Abstract
A considerable proportion of the world's bird species undertake seasonal long-distance migrations. These journeys are energetically demanding. Two major behavioural means to reduce energy expenditure have been suggested: the use of thermal uplifts for a soaring-gliding migration style and travelling in echelon or V-shaped formation. Both strategies have immediate consequences for the social organization of the birds as they either cause large aggregations or require travelling in small and stable groups. Here, we first discuss those consequences, and second present an analysis of formation flight in a flock of northern bald ibis on their first southbound migration. We observe clear correlations between leading and trailing on the dyadic level but only a weak correlation on the individual level during independent flight and no convincing correlation during the human guided part of the migration. This pattern is suggestive of direct reciprocation as a means for establishing cooperation during formation flight. In general, we conclude that behavioural adaptations for dealing with physiological constraints on long-distance migrations either necessitate or ultimately foster formation of social groups with different characteristics. Patterns and social organization of birds travelling in groups have been elusive to study; however, new tracking technology—foremost lightweight GPS units—will provide more insights in the near future.
This article is part of the themed issue ‘Physiological determinants of social behaviour in animals’.
Keywords: soaring, formation flight, bird migration, aggregations, flocks
1. Introduction
About 1800 bird species (almost 20% of all bird species) are long-distant migrants, undertaking seasonal long-distance migrations of many hundred and up to several thousand kilometres. The Arctic tern (Sterna paradisaea), for example, breeds in the Arctic, but in autumn the birds fly to the Antarctic, where they spend the winter at the edge of the pack ice before returning to the Arctic in late spring [1]. The Amur falcon (Falco amurensis) breeds in Siberia, Mongolia and northeast China and undertakes each autumn an 11 000 km journey to South Africa, crossing the Indian Ocean [2]. Yet, the longest non-stop migratory flights were reported from bar-tailed godwits (Limosa lapponica) which travel from Alaska across the Pacific to New Zealand, a distance of 11 600 km [3].
Most migrations are necessitated by temporal changes in the availability of key resources: migrants leave habitats where resources are declining to take refuge in habitats where resources are available [4]. In many species migration is a pre-emptive strategy, where individuals leave an area in time, before the resource is depleted or—in the case of migration to the breeding area—before the onset of the breeding season. Migration often involves major changes in the social organization, e.g. individuals that are territorial before migration are often seen in large social groups during migration [5]. Moore et al. [6] argue that the subtle consequences of en route competition can have significant impact on individuals and even population dynamics, including high mortality rates of immature birds and skewed sex ratios. Increased competition can result in increased risk of predation, delay in reaching the destination and nutritional deficits, which can severely affect survival probability as well as competitiveness upon arrival at the destination [7].
Forming flocks during migration has been suggested to improve navigational accuracy due to the influence of experienced individuals [8–10] or ‘wisdom of the crowd’ [11–16] and certain formation types might also allow energy savings through aerodynamic effects [17,18]. Furthermore, flocking benefits individuals by reducing the probability of being preyed upon by diluting the risk, confusing the predator, or detecting predators more efficiently [19–22]. The benefits of migrating in flocks may explain why many bird species first aggregate at staging sites before leaving together in larger groups [23,24]. The departure from the staging sites is reportedly under social influence, with individuals communicating their readiness for departure through specific vocalizations, pre-flight intention movements and repeated take-offs and short circle flights before departing [25].
Energetic demands are a limiting factor for the length of migratory flights. The more fuel reserves a flying bird carries and the heavier it becomes, the more induced power is required for countervailing gravity and keeping it airborne. Parasitic drag, too, can increase with increasing body size [26]. Alerstam [1] estimated that a bird flying with 50% body fat consumes about 40% more energy for covering a certain distance than a bird with a body fat load of only 10%. Many bird species cannot cover the complete travel route in one non-stop flight. A frequently observed migration strategy consists, therefore, of flight bouts interrupted by stopovers of one or more days for re-fattening [23]. Bird migration theory offers optimality models describing the relationship between flight length, re-fattening times and travel speed [27–30]. As suitable stop-over sites are often limited and migration takes place in a narrow time window, this can lead to large aggregations of birds at stopover sites. Non-stop flights usually occur if birds need to cross inhospitable areas, such as deserts or oceans.
Many migrating species show different flocking and travelling patterns during spring and autumn migration, which are mainly due to two factors: food availability and destination. As autumn migration is pre-emptive, birds leave the breeding grounds usually before resources are depleted and will encounter resource-rich stopover sites. In spring, however, birds time their migration so that they arrive at the breeding grounds early for breeding, which means that they might encounter low food availability on their way north [31,32]. When birds are travelling to their breeding grounds, birds arriving early are more likely to occupy high quality territories or to attract mating partners. Migration can take on the character of a race with the first arrivals reaping the greatest fitness benefits. Yet, various trade-offs might set limits to departure and arrival dates. On the other hand, migration to the wintering area is less competitive and economy and safety might have higher priority. O'Reilly and Wingfield [33] compared patterns of spring and autumn migration of Arctic shorebirds across North America. Spring migration was characterized by huge multi-species flocks at stopover sites as birds move within a narrow time window, dictated by the need to arrive at the breeding area in time. High densities of shorebirds at stopover or staging sites influence their social behaviour. As numbers increase, foraging competition increases [34–36] and rates of aggression have been observed to rise as well [37,38] (though see [39] for opposing effects). Not all birds are equally affected: young birds are usually less proficient foragers and suffer more from food shortage than adult birds. Furthermore, they are usually subordinate to adults and often displaced by adults from the best foraging sites [6,40]. Movements away from coastal areas by immature birds has been suggested as being a response to high competition at resource-rich areas [41,42]. In species with sexual size dimorphism, males and females are differently affected by competition [43,44].
Individuals start preparing for migration several weeks before departure by changing activity patterns, increasing foraging rates and lipid synthesis [45–47] and building up subcutaneous and visceral fat depots [1,48]. Long distance flights are primarily fuelled through fatty acid metabolism, as the caloric value of fat is much higher than that of carbohydrates or proteins [49–52]. Some bird species reduce the size of organs which are not essential for the migration briefly before departure or during the migration, mainly to reduce body mass but also to gain energy [53–55].
In addition to these physiological adaptations, birds have developed behavioural strategies that allow them to further cut on energy expenditure. Large bird species with large wing area and low wing loading use thermal updraughts to gain altitude and travel in a soaring–gliding fashion, while some large and intermediate-sized bird species (such as cranes, geese and ibises) travel in ordered V-shaped or echelon formation, presumably reducing energy requirements through aerodynamic effects. In both cases these strategies have direct consequences for the social organization of the species. A soaring–gliding travel style leads to aggregations of hundreds to hundred of thousands of birds at specific hot-spots with favourable soaring conditions, while formation flight requires the coordinated movement of a group of animals.
(a). Soaring–gliding flight
In active flapping flight the major flight muscles actively produce the power required to counteract gravity as well as the power to provide forward thrust. This flight mode is energetically demanding and the metabolic rate of birds flying by flapping their wings is estimated to be 8–30 times higher than the basal metabolic rate [23,56,57]. A number of bird species adopted a flight mode of soaring and gliding where they first circle up in the columns of rising air and then glide with loss of height to the next region providing lift. During soaring–gliding flight birds keep their wings in an outstretched position for most of the time. Lift comes largely from rising air and the forward motion mainly from losing altitude during gliding. As a consequence, the internally produced energy requirement for locomotion is only between 1.5 and 2 times the basal metabolic rate, or 5–25% of the requirement for active flapping flight [23,58]. Soaring is dependent on regions where air is moving upwards faster than the sink-rate of a gliding bird. Over land, columns of rising air caused by heating of the ground produce thermal updraughts. As those columns are often quite limited in their horizontal extension, birds have to fly tight rounds in order to stay within the thermal, leading to the typical circling behaviour. In addition, horizontal winds which are deflected upwards by landscape barriers such as hill and mountain slopes or cliffs can produce orographic lift [59].
Given the substantial differences in energy requirements between active flapping and gliding flight it might be surprising that only a small proportion of migrating bird species travel by soaring–gliding mode. One disadvantage of travelling by gliding–soaring flight is that it is usually slower than active flapping flight. Hedenström [60] compared optimality models for energy-selected migration and time-selected migration and concluded that minimization of transport costs is unlikely to be the only critical factor and that the requirement to reach the destination within a given time restricts the range of species that can travel by soaring–gliding mode. Due to this trade-off between energy expenditure and travel time, soaring–gliding travel is mainly observed in large species such as many raptors, pelicans and storks, because for heavier birds it becomes more difficult to create sufficient lift by muscle power alone [26,61].
When travelling by soaring–gliding flight, birds are restricted in their migration paths to areas where updraughts can be expected. They travel mainly over land and avoid long sea crossings where thermals are absent. The migration routes of soaring–gliding species are, therefore, often considerably longer than those of birds that fly a more direct route by active flapping flight. Soaring–gliding birds are also restricted to a certain time window, when thermal updraughts are created by the sun heating the ground. This leads to large aggregations of birds at migratory ‘hot-spots’ that offer favourable soaring conditions and to the formation of narrow migration corridors. For example, the migration route of a substantial proportion of western Palaearctic soaring birds, including storks, pelicans and 35 raptor species, passes over Israel, where they are funnelled between the Mediterranean Sea and the Rift Valley. Leshem & Yom-Tom [62] summarized counts from a close-meshed survey over a 4-year period. They observed both inter- and intraspecific spatio-temporal segregation of migrants. Interspecific variation has been suggested to be mainly driven by the timing and location of thermal convection. For example, honey buzzards (Pernis apivorus) start their migration early in the morning by active flapping, while the heavier lesser spotted eagles (Aquila pomarina) start later in the day, waiting for thermals before leaving the ground. As optimal conditions for soaring–gliding species depend on body mass and gliding ability, different species typically occupy different migration corridors through Israel, with most white storks (Ciconia ciconia) travelling more than 60 km east of the Mediterranean coast, honey buzzards 22–60 km, Levant sparrowhawks (Accipiter brevipes) 5–40 km, lesser spotted eagles 5–22 km, and white pelicans (Pelecanus onocrotalus) 5–10 km [62]. Intraspecific variation, in contrast, is supposed to reflect different breeding or wintering areas, with birds moving to the Arabian Peninsula and East Africa travelling on a more easterly route than birds moving to West, Central or South Africa [62].
So far, no convincing evidence has been brought forward that migrating raptors are attracted to each other, and the large aggregations of birds are usually explained as being caused by the limited availability of favourable updraughts, only. Yet, irrespectively of the mechanism that creates these aggregations, it seems likely that birds can profit from each other by using social cues about the location of thermals from conspecifics. Leshem & Bahat [63] reported that on peak migration days lines of columns with soaring raptors extend for more than 200 km with the next column being in sight of the previous. This allows the birds to directly glide from one thermal to the next without spending time and energy searching for regions of thermal uplift.
(b). Formation flight
Several larger bird species including geese, swans, cranes, pelicans, cormorants and ibis typically migrate in conspicuous echelon or V-shaped formations. It has been suggested that these formations allow the birds to save energy by using uplift produced by preceding birds [17–18,64,65]. During flight, high-pressure air under the wings flows around the tips to a region of low air pressure above the wings. This flow forms two wing-tip vortices in the bird's wake, producing regions of up-wash outboard of the wings, and regions of downwash closer to the flight axis. Photographs of the wake of birds flying through a cloud of helium bubbles and more recent studies using particle image velocimetry (PIV) confirmed the existence of such vortices [66–72]. This up-wash can provide a following bird with extra lift, reducing its requirements for mass support. Initial theoretical calculations suggest that by flying in this up-wash region at optimal wing-tip spacing, birds could save over 50% of their energy costs relative to unaccompanied solo flight [17,73,74]. However, these predictions were based on fixed-wing aerodynamics assuming optimal wing-tip spacing and have been repeatedly considered as overly optimistic [65,73,75–77]. Recently, Maeng et al. [78] modelled the fluid dynamics of flapping wings, estimating that Canada geese flying with an optimal depth of 4 m and optimal wing-tip spacing between −0.4 m and 0 m could reduce their energy costs of flying by 16%.
Analyses of photographs of goose formations showed that birds fly in positions where they avoid the downwash region directly behind other birds and where they can potentially profit from the beneficial up-wash [73,74,79,80]. Yet, wing-tip spacing and distance between birds was found to be rather variable, which means that the actually accrued energy savings will be clearly lower than the predicted value for optimal wing-tip spacing. The ‘energy savings hypothesis’ has not been uncontested and improved communication between individuals and reduced risk of mid-air collisions have been suggested as alternative explanations for the formation of echelons and Vs [75,76,81]. These potential benefits of formation flight are not mutually exclusive, though the energy savings hypothesis is currently the only hypothesis that is tentatively supported by empirical evidence. In a landmark study Weimerskirch et al. [82] demonstrated that heart rate, a proxy for energy expenditure, was lower in pelicans (Pelecanus onocrotalus) flying in the middle of a formation compared to the bird positioned at the front. Studying the migratory flight pattern of a group of northern bald ibises (Geronticus eremita) Portugal et al. [83] demonstrated that birds, when flying in formation, not only favoured positions that allowed them to profit from the up-wash, but also coordinated their wing flaps with a phase shift such that their wing tips followed the path of the preceding bird's wing tips through the air, allowing them to maximize the capture of beneficial up-wash.
While swans, cranes and large geese fly typically in acute V-formation, other species use more obtuse Vs or bow-like formations, in which the leading bird is only a little ahead of its neighbours. Hummel [84] proposed that in the latter case even the leading bird can profit from the wing-tip vortices of the following bird. Anderson & Wallander [85] suggested that differences in the shape of the flight formations between species might be explained by the specific composition of the travelling groups. In acute V-formations the leading bird will be at a disadvantage compared to the following birds, because it cannot profit from the up-wash produced by the others. This disadvantage can, however, be mitigated via kin selection [86] if birds in a flock are closely related; in this case leading birds can still gain indirect fitness benefits that outweigh their costs. If birds are not related, direct reciprocation in taking the lead, as suggested by Trivers [87], could ensure stable cooperation. Observational evidence supports both suggestions. First, small flocks of geese, swans or cranes, travelling in small family groups, typically form acute Vs [85,88–90]. Second, our own observations of northern bald ibis showed that birds frequently swap positions and match the time they are leading and following as expected for direct reciprocation ([91] and this work). Bow-formed or obtuse V-formations, in contrast, are found mainly in species such as Arctic geese, eider ducks and many waders [1,24], which travel usually in large flocks with presumably low relatedness between individuals. Yet, while observed patterns fit well with the idea that energy savings are more evenly distributed in obtuse formations, this idea hinges on the assumption that the vortices developing at the birds' wing tips advance sufficiently far ahead in order to support the preceding bird. Whether this is actually the case awaits being answered empirically.
Both hypotheses—cooperation during formation flight based on kin selection and cooperation based on reciprocity—predict group sizes of a few to a few dozen individuals. If groups are larger, then the average relatedness declines as clutch size determines how many offspring or siblings a group can contain. Also, reciprocation is easier to establish in small groups [92,93], because large groups would require extensive ‘book keeping’ of support given and received. The observation that most formations of birds are indeed of limited size—often a dozen individuals only and rarely exceeding one hundred individuals [23]—seems to support these hypotheses. However, Seiler [94] pointed out that size limitations exist also due to another reason. As birds do not fly in absolutely straight lines but permanently adjust their flight direction based on fluctuations in wind and air pressure, a bird trailing another bird has to follow these adjustments if it is to stay in an optimal trailing position. This, however, requires slightly stronger directional changes, so that adjustment movements increase with increasing rank in a formation while the time spent in the optimal position decreases for birds further back—up to a point where trailing does not bring an advantage any more.
(c). Autumn migration in northern bald ibis
Mortality is considerably higher during migration than at any other time of the year [23,46,95]. In greater snow geese (Chen caerulescens), for example, mortality during the autumn migration was estimated at 5% for adult birds but up to 35% for juvenile birds [96,97]. Consequently, there should be a strong selection pressure—especially on young birds during their first migration—to minimize energy expenditure during migratory flights (which is directly and indirectly contributing to their chance of survival). Apart from motor learning for mastering the course of motions for efficient flight performance, juvenile birds flying in flocks have also to learn how to coordinate their own behaviour with that of the other flock members. Coordination is required for overall flight direction and speed as well as for positional fine-tuning in order to avoid mid-air collisions and to take on specific positions within formations. As the latter constitutes a social dilemma, where participating animals have conflicting interests, the question arises how this social conflict is resolved. A previous study [91] suggested direct reciprocation as a potential mechanism for enabling cooperation during the formation flight. Yet, as that study provided only a ‘snapshot’ during a very early phase of the migration, the question how cooperation works over the entire migration remains to be answered.
Petit & Bildstein [98] observed the development of flight behaviour of juvenile white ibis (Eudocimus albus) during their daily flights from their roosting area to the foraging area. While juvenile birds were seen in formation in only 18% of the cases at the beginning of July, this increased to 80% only two month later, at the end of August. The authors hence concluded that the juvenile birds were already proficient formation-flyers at the onset of their first migration. Yet, it is not known how flight behaviour of juvenile birds develops further during their first migration.
We therefore make use of the positional data from all individuals of a migrating flock of juvenile northern bald ibis in order to ask whether (a) formation flight is consistently shown during the whole migration, (b) whether birds take turns in leading and trailing, and (c) whether the proportion birds spend in leading or trailing positions varies between individuals or over time.
2. Material and methods
(a). Subjects
Data were collected during a human-led migration with the aim of re-establishing a freely migrating population of bald ibis in Europe. Subjects were 14 juvenile northern bald ibis (Geronticus eremita) that were hand-reared by human foster parents and trained to follow an ultralight aircraft (powered parachute, figure 1) in order to learn a new migration route from Salzburg (Austria) to Orbetello (Tuscany, Italy) as part of an ongoing research and conservation programme by Waldrappteam, Austria. Observations from wild and semi-wild colonies show that juvenile ibis form cohesive flocks for travelling together, often accompanied by one or more experienced adults [99,100]. In this respect the human-led migration mimics the natural condition during the juvenile's first autumn migration. All birds hatched in March 2014 and were imprinted onto human foster parents. An extended discussion how this might influence the behaviour of the birds is given in the electronic supplementary material. The birds stem from nine nests of one breeding colony and were raised in four nests of four individuals each (table 1). Chicks of each nest had different age in order to mimic the natural situation of asynchronous hatching. Of the originally 16 raised birds, 14 were considered fit for the migration. From mid-June until mid-August, the birds were trained to follow the ultralight aircraft. The training followed a detailed protocol and consisted of a series of habituation and conditioning steps [101]. At the end of July birds were equipped with leg-loop harnesses and dummy loggers to habituate them to the equipment and the additional mass which they had to carry during the migration (approximately 3.5% of the body mass of the smallest bird).
Figure 1.

Northern bald ibis flying in formation parallel to the ultralight aircraft during the human-led migration in 2015. Image courtesy of Waldrappteam, Photographer: P. Prezesang. Inset: the flight path of the birds during the human led migration from Salzburg to Orbetello.
Table 1.
Properties and positional summary data of the 14 birds. % Lead, percentage of the time the bird was in the foremost position in the direction of flight of the core group; % in core area, percentage of time a bird was found in the core area of the flock defined as the area around the centre of gravity of the flock that contained 50% of the birds with the smallest inter-individual distances; MDC, median distance to the centre of gravity of the core area of the flock in metres; 98 k, 98 000 (due to two birds departing from the flock); % trail, percentage of time a bird was following another bird in its wake area; % front, percentage of time a bird had another bird following in its wake area; GF, guided flight; IF, independent flight (unaccompanied phase of leg 4).
| birds | Pi | Ed | Fl | Pe | Li | No | Am | To | So | Bl | Vi | Te | Ar | Au |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| nest ID | 1 | 4 | 3 | 3 | 1 | 4 | 2 | 4 | 3 | 3 | 1 | 4 | 2 | 2 |
| sex | M | F | F | F | M | M | M | M | F | F | F | F | F | F |
| mass: start (g) | 1519 | 1341 | 1413 | 1345 | 1509 | 1387 | 1445 | 1415 | 1301 | 1359 | 1403 | 1255 | 1259 | 1405 |
| mass: finish (g) | 1332 | 1140 | 1220 | 1164 | 1334 | 1228 | 1268 | 1224 | 1184 | 1170 | 1184 | 1102 | 1086 | 1174 |
| GF % lead | 3.3 | 4.6 | 0.7 | 2.9 | 3.0 | 6.8 | 2.7 | 41.1 | 1.1 | 18.0 | 4.3 | 3.0 | 5.8 | 2.6 |
| IF % lead | 4.9 | 4.5 | 8.7 | 3.1 | 11.2 | 9.5 | 5.9 | 15.6 | 0.1 | 15.0 | 7.8 | 8.5 | 0.4 | 4.9 |
| GF %: core area | 45 | 54 | 32 | 51 | 53 | 54 | 64 | 47 | 43 | 31 | 51 | 64 | 54 | 57 |
| IF %: core area | 52 | 58 | 62 | 49 | 65 | 60 | 59 | 44 | 1 | 51 | 64 | 70 | 1 | 65 |
| GF MDC | 4.2 | 3.5 | 3.5 | 3.8 | 3.6 | 3.5 | 2.9 | 3.9 | 4.5 | 5.2 | 3.8 | 2.9 | 3.4 | 3.4 |
| IF MDC | 4.6 | 4.2 | 3.8 | 5.0 | 3.7 | 3.9 | 4.0 | 5.2 | 98 k | 4.7 | 3.8 | 3.3 | 98 k | 3.6 |
| GF % front | 6.4 | 4.8 | 6.0 | 6.0 | 6.0 | 4.7 | 6.6 | 1.6 | 5.9 | 1.3 | 7.1 | 6.8 | 5.2 | 4.2 |
| GF % trail | 5.1 | 5.7 | 3.5 | 6.2 | 4.8 | 6.6 | 6.0 | 5.5 | 3.7 | 2.0 | 5.4 | 7.9 | 5.8 | 3.6 |
| IF % front | 3.9 | 4.9 | 4.9 | 4.8 | 4.4 | 4.2 | 4.3 | 2.4 | 2.2 | 1.6 | 4.6 | 6.1 | 1.3 | 4.0 |
| IF % trail | 3.6 | 3.8 | 4.4 | 2.6 | 4.6 | 4.9 | 4.0 | 3.9 | 1.2 | 2.4 | 5.8 | 6.1 | 2.2 | 3.5 |
(b). Data collection and processing
The human guided migration from Salzburg (47°48′ N 13°02′ E) to Orbetello (42°26′ N 11°11′ E, figure 1) lasted for 11 days (from 25 August to 4 September 2014). The whole journey was completed in four legs of length 241 km, 262 km, 127 km and 335 km on 25, 28, 30 August and 4 September, respectively. GPS loggers from e-obs, Germany, were attached with leg-loop harnesses to the backs of all 14 birds. The maximal altitude on the migration was 2250 m MSL, and the birds followed the paraplane at a distance of, on average, 38 m ± 17 m (s.d.), typically to the side of the ultralight. GPS was recorded at 1.0 Hz. The GPS data were logged in decimal degrees for longitude and latitude, using WGS84 as the ellipsoid. Each migratory leg started with a phase of circling over the airfield to gain initial altitude and motivate the birds to follow the aircraft by constant calling by the foster parent, followed by relatively straight flight thereafter. This initial starting phase, lasting between 3 and 10 min, was excluded from analysis. On the first migratory leg the GPS logger of one individual failed to record data, though on all other legs all loggers worked, giving an overall coverage of positional data of 97%. Binary log files of the GPS loggers were first extracted with decoder software (decoder_v6_2) provided by e-obs, and all further analyses were made with Mathematica 10 from Wolfram Research.
For each second, we calculated the relative position of each bird to every other bird in the flock. We considered a bird as flying in the wake of the preceding bird if (i) it was within 3 m behind the preceding bird, where behind refers to 180° relative to the flight direction of the preceding bird, (ii) it was between 1.0 and 1.55 m lateral to the preceding bird, where lateral refers to 90° or 270° relative to the flight direction of the preceding bird, and (iii) the difference in the altitude between the two birds was less than 0.5 m. If, based on this definition, the bird was in the wake of more than one individual, we considered it as being in the wake of the closest preceding individual.
The exact extent of the area behind a bald ibis, where another bald ibis can profit from the produced up-wash still awaits empirical verification. Yet, based on earlier studies on wake formation of a kestrel (Falco tinnunculus [66]), a thrush nightingale (Luscinia luscinia [68]), a jackdaw (Corvus monedula [69]) and common swifts (Apus apus [70–72]) it seems reasonable to assume that the wingtip vortex has a diameter of approximately one-quarter of the wingspan. Adding to this a buffer of 0.2 m (based on the observed inaccuracy for relative GPS positions) we arrive at a lateral range between 1.0 and 1.55 m behind a northern bald ibis where we assume a positive aerodynamic effect. The vortex decreases in strength over time, and hence with the distance to the bird. This effect can be assumed to be even stronger under natural conditions, where small-scale air turbulences and winds will interact with the created vortices. We have therefore chosen an arbitrary cut-off point at 3 m behind the preceding bird. In a previous study [91] we have shown that all findings were very robust to changes in this definition and the same patterns were found both for stricter and more generous definitions of the cut-off point. Given that the nearest trailing bird was found within the 3 m range approximately 80% of the time (see also figure 2), we assume this to be the case also in the current study. This definition is also in line with a study on white ibis by Petit and Bildsetin [98] who reported that birds in formation were usually found less than 2 m behind another bird.
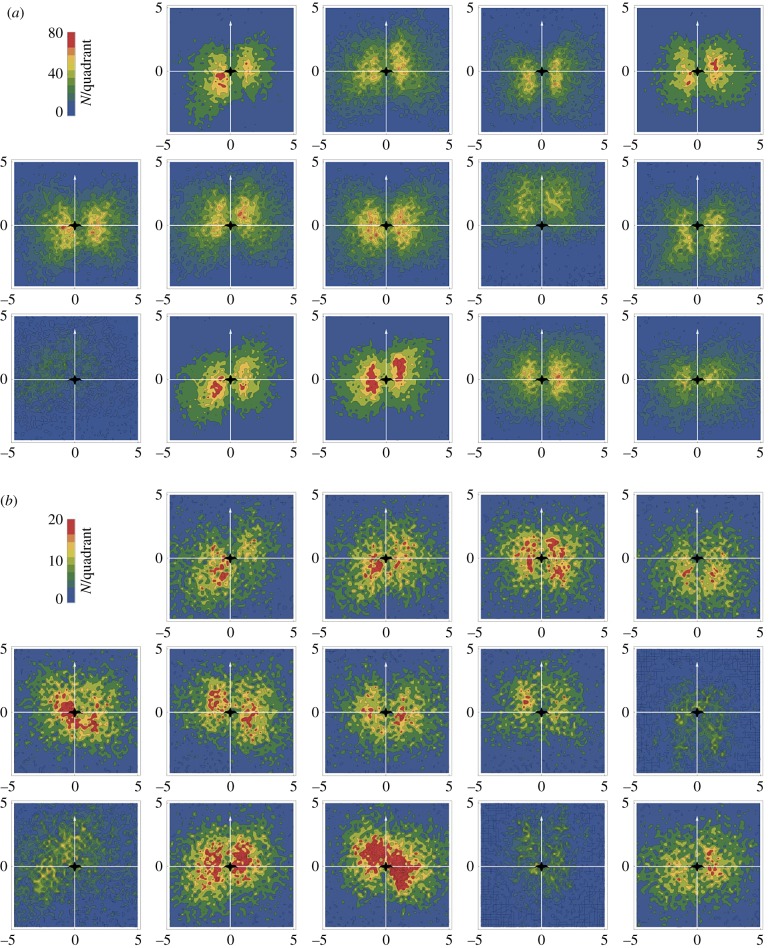
Figure 2.
(a) Relative positioning of the 14 birds during the human-guided flights of the migration. At each second the relative position of the nearest bird ahead and behind of each bird was evaluated. The upper part of each panel shows the density of nearest birds observed within a 5 m by 10 m area ahead of the focus bird. The lower part of each panel shows the density of nearest birds observed within a 5 m by 10 m area behind the focus bird. The arrow indicates the direction of flight of the focus bird. First line: birds ‘Pi’, ‘Ed’, ‘Fl’, ‘Pe’, ‘Li’, second line: birds ‘No’, ‘Am’, ‘To’, ‘So’, ‘Bl’, third line: birds ‘Vi’, ‘Te’, ‘Ar’, and ‘Au’. (b) Relative positioning of the same 14 birds during the independent flight.
(c). Matrix correlation
For dyadic leading and trailing data we calculated the Pearson product moment correlation coefficient. We used a non-parametric matrix permutation procedure introduced by Mantel [102] to produce a null-distribution for the correlation coefficient, because dyadic data involving the same individuals are not statistically independent. This procedure involved randomly reshuffling rows and columns of the data matrix according to the same permutation order and recalculating the correlation coefficient. This was repeated 104 times for each permutation test for deriving a null distribution.
3. Results
During the migration the birds formed a close flock for most of the time, though occasionally single individuals, pairs or small subgroups split off from the main group for some time, but joined the flock again. The core area of the flock, defined as the circular surface area around the centre of gravity of the flock that contained 50% of the birds, had a median radius of 3.5 m (IQR: 2.9–4.2 m). Individual birds spent between 31 and 64% of their flight time in this core area (table 1). On the fourth migratory leg 12 birds parted from the ultralight aircraft 60 min after take-off. Those birds stayed airborne for another 320 min (with two short stops), finally returning to the starting point of that leg. As this part of the migration differed from the rest in that flight direction, altitude and speed were not influenced by the ultralight aircraft, we analysed this flight bout separately and refer to it as ‘independent flight’ (in contrast to ‘guided flight’, which refers to the first three legs, and the first 60 min of the fourth leg).
During the guided flights, each bird was at some point in the foremost position in the direction of travel, though the time individual birds spent in this first position varied considerably with only two birds holding this position together for 59% of the time (table 1). During the independent flight proportions were more even, though the same two birds were in the lead position most often (31% of the flight time). Individuals differed also in their position within the flock, with some animals being overall closer to the centre of the flock, while others were more often in peripheral positions (table 1). Of special interest with respect to potential energy savings is the relative position of birds to the nearest bird ahead. Figure 2a shows for each bird the density of relative positions of the nearest bird ahead and the nearest bird behind, accumulated over the whole guided migration. Figure 2b shows the same data for the independent flight. Birds were only considered as neighbours when they were flying within an altitude range ±0.5 m of the focus bird. It can be seen that the highest density of observations is concentrated in two regions lateral and relatively close to the bird, while few birds were observed directly before or behind any other bird. This picture is in agreement with the predictions of the energy-savings hypothesis, according to which areas directly behind another bird should be avoided because of an expected downwash, while an up-wash is expected in the areas close behind the wing tip of a preceding bird. Defining an in-wake area behind another bird gives us estimates for the time birds spent in a position where they could presumably profit from the up-wash produced by the preceding bird. Likewise we can get estimates for the time that other birds in a trailing position could profit from the up-wash produced by the focus bird (table 1). Flying in the defined in-wake area was a phenomenon that was consistently observed over all legs of the migration (figure 3).
Figure 3.
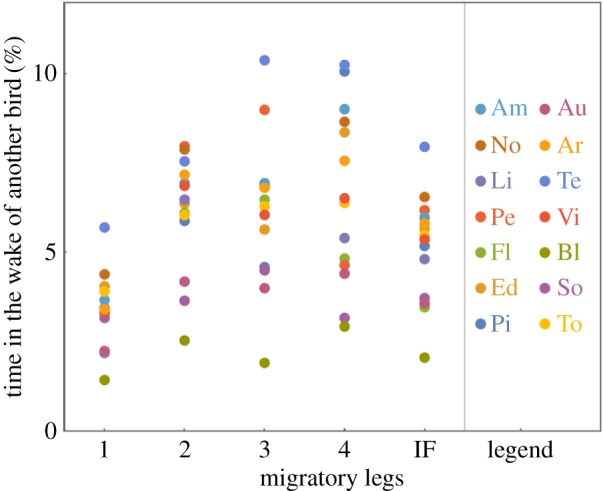
Percentage of time that birds were in the wake area of 0–3 m behind and 1.0–1.55 m lateral to another bird during the human guided flights of leg 1–4 and the independent flight (IF) of leg 4.
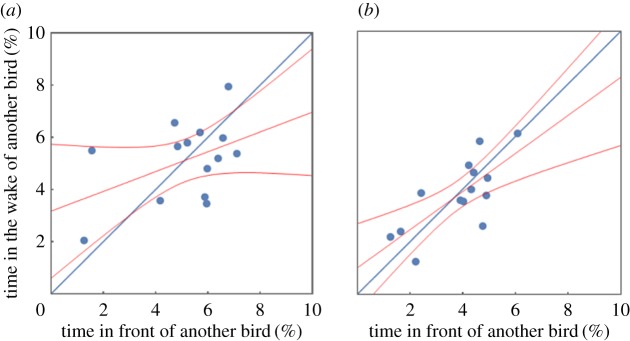
Interestingly, there is some variation in positional patterns between birds: while for most birds one sees both high densities of birds lateral ahead and behind the bird, two birds (‘No’ and ‘To’) were often flying in the wake of other birds, but rarely were other birds trailing them. If formation flight is considered as a multi-player cooperative behaviour, then these two individuals would be classified as free-riders or defectors, who profit from flying in the wake of others, but who do not provide lift for other birds. In [91] we observed that the time that birds spent in the wake of others and the time they are in front of others was highly correlated. Here, we find again a correlation between flying in the front and flying behind another bird (Pearson correlation r = 0.45, N = 14, figure 4a), though the confidence interval for this correlation is rather wide (CI95: −0.10 to 0.79), including a slope of zero, and hence delivering no additional support for this former finding. Yet, during the independent flight, we find a correlation between flying in front and flying in the wake of others (r= 0.74, CI95: 0.35–0.91, N = 14, figure 4b), corroborating findings of our previous study [91].
Figure 4.
(a) Percentage of time of the human guided flights that birds were in the wake area 0–3 m behind and 1.0–1.55 m lateral to another bird (corresponding to a wing tip spacing of −0.2 to 0.35 m) plotted against the percentage of time the same birds were trailed by another bird positioned in the in-wake area. Red lines: fit of a linear regression model and the corresponding 95% confidence interval. The blue line visualizes expected values for perfect reciprocation. (b) Percentage of time that birds were in the wake area of another bird plotted against the percentage of time another bird followed in their wake area during the independent flight phase of leg 4.
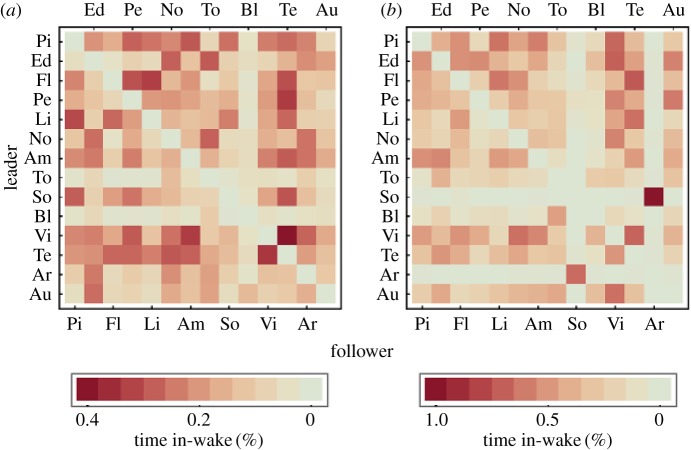
For reciprocation of in-wake flying on the dyadic level we get a Pearson product moment correlation coefficient of r = 0.60, and a Mantel matrix permutation procedure with 104 permutations shows that this value is clearly larger (4.9σ) than the expected value for randomized associations (figure 5a). This means that the time individual A spends in the wake of individual B is highly correlated with the time individual B spends in wake of individual A. For the independent flight the correlation between leading and trailing in pairs of birds was equally strong (r = 0.63, 6.6σ, figure 5b). Calculating matrix correlations for each leg separately we get r = 0.70, 4.9σ, r = 0.57, 5.0σ, r = 0.38, 3.8σ, and r = 0.27, 3.2σ, for legs 1–4, respectively, indicating that correlations between trailing and leading on a dyadic level are a consistent phenomenon over the entire migration.
Figure 5.
(a) Percentage of time of the human guided flights that specific birds were in the wake area 0–3 m behind and 1.0–1.55 m lateral to a specific bird plotted for each bird of the flock. (b) Percentage of time of the independent flight that specific birds were in the wake area 0–3 m behind and 1.0–1.55 m lateral to a specific bird.
On the mechanistic level two different cooperation strategies have been put forward as potential explanations for ensuring prolonged cooperation during the migration [91]: (i) direct reciprocity, where individuals immediately take turns on a dyadic level [87]—that is, after bird A has been trailing bird B for a while, individuals will swap positions and bird B will be allowed to fly in the wake of bird A for a time; (ii) indirect reciprocity [103], where animals do not keep track of who was trailing or leading. The latter is more likely to establish stable cooperation if groups are small [104]. These two strategies might lead to similar effects, though predictions for both are not entirely overlapping. In the case of indirect reciprocity we would expect to see high levels of reciprocation on the individual level (i.e. an individual that shows high levels of trailing other birds should also show high levels of leading), but not necessarily on the dyadic level, because different individuals could be found in the front and the wake area of a bird. In the case of dyadic reciprocity, we would expect correlations on both the dyadic and the individual level. In [91] the authors found evidence for both correlations, which did not allow them to distinguish between these two scenarios. Here, we see clear correlations on the dyadic level but only a weak correlation on the individual level during independent flight and no convincing correlation during the human guided part of the migration. This pattern hints at direct reciprocation being at work. Yet, as these are correlational data only and as the confidence intervals are rather wide, indirect reciprocity cannot be definitely excluded as a potentially involved mechanism.
4. Conclusion
Two behavioural adaptations to physiological constraints of long-distance flights—soaring–gliding and formation flight—are directly linked with social organization during migration. They either ultimately lead to the formation of large aggregations of birds—as it is the case for a soaring–gliding flight style—or implicitly necessitate the formation of groups—as it is the case for formation flight.
Flying in aerodynamically advantageous formations is a cooperative task. Establishing this task can be facilitated by relatedness between the individuals of a flock or by reciprocation. In both cases this requires small groups of birds staying together for a longer time or the whole migration. Here, we have provided evidence that cooperation during formation flight in northern bald ibis is based on dyadic reciprocation throughout the entire migration. Consistent correlations between leading and trailing times were also found during independent flight when animals were not guided by the ultralight aircraft.
While morphological and physiological adaptations to migration have been studied extensively, consequences for the social behaviour and social organization during the migration have received far less attention. Ten years ago Ramenofsky & Wingfield [105] summarized that almost nothing is known about the mechanisms underlying social adjustments associated with migration. The situation has improved little since then, though recent advances in monitoring techniques and several on-going research initiatives give reason to hope that this will change in the near future [106,107].
Supplementary Material
Acknowledgements
We thank Martin Wikelski for valuable advice and lending us equipment, all members of Waldrappteam, notably Anne-Gabriela Schmalstieg and Corinna Esterer, the foster parents of the hand-reared birds, and Christian Sperger for contributing to the data collection during the human guided migration. We are grateful to Ross Crates and three anonymous reviewers for comments on the manuscript.
Ethics
Study subjects were captive bred northern bald ibis (Geronticus eremita), in the property of the association Förderverein Waldrappteam (LIFE+12-BIO AT 000143). Valid CITES documents are held by Waldrappteam according to European regulations. All research measures adhere to Austrian legal regulations.
Data accessibility
Positional data (GPS) of the birds used in this manuscript are archived on Movebank: https://www.movebank.org/. Movebank is a free, online database of animal tracking data hosted by the Max Planck Institute for Ornithology, Germany.
Authors' contributions
B.V. and J.F. conceived the study, J.F. orchestrated the fieldwork, B.V. analysed the data and B.V. wrote the manuscript with input from J.F.
Competing interests
We declare no competing interests.
Funding
Supported with 50% contribution of the LIFE financial instrument of the European Union (LIFE+12-BIO_AT_000143, LIFE Northern Bald Ibis).
References
- 1.Alerstam T. (transl. Christie D.). 1990. Bird migration. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Ferguson-Lees J, Christie DA. 2001. Raptors of the world. Boston, MA: Houghton Mifflin Harcourt. [Google Scholar]
- 3.Gill RE, et al. 2009. Extreme endurance flights by landbirds crossing the Pacific Ocean: ecological corridor rather than barrier? Proc. R. Soc. B 276, 447–457. ( 10.1098/rspb.2008.1142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dingle H. 2014. Migration: the biology of life on the move. New York, NY: Oxford University Press. [Google Scholar]
- 5.Rappole JH, Warner DW. 1976. Relationships between behavior, physiology and weather in avian transients at a migration stopover site. Oecologia 26, 193–212. ( 10.1007/BF00345289) [DOI] [PubMed] [Google Scholar]
- 6.Moore F, Mabey S, Woodrey M. 2003. Priority access to food in migratory birds: age, sex and motivational asymmetries. In Avian migration (eds Berthold P, Gwinner E, Sonnenschein E), pp. 281–292. Heidelberg, Germany: Springer. [Google Scholar]
- 7.Piersma T. 1990. Pre-migratory ‘fattening’ usually involves more than the deposition of fat alone. Ringing Migr. 11, 113–115. ( 10.1080/03078698.1990.9673972) [DOI] [Google Scholar]
- 8.Schüz E. 1950. Früh-Auflassung ostpreussischer Jungstörche in West-Deutschland durch die Vogelwarte Rossitten 1933–1936. Bonner Zool. Beiträge 1, 239–253. [Google Scholar]
- 9.Williams CS, Kalmbach E. 1943. Migration and fate of transported juvenile waterfowl. J. Wildl. Manage. 7, 163–169. ( 10.2307/3795720) [DOI] [Google Scholar]
- 10.Ellis DH, Sladen WJ, Lishman WA, Clegg KR, Duff JW, Gee GF, Lewis JC. 2003. Motorized migrations: the future or mere fantasy? Bioscience 53, 260–264. ( 10.1641/0006-3568(2003)053%5B0260:MMTFOM%5D2.0.CO;2) [DOI] [Google Scholar]
- 11.Thorup K, Rabøl J. 2001. The orientation system and migration pattern of long-distance migrants: conflict between model predictions and observed patterns. J. Avian Biol. 32, 111–119. ( 10.1034/j.1600-048X.2001.320203.x) [DOI] [Google Scholar]
- 12.Biro D, Sumpter DJ, Meade J, Guilford T. 2006. From compromise to leadership in pigeon homing. Curr. Biol. 16, 2123–2128. ( 10.1016/j.cub.2006.08.087) [DOI] [PubMed] [Google Scholar]
- 13.Couzin ID, Krause J, Franks NR, Levin SA. 2005. Effective leadership and decision-making in animal groups on the move. Nature 433, 513–516. ( 10.1038/nature03236) [DOI] [PubMed] [Google Scholar]
- 14.Conradt L, Roper TJ. 2003. Group decision-making in animals. Nature 421, 155–158. ( 10.1038/nature01294) [DOI] [PubMed] [Google Scholar]
- 15.Sumpter DJ. 2006. The principles of collective animal behaviour. Phil. Trans. R. Soc. B 361, 5–22. ( 10.1098/rstb.2005.1733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simons AM. 2004. Many wrongs: the advantage of group navigation. Trends Ecol. Evol. 19, 453–455. ( 10.1016/j.tree.2004.07.001) [DOI] [PubMed] [Google Scholar]
- 17.Lissaman P, Schollenberger CA. 1970. Formation flight of birds. Science 168, 1003–1005. ( 10.1126/science.168.3934.1003) [DOI] [PubMed] [Google Scholar]
- 18.Hummel D. 1978. Die Leistungsersparnis in Flugformationen von Vögeln mit Unterschieden in Größe, Form und Gewicht. J. Ornithol. 119, 52–73. ( 10.1007/BF01642971) [DOI] [Google Scholar]
- 19.Hamilton WD. 1971. Geometry for the selfish herd. J. Theor. Biol. 31, 295–311. ( 10.1016/0022-5193(71)90189-5) [DOI] [PubMed] [Google Scholar]
- 20.Pulliam HR. 1973. On the advantages of flocking. J. Theor. Biol. 38, 419–422. ( 10.1016/0022-5193(73)90184-7) [DOI] [PubMed] [Google Scholar]
- 21.Pulliam HR, Caraco T. 1984. Living in groups: is there an optimal group size. In Behavioural ecology: an evolutionary approach (eds Davies NB, Krebs JR), pp. 122–147. Oxford, UK: Blackwell Scientific. [Google Scholar]
- 22.Krause J, Ruxton GD. 2002. Living in groups. Oxford, UK: Oxford University Press. [Google Scholar]
- 23.Newton I. 2010. The migration ecology of birds. London, UK: Academic Press. [Google Scholar]
- 24.Piersma T, Zwarts L, Bruggemann JH. 1990. Behavioural aspects of the departure of waders before long-distance flights: flocking, vocalizations, flight paths and diurnal timing. Ardea 78, 157–184. [Google Scholar]
- 25.Lorenz K. 1935. Der Kumpan in der Umwelt des Vogels. J. Ornithol. 83, 289–413. ( 10.1007/BF01905572) [DOI] [Google Scholar]
- 26.Pennycuick CJ. 2008. Modelling the flying bird. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 27.Alerstam T, Hedenström A. 1998. The development of bird migration theory. J. Avian Biol. 29, 343–369. ( 10.2307/3677155) [DOI] [Google Scholar]
- 28.Alerstam T, Lindström Å. 1990. Optimal bird migration: the relative importance of time, energy, and safety. In Bird migration: physiology and ecophysiology (ed. Gwinner E.), pp. 331–351. Berlin, Germany: Springer. [Google Scholar]
- 29.Lindström Å, Alerstam T. 1992. Optimal fat loads in migrating birds: a test of the time-minimization hypothesis. Am. Nat. 140, 477–491. ( 10.1086/285422) [DOI] [PubMed] [Google Scholar]
- 30.Weber TP, Houston AI. 1997. Flight costs, flight range and the stopover ecology of migrating birds. Anim. Ecol. 66, 297–306. ( 10.2307/5976) [DOI] [Google Scholar]
- 31.Both C, Van Turnhout CA, Bijlsma RG, Siepel H, Van Strien AJ, Foppen RP. 2009. Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proc. R. Soc. B 277, 1259–1266. ( 10.1098/rspb.2009.1525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rappole J. 2013. The avian migrant: the biology of bird migration. New York, NY: Columbia University Press. [Google Scholar]
- 33.O'Reilly KM, Wingfield JC. 1995. Spring and autumn migration in Arctic shorebirds: same distance, different strategies. Am. Zool. 35, 222–233. ( 10.1093/icb/35.3.222) [DOI] [Google Scholar]
- 34.Moore FR, Yong W. 1991. Evidence of food-based competition among passerine migrants during stopover. Behav. Ecol. Sociobiol. 28, 85–90. ( 10.1007/BF00180984) [DOI] [Google Scholar]
- 35.Carpenter FL, Hixon MA, Russell RW, Paton DC, Temeles EJ. 1993. Interference asymmetries among age-sex classes of rufous hummingbirds during migratory stopovers. Behav. Ecol. Sociobiol. 33, 297–304. ( 10.1007/BF00172927) [DOI] [Google Scholar]
- 36.Carpenter FL, Hixon MA, Temeles EJ, Russell RW, Paton DC. 1993. Exploitative compensation by subordinate age-sex classes of migrant rufous hummingbirds. Behav. Ecol. Sociobiol. 33, 305–312. ( 10.1007/BF00172928) [DOI] [Google Scholar]
- 37.Burger J, Hahn DC, Chase J. 1979. Aggressive interactions in mixed-species flocks of migrating shorebirds. Anim. Behav. 27, 459–469. ( 10.1016/0003-3472(79)90183-0) [DOI] [Google Scholar]
- 38.Recher HF, Recher JA. 1969. Some aspects of the ecology of migrant shorebirds. II. Aggression. Wilson Bull. 81, 140–154. [Google Scholar]
- 39.Stawarczyk T. 1984. Aggression and its suppression in mixed-species wader flocks. Ornis Scand. 15, 23–37. ( 10.2307/3675999) [DOI] [Google Scholar]
- 40.Cresswell W. 1994. Age-dependent choice of redshank (Tringa totanus) feeding location: profitability or risk? J. Anim. Ecol. 63, 589–600. ( 10.2307/5225) [DOI] [Google Scholar]
- 41.Alerstam T. 1978. Reoriented bird migration in coastal areas: dispersal to suitable resting grounds? Oikos 30, 405–408. ( 10.2307/3543491) [DOI] [Google Scholar]
- 42.Lindström Å, Alerstam T. 1986. The adaptive significance of reoriented migration of chaffinches Fringilla coelebs and bramblings F. montifringilla during autumn in southern Sweden. Behav. Ecol. Sociobiol. 19, 417–424. ( 10.1007/BF00300544) [DOI] [Google Scholar]
- 43.Alves JA, Gunnarsson TG, Potts PM, Sutherland WJ, Gill JA. 2013. Sex-biases in distribution and resource use at different spatial scales in a migratory shorebird. Ecol. Evol. 3, 1079–1090. ( 10.1002/ece3.503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Catry T, Alves JA, Gill JA, Gunnarsson TG, Granadeiro JP. 2012. Sex promotes spatial and dietary segregation in a migratory shorebird during the non-breeding season. PLoS ONE 7, e33811 ( 10.1371/journal.pone.0033811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berthold P. 1975. Migration: control and metabolic physiology. Avian Biol. 5, 77–128. ( 10.1016/B978-0-12-249405-5.50010-0) [DOI] [Google Scholar]
- 46.Berthold P. 1993. Bird migration: a general survey. Oxford, UK: Oxford University Press. [Google Scholar]
- 47.Klaassen M, Biebach H. 1994. Energetics of fattening and starvation in the long-distance migratory garden warbler, Sylvia borin, during the migratory phase. J. Comp. Physiol. B 164, 362–371. ( 10.1007/BF00302551) [DOI] [Google Scholar]
- 48.Bairlein F. 2003. Nutritional strategies in migratory birds. In Avian migration, pp. 321–332. Berlin, Germany: Springer. [Google Scholar]
- 49.Piersma T, Van Gils JA. 2011. The flexible phenotype: a body-centred integration of ecology, physiology, and behaviour. Oxford, UK: Oxford University Press. [Google Scholar]
- 50.McWilliams SR, Guglielmo C, Pierce B, Klaassen M. 2004. Flying, fasting, and feeding in birds during migration: a nutritional and physiological ecology perspective. J. Avian Biol. 35, 377–393. ( 10.1111/j.0908-8857.2004.03378.x) [DOI] [Google Scholar]
- 51.Pierce BJ, McWilliams SR, O'Connor TP, Place AR, Guglielmo CG. 2005. Effect of dietary fatty acid composition on depot fat and exercise performance in a migrating songbird, the red-eyed vireo. J. Exp. Biol. 208, 1277–1285. ( 10.1242/jeb.01493) [DOI] [PubMed] [Google Scholar]
- 52.Guglielmo CG. 2010. Move that fatty acid: fuel selection and transport in migratory birds and bats. Integr. Comp. Biol. 50, 336–345. ( 10.1093/icb/icq097) [DOI] [PubMed] [Google Scholar]
- 53.Piersma T, Lindström Å. 1997. Rapid reversible changes in organ size as a component of adaptive behaviour. Trends Ecol. Evol. 12, 134–138. ( 10.1016/S0169-5347(97)01003-3) [DOI] [PubMed] [Google Scholar]
- 54.Karason WH, Pinshow B.. 1998. Changes in lean mass and in organs of nutrient assimilation in a long-distance passerine migrant at a springtime stopover site. Physiol. Biochem. Zool. 71, 435–438. [DOI] [PubMed] [Google Scholar]
- 55.Battley PF, Piersma T, Dietz MW, Tang S, Dekinga A, Hulsman K. 2000. Empirical evidence for differential organ reductions during trans-oceanic bird flight. Proc. R. Soc. Lond. B 267, 191–195. ( 10.1098/rspb.2000.0986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Butler P, Woakes A. 1990. The physiology of bird flight. In Bird migration, pp. 300–318. Berlin, Germany: Springer. [Google Scholar]
- 57.Norberg UM. 1996. Energetics of flight. In Avian energetics and nutritional ecology (ed. Carey C.), pp. 199–249. Berlin, Germany: Springer. [Google Scholar]
- 58.Sapir N, Wikelski M, McCue MD, Pinshow B, Nathan R. 2010. Flight modes in migrating European bee-eaters: heart rate may indicate low metabolic rate during soaring and gliding. PLoS ONE 5, e13956 ( 10.1371/journal.pone.0013956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katzner TE, Turk PJ, Duerr AE, Miller TA, Lanzone MJ, Cooper JL, Brandes D, Tremblay JA, Lemaître J. 2015. Use of multiple modes of flight subsidy by a soaring terrestrial bird, the golden eagle Aquila chrysaetos, when on migration. J. R. Soc. Interface 12, 20150530 ( 10.1098/rsif.2015.0530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hedenstrom A. 1993. Migration by soaring or flapping flight in birds: the relative importance of energy cost and speed. Phil. Trans. R. Soc. Lond. B 342, 353–361. ( 10.1098/rstb.1993.0164) [DOI] [Google Scholar]
- 61.Pennycuick C. 1975. Mechanics of flight. Avian Biol. 5, 1–75. ( 10.1016/B978-0-12-249405-5.50009-4) [DOI] [Google Scholar]
- 62.Leshem Y, Yom-Tov Y. 1998. Routes of migrating soaring birds. Ibis 140, 41–52. ( 10.1111/j.1474-919X.1998.tb04539.x) [DOI] [Google Scholar]
- 63.Leshem Y, Bahat O. 1999. Flying with the birds. Tel-Aviv, Israel: Chemed. [Google Scholar]
- 64.Wieselsberger C. 1914. Beitrag zur Erklärung des Winkelfluges einiger Zugvögel. Z. Flugtechnik Motorluftschiffahrt 5, 225–229. [Google Scholar]
- 65.May RM. 1979. Flight formations in geese and other birds. Nature 282, 778–780. ( 10.1038/282778a0) [DOI] [Google Scholar]
- 66.Spedding G. 1987. The wake of a kestrel (Falco tinnunculus) in flapping flight. J. Exp. Biol. 127, 59–78. [Google Scholar]
- 67.Pennycuick CJ. 1989. Bird flight performance. Oxford, UK: Oxford University Press. [Google Scholar]
- 68.Spedding GR, Rosen MAH. 2003. A family of vortex wakes generated by a thrush nightingale in free flight in a wind tunnel over its entire natural range of flight speeds. J. Exp. Biol. 206, 2313–2344. ( 10.1242/jeb.00423) [DOI] [PubMed] [Google Scholar]
- 69.KleinHeerenbrink M, Warfvinge K, Hedenström A. 2016. Wake analysis of aerodynamic components for the glide envelope of a jackdaw (Corvus monedula). J. Exp. Biol. 219, 1572–1581. ( 10.1242/jeb.132480) [DOI] [PubMed] [Google Scholar]
- 70.Henningsson P, Hedenström A. 2011. Aerodynamics of gliding flight in common swifts. J. Exp. Biol. 214, 382–393. ( 10.1242/jeb.050609) [DOI] [PubMed] [Google Scholar]
- 71.Henningsson P, Muijres FT, Hedenström A. 2011. Time-resolved vortex wake of a common swift flying over a range of flight speeds. J. R. Soc. Interface 8, 807–816. ( 10.1098/rsif.2010.0533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spedding GR, Hedenström A. 2009. PIV-based investigations of animal flight. Exp. Fluids 46, 749–763. ( 10.1007/s00348-008-0597-y) [DOI] [Google Scholar]
- 73.Hainsworth FR. 1987. Precision and dynamics of positioning by Canada geese flying in formation. J. Exp. Biol. 128, 445–462. [Google Scholar]
- 74.Badgerow JP, Hainsworth FR. 1981. Energy savings through formation flight? A re-examination of the vee formation. J. Theor. Biol. 93, 41–52. ( 10.1016/0022-5193(81)90055-2) [DOI] [Google Scholar]
- 75.Cutts C, Speakman J. 1994. Energy savings in formation flight of pink-footed geese. J. Exp. Biol. 189, 251–261. [DOI] [PubMed] [Google Scholar]
- 76.Gould LL, Heppner F. 1974. The vee formation of Canada geese. Auk 91, 494–506. ( 10.2307/4084469) [DOI] [Google Scholar]
- 77.Higdon JJL, Corrsin S. 1978. Induced drag of a bird flock. Am. Nat. 112, 727–744. ( 10.1086/283314) [DOI] [Google Scholar]
- 78.Maeng J-S, Park J-H, Jang S-M, Han S-Y. 2013. A modeling approach to energy savings of flying Canada geese using computational fluid dynamics. J. Theor. Biol. 320, 76–85. ( 10.1016/j.jtbi.2012.11.032) [DOI] [PubMed] [Google Scholar]
- 79.Hainsworth FR. 1989. Wing movements and positioning for aerodynamic benefit by Canada geese flying in formation. Can. J. Zool. 67, 585–589. ( 10.1139/z89-084) [DOI] [Google Scholar]
- 80.Badgerow JP. 1988. An analysis of function in the formation flight of Canada geese. Auk 105, 749–755. [Google Scholar]
- 81.Speakman J, Banks D. 1998. The function of flight formations in greylag geese Anser anser; energy saving or orientation? Ibis 140, 280–287. ( 10.1111/j.1474-919X.1998.tb04390.x) [DOI] [Google Scholar]
- 82.Weimerskirch H, Martin J, Clerquin Y, Alexandre P, Jiraskova S. 2001. Energy saving in flight formation. Nature 413, 697–698. ( 10.1038/35099670) [DOI] [PubMed] [Google Scholar]
- 83.Portugal SJ, Hubel TY, Fritz J, Heese S, Trobe D, Voelkl B, Hailes S, Wilson AM, Usherwood JR. 2014. Upwash exploitation and downwash avoidance by flap phasing in ibis formation flight. Nature 505, 399–402. ( 10.1038/nature12939) [DOI] [PubMed] [Google Scholar]
- 84.Hummel D. 1983. Aerodynamic aspects of formation flight in birds. J. Theor. Biol. 104, 321–347. ( 10.1016/0022-5193(83)90110-8) [DOI] [Google Scholar]
- 85.Andersson M, Wallander J. 2004. Kin selection and reciprocity in flight formation? Behav. Ecol. 15, 158–162. ( 10.1093/beheco/arg109) [DOI] [Google Scholar]
- 86.Maynard-Smith J. 1974. The theory of games and the evolution of animal conflicts. J. Theor. Biol. 47, 209–221. ( 10.1016/0022-5193(74)90110-6) [DOI] [PubMed] [Google Scholar]
- 87.Trivers RL. 1971. The evolution of reciprocal altruism. Q. Rev. Biol. 46, 35–57. ( 10.1086/406755) [DOI] [Google Scholar]
- 88.Alonso JA, Alonso JC. 1993. Age-related differences in time budgets and parental care in wintering common cranes. Auk 110, 78–88. [Google Scholar]
- 89.Scott D. 1980. Functional aspects of prolonged parental care in Bewick's swans. Anim. Behav. 28, 938–952. ( 10.1016/S0003-3472(80)80156-4) [DOI] [Google Scholar]
- 90.Ely CR. 1993. Family stability in greater white-fronted geese. Auk 110, 425–435. ( 10.2307/4088407) [DOI] [Google Scholar]
- 91.Voelkl B, Portugal SJ, Unsöld M, Usherwood JR, Wilson AM, Fritz J. 2015. Matching times of leading and following suggest cooperation through direct reciprocity during V-formation flight in ibis. Proc. Natl Acad. Sci. USA 112, 2115–2120. ( 10.1073/pnas.1413589112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Voelkl B. 2010. The ‘Hawk-Dove’ game and the speed of the evolutionary process in small heterogeneous populations. Games 1, 103–116. ( 10.3390/g1020103) [DOI] [Google Scholar]
- 93.Noe R, Voelkl B. 2013. Cooperation and biological markets: the power of partner choice. In Cooperation and its evolution (ed. Sterelny K.), pp. 131–151. Cambridge, MA: MIT Press. [Google Scholar]
- 94.Seiler P, Pant A, Hedrick J. 2003. A systems interpretation for observations of bird V-formations. J. Theor. Biol. 221, 279–287. ( 10.1006/jtbi.2003.3191) [DOI] [PubMed] [Google Scholar]
- 95.Sillett TS, Holmes RT. 2002. Variation in survivorship of a migratory songbird throughout its annual cycle. J. Anim. Ecol. 71, 296–308. ( 10.1046/j.1365-2656.2002.00599.x) [DOI] [Google Scholar]
- 96.Menu S, Gauthier G, Reed A. 2005. Survival of young greater snow geese (Chen caerulescens atlantica). Auk 122, 479–496. ( 10.1642/0004-8038(2005)122%5B0479:SOYGSG%5D2.0.CO;2) [DOI] [Google Scholar]
- 97.Owen M, Black JM. 1991. The importance of migration mortality in non-passerine birds. In Bird population studies: relevance to conservation and management (eds Perrins CM, Lebreton JD, Hirons GJM), pp. 360–372. Oxford, UK: Oxford University Press. [Google Scholar]
- 98.Petit DR, Bildstein KL. 1986. Development of formation flying in juvenile white ibises (Eudocimus albus). Auk 103, 244–246. [Google Scholar]
- 99.Serra G, Lindsell JA, Peske L, Fritz J, Bowden CGR, Bruschini C, Welch G, Tavares J, Wondafrash M. 2014. Accounting for the low survival of the critically endangered northern bald ibis Geronticus eremita on a major migratory flyway. Oryx 49, 312–320. ( 10.1017/S0030605313000665) [DOI] [Google Scholar]
- 100.Fritz J, Riedler B. 2010. Neue Hoffnung für das Überleben einer hoch bedrohten Zugvogelart im Mittleren Osten: Freisetzung von Jungvögeln bei den letzten migrierenden Waldrappen in Syrien. Vogelwarte 48, 417–418. [Google Scholar]
- 101.Fritz J, Kramer R, Hoffmann W, Unsöld M. 2017. Project Life+reason for hope: the reintroduction of the northern bald ibis (Geronticus eremita) in Central Europe Intl. Zoo Yb. 51, 1–17. ( 10.1111/izy.12163) [DOI] [Google Scholar]
- 102.Mantel N. 1967. The detection of disease clustering and a generalized regression approach. Cancer Res. 27, 209–220. [PubMed] [Google Scholar]
- 103.Nowak MA, Sigmund K. 2005. Evolution of indirect reciprocity. Nature 437, 1291–1298. ( 10.1038/nature04131) [DOI] [PubMed] [Google Scholar]
- 104.Voelkl B. 2015. The evolution of generalized reciprocity in social interaction networks. J. Theor. Popul. Biol. 104, 17–25. ( 10.1016/j.tpb.2015.06.005) [DOI] [PubMed] [Google Scholar]
- 105.Ramenofsky M, Wingfield JC. 2007. Regulation of migration. Bioscience 57, 135–143. ( 10.1641/B570208) [DOI] [Google Scholar]
- 106.Chin DD, Lentink D. 2016. Flapping wing aerodynamics: from insects to vertebrates. J. Exp. Biol. 219, 920–932. ( 10.1242/jeb.042317) [DOI] [PubMed] [Google Scholar]
- 107.Herbert-Read JE. 2016. Understanding how animal groups achieve coordinated movement. J. Exp. Biol. 219, 2971–2983. ( 10.1242/jeb.129411) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Positional data (GPS) of the birds used in this manuscript are archived on Movebank: https://www.movebank.org/. Movebank is a free, online database of animal tracking data hosted by the Max Planck Institute for Ornithology, Germany.