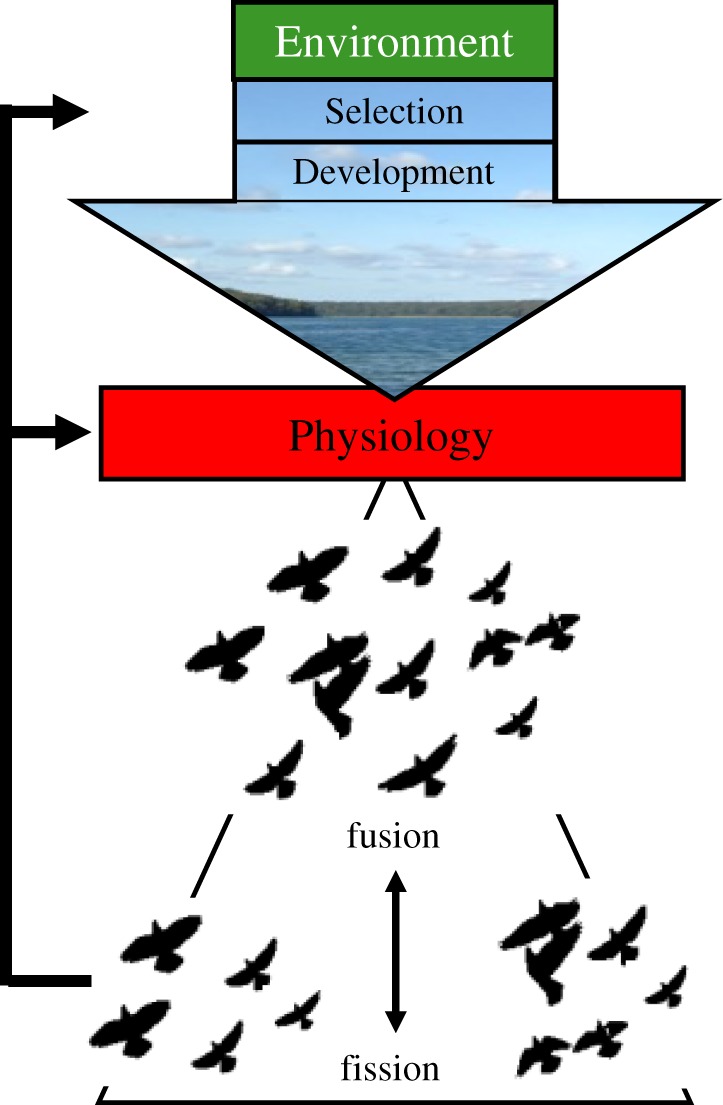
Schematic of the thesis presented in the text. Physiology acts as a filter between environmental selection pressures and developmental processes to influence fission and fusion dynamics in animals. Group composition and fission–fusion events may feed back to physiology if individuals within groups or populations are sorted according to their physiological characteristics, thereby altering gene flow and selection.
(a). Locomotor performance and metabolism
Similar environments may lead to significant differences in behavioural responses, which can be explained by phenotypic differences between individuals [6]. Differences between individuals in locomotor performance and metabolism represent proximate mechanisms that can alter individual behaviour [32–35]. The ensuing inter-individual behavioural differences may be important to understand social behaviour, because physiologically mismatched phenotypes could reduce group cohesion and limit the selective advantages gained from social behaviour, particularly across changing environments. On the other hand, physiological differences between individuals can also increase resilience of the species to environmental change, and may therefore be advantageous. Hence, fission–fusion events at a subspecies level may represent a cost resulting from the benefits of physiological diversity. However, fission and fusion events may be beneficial if the resulting groups comprise individuals with similar physiologies, so that group cohesion and the benefits of sociality are increased.
Maximal locomotor performance, voluntary movement speed, the energetic cost of locomotion and fatigue in individuals can influence their capacity to adopt the speed of the group and the resulting cost incurred. If the group moves at a speed that exceeds an individual's maximal speed, that individual will not be able to join the group. However, while animals move at maximal speeds during escape behaviour or while hunting prey, under most circumstances, movement occurs at a voluntary speed that is well below maximum [35–38].
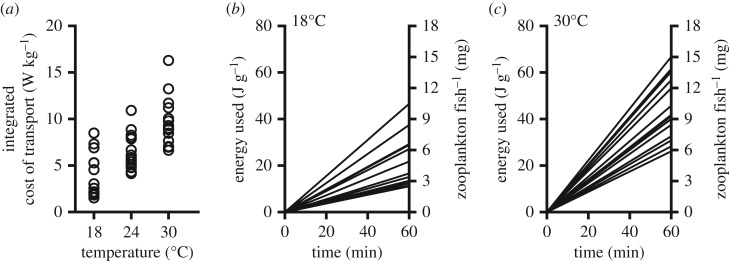
For example, the voluntary speed at which collared lizards (Crotophytus collaris) foraged was considerably lower than their maximum speeds [39]. In theory, voluntary speed reflects minimization of the cost of transport—that is, the energy used for a given distance travelled [12,27,28,40]. The energetic costs of staying within a group may differ between individuals if there are pronounced differences in the cost of transport between individuals within the group. For example, we re-analysed our data [41] on cost of transport of zebrafish (Danio rerio) to estimate differences in energetic costs incurred by individual fish when moving together at a slow speed of 0.05 m s−1 (approx. 1–2 body lengths s−1) at different temperatures (figure 2). There were substantial differences between individuals in energetic cost of transport and the cost incurred after moving for 60 min (J g−1 wet mass) increased with increasing temperature (n = 15 fish per temperature; permutational analysis, p < 0.001, see Seebacher et al. [41] for details of analysis). We converted these energetic data to the amount of zooplankton, the dominant food of zebrafish [42], that individuals of average mass (0.5 g) would need to eat to recover the energy spent on movement (assuming 2.3 J mg−1 zooplankton [43]). The fish with the highest cost of transport would need to consume an extra 1.5–2% of its own body mass in zooplankton compared with the fish with the lowest cost of transport after 1 h of moving at the same speed (figure 2). These data serve to demonstrate that physiological differences between individuals can translate into differences in ecological and behavioural costs, which can change with environmental variability and may lead to fission.
Figure 2.
Differential energetic costs of transport between individual zebrafish (D. rerio). We used previously published data (from Seebacher et al. [41]) to show that the cost of transport (integrated across speeds from 0 to 0.35 m s−1 in W kg−1 within each individual; see Seebacher et al. [41] for methods) varies considerably among individuals within groups and across different temperatures (n = 15 fish at each of 18, 24 and 30°C); (a) we used these data to determine individual variation in the energetic cost (J g−1 wet mass; b, c, left y-axis) of moving at a slow speed (0.05 ms−1 or approx). 1–2 body lengths s−1) for 1 h (each line represents one animal). The energetic cost of moving for 60 min increased significantly with increasing temperature from 18°C (b) to 30°C (c; data for 24°C not shown). We expressed energetic cost as the quantity of zooplankton an average-sized (0.5 g) fish would need to eat to recover the energy used for movement (right y-axis). After 1 h of moving together, the fish with the highest cost of transport would need to consume up to 10 mg more zooplankton (approx. 2% of body mass at 30°C) than the fish with the lowest cost of transport. Ultimately, these differences in energetic cost of movement may lead to fission of schools.
Inter-individual differences in intrinsic muscle functions that underlie locomotion can also translate into differences in fatigue resistance [44,45], and may force individuals with low fatigue resistance to separate from the group. Muscle contraction and relaxation are powered by the hydrolysation of ATP [46], so that locomotion can be closely associated with energy metabolism. Individual variation in metabolism, including metabolic demand (cost of transport) and maximal aerobic capacity, can thereby determine fission and fusion of groups, and lead to passive or active sorting of social groups according to physiological traits. As a result, individuals with similar phenotypes may cluster within groups. Alternatively, groups may segregate and fuse into new groups composed of individuals with similar physiological phenotypes, and these relationships are discussed further in this issue [30]. As a consequence, gene flow within populations can be non-random if reproductive events increase with proximity between individuals, and ultimately populations may diverge genetically.
Inter-individual differences in locomotor capacity within groups can also be caused by external influences. For example, new work presented in this issue shows that predation by sailfish (Istiophorus platypterus) on schools of sardines (Sardinella aurita) caused injury to individual sardines as a result of contact with the sailfish bill [47]. Injured sardines had reduced tailbeat frequencies and lower relative swimming speeds. As a consequence, the spatial composition of the school changed and injured fish were predominantly located at the rear and periphery of the shoal. The position of individuals within groups can determine the energetic cost of travelling within the group [48–50], and fish with lower maximum metabolic rates and metabolic scope can be found near the rear of the school [51].
One extreme example of animal movement is bird migration, where minimizing energetic cost of flight is essential for arrival at the destination [52,53], so that it is closely related to fitness [54]. Many bird species migrate seasonally because of temporarily changing resource availability and climate. Examples of long migrations include the bar-tailed godwit (Limosa lapponica) and sooty shearwaters (Puffinus griseus), which cross the Pacific to fly between New Zealand and Alaska [55,56]. Bird migration often involves changes in social organization in the lead up to, or during migration. For example, species that are territorial in their breeding grounds form large groups during migration. Interestingly, the strategy birds adopt to reduce energetic cost during migration also dictates social organization. Two major strategies are the use of thermal uplifts for soaring–gliding migration, or the formation of V-shaped echelons [54]. Similar to the intermittent locomotion in tuna, the advantage of soaring–gliding travel is that flapping flight is minimized by exploiting thermal uplifts [49,57]. As a result, large aggregations of birds often form, particularly in areas with favourable soaring conditions. A disadvantage of soaring–gliding migration is that the migratory path is dictated by the occurrence of thermal uplifts, so that the migratory route and the duration of migration are less predictable [58]. Migration in V-shaped formation, on the other hand, can lead to reductions in the cost of flapping flight as a result of the aerodynamic advantages of flying in the wake of preceding birds [49,57], which is similar to the hydrodynamic advantage of swimming in fish schools [50,59]. In contrast with soaring–gliding migration, V-shaped echelons lead to the formation of small and stable groups. New data presented here show that in bald ibis (Geronticus eremita), V-shaped echelons are maintained and the energetic advantage is distributed across the group because individuals reciprocally swap between the energetically disadvantageous leading position and energetically advantageous trailing positions [54]. Energetic considerations can therefore determine interactions between individuals within the group and their spatial position. The energetic advantages of flying in a group are not universal, and may be restricted to relatively large species. Pigeons (Columba livia), for example, do not gain an energetic advantage from flying in a cluster with conspecifics [60]. Energetic costs may even increase by flying in a cluster, because flap frequency increases when flying close behind another bird as a result of aerodynamic interactions [60]. Interestingly, new data in this issue demonstrate that personality of individuals can affect movement patterns and energy expenditure. Neophilic pigeons (C. livia) chose more efficient flight routes, and presumably incurred lower energetic costs, than neophobic conspecifics [61]. However, neophilia did not lead to dominance within the social hierarchy [61], so that the energy saving during flight may be counterbalanced by decreased access to resources.
(b). Environmental impacts: hypoxia and nutrition
In addition to predation and other biotic interactions, the physical environment, such as temperature and aquatic oxygen availability, can influence fission and fusion events. Temperature has a direct effect on muscle function and locomotion [62], but it also increases organisms' oxygen use and reduces oxygen solubility in water. Hence, the combination of global warming and increased eutrophication resulting from human activity is likely to increase the extent of hypoxia in aquatic ecosystems [63–65]. Fish have a number of physiological responses to hypoxia [50,66]. However, long-term hypoxia will decrease scope for activity by decreasing maximal metabolic rates, thereby limiting locomotor performance. Fish may become less active in response to hypoxia to conserve energy, or become more active to seek out more favourable environments, as reviewed in this issue [50]. Differences in activity are related to differences in metabolism between individuals [67], and these inter-individual differences could lead to fission of a school if responses to hypoxia override schooling behaviour. The distance between individuals within schools also increases in response to hypoxia, so that each fish experiences greater oxygen supply. However, there are trade-offs associated with these school-level responses to hypoxia because hydrodynamic advantages that reduce energetic costs as a result of swimming in the wake of the preceding fish [68] decrease in less compact schools, and less compact schools are also less effective in performing anti-predator manoeuvres [50].
Schools of fish themselves create hypoxic environments, and fish at the rear of the school are more likely to experience hypoxia as a result of the oxygen consumption of fish at the front. Hence, spatial position within schools determines exposure to hypoxia and this can lead to fission of the school if fish towards the rear segregate to avoid hypoxic conditions created by the front of the school [50]. There exists, therefore, an upper size limit for schools of fish beyond which the benefits of group membership decrease.
Similar to hypoxia responses in fish schools, there are optimal group sizes that are dictated by foraging and nutrient availability. Animals often trade-off group cohesion with individual nutritional requirements [13,69–71]. The spatial distribution of food determines the effort necessary to achieve nutritional balance, which can thereby also impact group cohesion. A new spatially explicit model of foraging behaviour in socially interacting individuals presented in this issue [70] shows that there is a trade-off between individual nutrition and the collective need of the group, and that the predictability of the nutritional environment can affect synchronization and cohesion of the group. The increase in information collected by the group means that individuals should rely more on social information in uncertain environments, where food sources are rare or patchy, to enhance food finding. On the other hand, individual sampling rather than reliance on social information would be advantageous in predictable environments, where food is abundant and scattered evenly, because competition is reduced.
Individual foraging reduces competition for food in social animals, and this may be particularly important in animals with high energy demands such as pregnant primates, reviewed in this issue [71]. For example, group size in female primates, such as baboons (Papio cynocephalus), varies to modulate competition as a function of energy demand and food availability. If food availability is low, animals can increase time spent on foraging and travel distance to increase the number of food patches encountered, but both strategies increase energy demands [13]. Alternatively, individuals can increase the distance to other group members, which decreases competition but reduces the benefits of the group. Competition also decreases in smaller groups. Hence, balancing the trade-offs associated with energy needs and the quality of the nutritional landscape on the one hand, and competition on the other can determine group cohesion and group size. Competition between groups in addition to competition between individuals within groups complicates these dynamics, because smaller groups are less competitive than larger groups [71]. In primates, group size equates to group dominance and larger groups occupy better-quality habitats. For example, a low-ranking female in a large group experiences similar nutritional and energy gains to a high ranking female in a small group. These dynamics determine whether or not individuals switch between groups, and energy needs of individuals and the nutritional quality of the habitat thereby cause fission and fusion of groups [71,72].
(c). Neuroendocrine signalling and information processing
The relationships discussed above can be viewed in terms of allostasis [73,74]. The allostatic state is regulated by hormones and neural signalling systems in response to changing requirements such as those resulting from reproduction or seasonality. An allostatic load occurs in response to an unpredictable event, and if the energetic and nutritional demands necessary to restore homeostasis exceed supply an individual experiences allostatic overload. Group size and composition would determine allostatic state and load in the context of competition and energetic needs, so that endocrine signalling and neural responses become central to the interactions between group-living animals and, ultimately, their fitness [71,75–77]. Animals within groups often interact non-randomly with each other, and their relations with each other may be described by social networks [1]. The social network structure captures direct and indirect interactions between individuals, which may be related to animal personality [78], and which have pronounced consequences for ecology and evolution [3]. For example, social network structure in non-breeding long-tailed tits (Aegithalos caudatus) was determined by kinship, and social network structure during the non-breeding season influenced reproductive patterns during the breeding season [79]. Sociality depends on information processing by individuals, which is essential for the coordination of the group as a whole and to form more or less complex relationships between members of the group. Group size may be limited by the information processing capacity of individuals, which is linked to cognitive capacity and brain function [80]. Within groups, individuals must compromise between their own interests and those of the group, which relies on processing information related to other group members and the environment. The complexity of information, and the necessary processing capacity, increase as group size increases, so that the evolution of brain capacity may be intrinsically linked to the evolution of social complexity [81,82]. Ultimately, this problem of coordination may be solved and total group size may be increased by forming several smaller groups with stronger bonds within the one larger group. In this case, and as discussed in this issue, each individual would maintain complex relationships with a small number of conspecifics, and a larger number of relatively loose relationships that require less information processing [82]. Note, however, that the relationship between brain capacity and group size did not hold for birds, and it may be that this relationship does not exist for non-competitive groups that form for cooperative advantages [83].
There is an intrinsic link between information processing and the endocrine system in mediating responses of animals to environmental stimuli. This link is particularly pronounced in stressful situations. The brain coordinates stress responses, from perception of stressful states and events to mediating responses via the neural and endocrine systems [75]. Often, ‘stress’ is chronic and represents the accumulation of daily challenging or stressful situations, which together represent an allostatic load. In response, the brain communicates with physiological systems, thereby eliciting a compensatory response to promote allostasis [75]. The acute response to a stressor is the autonomically mediated ‘fight or flight’ response, which is necessary to survive immediate threats. However, many environmental changes represent challenges that require more chronic phenotypic responses to adjust behaviour to novel contexts, particularly within social systems [84,85]. Interestingly, exposure to stress early in life can influence the ability of individuals to interact in social networks later, and these responses are mediated by the way the hypothalamic–pituitary–adrenal (HPA) axis is regulated within the brain [85]. The HPA axis affects the action of nonapeptides, which are a group of hormones that are closely linked to social behaviour [77,85].
Social behaviours, such as aggression in an attempt to achieve higher rank, are characteristic of many animals living in social groups. Sociality and aggression are complementary behavioural dimensions and may be regulated by the same neural systems [86]. Additionally, social groups are rarely stable because the context of the group changes frequently. For example, and as briefly outlined above, the trade-offs inherent in fulfilling nutritional demands in different environments affect cohesion and group size. Other environmental changes such as seasonality and intruders, as well as life-history characteristics such as pregnancy and age structure, impact on group dynamics and optimal group sizes. Hence, individuals need to respond rapidly to adjust their phenotype to the changing contexts of the group. Such ‘activational plasticity’ [84] is important both for individuals and the group to function in variable environments. A review in this issue summarizes how endocrine and neuroendocrine mechanisms mediate these rapid phenotypic responses to environmental changes [77]. Nonapeptides and steroid hormones are particularly important mechanisms that enable rapid changes in behavioural phenotype in response to environmental stimuli [77,87]. Nonapeptides are produced by the limbic system and include vasotocin, vasopressin and oxytocin (isotocin in fish). Nonapeptides modulate sociality, aggression and stress responses, and may mediate a fast and focused response to an external stimulus, as well as a slower, more diffuse response that maintains a more stable phenotype [77]. Steroid hormones such as glucocorticoids are generally slower-acting and mediate longer-lasting responses than nonapeptides. Glucocorticoids may have genomic and non-genomic actions that adjust social and aggressive behaviour, and may also act as central regulators of nonapeptides. In stressful situations, glucocorticoids can reduce expression of affiliative behaviours leading to a weakening of social bonds [88], which can adjust social behaviour permanently [77] and possibly trigger fission events.
Individual responses within groups are often reactionary in response to external stimuli or to the behaviour of other individuals. However, effective group function and cohesion also require predictions of the internal state of other members. Social behaviour is closely tied to brain structures that are important in processing emotions [89]. Perception of stimuli in the sensory cortices is processed and associated with an emotional response in the amygdala among other brain regions. This information is then used by the higher cortical regions to construct an internal model of the social environment, including the relationship between ‘self’ and ‘other’ individuals [89]. The experience of interacting with others stimulates the internal reward system, thereby encouraging further interactions and sociality as reviewed in this issue [90]. Failure to evaluate socially relevant information correctly limits social interactions, which is seen, for example, in autism [90]. Hence, group cohesion relies on the perception and appropriate processing of signals from conspecifics.
2. Conclusion
The size and composition of groups are of fundamental importance in determining movement of animals across their environment and for spreading genetic and cultural information. Hence, sociality is at the core of the ecology and evolution of many species [4]. Fission of groups may decrease the benefits associated with group membership, such as protection from predators. On the other hand, smaller group sizes may also decrease competition and therefore increase access to resources. We suggested here that physiological capacities and physiological differences between individuals modify social behaviour and group dynamics. Hence, physiology acts as a filter between evolutionary and developmental processes and their adaptive consequences for social groups (figure 1). The environment impacts physiology directly, and it can thereby have secondary effects on fission–fusion dynamics and group sizes. Fission–fusion events may also sort individuals to form groups according to particular physiological traits, and the resulting altered patterns of gene flow may feed back to the evolution of physiological traits. Many, if not all physiological systems are mechanistically linked, and therefore have synergistic effects on social behaviour. A challenge for the future lies in understanding these interactive effects, which will improve understanding of group dynamics, particularly in changing environments. Physiology links behaviour to the abiotic, nutritional and cognitive environments. Every aspect of these environments is changing at a dramatic rate, driven by factors such as epidemiology of human disease, range shifts of food species, habitat modification and global warming. Considering the importance of physiological interactions in group dynamics will, therefore, increase understanding of sociality itself, as well as the impact of environmental change on social species.
Authors' contributions
F.S. and J.K. conceived the ideas, F.S. wrote the paper and J.K. edited the paper.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by Australian Research Council Discovery grant DP160102260 to F.S., and by funding from the Leibnitz Institute for Freshwater Ecology to J.K.
References
- 1.Krause J, Lusseau D, James R. 2009. Animal social networks: an introduction. Behav. Ecol. Sociobiol. 63, 967–973. ( 10.1007/s00265-009-0747-0) [DOI] [Google Scholar]
- 2.NESCent Working Group on Integrative Models of Vertebrate Sociality: Evolution, Mechanisms, and Emergent Properties et al. 2014. An evolutionary framework for studying mechanisms of social behavior. Trends Ecol. Evol. 29, 581–589. ( 10.1016/j.tree.2014.07.008) [DOI] [PubMed] [Google Scholar]
- 3.Kurvers RHJM, Krause J, Croft DP, Wilson ADM, Wolf M. 2014. The evolutionary and ecological consequences of animal social networks: emerging issues. Trends Ecol. Evol. 29, 326–335. ( 10.1016/j.tree.2014.04.002) [DOI] [PubMed] [Google Scholar]
- 4.Farine DR, Montiglio P-O, Spiegel O. 2015. From individuals to groups and back: the evolutionary implications of group phenotypic composition. Trends Ecol. Evol. 30, 609–621. ( 10.1016/j.tree.2015.07.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tinbergen N. 1963. On aims and methods of ethology. Zeitschr. Tierpsych. 20, 410–433. ( 10.1111/j.1439-0310.1963.tb01161.x) [DOI] [Google Scholar]
- 6.Barrett L, Blumstein DT, Clutton-Brock TH, Kappeler PM. 2013. Taking note of Tinbergen, or: the promise of a biology of behaviour. Phil. Trans. R. Soc. B 368, 20120352 ( 10.1098/rstb.2012.0352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nesse RM. 2013. Tinbergen's four questions, organized: a response to Bateson and Laland. Trends Ecol. Evol. 28, 681–683. ( 10.1016/j.tree.2013.08.001) [DOI] [PubMed] [Google Scholar]
- 8.Bateson P, Laland KN. 2013. Tinbergen's four questions: an appreciation and an update. Trends Ecol. Evol. 28, 712–718. ( 10.1016/j.tree.2013.09.013) [DOI] [PubMed] [Google Scholar]
- 9.von Rueden C, Gavrilets S, Glowacki L. 2015. Solving the puzzle of collective action through inter-individual differences. Phil. Trans. R. Soc. B 370, 20150002 ( 10.1098/rstb.2015.0002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conradt L, Roper TJ. 2000. Synchrony and social cohesion: a fission–fusion model. Proc. R. Soc. Lond. B 267, 2213–2218. ( 10.1098/rspb.2000.1271) [DOI] [Google Scholar]
- 11.Herbert-Read JE, Perna A, Mann RP, Schaerf TM, Sumpter DJ, Ward AJ. 2011. Inferring the rules of interaction of shoaling fish. Proc. Natl Acad. Sci. USA 108, 18 726–18 731. ( 10.1073/pnas.1109355108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claireaux G, Couturier C, Groison A-L. 2006. Effect of temperature on maximum swimming speed and cost of transport in juvenile European sea bass (Dicentrarchus labrax). J. Exp. Biol. 209, 3420–3428. ( 10.1242/jeb.02346) [DOI] [PubMed] [Google Scholar]
- 13.Markham AC, Gesquiere LR, Alberts SC, Altmann J. 2015. Optimal group size in a highly social mammal. Proc. Natl Acad. Sci. USA 112, 14 882–14 887.. ( 10.1073/pnas.1517794112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbert-Read JE. 2016. Understanding how animal groups achieve coordinated movement. J. Exp. Biol. 219, 2971–2983. ( 10.1242/jeb.129411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collignon B, Séguret A, Halloy J. 2016. A stochastic vision-based model inspired by zebrafish collective behaviour in heterogeneous environments. R. Soc. open sci. 3, 150473 ( 10.1098/rsos.150473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfeiffer UJ, Timmermans B, Vogeley K, Frith CD, Schilbach L. 2013. Towards a neuroscience of social interaction. Front. Hum. Neurosci. 7, 22 ( 10.3389/fnhum.2013.00022/full) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfeiffer UJ, Schilbach L, Timmermans B, Kuzmanovic B, Georgescu AL, Bente G, Vogeley K. 2014. Why we interact: on the functional role of the striatum in the subjective experience of social interaction. Neuroimage 101, 124–137. ( 10.1016/j.neuroimage.2014.06.061) [DOI] [PubMed] [Google Scholar]
- 18.Couzin ID, Laidre ME. 2009. Fission–fusion populations. Curr. Biol. 19, R633–R635. ( 10.1016/j.cub.2009.05.034) [DOI] [PubMed] [Google Scholar]
- 19.Ma Q, Johansson A, Sumpter DJT. 2011. A first principles derivation of animal group size distributions. J. Theor. Biol. 283, 35–43. ( 10.1016/j.jtbi.2011.04.031) [DOI] [PubMed] [Google Scholar]
- 20.Conradt L, Roper TJ. 2003. Group decision-making in animals. Nature 421, 155–158. ( 10.1029/2001GC000200) [DOI] [PubMed] [Google Scholar]
- 21.Heinz SK, Wissel C, Conradt L, Frank K. 2007. Integrating individual movement behaviour into dispersal functions. J. Theor. Biol. 245, 601–609. ( 10.1016/j.jtbi.2006.12.009) [DOI] [PubMed] [Google Scholar]
- 22.Kelley JL, Morrell LJ, Inskip C, Krause J, Croft DP. 2011. Predation risk shapes social networks in fission–fusion populations. PLoS ONE 6, e24280 ( 10.1371/journal.pone.0024280.s002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palla G, Barabási A-L, Vicsek T. 2007. Quantifying social group evolution. Nature 446, 664–667. ( 10.1038/nature05670) [DOI] [PubMed] [Google Scholar]
- 24.Krause J, Ruxton GD. 2002. Living in groups. Oxford, UK: Oxford University Press. [Google Scholar]
- 25.Conradt L, Roper TJ. 2010. Deciding group movements: where and when to go. Behav. Proc. 84, 675–677. ( 10.1016/j.beproc.2010.03.005) [DOI] [PubMed] [Google Scholar]
- 26.Noda T, Fujioka K, Fukuda H, Mitamura H, Ichikawa K, Arai N. 2016. The influence of body size on the intermittent locomotion of a pelagic schooling fish. Proc. R. Soc. B 283, 20153019 ( 10.1098/rspb.2015.3019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weihs D. 1973. Optimal fish cruising speed. Nature 245, 48–50. ( 10.1038/245048a0) [DOI] [Google Scholar]
- 28.Pettersson LB, Hedenstrom A. 2000. Energetics, cost reduction and functional consequences of fish morphology. Proc. R. Soc. B 267, 759–764. ( 10.1098/rspb.2000.1068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palstra A, van Ginneken V, van den Thillart G. 2008. Cost of transport and optimal swimming speed in farmed and wild European silver eels (Anguilla anguilla). Comp. Biochem. Physiol. A 151, 37–44. ( 10.1016/j.cbpa.2008.05.011) [DOI] [PubMed] [Google Scholar]
- 30.Killen SS, Marras S, Nadler L, Domenici P. 2017. The role of physiological traits in assortment among and within fish shoals. Phil. Trans. R. Soc. B 372, 20160233 ( 10.1098/rstb.2016.0233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gersick AS, Rubenstein DI. 2017. Physiology modulates social flexibility and collective behaviour in equids and other large ungulates. Phil. Trans. R. Soc. B 372, 20160241 ( 10.1098/rstb.2016.0241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Killen SS, Mitchell MD, Rummer JL, Chivers DP, Ferrari MCO, Meekan MG, McCormick MI. 2014. Aerobic scope predicts dominance during early life in a tropical damselfish. Funct. Ecol. 28, 1367–1376. ( 10.1111/1365-2435.12296) [DOI] [Google Scholar]
- 33.Killen SS, Marras S, Metcalfe NB, McKenzie DJ, Domenici P. 2013. Environmental stressors alter relationships between physiology and behaviour. Trends Ecol. Evol. 28, 651–658. ( 10.1016/j.tree.2013.05.005) [DOI] [PubMed] [Google Scholar]
- 34.Sinclair ELE, de Souza CRN, Ward AJW, Seebacher F. 2014. Exercise changes behaviour. Funct. Ecol. 28, 652–659. ( 10.1111/1365-2435.12198) [DOI] [Google Scholar]
- 35.Seebacher F, Little AG, James RS. 2015. Skeletal muscle contractile function predicts activity and behaviour in zebrafish. J. Exp. Biol. 218, 3878–3884. ( 10.1242/jeb.129049) [DOI] [PubMed] [Google Scholar]
- 36.Irschick DJ, Herrel A, Vanhooydonck B, Huyghe K, Van Damme R. 2005. Locomotor compensation creates a mismatch between laboratory and field estimates of escape speed in lizards: a cautionary tale for performance-to-fitness studies. Evolution 59, 1579–1587. ( 10.1111/j.0014-3820.2005.tb01807.x) [DOI] [PubMed] [Google Scholar]
- 37.Husak JF, Fox SF. 2006. Field use of maximal sprint speed by collared lizards (Crotaphytus collaris): compensation and sexual selection. Evolution 60, 1888–1895. ( 10.1111/j.0014-3820.2006.tb00532.x) [DOI] [PubMed] [Google Scholar]
- 38.Wilson RS, Husak JF, Halsey LG, Clemente CJ. 2015. Predicting the movement speeds of animals in natural environments. Integr. Comp. Biol. 55, 1125–1141. ( 10.1093/icb/icv106) [DOI] [PubMed] [Google Scholar]
- 39.Husak JF. 2006. Does survival depend on how fast you can run or how fast you do run? Funct. Ecol. 20, 1080–1086. ( 10.1111/j.1365-2435.2006.01195.x) [DOI] [Google Scholar]
- 40.Palstra AP, Tudorache C, Rovira M, Brittijn SA, Burgerhout E, van den Thillart GEEJM, Spaink HP, Planas JV. 2010. Establishing zebrafish as a novel exercise model: swimming economy, swimming-enhanced growth and muscle growth marker gene expression. PLoS ONE 5, e14483 ( 10.1371/journal.pone.0014483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seebacher F, Borg J, Schlotfeldt K, Yan Z. 2016. Energetic cost determines voluntary movement speed only in familiar environments. J. Exp. Biol. 219, 1625–1631. ( 10.1242/jeb.136689) [DOI] [PubMed] [Google Scholar]
- 42.Spence R, Gerlach G, Lawrence C, Smith C. 2007. The behaviour and ecology of the zebrafish, Danio rerio. Biol. Rev. 83, 13–34. ( 10.1111/j.1469-185X.2007.00030.x) [DOI] [PubMed] [Google Scholar]
- 43.James DA, et al. 2012. A generalized model for estimating the energy density of invertebrates. Freshw. Sci. 31, 69–77. ( 10.1899/11-057.1) [DOI] [Google Scholar]
- 44.Wilson RS, James RS. 2004. Constraints on muscular performance: trade-offs between power output and fatigue resistance. Proc. R. Soc. Lond. B 271, S222–S225. ( 10.1098/rsbl.2003.0143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allen DG, Lamb GD, Westerblad H. 2008. Skeletal muscle fatigue: cellular mechanisms. Physiol. Rev. 88, 287–332. ( 10.1152/physrev.00015.2007) [DOI] [PubMed] [Google Scholar]
- 46.Woledge RC, Barclay CJ, Curtin NA. 2009. Temperature change as a probe of muscle crossbridge kinetics: a review and discussion. Proc. R. Soc. B 276, 2685–2695. ( 10.1098/rspb.2009.0177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krause J, et al. 2017. Injury-mediated decrease in locomotor performance increases predation risk in schooling fish. Phil. Trans. R. Soc. B 372, 20160232 ( 10.1098/rstb.2016.0232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liao JC. 2007. A review of fish swimming mechanics and behaviour in altered flows. Phil. Trans. R. Soc. B 362, 1973–1993. ( 10.1098/rstb.2007.2082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Portugal SJ, Hubel TY, Fritz J, Heese S, Trobe D, Voelkl B, Hailes S, Wilson AM, Usherwood JR. 2014. Upwash exploitation and downwash avoidance by flap phasing in ibis formation flight. Nature 505, 399–402. ( 10.1038/nature12939) [DOI] [PubMed] [Google Scholar]
- 50.Domenici P, Steffensen JF, Marras S. 2017. The effect of hypoxia on fish schooling. Phil. Trans. R. Soc. B 372, 20160236 ( 10.1098/rstb.2016.0236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Killen SS, Marras S, Steffensen JF, McKenzie DJ. 2012. Aerobic capacity influences the spatial position of individuals within fish schools. Proc. R. Soc. B 279, 357–364. ( 10.1098/rspb.2011.1006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hedenstrom A, Alerstam T. 1997. Optimum fuel loads in migratory birds: distinguishing between time and energy minimization. J. Theor. Biol. 189, 227–234. ( 10.1006/jtbi.1997.0505) [DOI] [PubMed] [Google Scholar]
- 53.Alerstam T, Hedenström A, Åkesson S. 2003. Long-distance migration: evolution and determinants. Oikos 103, 247–260. ( 10.1034/j.1600-0706.2003.12559.x) [DOI] [Google Scholar]
- 54.Voelkl B, Fritz J. 2017. Relation between travel strategy and social organization of migrating birds with special consideration of formation flight in the northern bald ibis. Phil. Trans. R. Soc. B 372, 20160235 ( 10.1098/rstb.2016.0235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shaffer SA, et al. 2006. Migratory shearwaters integrate oceanic resources across the Pacific Ocean in an endless summer. Proc. Natl Acad. Sci. USA 103, 12 799–12 802. ( 10.1073/pnas.0603715103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gill RE, et al. 2009. Extreme endurance flights by landbirds crossing the Pacific Ocean: ecological corridor rather than barrier? Proc. R. Soc. B 276, 447–457. ( 10.1098/rspb.2008.1142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weimerskirch H, Martin J, Clerquin Y, Alexandre P, Jiraskova S. 2001. Energy saving in flight formation. Nature 413, 697–698. ( 10.1038/35099670) [DOI] [PubMed] [Google Scholar]
- 58.Shamoun-Baranes J, Bouten W, van Loon EE, Meijer C, Camphuysen CJ. 2016. Flap or soar? How a flight generalist responds to its aerial environment. Phil. Trans. R. Soc. B 371, 20150395 ( 10.1098/rstb.2015.0395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marras S, Killen SS, Lindström J, McKenzie DJ, Steffensen JF, Domenici P. 2015. Fish swimming in schools save energy regardless of their spatial position. Behav. Ecol. Sociobiol. 69, 219–226. ( 10.1007/s00265-014-1834-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Usherwood JR, Stavrou M, Lowe JC, Roskilly K, Wilson AM. 2011. Flying in a flock comes at a cost in pigeons. Nature 474, 494–497. ( 10.1038/nature10164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Portugal SJ, Ricketts RL, Chappell J, White CR, Shepard EL, Biro D. 2017. Boldness traits, not dominance, predict exploratory flight range and homing behaviour in homing pigeons. Phil. Trans. R. Soc. B 372, 20160234 ( 10.1098/rstb.2016.0234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.James RS. 2013. A review of the thermal sensitivity of the mechanics of vertebrate skeletal muscle. J. Comp. Physiol. B 183, 723–733. ( 10.1007/s00360-013-0748-1) [DOI] [PubMed] [Google Scholar]
- 63.Portner HO, Knust R. 2007. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315, 95–97. ( 10.1126/science.1135013) [DOI] [PubMed] [Google Scholar]
- 64.Diaz RJ, Rosenberg R. 2008. Spreading dead zones and consequences for marine ecosystems. Science 321, 926–929. ( 10.1126/science.1156401) [DOI] [PubMed] [Google Scholar]
- 65.McBryan TL, Anttila K, Healy TM, Schulte PM. 2013. Responses to temperature and hypoxia as interacting stressors in fish: implications for adaptation to environmental change. Integr. Comp. Biol. 53, 648–659. ( 10.1093/icb/ict066) [DOI] [PubMed] [Google Scholar]
- 66.Zhu C-D, Wang Z-H, Yan B. 2013. Strategies for hypoxia adaptation in fish species: a review. J. Comp. Physiol. B 183, 1005–1013. ( 10.1007/s00360-013-0762-3) [DOI] [PubMed] [Google Scholar]
- 67.Killen SS, Marras S, Ryan MR, Domenici P, McKenzie DJ. 2012. A relationship between metabolic rate and risk-taking behaviour is revealed during hypoxia in juvenile European sea bass. Funct. Ecol. 26, 134–143. ( 10.1111/j.1365-2435.2011.01920.x) [DOI] [Google Scholar]
- 68.Liao JC, Beal DN, Lauder GV, Triantafyllou MS. 2003. Fish exploiting vortices decrease muscle activity. Science 302, 1566–1569. ( 10.1126/science.1088295) [DOI] [PubMed] [Google Scholar]
- 69.Wrangham RW. 1980. An ecological model of female-bonded primate groups. Behaviour 75, 262–300. ( 10.1163/156853980X00447) [DOI] [Google Scholar]
- 70.Lihoreau M, Charleston MA, Senior AM, Clissold FJ, Raubenheimer D, Simpson SJ, Buhl J. 2017. Collective foraging in spatially complex nutritional environments. Phil. Trans. R. Soc. B 372, 20160238 ( 10.1098/rstb.2016.0238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Markham AC, Gesquiere LR. 2017. Costs and benefits of group living in primates: an energetic perspective. Phil. Trans. R. Soc. B 372, 20160239 ( 10.1098/rstb.2016.0239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown JL. 1982. Optimal group size in territorial animals. J. Theor. Biol. 95, 793–810. ( 10.1016/0022-5193(82)90354-X) [DOI] [Google Scholar]
- 73.McEwen BS, Wingfield JC. 2003. The concept of allostasis in biology and biomedicine. Horm. Behav. 43, 2–15. ( 10.1016/S0018-506X(02)00024-7) [DOI] [PubMed] [Google Scholar]
- 74.Wingfield JC. 2005. The concept of allostasis: coping with a capricious environment. J. Mammal. 86, 248–254. ( 10.1644/BHE-004.1) [DOI] [Google Scholar]
- 75.McEwen BS. 2007. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 87, 873–904. ( 10.1152/physrev.00041.2006) [DOI] [PubMed] [Google Scholar]
- 76.Angelier F, Parenteau C, Ruault S, Angelier N. 2016. Endocrine consequences of an acute stress under different thermal conditions: a study of corticosterone, prolactin, and thyroid hormones in the pigeon (Columbia livia). Comp. Biochem. Physiol. A 196, 38–45. ( 10.1016/j.cbpa.2016.02.010) [DOI] [PubMed] [Google Scholar]
- 77.Kelly AM, Vitousek MN. 2017. Dynamic modulation of sociality and aggression: an examination of plasticity within endocrine and neuroendocrine systems. Phil. Trans. R. Soc. B 372, 20160243 ( 10.1098/rstb.2016.0243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wolf M, Krause J. 2014. Why personality differences matter for social functioning and social structure. Trends Ecol. Evol. 29, 306–308. ( 10.1016/j.tree.2014.03.008) [DOI] [PubMed] [Google Scholar]
- 79.Napper CJ, Hatchwell BJ. 2016. Social dynamics in nonbreeding flocks of a cooperatively breeding bird: causes and consequences of kin associations. Anim. Behav. 122, 23–35. ( 10.1016/j.anbehav.2016.09.008) [DOI] [Google Scholar]
- 80.David-Barrett T, Dunbar RIM. 2013. Processing power limits social group size: computational evidence for the cognitive costs of sociality. Proc. R. Soc. B 280, 20131151 ( 10.1098/rspb.2013.1151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Freeberg TM, Dunbar RIM, Ord TJ. 2012. Social complexity as a proximate and ultimate factor in communicative complexity. Phil. Trans. R. Soc. B 367, 1785–1801. ( 10.1098/rstb.2011.0213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dunbar RIM, Shultz S. 2017. Why are there so many explanations for primate brain evolution? Phil. Trans. R. Soc. B 372, 20160244 ( 10.1098/rstb.2016.0244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fedorova N, Evans CL, Byrne RW. 2017. Living in stable social groups is associated with reduced brain size in woodpeckers (Picidae). Biol. Lett. 13, 20170008 ( 10.1098/rsbl.2017.0008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Snell-Rood EC. 2013. An overview of the evolutionary causes and consequences of behavioural plasticity. Anim. Behav. 85, 1004–1011. ( 10.1016/j.anbehav.2012.12.031) [DOI] [Google Scholar]
- 85.Spencer KA. 2017. Developmental stress and social phenotypes: integrating neuroendocrine, behavioural and evolutionary perspectives. Phil. Trans. R. Soc. B 372, 20160242 ( 10.1098/rstb.2016.0242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Albers HE. 2012. The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Horm. Behav. 61, 283–292. ( 10.1016/j.yhbeh.2011.10.007) [DOI] [PubMed] [Google Scholar]
- 87.Godwin J, Thompson R. 2012. Nonapeptides and social behavior in fishes. Horm. Behav. 61, 230–238. ( 10.1016/j.yhbeh.2011.12.016) [DOI] [PubMed] [Google Scholar]
- 88.Barik J, et al. 2013. Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science 339, 332–335. ( 10.1126/science.1226767) [DOI] [PubMed] [Google Scholar]
- 89.Adolphs R. 2003. Cognitive neuroscience of human social behaviour. Nat. Rev. Neurosci. 4, 165–178. ( 10.1038/nrn1056) [DOI] [PubMed] [Google Scholar]
- 90.Vogeley K. 2017. Two social brains: neural mechanisms of intersubjectivity. Phil. Trans. R. Soc. B 372, 20160245 ( 10.1098/rstb.2016.0245) [DOI] [PMC free article] [PubMed] [Google Scholar]