Abstract
The costs and benefits of group living often depend on the spatial position of individuals within groups and the ability of individuals to occupy preferred positions. For example, models of predation events for moving prey groups predict higher mortality risk for individuals at the periphery and front of groups. We investigated these predictions in sardine (Sardinella aurita) schools under attack from group hunting sailfish (Istiophorus platypterus) in the open ocean. Sailfish approached sardine schools about equally often from the front and rear, but prior to attack there was a chasing period in which sardines attempted to swim away from the predator. Consequently, all sailfish attacks were directed at the rear and peripheral positions of the school, resulting in higher predation risk for individuals at these positions. During attacks, sailfish slash at sardines with their bill causing prey injury including scale removal and tissue damage. Sardines injured in previous attacks were more often found in the rear half of the school than in the front half. Moreover, injured fish had lower tail-beat frequencies and lagged behind uninjured fish. Injuries inflicted by sailfish bills may, therefore, hinder prey swimming speed and drive spatial sorting in prey schools through passive self-assortment. We found only partial support for the theoretical predictions from current predator–prey models, highlighting the importance of incorporating more realistic predator–prey dynamics into these models.
This article is part of the themed issue ‘Physiological determinants of social behaviour in animals’.
Keywords: group-living, fish schools, predation, spatial positions, locomotion
1. Introduction
The costs and benefits of group living are not equally distributed across spatial positions in groups [1]. Individuals at the front of moving groups often have higher foraging rates [2,3], but front positions can also incur higher energy expenditures (fish schools [4–6]; bird flocks [7]). Predation risk has also received considerable attention in this context [8,9]. A number of theoretical models make predictions for which spatial positions are particularly at risk [10–13]. Hamilton's model for stationary groups is based on the assumption that a predator strikes at the closest prey and predicts higher risk at the periphery than the centre of groups. For moving prey groups, Bumann et al.'s [11] simulations suggest an additional risk gradient from the front to the rear of the group (in addition to the periphery-to-centre gradient) because front individuals have a higher encounter probability with predators. If both predator and prey groups are moving, the front-to-rear gradient is predicted to be weaker, albeit still present, since predators are more likely to attack the rear of the groups compared to a situation with stationary predators.
There is strong empirical support for Hamilton's original prediction of higher predation risk at peripheral compared with central positions within stationary groups [1,14]. However, which prey are targeted in moving groups has received considerably less attention and the available evidence is ambiguous: Krause [15] reported that alarmed minnows (Phoxinus phoxinus) moved to central positions within shoals and concluded that these positions are perceived to be safer. By contrast, when sea-bass (Centropristis striata) attack silverside schools (Medidia menidia), central positions received higher per capita attacks than peripheral ones [16]. Another laboratory study by Krause et al. [17] reported that rock bass (Ambloplites rupestris) attacking from a refuge, targeted creek chub (Semotilus atromaculatus) more often at the front than the rear of shoals.
If certain positions within groups are associated with increased risk, then individuals currently occupying those positions should attempt to move out of them. However, there may be two reasons why individuals may not move out of these positions. First, individuals may be unaware they are occupying these riskier positions, owing to only local knowledge of their whereabouts with respect to other near neighbours, but not the entire group. This seems unlikely, however, for individuals in small groups, or individuals that occupy positions on the edge of groups. Second, individuals may be unable to remove themselves from these positions owing to energetic or other movement constraints. For example, individuals with lower aerobic capacity may not be able to maintain pace with individuals that have higher capacities without the aero- or hydrodynamic advantages that rear positions afford [18]. Constraints on individuals' ability to occupy particular positions, therefore, may also affect the spatial sorting of individuals within groups.
Almost all studies testing the effect of spatial positioning and predation risk in fish schools have been conducted in laboratory settings. Although these studies have rendered important insights, they also suffer from several constraints. In captivity, prey usually have little to no opportunity to (i) avoid approaching predators (due to space limitations), likewise they cannot (ii) form large groups (a common anti-predator behaviour). Studying predation in the wild, however, is challenging since predation events are rarely observed and often occur under conditions in which quantifying the entire capture sequence including predator approach and attack behaviour and prey responses and spatial positioning is difficult [1,19].
Here we report on attacks of group hunting sailfish (Istiophorus platypterus) on schools of sardines (Sardinella aurita) in the open ocean, with the aim of quantifying predator behaviour, prey behaviour and spatial positions of prey within groups. We used high-speed video to record sailfish–sardine interactions and image analysis of sardine schools to quantify injury levels across schools. Different groups of sailfish were observed attacking prey schools for prolonged periods (hours), whereby both predator and prey schools were highly mobile. During these attacks, sailfish alternate attacks on the prey school, usually not attacking simultaneously [20]. We used this predator–prey system to test the predictions by Bumann et al. [11] that predation risk is higher for fish (i) at the periphery than in the centre of the school and (ii) at the front compared to the rear. The risk at different spatial positions within the school was then linked to tail-beat frequency (TBF) and positioning behaviour of sardines within the school. Prey schools contained individuals with different injury levels from previous attacks [20] and we investigated the consequences of these injuries on spatial positioning and swimming characteristics. We hypothesized that during chases by the sailfish, sardine schools get sorted by swimming speed with injured individuals being slower and more often found at the rear of groups.
2. Material and methods
Research was conducted offshore of Cancun in the Gulf of Mexico. To find sardine schools, we boated to locations where frigate birds (Fregata magnificiens) were observed feeding at the water surface. Under snorkel, we used Casio EX-FH100 cameras and GoPro HD HERO cameras to film the sardine schools under sailfish attack.
Over a 3-year period (2011–2013), we filmed 11 schools of sardines (estimated school size range: approx. 30–1000 individuals) that were under attack by sailfish (estimated group size range: 1–14). To obtain information about the approach and attack stage of the predator–prey interaction, we recorded whether the sailfish was at the front or the rear of the sardine school (relative to the swimming direction of the school) (i) during an approach of a sailfish and (ii) immediately prior to an attack with the bill. Sailfish are known to take turns when making their approaches and attacks [20] and an approach started when a given sailfish was replaced by another.
(a). School positions
We divided sardine schools into a front and rear half based on our video footage such that similar numbers of fish were present in each half, thereby making this approach independent from the school's absolute size. Peripheral positions were defined as those that were within one sardine body length (19 cm) of the outline of the school as defined by the vertices of a convex polygon [1], a commonly used criterion for defining peripheral and central positions [13]. Additionally, we recorded how far the sailfish's bill entered into the prey school during attacks.
In addition to front versus rear and centre versus periphery we recorded in how many cases the sailfish attacked and captured a sardine that was in the last row of fish in the school. In those cases, we counted the number of fish in the last row (whenever possible, N = 16) and divided this number by the overall school size to obtain an estimate of the proportion of fish that were a potential target during an attack.
Sailfish typically capture their prey using a slash (a forceful, rapid lateral movement of the bill often through a large section of the school) or a tap (a targeted short-range bill movement) [21]. There is usually a time delay between the attack with the bill that destabilizes the sardine and the moment when the sardine is captured (clasped in the sailfish's mouth). Therefore, we recorded the two-dimensional spatial position of sardines (i.e. front or rear half, and periphery or centre) both during (i) first contact with the bill (i.e. hit) and (ii) capture. To test for significant differences in the likelihood of being hit or captured between the different school positions, we used binomial tests. Furthermore, we also quantified how often sardines were turned upside down or sideways as a result of bill contact, and how far they were displaced (in body lengths) from their original school position because of this destabilization.
In all our analyses, we only included attacks which resulted in capture thereby restricting our analyses to sailfish from eight different sailfish groups across 3 years, with a total of 36 capture events. Since not all behavioural/positioning data could be recorded for each capture event (due to, for example, obstruction by other school members), we report final sample sizes for each category in the caption of figure 3.
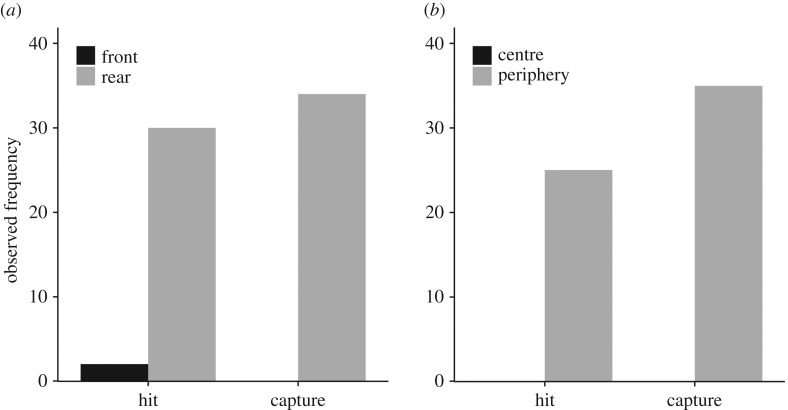
Figure 3.
(a,b) Observed frequency of hits and captures for different school positions; front versus rear half (hits: 30 versus 2, captures: 34 versus 0) of the school and periphery versus centre (hits: 25 versus 0, captures: 35 versus 0).
(b). Injury analysis of sardines
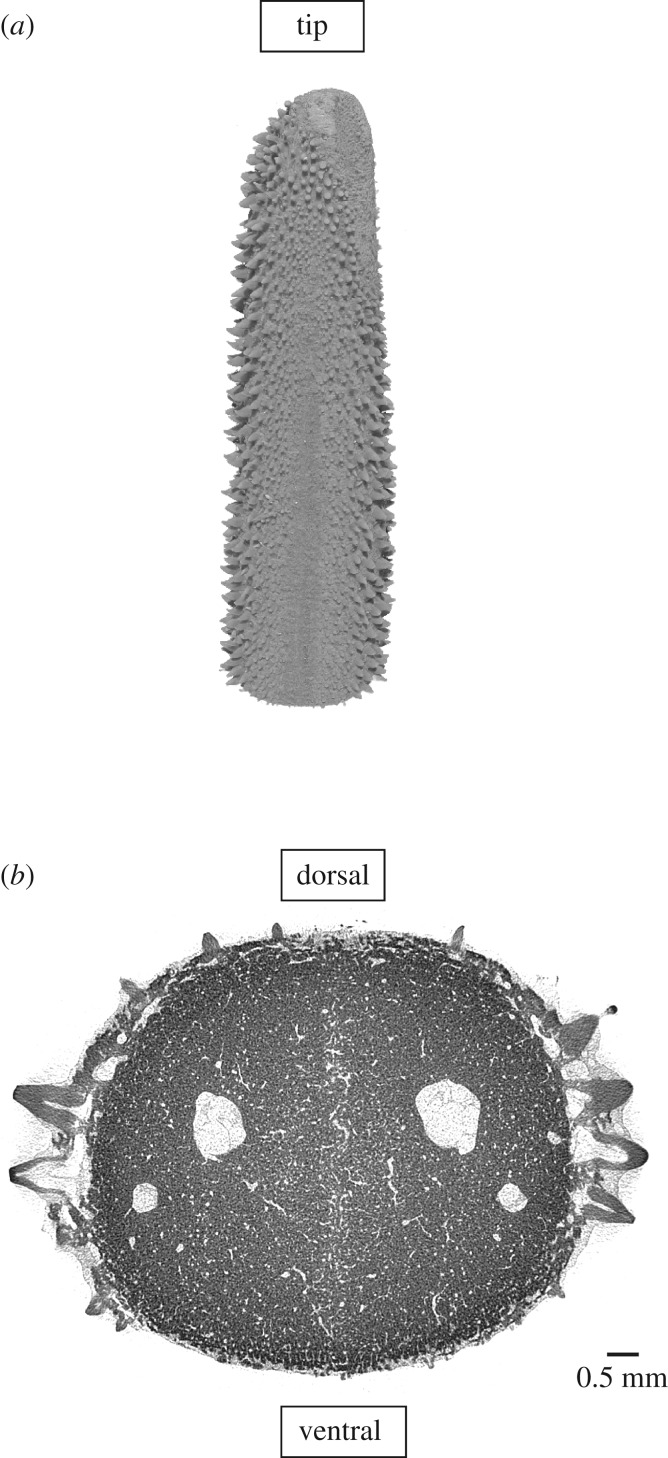
Individual sailfish alternate (i.e. one sailfish at a time) attacking the sardine school and each attack yields a maximum of one captured sardine [21]. Only 24% of attacks are successful, but multiple sardines are injured in 95% of attacks, resulting in an accumulation of injuries in the sardine school over time [20]. Injuries to the sardines result from abrasion by the micro-teeth of the sailfish rostrum (figure 1). These white/pinkish injury marks have a distinctive colour, different from uninjured parts of the fishes' bodies or surrounding water (figure 2a). This allowed us to use image analysis to determine where injured fish were within the school.
Figure 1.
Micro-CT image of a 5 cm bill-tip showing (a) the dorsal surface with curved micro-teeth and (b) a cross-section with micro-teeth mainly on the sides of the bill. Sailfish bills were scanned on a Skyscan 1172 (Brucker-CT, Kontich, Belgium) with an effective pixel size of 7 µm, an energy of 65 KeV, 360° scans using an Al filter 500 µm thick. Reconstruction was performed using manufacturer software (NRecon v. 1.6.9.4) and data were visualized (CTvox) and further processed using Fiji [22].
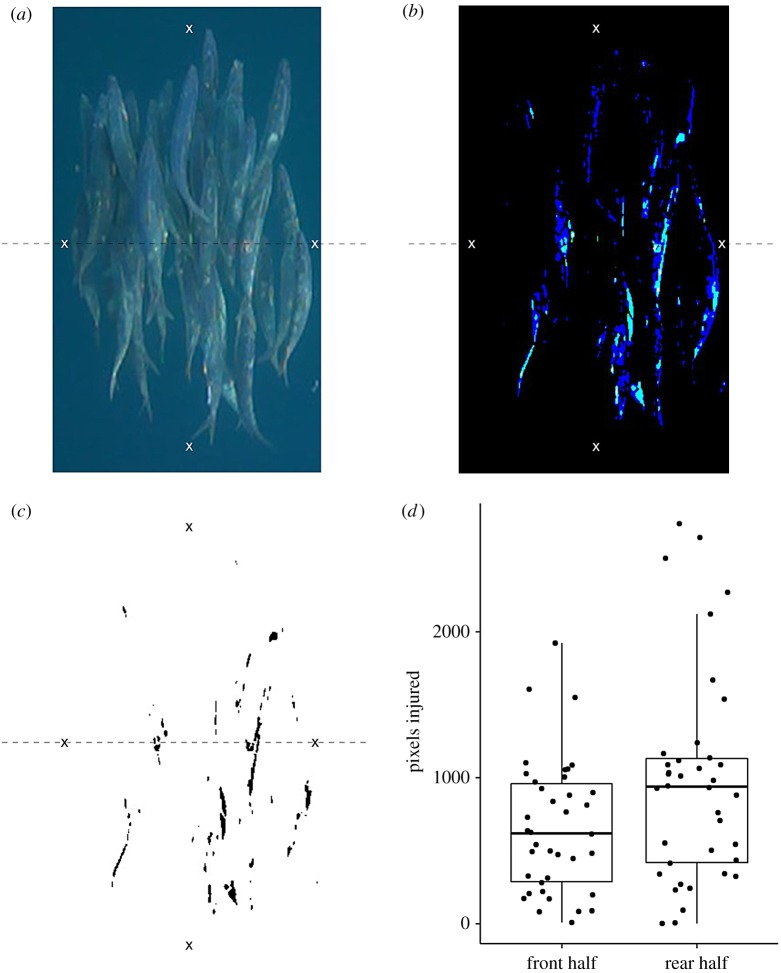
Figure 2.
(a) Still-frame image taken from one of the videos showing the white injury marks on the sardines caused by damage from sailfish bills. Markings show the front, rear and two sides of the school. Dashed line shows the division between front and rear half of the school. The original image has its contrast and brightness adjusted; (b) before binary thresholding; (c) which reveals the positions of the injuries on the fish in the front and rear halves. (d) The number of pixels indicating injury in the front and rear halves of schools. Data points are jittered to reveal overlapping data points. Boxplots show median, interquartile ranges and whiskers show highest and lowest values (excluding outliers).
We selected 39 still frames where there was no obvious glare or reflections off the sardines' bodies and where light contrast across the schools was minimal. If multiple frames were selected from the same video, at least 1 min worth of footage had passed between successive frames of interest. We marked a polygon around the edge of the school, and then cleared all pixels from outside the marked polygon (setting each of their RGB channels to 255). We then adjusted the brightness and contrast of each image so that only the injury marks on the fish became pronounced. By adjusting the brightness and contrast for each image appropriately (figure 2b), we could perform a binary threshold on the images to reveal the pixels in each image where injuries had occurred (figure 2c). Binary thresholding converted the images into black and white images by setting each pixel's RGB channels either to 255 (white) or 0 (black). We rotated and cropped each image so that all schools faced the same direction relative to one another (see the electronic supplementary material). We imported these images into MATLAB (2012b) where each image was represented by a matrix with cells equal to zero (black pixels) representing injured parts of the school, and cells equal to 255 (white pixels) representing uninjured parts of the school. We then counted the number of cells depicting injuries, separately for the front and rear halves of each school.
To test for differences in the injury level between front and rear half of the school, we used a generalized linear mixed model (GLMM) with ‘number of cells injured’ as response variable and school half (front/rear) as fixed effect. As random terms we included observation event (39 observation events, corresponding to the 39 frames) and sardine school. Since these data are count data, we used a Poisson link function. One observation event was excluded because it was an extreme outlier (although including it did not change the main results). We used the glmer function from the lme4 package in R (v. 3.2.2). We visually confirmed that model residuals exhibited homogeneity of variance and normality. R code as well as raw data can be found as the electronic supplementary material.
(c). Swimming characteristics of sardines
To quantify potential behavioural effects of injury, we compared the TBF and relative positions of injured and uninjured fish. To control for school position we always selected ‘pairs of fish’ (i.e. one injured and uninjured fish, N = 41 pairs) from either the front or rear half of a school. We defined injured fish as fish with clear white injury marks on their body and uninjured fish as fish without large white marks, which could, however, include individuals with internal (but unobservable) injuries. For the TBF analysis, we selected parts of a video where the tail beats of an injured and an uninjured fish could be observed for three to five complete tail-beat oscillations. We recorded the total number of frames taken for each fish to complete three to five complete tail-beat oscillations and converted this measure into oscillations per second. To test the effect of injury on TBF we used a linear mixed model with TBF as response variable and as fixed effects school position (front/rear), injury (yes/no) and their interaction. We also included the number of sardines in the school and the number of sailfish in the group as fixed effects. As random effect we used ‘pair of fish’ nested in sardine school. We used the lme function from the nlme package in R (v. 3.2.2). Non-significant interactions were removed from the model. We visually confirmed that model residuals exhibited homogeneity of variance and normality. We did not include sardines' body size; previous work showed that such differences are very small (mean total body length ± s.d. = 19.0 ± 0.2; N = 14; [21]).
To measure relative positions of injured and uninjured fish, we estimated for a given pair of injured and uninjured fish, and from a given starting position, the distance fish were gaining on each other measured in terms of body lengths in the swimming direction of the school, with positive values indicating that the uninjured fish was gaining relative to the injured fish, negative values indicating that the injured fish was gaining relative to the uninjured fish, and zero indicating no change in positional gain between injured and uninjured fish. We used a one-sample Wilcoxon signed-rank test to test whether there was a statistical difference in relative positional gain between injured and uninjured fish. As test value we used zero (i.e. no difference in positional gain between injured and uninjured fish).
3. Results
Sailfish approached (N = 25) the sardine school from the front in 52% of the cases and in 48% from the rear. On being approached sardines usually turned away from the predator resulting in a chase. As a result, all sailfish attacks (N = 36) took place with the sailfish being at the rear of the sardine school.
Sardines experienced more first contact by the sailfish bill (i.e. hits) and more captures in the rear half/periphery of the school than in the front half/centre (binomial tests: all p < 0.01, figure 3). Since the front and rear halves consisted of approximately equal numbers of sardines, the per capita predation risk of sardines was thus considerably higher in the rear of the school compared to the front. Exact fish numbers were difficult to determine in the centre and periphery because schools changed shape quickly. However, in all attacks (for which this could be determined, N = 26) sailfish entered at most the first third of their bill into the school which corresponds to circa 16.5 cm (mean length sailfish bill: 49.6 ± s.d. = 5.50 cm, N = 25) which approximates the typical length of a sardine (19 cm). For a sailfish attacking directly from behind this would only make the outermost layer of a sardine school accessible. More detailed analysis showed that in 25 of 36 captures, sailfish hit sardines that were in the last row of fish in the school (see the electronic supplementary material; in the remaining 11 cases it could not be determined whether a fish got hit in the last row or not). The percentage of fish from the school being in the last row (for cases in which this could be determined, N = 17) was on average 4.2% (± 3.57 s.d.), suggesting that the per capita predation risk was highest in the rear periphery of the school.
On average, 0.33 s (s.d. = 0.144, N = 30) elapsed between a sardine getting hit and the subsequent capture. In 80.8% of the attacks, the sardine's body was destabilized and turned upside down or sideways, thereby considerably slowing it down relative to its neighbours. This resulted in an average displacement (compared with its original position relative to other sardines in the school) of 2.2 ± 1.54 (mean ± s.d.) body lengths, which can leave the sardine in an isolated position behind the school (electronic supplementary material, video S1).
Although injured fish were present throughout the school (figure 2a), the injury level was significantly higher in the rear half of the school than in the front half of the school (GLMM: estimate (est) ± s.e. = 0.38 ± 0.008, z = 46.6, p < 0.01; figure 2d). Additionally, we verified that there were no differences in the total number of pixels representing fish (both injured and uninjured parts) in the front and the rear half of the schools (see electronic supplementary material).
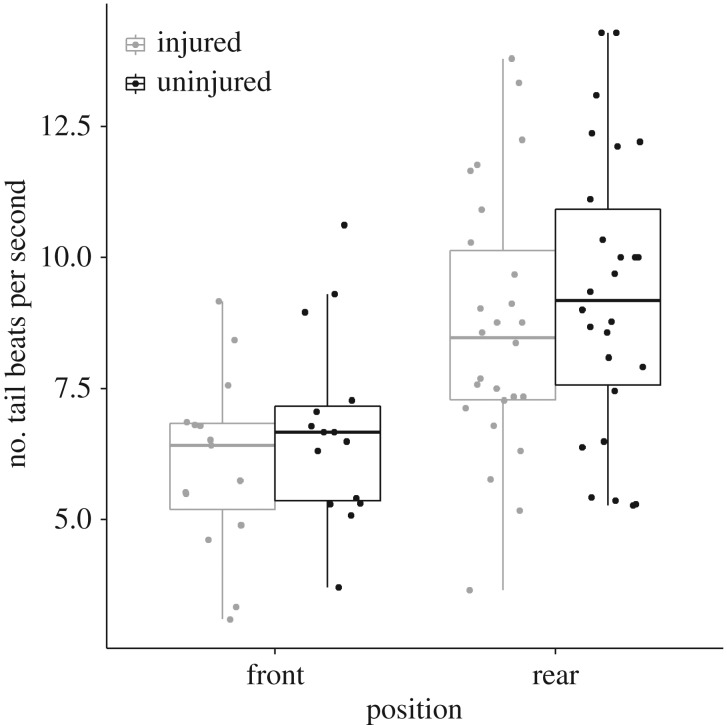
There was no significant effect of the interaction of school position and injury on TBF (est ± s.e. = −0.04 ± 0.35, t = −0.11, p = 0.91) so we removed the interaction term from the model. After removal, we found that individuals at the rear half of the school had a significantly higher TBF than individuals at the front half (est ± s.e. = 2.29 ± 0.60, t = 3.87, p < 0.001; figure 4), and that injured fish had a significantly lower TBF than uninjured fish (est ± s.e. = 0.62 ± 0.17, t = 3.68, p < 0.001; figure 4). Neither the number of sardines in the school, nor the number of sailfish in the group, significantly affected the TBF (both p > 0.4). Uninjured fish gained about 0.38 body lengths over three full tail-beat oscillations on injured ones (one-sample Wilcoxon signed-rank test, V = 178, p = 0.005, N = 25). School position (front/rear) did not affect the difference in relative position gain between uninjured and injured fish (Wilcoxon signed-rank test, W = 88.5, p = 0.52, N = 25). Additional analyses showed that injured fish rarely moved from the rear half to the front half of the school (17% of cases) compared with uninjured fish (75%) and fell back from the front half to the rear half (70%) more often than uninjured fish (0%) (see electronic supplementary material). Thus, uninjured fish significantly outperformed injured ones, either advancing or maintaining their position in the front half of the schools (Wilcoxon signed-rank test: W = −3.357, p = 0.001).
Figure 4.
Comparison of TBFs of injured and uninjured fish in the front (N = 30) and rear half (N = 52) of the sardine schools. Data points are jittered to reveal overlapping data points. Boxplots show median, interquartile ranges and whiskers show highest and lowest values (excluding outliers).
4. Discussion
Sailfish attacked mostly the rear half and peripheral positions of the sardine schools. Injured fish were more frequently observed in these riskier positions than in the safer positions at the front of the schools. Our data on TBFs, relative positions and switching probabilities between rear and front halves (see electronic supplementary material), all suggest that reduced swimming speed of injured fish is the underlying mechanism explaining the higher proportion of injured fish in the rear of the school compared with the front.
Most of the predator–prey models that simulated predation events assumed instantaneous attacks of predators and do not distinguish between predator approach and attack [10–12]. We identified these as two separate stages in the attack sequence because an approach triggered countermeasures by the prey before an attack could be initiated (see below). In contrast with Bumann et al.'s [11] model, sailfish approached the school from the front and rear about equally often. This discrepancy is probably due to the fact that sailfish often surround their prey schools and approaches take place in quick succession. A sailfish will approach the moment it has free access to a nearby prey when no other sailfish is with the sardine school, resulting in approaches from any direction. The second discrepancy with Bumann et al. was that most attacks and captures occurred in the rear half of the school. Upon a sailfish's approach, the sardines usually turned away from the sailfish as an anti-predator response and a period of chase followed. The smaller size of the sardines (approx. 19 cm) compared with the sailfish (mean ± s.d. = 184.7 ± 20.9 cm; range: 143.5–242.0 cm) gives sardines considerably higher relative manoeuvrability [23,24], a factor unaccounted for by Bumann et al. Taken together, this illustrates the importance of incorporating more realistic features, such as predator attack strategy and prey response upon predator detection into models of predator–prey dynamics. Which prey are targeted in moving groups, therefore, likely depends on both predator attack strategy and prey response.
Several studies on interactions between marine teleost predators and their schooling prey have focused on predators that attack at high speeds, thereby rapidly moving along the periphery or right through the prey schools [16,25–28]. By contrast, sailfish swim at approximately the same speed as their prey when they enter their bill into the school to launch an attack [21,29]. This difference in attack strategy may explain why peripheral and rear positions were primarily targeted in our study making front and presumably also central positions safer. We were not able to record fish numbers exactly in the periphery and centre. However, it seems highly likely that peripheral positions are more risky given the attack strategy of the sailfish to enter at most the first third of the bill into the school. Furthermore, we did not observe any attacks on central positions (figure 3b). More research on free-swimming predators using different attacking strategies is needed to better understand the effect of predator attack strategies on spatial gradients of predation risk in prey groups [13].
Previous studies found that individuals in rear positions in groups require a lower TBF than individuals in the front to maintain position within the group, and therefore possibly less energy for locomotion ([4] and [6]). By contrast, we observed the highest TBF in the rear of the school. Our results have to be interpreted in the context of the predatory–prey dynamics of this system. On detection of an approaching sailfish, the sardine school often performed a turn and accelerated in the opposite direction. These manoeuvres also often resulted in an elongated school shape, with fish at the back attempting to catch up with the rest of the school, and therefore temporarily using higher TBF than those at the front. Fish at the rear thus likely prioritized predator escape above potential hydrodynamics benefits of rear positions, and increased swimming effort to reach more frontal positions. The higher presence of injured fish at the rear of the group is likely to be the result of reduced locomotor performance, because uninjured fish had higher TBFs and gained (in terms of body lengths) on injured fish.
Differences in locomotion speed/acceleration might, however, only partly explain the spatial differences between injured and uninjured fish. Theoretical work suggests that a set of local interaction rules can influence the spatial position of individuals within a group (e.g. to move to the centre, the front, or the periphery) in the absence of information on their current position within the group as a whole [30–32]. For example, differences in turning rates and inter-individual distances may play an important role in positioning behaviour [30,33]. Little is known about how these factors are affected by injury. Injuries could also hinder the ability to coordinate movements with neighbours (for example, because of longer response latencies regarding directional changes of school mates) which may not only affect an injured fish's predation risk but also information transmission within schools. Indeed, social information is key for allowing group members to respond to threats that have not been privately detected [34–36]. A recent study [37] found that fish at the front and centre were the first to respond to aerial threats, while individuals at peripheral and rear positions responded later. Detailed studies of schools containing different ratios of injured and uninjured fish could extend the investigation by Marras & Domenici [37] on heterogeneity of individual responses within fish schools to predation stimuli.
In our system, injured fish may have a reduced ability to detect the sailfish's bill due to injury to their lateral line system. This may reduce the likelihood of socially transmitted information, which could increase predation risk not only for themselves, but for other school members as well. This type of research could shed light on how predator attack strategies might have evolved to disrupt collective prey defences.
In addition to injuring sardines, sailfish also used their bill to separate sardines from the school. Isolation of prey (from the rest of the school) for which many other predators need coordinated group attacks [25,28] or high-speed attacks, were instead accomplished by taps and slashes with the bill. This is similar to the tail slaps of thresher sharks (Alopius pelagicus, [38]) and killer whales (Orcinus orca [39]), but can be a lot more controlled in targeting specific individuals. How sailfish select their target and the attack strategy (e.g. tap or slash), is currently not understood. Future work could investigate whether sailfish actively select injured individuals as they might be easier to identify, hit and capture given that injuries may make them behaviourally and morphologically conspicuous, because of an oddity effect [1,40,41]. Predators are known to focus on odd individuals in groups. Interestingly, the presence of odd individuals can also reduce the overall predator confusion effect thus increasing predator risk for all group members [1,40]—an aspect which warrants further empirical research to understand the underlying mechanism.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
We thank Rodrigo Friscione Wyssmann and the staff of Solo Buceo for logistic assistance during fieldwork, and Thomas Poirout and Emmanuel Caro for collecting sailfish bills.
Ethics
All research was conducted in line with laws and legislation of Secretaria de Medio Ambiente y Recursos Naturales, Mexico.
Authors' contributions
All authors except F.S., D.S., S.K., P.B., P.S.S. and P.Z. collected field data. R.H.J.M.K., J.E.H.-R. and J.K. analysed the empirical data and performed statistical analyses. P.Z. performed the CT-analysis of the bills. All authors wrote the paper. All authors gave final approval for publication.
Data accessibility
All data are available as the electronic supplementary material.
Competing interests
We have no competing interests.
Funding
J.E.H.-R. was supported by a Knut and Alice Wallenberg Foundation grant no. 829 2013.0072 awarded to David J.T. Sumpter and J.K. acknowledges financial support from the IGB.
References
- 1.Krause J, Ruxton GD. 2002. Living in groups. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.DeBlois EM, Rose GA. 1996. Cross-shoal variability in the feeding habits of migrating Atlantic cod (Gadus morhua). Oecologia 108, 192–196. ( 10.1007/BF00333231) [DOI] [PubMed] [Google Scholar]
- 3.Hansen MJ, Schaerf TM, Krause J, Ward AJW. 2016. Crimson-spotted rainbowfish (Melanotaenia duboulayi) change their position within shoals according to nutritional requirement. PLoS ONE 11, e0148334 ( 10.1371/journal.pone.0148334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herskin J, Steffensen JF. 1998. Energy savings in sea bass swimming in a school: measurements of tail beat frequency and oxygen consumption at different swimming speeds. J. Fish Biol. 53, 366–376. ( 10.1111/j.1095-8649.1998.tb00986.x) [DOI] [Google Scholar]
- 5.Killen SS, Marras S, Steffensen JF, McKenzie DJ.. 2012. Aerobic capacity influences the spatial position of individuals within fish schools. Proc. R. Soc. B 279, 357–364. ( 10.1098/rspb.2011.1006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marras S, Killen SS, Lindström J, McKenzie DJ, Steffensen JF, Domenici P. 2015. Fish swimming in schools save energy regardless of their spatial position. Behav. Ecol. Sociobiol. 69, 219–226. ( 10.1007/s00265-014-1834-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voelkl B, Portugal SJ, Unsölde M, Usherwoodd JR, Wilson AM, Johannes F. 2015. Matching times of leading and following suggest cooperation through direct reciprocity during V-formation flight in ibis. Proc. Natl Acad. Sci. USA 112, 2115–2120. ( 10.1073/pnas.1413589112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romey WL, LaBuda S. 2010. Predator type, not body condition, influences positioning within whirligig groups. Behav. Ecol. Sociobiol. 64, 665–673. ( 10.1007/s00265-009-0884-5) [DOI] [Google Scholar]
- 9.King AJ, Wilson AM, Wilshin SD, Lowe J, Haddadi H, Hailes S, Morton AJ. 2012. Selfish-herd behaviour of sheep under threat. Curr. Biol. 22, 561–562. ( 10.1016/j.cub.2012.05.008) [DOI] [PubMed] [Google Scholar]
- 10.Hamilton WD. 1971. Geometry of the selfish herd. J. Theor. Biol. 31, 295–311. ( 10.1016/0022-5193(71)90189-5) [DOI] [PubMed] [Google Scholar]
- 11.Bumann D, Krause J, Rubenstein DI. 1997. Mortality risk of spatial positions in animal groups: the danger of being in the front. Behaviour 134, 1063–1076. ( 10.1163/156853997X00403) [DOI] [Google Scholar]
- 12.James R, Bennett PG, Krause J. 2004. Geometry for mutualistic and selfish herds: the limited domain of danger. J. Theor. Biol. 228, 107–113. ( 10.1016/j.jtbi.2003.12.005) [DOI] [PubMed] [Google Scholar]
- 13.Hirsch BT, Morrell LJ. 2011. Measuring marginal predation in animal groups. Behav. Ecol. 22, 648–656. ( 10.1093/beheco/arr026) [DOI] [Google Scholar]
- 14.Rieucau G, Fernö A, Ioannou CC, Handegard NO. 2015. Towards of a firmer explanation of large shoal formation, maintenance and collective reactions in marine fish. Rev. Fish Biol. Fish. 25, 21–37. ( 10.1007/s11160-014-9367-5) [DOI] [Google Scholar]
- 15.Krause J. 1993. The effect of ‘Schreckstoff’ on the shoaling behaviour of the minnow—a test of Hamilton's selfish herd theory. Anim. Behav. 45, 1019–1024. ( 10.1006/anbe.1993.1119) [DOI] [Google Scholar]
- 16.Parrish JK. 1989. Re-examining the selfish herd: are central fish safer? Anim. Behav. 38, 1048–1053. ( 10.1016/S0003-3472(89)80143-5) [DOI] [Google Scholar]
- 17.Krause J, Ruxton GD, Rubenstein DI. 1998. Is there an influence of group size on predator hunting success? J. Fish Biol. 52, 494–501. ( 10.1111/j.1095-8649.1998.tb02012.x) [DOI] [Google Scholar]
- 18.Killen SS, Marras S, Steffensen JF, McKenzie DJ. 2011. Aerobic capacity influences the spatial position of individuals within fish schools. Proc. R. Soc. B 279, 357–364. ( 10.1098/rspb.2011.1006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krause J, Ruxton GD. 2010. Important topics in group living. In Social behaviour: genes, ecology and evolution (eds Szekeley T, Moore A, Komdeur J), pp. 203–225. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 20.Herbert-Read JE, et al. 2016. Proto-cooperation: group hunting sailfish improve hunting success by alternating attacks on grouping prey. Proc. R. Soc. B 283, 20161671 ( 10.1098/rspb.2016.1671) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domenici P, et al. 2014. How sailfish use their bill to capture schooling prey. Proc. R. Soc. B 281, 20140444 ( 10.1098/rspb.2014.0444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schindelin J, et al. 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. ( 10.1038/nmeth.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webb PW, Debuffrenil V. 1990. Locomotion in the biology of large aquatic vertebrates. Trans. Am. Fish. Soc. 119, 629–641. ( 10.1577/1548-8659(1990)119%3C0629:LITBOL%3E2.3.CO;2) [DOI] [Google Scholar]
- 24.Domenici P. 2001. The scaling of locomotor performance in predator–prey encounters: from fish to killer whales. Comp. Biochem. Physiol. A 131, 169–182. ( 10.1016/S1095-6433(01)00465-2) [DOI] [PubMed] [Google Scholar]
- 25.Major PF. 1978. Predator-prey interactions in two schooling fishes, Caranx ignobilis and Stolephorus purpureus. Anim. Behav. 26, 760–777. ( 10.1016/0003-3472(78)90142-2) [DOI] [Google Scholar]
- 26.Parrish JK. 1993. Comparisons of the hunting behavior of four piscine predators attacking schooling prey. Ethology 95, 233–246. ( 10.1111/j.1439-0310.1993.tb00473.x) [DOI] [Google Scholar]
- 27.Clua E, Grosvalet F. 2001. Mixed-species feeding aggregation of dolphins, large tunas and seabirds in the Azores. Aquat. Living Resour. 14, 11–18. ( 10.1016/S0990-7440(00)01097-4) [DOI] [Google Scholar]
- 28.Handegard NO, Boswell KM, Ioannou CC, Leblanc SP, Tjostheim DB, Couzin ID. 2012. The dynamics of coordinated group hunting and collective information transfer among schooling prey. Curr. Biol. 22, 1213–1217. ( 10.1016/j.cub.2012.04.050) [DOI] [PubMed] [Google Scholar]
- 29.Marras S, et al. 2015. Not so fast: swimming behavior of sailfish during predator–prey interactions using high-speed video and accelerometry. Integr. Comp. Biol. 55, 719–727. ( 10.1093/icb/icv017) [DOI] [PubMed] [Google Scholar]
- 30.Couzin ID, Krause J, James R, Ruxton GD, Franks NR. 2002. Collective memory and spatial sorting in animal groups. J. Theor. Biol. 218, 1–11. ( 10.1006/jtbi.2002.3065) [DOI] [PubMed] [Google Scholar]
- 31.Couzin ID, Krause J. 2003. Self-organisation and collective behaviour of vertebrates. Adv. Study Behav. 32, 1–75. ( 10.1016/S0065-3454(03)01001-5) [DOI] [Google Scholar]
- 32.Hemelrijk CK, Hildenbrandt H. 2012. Schools of fish and flocks of birds: their shape and internal structure by self-organization. Interface Focus 2, 726–737. ( 10.1098/rsfs.2012.0025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaerf T, Herbert-Read JE, Myerscough MR, Sumpter DJ, Ward AJ. 2016. Identifying differences in the rules of interaction between individuals in moving animal groups. (http://arxiv.org/abs/1601.08202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herbert-Read JE, Buhl J, Hu F, Ward AJW, Sumpter DJ. 2015. Initiation and spread of escape waves within animal groups. R. Soc. open sci. 2, 140355 ( 10.1098/rsos.140355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurvers RHJM, Wolf M, Naguib M, Krause J. 2015. Self-organized flexible leadership promotes collective intelligence in human groups. R. Soc. Open Sci. 151, 1346–1353. ( 10.1098/rsos.150222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenthal SB, Twomey CR, Hartnetta AT, Wub HS, Couzin ID. 2015. Revealing the hidden networks of interaction in mobile animal groups allows prediction of complex behavioral contagion. Proc. Natl Acad. Sci. USA 112, 4690–4695. ( 10.1073/pnas.1420068112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marras S, Domenici P. 2013. Schooling fish under attack are not all equal: some lead, others follow. PLoS ONE 8, e65784 ( 10.1371/journal.pone.0065784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oliver SP, Turner JR, Gann K, Silvosa M, D'Urban Jackson T. 2013. Thresher sharks use tail-slaps as a hunting strategy. PLoS ONE 8, e67380 ( 10.1371/journal.pone.0067380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Domenici P, Batty RS, Simila T, Ogam E. 2000. Killer whales (Orcinus orca) feeding on schooling herring (Clupea harengus) using underwater tail-slaps: kinematic analyses of field observations. J. Exp. Biol. 203, 283–294. [DOI] [PubMed] [Google Scholar]
- 40.Landeau L, Terborgh J. 1986. Oddity and the confusion effect in predation. Anim. Behav. 34, 1372–1380. ( 10.1016/S0003-3472(86)80208-1) [DOI] [Google Scholar]
- 41.Ohguchi O. 1981. Prey density and selection against oddity by three-spined sticklebacks. Z. Tierpsychol. Suppl. 23, 1–79. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available as the electronic supplementary material.