Abstract
Though morphologically very similar, equids across the extant species occupy ecological niches that are surprisingly non-overlapping. Occupancy of these distinct niches appears related to subtle physiological and behavioural adaptations which, in turn, correspond to significant differences in the social behaviours and emergent social systems characterizing the different species. Although instances of intraspecific behavioural variation in equids demonstrate that the same body plan can support a range of social structures, each of these morphologically similar species generally shows robust fidelity to its evolved social system. The pattern suggests a subtle relationship between physiological phenotypes and behavioural flexibility. While environmental conditions can vary widely within relatively short temporal or spatial scales, physiological changes and changes to the behaviours that regulate physiological processes, are constrained to longer cycles of adaptation. Physiology is then the limiting variable in the interaction between ecological variation and behavioural and socio-structural flexibility. Behavioural and socio-structural flexibility, in turn, will generate important feedbacks that will govern physiological function, thus creating a coupled web of interactions that can lead to changes in individual and collective behaviour. Longitudinal studies of equid and other large-bodied ungulate populations under environmental stress, such as those discussed here, may offer the best opportunities for researchers to examine, in real time, the interplay between individual behavioural plasticity, socio-structural flexibility, and the physiological and genetic changes that together produce adaptive change.
This article is part of the themed issue ‘Physiological determinants of social behaviour in animals’.
Keywords: equids, behavioural flexibility, plains zebra, Grevy's zebra, socioecological model, social network analysis
1. Introduction
Socioecology is concerned with the ways that environments shape the evolution of animal societies. In its early days, this meant that the field's theories focused largely on unidirectional relationships between environmental conditions, such as resource distribution or predation pressure, and social characteristics such as group size or mating system [1]. Over time, however, this picture of environmental inputs and social outputs grew ever more complicated, as socioecologists increasingly agreed that animal societies are influenced by complex, multidirectional interactions among genetic [2,3], environmental [4], life-historical [5] and phylogenetic [6,7] factors. Like all models, socioecological models threatened to become less straightforwardly predictive as they gained descriptive accuracy and intricacy [8,9]. The field therefore matured towards an ongoing tension between the search for a clean, unified theory of social-system evolution, and the requirement that any such theory should address the apparently messy inconsistencies of real natural history [10–12].
Behavioural flexibility is one of the greatest sources of this apparent messiness. Some of the individual behaviours that compose social structures—such as the ritualized displays that facilitate mate-choice in many species [13]—have long appeared to be largely genetically fixed. However, the more carefully field scientists have examined these and other features of animal societies such as mating systems, grouping and ranging patterns [14,15], the more examples have emerged of individuals, subgroups or populations that flexibly alter individual or collective social behaviours in response to short-term contextual variation [14]. A classic example is the golden-winged sunbird (Drepanorhynchus reichenowi), whose mating system shifts from resource-defence polygyny to non-territorial, dominance-based male–male competition when the availability of nectar-rich flowers drops [16]. Such shifts simultaneously ratify and scramble the socioecological model. On one hand, the sunbird's system of male–male competition reshapes itself in direct response to changes in resource abundance; on the other hand, the speed of the response challenges the feasibility of tracing a clear relationship between environmental features and evolved social structures. The sunbird is far from unique. Contextually plastic individual behaviour, cascading into population-wide variation of social structures such as mating systems, has been found in a vast array of species (e.g. in prairie voles (Microtus ochrogaster) [17]; in burying beetles (Nicrophorus vespilloides) [18]; in oribi (Ourebia ourebi) [19]). Such widespread flexibility complicates any potential model of the evolutionary relationship between ecology and sociality [10]. How do selective forces shape animal societies if individuals and populations can flexibly accommodate environmental variation within a single generation, lifespan or moment?
Insofar as species are flexible enough to reconfigure social structures when environmental conditions turn unfavourable, the challenge is to identify the targets that natural selection can act upon to shape component behaviours that form social systems. Traits that constrain within-lifespan malleability would be sites of more intense selective pressure and, therefore, key elements of adaptation and speciation. Comparative studies within taxa, where patterns of variation in habitat, social system and physiology can be mapped onto phylogenetic relationships [20,21], have attempted to isolate those targets of selection. The ungulate clade—comprising species with diverse body sizes and social systems, occupying equally diverse habitats—is a natural testbed for this kind of comparative study [22–27]. Following Jarman's foundational study relating aspects of antelope social structures (group size, reproductive behaviour, range) to variation in habitats, diets and morphological traits [23], behavioural ecologists used the ungulates to test increasingly complex socioecological models [26–29]. And, as in the rest of the field, those seeking to model causal evolutionary relationships between ungulate ecologies, physiologies and social structures have grappled with confoundingly complex intra- and interspecific variation in social behaviour [27].
To narrow the scope of species studied without losing access to the full range of that confounding variability, the genus Equus offers a valuable model comparative system. The seven extant equid species share a common body plan, occupy broadly similar habitats and consume mostly grasses and herbaceous vegetation, yet there is strong across-species variation in social systems. Equids have demonstrated intraspecific social variability much like that of the sunbird, with populations adopting fundamentally different social systems in response to fine-scale ecological variation [30]. At the same time, a small number of characteristic physiological traits, ranging and foraging behaviours, and corresponding patterns of niche exploitation and mating behaviour subdivide the equids into species with one of two basic social systems: those that form stable, closed-membership groups and one-male multi-female reproductive units; and those with looser, more ephemeral associations and mating systems based on territoriality [30,31]. Here, we will discuss findings from long-term research programmes on wild equid populations in Africa, Asia and North America. While behavioural flexibility allows all of the equids to contend with some environmental variation, broad species-typical social structures are persistent, and insights from hybrids, human-modified landscapes and anthropogenic re-introductions suggest that complex interactions between flexible social behaviours and more constrained physiological and genetic traits shape the nature and degree to which these species can shift their behaviours in response to ecological pressure.
2. Environments and social structures of equid populations and species
Alongside the golden sunbird, another striking example of intraspecific variation in social structure comes from the population of feral horses (Equus caballus) on Shackleford Banks, a coastal barrier island in North Carolina [30,32,33]. The 15 km long, narrow island (1.5 km wide at its widest point) is free of predators and features an even distribution of water but two distinct foraging habitats: grass on its eastern end is evenly distributed while in the west it is restricted to discrete patches interspersed with dense maritime forest and high dunes. The horses of Shackleford Banks have responded to this stark variation in resource distribution by varying their social behaviour to match each habitat. In the east, female horses form long-term associations in stable, closed-membership groups, and males defend harems and, in some cases, territories. In the west, female relationships are ephemeral, group sizes and their weak persistence are governed by the size of grazing patches, and wandering males pursue short-term mating opportunities with oestrus females. Though the intraspecific aspect of the Shackleford Banks example demonstrates ‘surprising’ behavioural flexibility, these micro-societies appear to follow straightforwardly from their native environmental constraints, especially since there are no physical barriers preventing individuals from moving from one end of the island to the other. In the comparatively harsh environment of the western island before the vegetation matured, the imperative to access patchily distributed food prohibited females from associating in stable groups and required males to follow individual receptive females from patch to patch. In the east, where food was and remains continuous, females can forage uninterrupted and consequently can form stable groups. They then benefit, in increased foraging time, from the vigilance and protection-from-harassment provided by their group's stallion. Those stallions that defend territories further monopolize access to their females and enjoy enhanced reproductive benefits themselves. Specific details of this ecologically driven variation in social structures also illustrate the socio-ecological model's [7] definition of social systems as emergent properties of individual behaviours: females in the weakly associating western population perform less affiliative mutual grooming; males in the harem-forming eastern population demarcate territories with dung [32], etc. In other words, the horses of Shackleford Banks exemplify the puzzling malleability of individual and emergent collective behaviour in response to ecological variation. At the same time, the contours of their habitat-dependent sociality show the Shackleford horses behaving like good socioecologists—adapting social structure to meet first the constraints of resource distribution, then the distribution of females, and finally influences of inter- and intrasexual competition [30].
While the Shackleford horses exemplify intraspecific flexibility with regard to mating systems and association patterns, equids as a clade show a robust fidelity to the social structures that characterize each member species [30,34]. Wild equids occupy two major categories of social system. The horses (Equus ferus przewalskii; E. caballus) and plains (E. quagga) and mountain zebras (E. zebra) form single- or multilevel societies based on stable, closed-membership groups of females and their immature offspring, bonded to a single reproductive male [30,33]. In these species, both male and female juveniles disperse from their natal groups, females to join a harem or pair with a single male, males typically to join groups of bachelors. Grevy's zebras (E. grevyi) and wild asses (E. africanus, E. hermionus, E. asinus) live in more open societies wherein relationships beyond those between mothers and foals are ephemeral. Males in these species defend territories based on access to resources, and female–female bonds, when present, are similarly resource-based, predicated on the increased watering requirements of lactating females. Those females aggregate near water sources until foals mature and they are freed to range away from both the water-point and the group in search of more abundant food [35].
Like the flexible horses of east and west Shackleford, the various equid species appear to conform to expected socioecological patterns in their evolved social systems. Those that form long-term social bonds and stable groups occupy more mesic habitats where resources are more evenly distributed and distances between sources of food and water are comparatively low. Female horses and plains zebras remain loyal to stallions that provide benefits in the form of antipredator vigilance and protection from male harassment. Plains zebra stallions form coalitions to counter pressure from bachelor groups, motivating the second (multi-harem herd) level of that species' social organization [35,36]. The more loosely structured equid societies of Grevy's zebras and wild asses reflect the more desert-like conditions in those species' ancestral ranges. In the xeric northeast African landscape where Grevy's zebra were once most abundant, the hot, dry deserts where African asses are found, and the hot and cold Near Eastern and Asian deserts where most populations of Asiatic asses range, highly dispersed water sources and patchily distributed forage seem to have dictated these species' adaptive social structures. Female sociality is largely driven by the differing water requirements of individuals in different reproductive states. Lactating females with young offspring must remain near water and form their most stable (though still impermanent) aggregations during this period of constrained ranging. Those without young foals range widely and without loyalty to stable groups in search of the best possible forage. Mature males therefore compete for territories based on those territories' proximity to either travel routes to and from water or, for less dominant males, the presence of high-quality forage farther from a water source [33].
At the level of these large-scale patterns, the social behaviour and social structures of equid species appear to illustrate a clean, proto-socioecological model of environmental drivers and social outcomes. But that simple picture cannot be the whole story. For one thing, the apparent symmetry between the Shackleford Banks phenomenon and the larger pattern of species-level sociality belies an inherent conflict. If equids are as flexibly responsive to environmental conditions as they appear to be on Shackleford, then across multiple populations of equids we should expect to see—not tidy species-typical sociality—but a range of variation in response to ecological variance. As environmental challenges push individuals to physiological limits, do changes in individual and collective behaviour lead to novel social variants, or those that predictably emerge from existing rules? If the general rule of equid sociality is that each species sticks largely to its species-typical social system (as argued by Linklater [34]), then we should ask what sorts of traits constrain behavioural flexibility, anchoring equid populations to their ancestral social systems in most instances. A closer look at some of the distinct adaptations that equids have made to their respective environments, and a number of special cases of equid populations under unusual ecological circumstances, will reveal more about the interactions between environments and equids' physiological and behavioural traits.
3. Same general body plan, surprisingly distinct niches
The range of morphological variation across the seven extant equid species is slender. All equids are large-headed, long-necked and medium-to-large bodied. Long legs end in a single hoof and contain a network of ligaments, muscles and tendons called a ‘stay apparatus’ which allows the leg to lock while extended, permitting equids to stand for long stretches while relaxing the muscles and expending comparatively little energy. As hindgut fermenters, equids extract nutrients from vegetation in a single chambered-stomach followed by a caecum. Though relatively inefficient compared to ruminant digestion, hindgut fermentation, combined with the energetic savings afforded by the stay apparatus, permits equids to consume large volumes of low-quality food to fuel and transport large bodies over long distances [37,38].
This common physiology corresponds to broadly similar habitat use. All equids are generally grass eaters, though some incorporate a moderate amount of browse into their diets [39], and all inhabit relatively open landscapes—savannahs, grasslands and deserts. However, a recent application of principal component analysis (PCA) characterizing the niches occupied by each wild equid species found surprisingly little overlap in those niches as defined by the environmental features that shape the abundance and quality of water and vegetation: temperature and precipitation [36] (figure 1).
Figure 1.
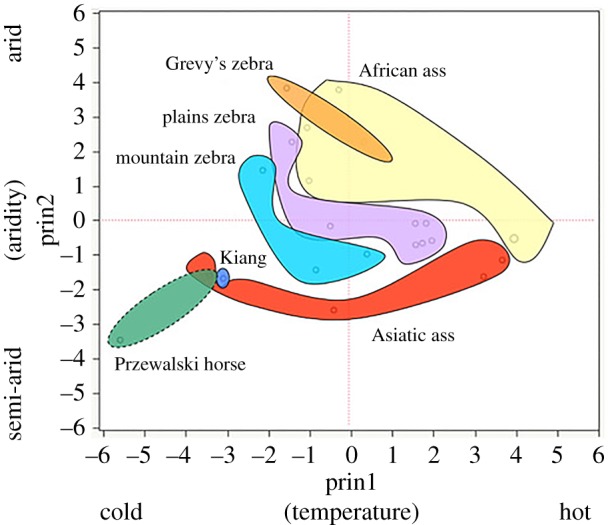
Equid niches as characterized by PCA analysis of the monthly temperatures and precipitation together with the number of rainy days in areas occupied by wild populations of each species during four months (January, May, August and October). Niche boundaries are depicted with minimum convex polygons (figure 1 adapted from Rubenstein et al. [36]).
In a two-dimensional niche space defined by aridity and temperature, the fundamental niches of the seven equid species were strikingly distinct. Only two species pairings—African asses with Grevy's zebra and Przewalski's horses with Asiatic asses—showed any overlap at all. And only one of these cases—that of the Asiatic asses and reintroduced populations of Przewalski's horses in the Gobi and Dzungarian deserts—corresponds to actual sympatry of two species. In other cases of sympatry, the co-occurring species' distinct adaptations related to diet, ranging patterns and water use appear to mitigate competition.
Though Grevy's and plains zebras overlap in the mesic scrub- and grasslands of central Kenya, this overlap is partly due to anthropogenic pressures of extensive livestock herding that have largely pushed Grevy's zebras south from an ancestral range that once covered northern Ethiopia, southwest Somalia and South Sudan [40,41]. Their more northern, ancestral range was more xeric, characterized by dispersed sources of water and vegetation. Grevy's are consequently more arid-adapted than plains zebras, possessing a suite of traits and behaviours that facilitate long travel between dispersed resources. Larger bodies permit longer stretches between drinks of water—non-lactating females only need to drink every other day [42]. Their more species-rich diets [39] reflect the need to forage broadly when food is sparser and less predictable. Behaviours related to resource consumption further enhance Grevy's zebras' abilities to thrive in relative austerity: Grevy's foals suckle less frequently than other equid foals, are less active and accelerate the transition to adult food relative to other equids—adaptations that somewhat reduce lactating females' dependence on abundant water [42]. Territorial Grevy's males will also guard ‘kindergarten’ groups of young foals, further freeing lactating females to travel to water while conserving the energy of offspring [31].
Like the Grevy's zebra in Africa, khulan (E. h. hemionus)—the Asiatic asses that share territory with reintroduced Przewalski's horses in China and Mongolia—are the more arid-adapted species in that sympatric dyad. While Przewalski's horses drink once per day in mesic grasslands and at least two times per day in more arid regions [43], desert dwelling khulan have been observed to go up to 2.2 days between visits to waterpoints during the hot, dry season [44]. Asiatic asses also mirror the Grevy's zebra in their adaptive foraging behaviour. They have the most species-rich diets of the Asian equids [36].
Looking closely at traits like these indicates at least two ways that the species-level interaction between environment and social structure is unlike that demonstrated by the Shackleford horses. First: deep ‘adaptive investments’, in the form of physiological traits (body size, accelerated foal development, water dependence) and behavioural traits that regulate resource exploitation (‘kindergartens’) do not only enhance the arid-adapted equids' capacities to survive in their ancestral environments. These sorts of adaptive investments also make possible the characteristic social behaviours and structures we observe in those species' social systems. Water-independence and diet diversity, for example, enable females' wide-ranging foraging strategies and permit the territorial defence behaviours of Grevy's zebra, onager or khulan males that must sometimes guard territories far from water. Second: even less malleable physiological constraints—in the form of differences among females that are lactating and those that are not—have further determined the shape of socio-structural adaptation among these arid-adapted equids. Water requirements constrain the ability of lactating females to wander widely in search of abundant forage. Consequently, the social cohesion exhibited by all female plains zebras is absent in the Grevy's zebra, forcing males to abandon bonding to either class of females in establishing territories. For dominant males, these are located near watering points so they can associate with lactating females showing postpartum oestrus and intercept continuously cycling non-lactating females periodically coming to water. The strong niche differentiation seen in the PCA analysis reflects each species' capacity to occupy and exploit a unique slice of its environment. Additionally, some of these species-specific differences result from individuals in differing reproductive states having different physiologies that modulate the way environments shape their behaviour. The behaviourally flexible Shackleford horses have tailored their social behaviour to variation in their relatively forgiving physical environment over several generations. Evolutionary time and a harsher selective regime appear to have outfitted each equid species with physiological and behavioural adaptations that further support distinct, environmentally adaptive social structures.
4. Physiology, resource uncertainty and variations on common themes
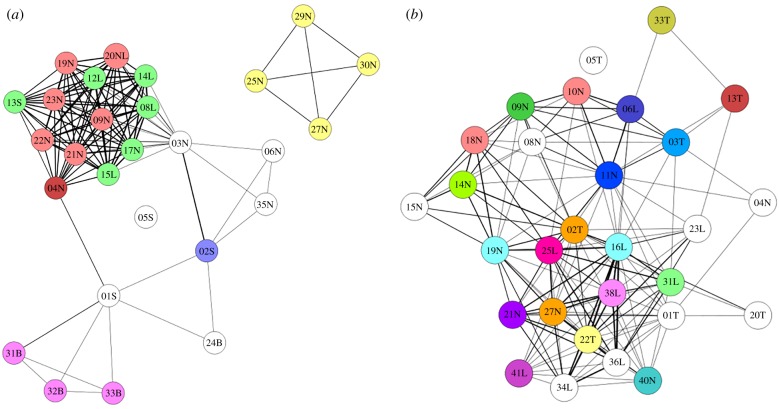
Evolved traits such as those supporting water-dependence or -independence are not likely to shift as quickly and flexibly as do more purely social behaviours. This is clearly seen when fine-grained details of two classic fission–fusion equids—Grevy's zebras and Asiatic wild asses (onagers)—are examined with respect to differences in the availability of water. With the advent of social network analysis, it is possible to examine the connectivity of individuals within social groups. In static networks constructed by aggregating large numbers of associations among individuals over time, metrics can measure the number of individuals with which particular individuals associate (degree centrality), or the fraction of close associates who themselves are close associates (cluster coefficient), or which individuals have the number of shortest paths between others flowing through them (betweenness centrality) or even the number of distinct communities that exist within a society (connected components) [45]. Dynamic networks, in which time is not aggregated, can also be created and parallel metrics to those of static networks and more can be computed. When both static [35] and dynamic networks [46] of Grevy's zebras and onagers are generated and combined on a static network graph (figure 2), what previously superficially looks similar becomes markedly different. Grevy's zebras show more sub-structuring and modularity than do onagers, which show each individual as essentially being in a module by itself. Although both Grevy's zebras and onagers are considered fission–fusion species, Grevy's individuals show more social viscosity than onagers who tend to be more individualistic, changing close associates routinely. Both live in arid lands, but in India government officials have eradicated predators and have provided reliable and regularly spaced watering points for livestock that the wild asses also use. In Kenya, no such water provisioning occurs. In fact, droughts and the unpredictable temporal and spatial availability of water remain high. When the network graphs are used to simulate the spread and retention of information about the location of critical resources, graphs with high modularity spread and prevent the loss of information best. Thus, network analyses reveal that even for two water-independent species, those that live in more unpredictable environments develop variants of fission–fusion social relationships and social structures that facilitate information-sharing, and collective actions that increase survival prospects.
Figure 2.
Static social networks of Grevy's zebras (a) and onagers (b). Lines depict edges showing social connections among individuals identified by numbers and reproductive state (S, stallion; B, bachelor male; T, territorial (breeding) male; N, non-lactating female; L, lactating female). The colourings represent communities identified by dynamic social network analysis [46]. In a dynamic network, a community is very similar to a module in a static network.
The interplay between less-flexible adaptive traits (such as water-independent physiology) and more malleable social traits, like the grouping behaviour seen in these social network analyses, has implications for the overall social flexibility of a species, especially when equids are moved to novel environments.
5. Behavioural flexibility and physiological constraint in two cases of anthropogenically induced sympatricity
If ecologically driven physiological and behavioural adaptations support and anchor equids' social systems in their ancestral environments, what happens to sociality when species leave those environments and encounter ecological conditions for which they are less well suited? The case of onagers and Grevy's zebras described in §4 suggests that behavioural flexibility allows species to tune social relationships to minimize harm when their abilities to meet physiological needs are challenged by unpredictability. At the same time, two cases of anthropogenically induced sympatry—one in the Asian equids and the other in African zebras—suggest the constraints that physiology may place on behavioural flexibility.
Wild Przewalski's horses were extirpated from Asiatic rangelands in the 1960s, and have subsequently been reintroduced in a number of ecologically distinct areas where pre-extinction sightings occurred. Of these, the habitat type that appears to best support its reintroduced population is that of the cold, mesic Mongolian steppes [47]. However, other populations have been placed in the arid Kalamaili Nature Reserve, China, and the Great Gobi B Strictly Protected Area, Mongolia, where they are sympatric with native khulan (wild ass) populations. These sites are proximate to the locations where Przewalski's horses were last sighted in the wild before extirpation, but it is generally accepted that those last desert populations were making the best of marginal habitat at the edge of human disturbance [48–50]. Where they overlap with khulan, the larger-bodied and more water-dependent Przewalski's horses seemingly outcompete and displace the smaller Asiatic asses at waterpoints [36,43]. Desert conditions force the mesic-adapted horses to drink multiple times during the day [43], and so they restrict their ranging to the areas around preferred waterholes which they appear to entirely monopolize during daylight hours—the less formidable khulan avoid waterpoints when horses are present and shift their drinking to poorer, more saline waterpoints during the day, visiting the preferred sites only at night and only when horses are not present. To this extent, competition from the Przewalski's horses effectively forces the khulan to shift their ranging and foraging patterns. From another angle, however, it is the horses that are more drastically affected by the combination of competition and unfavourable environmental conditions. The arid-adapted khulan, that can often go without water for days, can ‘afford’ to shift their drinking schedule and ranging patterns to avoid direct competition; their diverse diet and low water-dependency equips them to range farther in order to drink, and to feed on whatever plant species are present within that wider ranging pattern. Przewalski's horses, by contrast, are strictly water-limited in this desert environment. Constrained to stay in close proximity to water, their daytime diet shifts to disproportionate consumption of the rare and normally avoided plant community growing near their favoured water-point. These water-yoked Przewalski's-horse groups also alter their ranging patterns significantly, conditioning them on intensity of heat loading. Only when the sun has set and night-time temperatures have dropped can these horses move away from water and consume more typical grasses. Moreover, when summer rains cool daytime temperatures and deposit drinkable water widely across the desert, horses abruptly double or triple the distances they travel from waterholes on daily foraging trips (from 2–3 km to approx. 6 km [36]). But individuals' responses to these physiological constraints vary depending on social factors, in particular dominance status. In these periods of expanded foraging, groups led by mid-ranking males reach and exploit the richest patches of new vegetation farthest from the permanent watering points. The groups led by the most dominant males and the most subordinate males associate and move in concert, staying closer to the permanent watering points where dominants control the order and duration of drinking [36]. Although the shorter-ranging dominant-subordinate groups forego access to some bursts of high-quality vegetation that is only accessible when temporary water appears in the desert, they also reduce the risk of ever going without water. Thus, it appears that for the Przewalski's horse, the environmental pressure attendant to reintroduction in xeric habitats has not produced a universal, simply ‘flexible’, behavioural adjustment to new conditions. Rather the constraints imposed by water-dependency have limited their ranging behaviour, reconfigured their drinking behaviour and diets, and elevated the prevalence of dominance-mediated skew in resource access. The current contours of Przewalski's-horse sociality in this environment appear to be shaped equally by constraint and flexibility. Being a water-dependent species in a stochastically modulated and generally water-limited environment has not radically changed the overall horse social system of uni-male, multi-female living. But it has changed drinking frequency, ranging behaviour, diet and heightened differences among groups based on social status. In doing so, it has also altered the collective behaviour of groups. Normally, horses do not form herds, yet one of the coping mechanisms of mesic-adapted horses to extremely arid conditions is greater male–male tolerance—albeit at the extremes of the social hierarchy—which results in the emergence of male coalitions and nascent proto-herding tendencies.
While the example of reintroduced Przewalski's horses appears to point to the influence of ecological adaptations on social flexibility, a study of Grevy's × plains zebra hybrids suggests limits to the flexibility of traits that are directly implicated in social behaviours. Under purely natural conditions, Grevy's and plains zebras are not expected to mate—the ranges of the two species have overlapped at the southern edge of the Grevy's range for centuries without interbreeding. However such hybridization has recently been described [51,52] on the Ol Pejeta Reserve in Laikipia, Central Kenya, where interactions between a large (approx. 5000 member) resident plains zebra population and a small, heavily male-biased population of translocated Grevy's zebras (nine males and four females at the time of introduction to the reserve in the 1980s) produced 25 male and female F1 hybrids, all of them sired by Grevy's males on plains females. Because all hybrids were born to plains zebra mothers, all were raised exclusively within plains zebra social groups. Behavioural observations of the individual hybrids—10 females and 15 males—showed that individuals of both sexes performed ‘compromised’ versions of some key social behaviours, pointing to the heritable (though not simply ‘fixed’) nature of behavioural traits that differentiate Grevy's- and plains-like social systems [52]. Hybrid females in plains zebra harems were more vigilant than their plains zebra counterparts, mirroring the behaviour of their more vigilant Grevy's zebra relatives. This higher vigilance is a non-trivial component of Grevy's zebras' more open and transitory social structure where females are often on their own apart from males. While plains females increase their feeding rates via long-term bonds with highly vigilant stallions, Grevy's females do not form such long-term associations and must perform vigilance duties themselves. That hybrid females retained a Grevy's-like behavioural phenotype, even when ensconced in plains zebra social context, suggests the relative inflexibility of this component of their ancestral social system.
Male hybrids, meanwhile, showed that genes and socialization interact in complex ways. These males dispersed to join bachelor groups (though somewhat later than their plains zebra counterparts), as is typical of both Grevy's and plains zebra males. As they matured, however, hybrid males occupied a range of behavioural statuses—from wandering and asocial behaviour to bonded and tending behaviour; from solitary territoriality to satellite-male status to solo stallionhood—and they shifted frequently between them. Yet, as these males matured, individuals became set in particular ways. While some tended to join harems creating two-stallion groups, more simply moved in and out of harems inspecting individual females for oestrus as typical Grevy's males do when females enter their territories. Either strategy involved frequent shifting among individuals and groups and thus limited the feasibility of the hybrids' more plains-like behaviour, since the formation of stable harem bonds is essential to success as a stallion. While hybrid males did occasionally take over harems of females, they were never sighted as long-term bonded stallions on more than a few contiguous occasions, suggesting they did not possess the full suite of behaviours necessary to occupy the role of a long-term social dominant in a plains zebra social structure even when life history (being raised within a plains zebra harem) and ecological context (a mesic habitat, and access to only plains-like, harem-based mating opportunities) would support this behaviour [52]. Thus, while the Grevy's × plains hybrids possessed an equal share of plains zebra genes, were raised in exclusively plains zebra social groups, and matured in an almost homogeneous plains zebra social environment, they nevertheless retained persistent behavioural traits apparently inherited from their Grevy's fathers: comparatively increased vigilance and the tendency towards transitory social bondedness after dispersal. At the same time, the fact that different males revealed mostly Grevy's or plains zebra tendencies suggests that social or epigenetic feedbacks may play important roles in determining male mating propensities.
6. Implications beyond equids: an argument for long-term socioecological studies
The work discussed here suggests a different sort of relationship between a species' ecology and its behaviour or social structure than one in which the former ‘pushes’ the latter (as in the early precursors to socioecological models) or even a bidirectional relationship wherein environments shape behaviours of species that, in turn, reshape those environments. The equids' multiplicity of responses to environmental stressors suggests that physiology acts like a prism, splitting environmental influences into variants-on-theme depending on how physiology and other constraints interact (figure 3).
Figure 3.
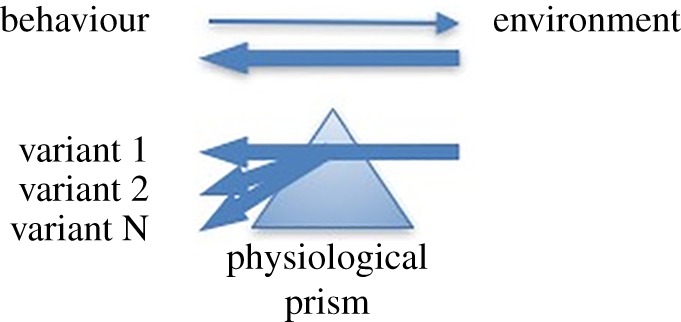
Physiological constraints can bend the influence of the environment toward a variety of behavioural phenotypes. (Online version in colour.)
Our equid work has been useful for probing this interaction because the sympatric species and populations are so similar in basic physiology. Individuals, however, often find themselves in different states based on particular physiological needs. These differences often interact with other state-dependent responses to environmental change which in turn highlights how strongly divergent behavioural outcomes can arise from subtle physiological differences. In highlighting this work, we propose a slight variation on the usual argument for the ‘optimality’ of any given study system. A key feature of equids' utility is simply that our subject populations of feral and wild horses, wild asses and zebras have been continuously studied for over 40 years. Long-term data collection on the demography of these groups and their interactions with sympatric competitors, predators and humans, as well as data collection on changes in their local environments, are preconditions that are now allowing us to examine long-term patterns, as well as their underlying mechanisms, in these species' adaptations to ecological stressors. Surveying research on behavioural flexibility and physiological constraint in other ungulate species, we find examples of many potentially ‘optimal’ taxa for examining the influence of slow-changing physiological constraints on interactions between ecological variation and behavioural and socio-structural adaptation (table 1).
Table 1.
Flexibility and constraint in response to ecological stressors (ungulate studies).
| references | species | flexible trait(s) | constraint(s) | stressor/response |
|---|---|---|---|---|
| Valeix et al. [53,54] Valls-Fox et al. [55] |
giraffe (Giraffa camelopardalis); impala (Aepyceros melampus), greater kudu (Tragelaphus strepsiceros), waterbuck, (Kobus ellipsiprymnus), blue wildebeest (Connochaetes taurinus), common zebra (Equus burchelli), roan antelope (Hippotragus equinus), sable antelope (Hippotragus niger), cape buffalo (Syncerus caffer) and warthog (Phacochoerus africanus) African elephant (Loxodonta Africana), cape buffalo (Syncerus caffer) |
day–night temporal activity pattern/tolerance of interspecies competition/antipredator behaviour | water dependency | dry-season conditions alter by-species patterns of sensitivity to interference competition, antipredator behaviour and day–night time-shifting as water dependency increases |
| Crosmary et al. [56] | impala (Aepyceros melampus), greater kudu (Tragelaphus strepsiceros), sable antelope (Hippotragus niger) | day–night temporal activity pattern | water dependency | under anthropogenic hunting pressure, differential vulnerability to natural sympatric predators mediates flexible time-shifting behaviour in three ungulate species |
| Jin & Ma [57]; Luo et al. [58] | Mongolian gazelle (Procapra gutturosa) | habitat selection/antipredator behaviour | feeding constraints | winter conditions reduce flexible, predator-sensitive habitat selection as feeding constraint increases |
| de Silva et al. [59,60] Wittemayer et al. [61] |
Asian elephant (Elephas maximus), African elephant (Loxodonta africana) African elephant (Loxodonta africana) |
social networks/grouping | feeding constraints | under conditions of more uniform and abundant forage, Asian elephants show more fluid grouping patterns and hierarchical structuring than do African elephants |
| Lone et al. [62] | roe deer (Capreolus capreolus) | day–night temporal activity pattern/habitat selection | feeding constraints | winter conditions reduce flexible, predator sensitive day–night time-shifting and habitat selection as feeding constraint increases |
| Loehr et al. [63] | Stone's sheep (Ovis dalli stonei), thinhorn sheep (O. dalli), bighorn sheep (O. canadensis) | inherited behavioural traits | a subspecies descended from hybrids shows variable expression of phenotypic and behavioural traits inherited from the parent species and subsequently refined by selection | |
| Endicott-Davies et al. [64] | red deer (Cervus elaphus), ¾ red deer × ¼ Père David's deer (Elaphurus davidianus) hybrids | inherited behavioural traits | hybrid calves show intermediate inherited behavioural traits |
(a). Water dependency
For the equids, the key physiological need that shapes behavioural responses to environmental change is the need for water in relation to its availability. Crosmary et al. [56,65], investigating how three sympatric ungulates in and around Hwange National Park, Zimbabwe, respond to anthropogenic hunting pressure, found intriguing variation in the behaviour of impala (Aepyceros melampus), greater kudu (Tragelaphus strepsiceros) and sable antelope (Hippotragus niger). All three species share an inflexible physiological constraint of high water dependency and all respond to some degree with the same flexible, behavioural strategy—time-shifting to nocturnal drinking. The strategy allows the ungulates to avoid the additional ecological pressure of diurnal human hunting pressure, but increases exposure to nocturnally active natural predators (lion, spotted hyena and leopard). This second inflexible predation constraint, however, varies across the three species: concurrent studies have found that sable are less vulnerable to these sympatric predators than kudu or impala [56,66]. Of the three, sable show the most time-shifting to nocturnal use of waterholes. The flexible behaviour that best alleviates one pressure (human hunting) is only partly available to those species constrained by inherent vulnerability to another pressure (natural predation). Presumably allometric body-size scaling differences and differences in defensive armament are affecting nocturnal anti-predator outcomes. In another group of studies, water-dependency again appears to be the fracture-point for varying behavioural responses of an entire ungulate guild (table 1) studied by Valeix et al. [53,54] and Valls-Fox et al. [55] (again in Hwange) to a host of other pressures. As temperature and aridity increase in the hottest months, each species displays different degrees or forms of flexibility with respect to behaviours such as day–night shifting, tolerance of humans and livestock, habitat selection and antipredator strategies, as the constraint of water dependency becomes increasingly determinative. These studies all mirror our findings on the divergent short- and long-term strategies adopted by khulan and Przewalski's horses, Grevy's and plains zebras in response to the pressure of water scarcity.
(b). Feeding constraints
Another critical physiological influence on the varied responses of sympatric equids to common pressures are constraints of dietary composition and intake rate. Here, again, we find ample parallels in the broader ungulate literature. Lone et al. [62] found that roe deer flexibly time-shift their habitat selection to reduce their risk from human hunters during the day and wild lynx at night. However, the pattern breaks down in winter, when forage scarcity and correspondingly determinative feeding pressure appears to constrain the deer from avoiding lynx risk at night. Similarly, a pair of studies by Luo et al. [58] and Jin & Ma [57] found that Mongolian gazelle (Procapra gutturosa) tend to flexibly select among a range of foraging habitats in order to avoid human disturbance, but that snow depth and above-ground biomass become determinative in winter. And a suite of social-network studies on Asian and African elephants by de Silva et al. [59,60] and Wittemyer et al. [61] suggest patterns similar to our findings comparing onager and Grevy's zebra networks (though specific methods and species-typical social structuring varied)—namely that the predictability and evenness of forage distribution may shape differences in the comparative cohesion and fluidity of social networks in these closely related species.
(c). Heritability of behavioural traits
Finally, our study of Grevy's × plains zebra hybrids suggests a complex interplay between social and genetic or epigenetic influences on individual behaviour. Our exploration of how this interaction might ultimately shape the evolution of the two sympatric species (and their hybrid offshoots) is just beginning, but examples from research on other ungulates point to the value of continuing to monitor this population. Endicott-Davies's [64] study of F1 hybrids—between red (Cervus elaphus) and Père David's deer (Elaphurus davidianus)—showed a comparable pattern of retained behaviours in the offspring (¾ red deer × ¼ Père David's hybrids), which were precocious and engaged in less antipredator behaviour (hiding) compared with their red deer equivalents. More intriguing is Loehr et al.'s [63] work on the Stone's (Ovis dalli stonei) subspecies of mountain sheep which seems to offer a window onto the possible future of Grevy's × plains zebras if interbreeding persists. Stone's sheep arose from hybridization of bighorn (O. canadensis) and thinhorn sheep (O. dalli) before the last ice age [63]. The modern Stone's subspecies has a dark-pelaged variant comparable to bighorn but not thinhorn sheep (which are all white). Mountain sheep have three distinct mating strategies (coursing, blocking and tending); the dark-type Stone's males are also more likely than light-type males to perform the mate-tending (guarding) behaviour—a strategy also associated with greater dominance rank. Hybridization appears, in this case, to have produced a subspecies with variable expression of phenotypic and behavioural traits inherited from the parent species and subsequently refined by selection.
These (and many other comparable studies) represent cases, like those from our equid research, where only long-term research using multiple methodologies can clarify the series of interactions between physiology, behaviour and social structure that constitute an adaptive response to ecological change.
7. Conclusion
In the context of evolutionary socioecology, concepts like ‘flexibility’ are inherently problematic. For living organisms, all traits are potentially flexible. The critical difference in the flexibility of behavioural, epigenetic, genetic or morphological traits is not in the degree to which they can change but the timescale over which those changes can occur. Our studies of equid populations under conditions of environmental variability illustrate the complicated interplay—between slow-changing physiological adaptations and faster-moving social flexibility—that shapes equid sociality in the face of novel ecological challenges. Given a common suite of physiological traits and requirements, different populations of feral horses on Shackleford Banks appear to have broad behavioural flexibility with respect to mating system—they can adopt territorial or harem-defence mating behaviours in more or less immediate response to ecological conditions. But the territoriality that forms involves strong and relatively permanent bonds among females and males. Thus, in one locale where significant environmental differences exist, but do not create strong physiological challenges, the behavioural adjustments that occur generally follow socioecological cost–benefit strictures. When species are pushed to their physiological limits, as was the case when mesic-adapted Przewalski's horses were translocated to deserts, then more idiosyncratic behavioural adjustments must occur if the species is to survive. Fundamental changes in time budgets and movements emerged, but were not universal. Rather they varied depending on social factors and how they interact with physiological needs (sensu figure 3). Dominance status both within and between species skewed the responses that led to variations in ranging behaviour and even pushed the social system from one in which groups generally stayed apart to one where some groups formed close associations, thus moving the social system from a one- to a two-tiered system. Ultimately, individuals with the ability to minimize the risk of water-deprivation changed their behaviour to exercise control of that essential physiological resource. The horses' water-dependent physiology was the chief constraint upon other, more flexible behavioural adaptations.
The same theme emerged even among two water-independent species both exhibiting fission–fusion social dynamics. The species faced with more uncertain spatial and temporal water dynamics formed more societies with more cohesive subgroups that simulations show increases the spread and reduces the loss of important memes. The ability to adjust behaviour to cope with small and large environmental changes is not without constraint. Ancestral tendencies encoded in genes still operate as the hybrid offspring of Grevy's and plains zebras showed. Even when socialized in the environment of one parent, traits derived from the parent not of that social environment emerge. But just as social differences in dominance status skewed the responses of horses challenged by extreme aridity, not all hybrid sons of Grevy's fathers showed similar social personalities as they matured. Thus, the interplay between behaviour and the constraining roles of physiology and genetics is subtle, often involving indirect influences and feedbacks mediated by social differences, the magnitude of the environmental challenge and the ability of behavioural changes to reduce uncertainty.
If physiological change is a bottleneck that constrains the pace or shapes the form of adaptation in behavioural or socio-structural domains, nevertheless phenotypic evolution, too, can occur rapidly. Though the best known and most comprehensive work on rapid vertebrate evolution has been done on avian [67,68] and piscine [69] species, rapid phenotypic change has also been studied in multiple species of ungulates [70]. The equid studies discussed here illustrate the behavioural and socio-structural changes that ungulates undergo when confronted with novel or enhanced ecological challenges. However, these populations of horses, asses and zebras are not static, and may yet show changes to comparatively inflexible physiological traits such as species-typical water-dependency. Our longitudinal field studies have already allowed us to document and quantify some examples of behavioural adaptation, while perhaps glimpsing—for example, in the hybridization of Grevy's and plains zebras—one facet of more fundamental genetic and morphological changes to come. By continuing these extended empirical studies in equids as well as other ungulates (table 1), we may have opportunities to observe and illuminate the interplay between behavioural flexibility and evolutionary change, bringing us closer to a socioecological model that encompasses the messy complexity of natural systems.
Acknowledgements
We would also like to thank Ilya Fischhoff, Siva Sundaresan, Qing Cao, Kaia Tomback and Emily Nonnamaker for help in the field and three anonymous reviewers for their valuable comments.
Competing interests
We declare we have no competing interests.
Funding
This work was supported over the years by the National Science Foundation (IBN-0309233, CNS-025214, IOB-9874523, IIS-0705822 and IIS-0747369).
References
- 1.Petrucci R. 1906. Origine polyphylétique, homotypie & non Comparabilité directe Des sociétés Animales. Brussels, Belgium: Misch & Thron. [Google Scholar]
- 2.Hamilton WD. 1964. The genetical evolution of social behaviour. I. J. Theor. Biol. 7, 1–16. ( 10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 3.Hamilton WD. 1964. The genetical evolution of social behaviour. II. J. Theor. Biol. 7, 17–52. ( 10.1016/0022-5193(64)90039-6) [DOI] [PubMed] [Google Scholar]
- 4.Brown JL. 1964. The evolution of diversity in avian territorial systems. Wilson Bull. 76, 160–169. [Google Scholar]
- 5.Orians GH. 1969. On the evolution of mating systems in birds and mammals. Am. Nat. 103, 589–603. ( 10.1086/282628) [DOI] [Google Scholar]
- 6.Verner J. 1964. Evolution of polygamy in the long-billed marsh wren. Evolution 18, 252–261. ( 10.2307/2406398) [DOI] [Google Scholar]
- 7.Emlen ST, Oring LW. 1977. Ecology, sexual selection, and the evolution of mating systems. Science 197, 215–223. ( 10.1126/science.327542) [DOI] [PubMed] [Google Scholar]
- 8.Rubenstein DI, Wrangham RW. 1986. Ecological aspects of social evolution: birds and mammals. Princeton, NJ: Princeton University Press; (http://library.wur.nl/WebQuery/clc/257624). [Google Scholar]
- 9.Rubenstein DI. 2009. Social behavior and sociobiology. In Evolution: first four billion years (eds Ruse M, Travis J), pp. 237–255. Cambridge, MA: Harverd University Press. [Google Scholar]
- 10.Clutton-Brock T, Janson C. 2012. Primate socioecology at the crossroads: past, present, and future. Evol. Anthropol. Issues News Rev. 21, 136–150. ( 10.1002/evan.21316) [DOI] [PubMed] [Google Scholar]
- 11.Koenig A, Borries C. 2009. The lost dream of ecological determinism: time to say goodbye?… or a white queen's proposal? Evol. Anthropol. Issues News Rev. 18, 166–174. ( 10.1002/evan.20225) [DOI] [Google Scholar]
- 12.Sterck EH, Watts DP, van Schaik CP. 1997. The evolution of female social relationships in nonhuman primates. Behav. Ecol. Sociobiol. 41, 291–309. ( 10.1007/s002650050390) [DOI] [Google Scholar]
- 13.Searcy WA, Nowicki S. 2005. The evolution of animal communication: reliability and deception in signaling systems. Princeton, NJ: Princeton University Press. [Google Scholar]
- 14.Lott DF. 1991. Intraspecific variation in the social systems of wild vertebrates. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 15.Oliveira RF, Taborsky M, Brockmann HJ. 2008. Alternative reproductive tactics: an integrative approach. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 16.Gill FB, Wolf LL. 1975. Economics of feeding territoriality in the golden-winged sunbird. Ecology 56, 333–345. ( 10.2307/1934964) [DOI] [Google Scholar]
- 17.Lucia KE, Keane B, Hayes LD, Lin YK, Schaefer RL, Solomon NG. 2008. Philopatry in prairie voles: an evaluation of the habitat saturation hypothesis. Behav. Ecol. 19, 774–783. ( 10.1093/beheco/arn028) [DOI] [Google Scholar]
- 18.Müller JK, Braunisch V, Hwang W, Eggert A-K. 2007. Alternative tactics and individual reproductive success in natural associations of the burying beetle, Nicrophorus vespilloides. Behav. Ecol. 18, 196–203. ( 10.1093/beheco/arl073) [DOI] [Google Scholar]
- 19.Brashares JS, Arcese P. 2002. Role of forage, habitat and predation in the behavioural plasticity of a small African antelope. J. Anim. Ecol. 71, 626–638. ( 10.1046/j.1365-2656.2002.00633.x) [DOI] [Google Scholar]
- 20.Kappeler PM, van Schaik CP. 2002. Evolution of primate social systems. Int. J. Primatol. 23, 707–740. ( 10.1023/A:1015520830318) [DOI] [Google Scholar]
- 21.Connor RC. 2000. Group living in whales and dolphins. Cetacean societies: field studies of dolphins and whales, pp. 199–218. Chicago, IL: University of Chicago Press. [Google Scholar]
- 22.Janis C. 1976. The evolutionary strategy of the Equidae and the origins of rumen and cecal digestion. Evolution 30, 757–774. ( 10.2307/2407816) [DOI] [PubMed] [Google Scholar]
- 23.Jarman P. 1974. The social organisation of antelope in relation to their ecology. Behaviour 48, 215–267. ( 10.1163/156853974X00345) [DOI] [Google Scholar]
- 24.Owen-Smith N. 1977. On territoriality in ungulates and an evolutionary model. Q. Rev. Biol. 52, 1–38. ( 10.1086/409720) [DOI] [Google Scholar]
- 25.East R. 1984. Rainfall, soil nutrient status and biomass of large African savanna mammals. Afr. J. Ecol. 22, 245–270. ( 10.1111/j.1365-2028.1984.tb00700.x) [DOI] [Google Scholar]
- 26.Pérez-Barbería FJ, Gordon IJ, Pagel M, Huelsenbeck J. 2002. The origins of sexual dimorphism in body size in ungulates. Evolution 56, 1276–1285. ( 10.1111/j.0014-3820.2002.tb01438.x) [DOI] [PubMed] [Google Scholar]
- 27.Brashares JS, Garland T, Arcese P. 2000. Phylogenetic analysis of coadaptation in behavior, diet, and body size in the African antelope. Behav. Ecol. 11, 452–463. ( 10.1093/beheco/11.4.452) [DOI] [Google Scholar]
- 28.Hofmann RR. 1989. Evolutionary steps of ecophysiological adaptation and diversification of ruminants: a comparative view of their digestive system. Oecologia 78, 443–457. ( 10.1007/BF00378733) [DOI] [PubMed] [Google Scholar]
- 29.Lundrigan B. 1996. Morphology of horns and fighting behavior in the family Bovidae. J. Mammal. 77, 462–475. ( 10.2307/1382822) [DOI] [Google Scholar]
- 30.Rubenstein DI. 1986. Ecology and sociality in horses and zebras. In Ecological aspects of social evolution (eds Rubenstein DI, Wrangham RW), ch. 13, pp. 282–302. Princeton, NJ: Princeton University Press. [Google Scholar]
- 31.Klingel H. 1974. Social organization and behavior of Grevy's zebra (Equus grevyi). Z. Tierpsychol. 36, 37–70. ( 10.1111/j.1439-0310.1974.tb02127.x) [DOI] [PubMed] [Google Scholar]
- 32.Rubenstein DI. 1981. Behavioural ecology of island feral horses. Equine Vet. J. 13, 27–34. ( 10.1111/j.2042-3306.1981.tb03443.x) [DOI] [Google Scholar]
- 33.Rubenstein DI. 1994. The ecology of female social behavior in horses, zebras, and asses. In Animal societies: individuals, interactions and organization, pp. 13–28. Kyoto, Japan: Kyoto University Press. [Google Scholar]
- 34.Linklater WL. 2000. Adaptive explanation in socio-ecology: lessons from the Equidae. Biol. Rev. 75, 1–20. ( 10.1017/S0006323199005411) [DOI] [PubMed] [Google Scholar]
- 35.Sundaresan SR, Fischhoff IR, Dushoff J, Rubenstein DI. 2007. Network metrics reveal differences in social organization between two fission–fusion species, Grevy's zebra and onager. Oecologia 151, 140–149. ( 10.1007/s00442-006-0553-6) [DOI] [PubMed] [Google Scholar]
- 36.Rubenstein DI, Cao QS, Chiu J. 2016. Equids and ecological niches: behavioral and life history variations on a common theme. In Wild equids: ecology, management and conservation, p. 58–68. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 37.Groves CP. 1974. Horses, asses and zebras in the wild. London, UK: David & Charles. [Google Scholar]
- 38.Rubenstein DI. 2001. Horses, zebras, and asses. In Encyclopedia of mammals (ed. MacDonald DW.), pp. 482–487. Oxford, UK: Oxford University Press. [Google Scholar]
- 39.Kartzinel TR, Chen PA, Coverdale TC, Erickson DL, Kress WJ, Kuzmina ML, Rubenstein DI, Wang W, Pringle RM. 2015. DNA metabarcoding illuminates dietary niche partitioning by African large herbivores. Proc. Natl Acad. Sci. USA 112, 8019–8024. ( 10.1073/pnas.1503283112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubenstein DI. 2010. Ecology, social behavior, and conservation in zebras. Adv. Study Behav. 42, 231–258. ( 10.1016/S0065-3454(10)42007-0) [DOI] [Google Scholar]
- 41.Rubenstein DI, Low Mackey B, Davidson ZD, Kebede F, King S. 2016. Equus grevyi. The IUCN red list of threatened species, e.T7950A89624491 ( 10.2305/IUCN.UK.2016-3.RLTS.T7950A89624491.en) [DOI] [Google Scholar]
- 42.Becker CD, Ginsberg JR. 1990. Mother-infant behaviour of wild Grevy's zebra: adaptations for survival in semidesert East Africa. Anim. Behav. 40, 1111–1118. ( 10.1016/S0003-3472(05)80177-0) [DOI] [Google Scholar]
- 43.Zhang Y, et al. 2015. Water use patterns of sympatric Przewalski's horse and khulan: interspecific comparison reveals niche differences. PLoS ONE 10, e0132094 ( 10.1371/journal.pone.0132094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaczensky P, Dresley V, Vetter D, Otgonbayar H, Walzer C. 2010. Water use of Asiatic wild asses in the Mongolian Gobi Explor. Biol. Resour. Mong. 11, 291–298. [Google Scholar]
- 45.Rubenstein DI. 2015. Networks of terrestrial ungulates: linking form and function. In Animal social networks (eds Krause J, James R, Franks DW, Croft DP), pp. 184–196. Oxford, UK: Oxford University Press. [Google Scholar]
- 46.Rubenstein DI, Sundaresan SR, Fischhoff IR, Tantipathananandh C, Berger-Wolf TY. 2015. Similar but different: dynamic social network analysis highlights fundamental differences between the fission-fusion societies of two equid species, the onager and Grevy's zebra. PLoS ONE 10, e0138645 ( 10.1371/journal.pone.0138645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dorj U, Namkhai B. 2013. Reproduction and mortality of re-introduced Przewalski's horse Equus przewalskii in Hustai National Park, Mongolia. J. Life Sci. 7, 623. [Google Scholar]
- 48.Gao X, Gu Zhou J. 1989. The change on the distribution area of the wild horse in the modern times. Arid Zone Res. 2, 10. [Google Scholar]
- 49.Paklina N, Pozdnyakova M. 1989. Why the Przewalski horses of Mongolia died out. Przewalski Horse 24, 30–34. [Google Scholar]
- 50.Wakefield S, Knowles J, Zimmermann W, Van Dierendonck M. 2002. Status and action plan for the Przewalski's horse (Equus ferus przewalskii). In Equids, zebras, asses and horses status survey and conservation action plan (ed. PD Moehlman), pp. 82–92. Cambridge, UK: IUCN/SCC Equid Specialist Group. [Google Scholar]
- 51.Cordingley JE, Sundaresan SR, Fischhoff IR, Shapiro B, Ruskey J, Rubenstein DI. 2009. Is the endangered Grevy's zebra threatened by hybridization? Anim. Conserv. 12, 505–513. ( 10.1111/j.1469-1795.2009.00294.x) [DOI] [Google Scholar]
- 52.Schieltz JM, Rubenstein DI. 2015. Caught between two worlds: genes and environment influence behaviour of plains × Grevy's zebra hybrids in central Kenya. Anim. Behav. 106, 17–26. ( 10.1016/j.anbehav.2015.04.026) [DOI] [Google Scholar]
- 53.Valeix M, Chamaillé-Jammes S, Fritz H. 2007. Interference competition and temporal niche shifts: elephants and herbivore communities at waterholes. Oecologia 153, 739–748. ( 10.1007/s00442-007-0764-5) [DOI] [PubMed] [Google Scholar]
- 54.Valeix M, Fritz H, Matsika R, Matsvimbo F, Madzikanda H. 2008. The role of water abundance, thermoregulation, perceived predation risk and interference competition in water access by African herbivores. Afr. J. Ecol. 46, 402–410. ( 10.1111/j.1365-2028.2007.00874.x) [DOI] [Google Scholar]
- 55.Valls Fox H. 2015. To drink or not to drink? The influence of resource availability on elephant foraging and habitat selection in a semi-arid savanna. PhD thesis, Université de Montpellier, Montpellier, France.
- 56.Crosmary W-G, Valeix M, Fritz H, Madzikanda H, Côté SD. 2012. African ungulates and their drinking problems: hunting and predation risks constrain access to water. Anim. Behav. 83, 145–153. ( 10.1016/j.anbehav.2011.10.019) [DOI] [Google Scholar]
- 57.Jin K, Ma J. 2003. Distribution quantity threatening factors and protection of Mongolian gazelle. J. Northeast For. Univ. 32, 104–106. [Google Scholar]
- 58.Luo Z, Liu B, Liu S, Jiang Z, Halbrook RS. 2014. Influences of human and livestock density on winter habitat selection of Mongolian gazelle (Procapra gutturosa). Zoolog. Sci. 31, 20–30. ( 10.2108/zsj.31.20) [DOI] [PubMed] [Google Scholar]
- 59.de Silva S, Schmid V, Wittemyer G. 2017. Fission–fusion processes weaken dominance networks of female Asian elephants in a productive habitat. Behav. Ecol. 28, 243–252. ( 10.1093/beheco/arw153) [DOI] [Google Scholar]
- 60.de Silva S, Ranjeewa AD, Kryazhimskiy S. 2011. The dynamics of social networks among female Asian elephants. BMC Ecol. 11, 17 ( 10.1186/1472-6785-11-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wittemyer G, Douglas-Hamilton I, Getz WM. 2005. The socioecology of elephants: analysis of the processes creating multitiered social structures. Anim. Behav. 69, 1357–1371. ( 10.1016/j.anbehav.2004.08.018) [DOI] [Google Scholar]
- 62.Lone K, Mysterud A, Gobakken T, Odden J, Linnell J, Loe LE. 2016. Temporal variation in habitat selection breaks the catch-22 of spatially contrasting predation risk from multiple predators. Oikos 126, 624–632. ( 10.1111/oik.03486) [DOI] [Google Scholar]
- 63.Loehr J, Carey J, Ylönen H, Suhonen J. 2008. Coat darkness is associated with social dominance and mating behaviour in a mountain sheep hybrid lineage. Anim. Behav. 76, 1545–1553. ( 10.1016/j.anbehav.2008.07.012) [DOI] [Google Scholar]
- 64.Endicott-Davies DR, Barrie AN, Fisher MW. 1996. Differences in the hiding behaviour of new-born red deer and hybrid 1/4 Père David's × 3/4 red deer calves. Anim. Sci. 62, 363–367. ( 10.1017/S1357729800014685) [DOI] [Google Scholar]
- 65.Crosmary W-G, Makumbe P, Côté SD, Fritz H. 2012. Vulnerability to predation and water constraints limit behavioural adjustments of ungulates in response to hunting risk. Anim. Behav. 83, 1367–1376. ( 10.1016/j.anbehav.2012.03.004) [DOI] [Google Scholar]
- 66.Drouet-Hoguet N. 2007. Influence des activités anthropogéniques sur le régime alimentaire et la réponse numérique de la hyène tachetée en savane arborée dystrophique dominée par l’éléphant. PhD thesis, Université Claude Bernard Lyon 1, Lyon, France.
- 67.Grant PR. 1999. Ecology and evolution of Darwin's finches. Princeton, NJ: Princeton University Press. [Google Scholar]
- 68.Grant PR, Grant BR. 2002. Unpredictable evolution in a 30-year study of Darwin's finches. Science 296, 707–711. ( 10.1126/science.1070315) [DOI] [PubMed] [Google Scholar]
- 69.Reznick DN, Shaw FH, Rodd FH, Shaw RG. 1997. Evaluation of the rate of evolution in natural populations of guppies (Poecilia reticulata). Science 275, 1934–1937. ( 10.1126/science.275.5308.1934) [DOI] [PubMed] [Google Scholar]
- 70.Ezard THG, Côté SD, Pelletier F. 2009. Eco-evolutionary dynamics: disentangling phenotypic, environmental and population fluctuations. Phil. Trans. R. Soc. B 364, 1491–1498. ( 10.1098/rstb.2009.0006) [DOI] [PMC free article] [PubMed] [Google Scholar]