Abstract
It is the aim of this article to present an empirically justified hypothesis about the functional roles of the two social neural systems, namely the so-called ‘mirror neuron system’ (MNS) and the ‘mentalizing system’ (MENT, also ‘theory of mind network’ or ‘social neural network’). Both systems are recruited during cognitive processes that are either related to interaction or communication with other conspecifics, thereby constituting intersubjectivity. The hypothesis is developed in the following steps: first, the fundamental distinction that we make between persons and things is introduced; second, communication is presented as the key process that allows us to interact with others; third, the capacity to ‘mentalize’ or to understand the inner experience of others is emphasized as the fundamental cognitive capacity required to establish successful communication. On this background, it is proposed that MNS serves comparably early stages of social information processing related to the ‘detection’ of spatial or bodily signals, whereas MENT is recruited during comparably late stages of social information processing related to the ‘evaluation’ of emotional and psychological states of others. This hypothesis of MNS as a social detection system and MENT as a social evaluation system is illustrated by findings in the field of psychopathology. Finally, new research questions that can be derived from this hypothesis are discussed.
This article is part of the themed issue ‘Physiological determinants of social behaviour in animals’.
Keywords: social cognition, mentalizing system, mirror neuron system, functional neuroimaging, autism spectrum disorder
1. Introduction
As human beings we seemingly effortlessly interact with others by perceiving and responding to signals exchanged between oneself and an interaction partner both verbally and non-verbally. It is estimated that the exchange of non-verbal cues for the purpose of communication is at least as important as the verbal domain [1,2]. Non-verbal behaviour serves four different purposes, namely: (i) modelling and coordination functions in the sense of a ‘motor contagion’ [3], (ii) discourse functions, (iii) dialogue functions, and (iv) socio-emotional functions [4]. They have a deep impact on the process and outcome of our communication [5,6] and can contribute to our impression formation very early during social encounters [7].
In the field of social neuroscience essentially two different neural systems have been established as the fundamental neural correlates of all processes related to social information processing. These two systems comprise the so-called ‘mirror neuron system’ (MNS) and the ‘mentalizing’ system (MENT), also referred to as ‘social neural network’ or ‘theory of mind network’. Both systems are recruited during interaction or communication with other human beings in social encounters, thereby constituting intersubjectivity. Intersubjectivity is defined as information processing performed by cognitive systems that serve interaction or communication between persons or interactants within and even between species. However, although it is claimed that both systems have social functions, it is still today unclear which differential functional roles have to be assigned to both neural systems. This article aims to develop and defend a speculative, but empirically justifiable differential hypothesis about their functional roles.
The hypothesis is developed in different steps. First, we have to make explicit the fundamental, but usually implicit distinction that we make between persons and things. Whereas persons have inner experiences, reasons, motivations or intentions-to-act that are usually described in the framework of ‘folk psychology’, the behaviour of physical things is completely explained by the impact of physical forces in the framework of ‘folk physics’ (at least in the scope of Newtonian mechanics). In a second step, this leads to the phenomenon of communication that is only applicable for the interaction between two persons or two cognitive systems, but not between things, who can exchange information for the purpose of an adaptive survival in their environment. Third, the capacity to communicate with others crucially depends on the capacity to ‘mentalize’ or to understand, simulate, imagine or model the inner experience of other persons. This field has been extensively studied in the research domain of social neuroscience and has identified the two different brain systems MNS and MENT. On this basis, the hypothesis of MNS as a social detection system and MENT as a social evaluation system can be developed and defended. Finally, this hypothesis is illustrated by empirical findings in the field of psychopathological conditions and research questions derived from this hypothesis are discussed.
2. Persons and things
Successful social encounters between two interaction partners crucially depend on the adequate mutual understanding of both interactants. We seemingly without any effort ascribe mental states to other persons. This ‘mentalizing’ capacity [8] has its roots in the ability to predict or explain the behaviour of other persons referred to as ‘theory of mind’ [9]. Mentalizing allows us to develop a ‘folk psychology’ as a set of psychological rules on how other persons will presumably experience and behave in given situations. The complex phenomenon of intersubjectivity as information processing allowing the exchange of inner experience in communicative acts and the question of how we can attribute mental states to others has been thoroughly studied by Fritz Heider in his canonical work ‘Psychology of interpersonal relations’ [10]. We cannot fully understand the other person as our understanding of others is often enough only vague and ambiguous, which limits our capacity to predict the behaviour of other persons substantially [10, p. 2] and leads to an inherent ambiguity and uncertainty [10, p. 29].
In contrast to persons, we are also confronted with things or physical objects which require a concept of ‘folk physics’. The behaviour of things follows the influence of physical forces and relies on natural laws that are valid in the framework of Newtonian mechanics. For instance, things reproducibly fall to the ground if we let them go because of gravity. In the case of persons, on the contrary, one cannot expect another person to reliably respond to one's own directed gaze towards the other in the same, highly predictable manner, because persons have inner experiences including motivations, reasons or intentions for their behaviour that let them decide whether better to respond or not to another persons gaze. This constitutes the fundamental difference between ‘thing perception’ (or ‘non-social perception’) contrasting ‘person perception’ (or ‘social perception’) ([10, p. 21]; table 1). Although both persons and things can be described in physical terms, persons are not merely physical things. The decisive differences are: (i) persons have inner experiences such as perceptions, imaginations, thoughts, feelings, etc., that let them act as ‘action centers’ on the basis of their internal reasoning [10, p. 21], (ii) persons can establish a ‘peculiar functional closeness and interaction’ in social encounters if they interact with each other, for instance, on the basis of exchanging non-verbal communication cues [10, p. 77]. This is, of course, associated with a high degree of unpredictability in the case of behaviour of persons ([10, p. 29]; table 1).
Table 1.
Differences between persons and things (according to [10]).
| criteria | persons | things |
|---|---|---|
| inner experience | existent; relevant for behaviour | non-existent |
| behaviour | internal intentions to act (action centers); external physical forces |
external physical forces (mere manipulanda) |
| predictability | low (less perfect constancy) | high (perfect constancy) |
Reading other persons inner mental states relates to the ‘problem of other minds’, this is one of the essential sceptical questions in epistemology and philosophy of mind [11]. More than a century ago, it was explicitly put forward by the philosopher John Stuart Mill: ‘By what evidence do I know, or by what considerations am I led to believe, that there exist other sentient creatures; that the walking and speaking figures which I see and hear, have sensations and thoughts, or in other words, possess Minds?’ [12, p. 243]. Evolution has provided a solution for the problem of other minds: learning from and adapting to the behaviour of others is an indispensible prerequisite for navigating and living in social groups [13,14]. One can speculate that the unique capacity to create, to process and to make use of social information that humans share with other conspecifics constitutes a remarkable evolutionary advantage, enabling us to communicate with others, and to join in complex forms of collaboration. This has also become well known as the so-called ‘cultural intelligence hypothesis’ stating that it was essentially our social-cognitive capacities—rather than general cognitive capacities—that provided this evolutionary advantage for the human species. The comparison of non-human primates with young children at the age of 2.5 years showed that children were superior in social learning, in communication and theory of mind tasks, whereas physical cognitive capacities related to space, quantities and causality were comparable between species [15]. This unique capacity to navigate the social world, to adjust to social affordances and to adapt and coordinate intentions, feelings and actions with others [16] could even have substantially promoted the evolutionary development of culture in a universal sense including sciences, technology, arts and philosophy within the human species [17,18].
3. Communication
On the background of this basic distinction between persons and things, we now proceed to the necessary core features of communication. Our human communicative capacities are necessary for our survival, our navigation in the social world and the full participation in culture and society. Research during the last decade has provided ample evidence that—beyond language—social understanding, rapport and successful collaboration heavily depend on non-verbal bodily communication. This implies dynamic decoding of social behavioural cues from others and dynamic, online synchronization with others [19]. Simple rhythmic alignment and motor synchronization can influence cooperativeness and group entitativity [20,21]. Non-verbal mimicry increases affiliation, fosters interdependent self-construal and supports the generation of collaborative goals [22,23]. In other words, communication serves as ‘social glue’ in human interactions and groups [22]. Non-verbal communication is essential to establish, maintain and monitor social interactions comprising the interpersonal adjustment of higher-level psychological phenomena, such as self-construal [24], cooperativeness [17] and group entitativity referred to as the so-called ‘we-mode’ [16].
Conceptually, this perspective has been supported by emphasizing the intersubjective relationship and communication in dyadic interactions between humans [25]. Innovative approaches in cognitive science turn to conceptions of communication as co-creation and negotiation of social reality in the framework of social interactionism [26]. Already early concepts in communication theory defined communication as a closed loop constituted by three different elements: (i) the signal sent out by the first interaction partner (addresser), (ii) the adequate processing by the interaction partner (addressee), and (iii) his/her reaction to the signal of the addresser which demonstrates that the sent signal was perceived and understood by the addressee ([27, p. 15]; [28, p. 34 and p. 189]). By combining these three elements a ‘social situation’ [27, p. 23 and p. 28] is constituted (figure 1). Obviously, the feedback signal in the third step of the communicative loop can itself stimulate a reaction of the original addresser who was asked to respond to the feedback signal of the addressee. This can launch a series of communicative events also known as turn-taking that might possibly end up in an ongoing conversation of two persons.
Figure 1.
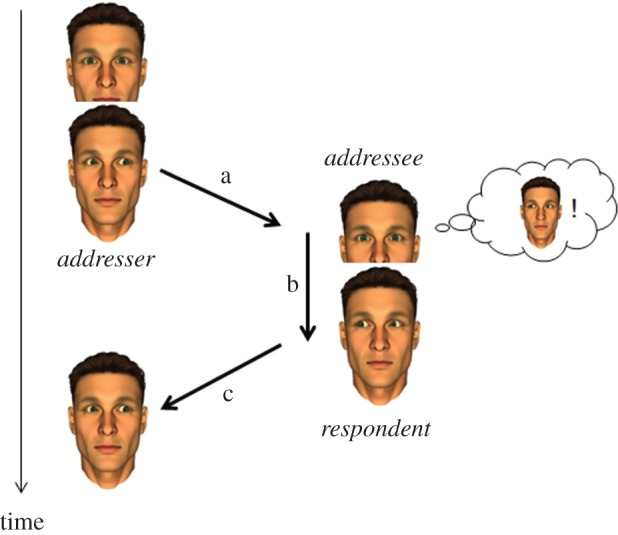
Two persons establish a ‘social situation’ as soon as the ‘loop is closed’. In a very simple social encounter on the basis of gaze as a non-verbal cue the communicative loop is constituted by three different components: (a) one person, the addresser, decides to send a signal to another person, the addressee, by looking at him, (b) the addressee perceives this signal, processes this information and—after a certain latency—decides to prepare an answer, (c) the addressee responds with mutual gaze, thereby becoming the respondent and closing the loop (according to [27]). (Online version in colour.)
Although the basic format always comprises in the one or the other way the elements of sending an information, receiving and processing it and back-channelling an adequate signal back to the sender, the variance of the individual configuration or design of communicative encounters can vary massively and is virtually infinite. Even unintended signals sent out to other persons can be perceived and are usually interpreted as a communicative signal. In other words, ‘all actions and events have communicative aspects, as soon as they are perceived by a human being’ [27, p. 6 and p. 31], ‘one cannot not communicate’ [29, p. 51].
4. Understanding the inner experience of others
Equipped by nature with unique prerequisites for social information processing for the purpose of communication and interaction with others, humans already in early childhood develop the capability to differentiate between self and others [30], to infer emotional and cognitive states of other peron's minds [8], to form social impressions and to adjust actions and communicative behaviour accordingly [8,18,30]. According to the attribution theory [31] based on Fritz Heider's account [10], we are generating hypotheses about the inner mental states of others based on three different types of data: (i) the provided actual stimuli, e.g. a smile or directed gaze of another person, (ii) the situational context of the social encounter, e.g. allowing to apply generally accepted rules for the interaction (formal encounters) or not (informal encounters), and (iii) previously acquired knowledge about the interaction partner. We use these different sources of information to reconstruct the inner experience of others, and we interact with other persons on the basis of our assumptions of their inner experience.
As already introduced, non-verbal cues including facial expressions, gaze behaviour, gestures, postures and body movements substantially influence our communication [5,6] by their coordination functions, discourse functions, dialogue functions and socio-emotional functions [4]. A highly relevant non-verbal cue to prepare for imitation and/or coordination before the onset of action is the observed gaze direction of others: ‘the complexity of feelings and actions that can be understood at a glance is surprisingly great’ [10, p. 2]. However, as a deictic cue, gaze can also be used to direct the attention of another person to an object, ‘even the direction of a glance may provide a strong hint as to what the person is thinking, feeling and wishing’ [10, p. 43]. Following another person's gaze teaches us about the attentional focus of this person and hence about her/his inner experience. Although gaze behaviour does not make use of an explicit semantic code as compared to language-based utterances, looking at each other is a very prominent and important signal system [10, p. 77]. It could be shown, for instance, that even independent from cultural backgrounds mutual gaze contact between persons has a constant duration of approximately 3 s [32,33]. Gaze helps to regulate dyadic encounters, and its coordination can help to establish three-way relations or triadic interactions between self, other and the object world [34].
A particularly interesting phenomenon is the experience of ‘joint attention’ that is established as soon as a given person follows another individual's gaze to a novel focus of visual attention which could be an object in the environment or the other person her/himself in the case of mutual gaze ([35]; figure 1). From early infancy onwards, the eyes are the primary and most consistent target of visual attention [36]. Despite the development of other tools to navigate the social world (e.g. language), gaze remains a crucial cue system for our understanding of others and serves a variety of social-cognitive functions comprising seeking of information, signalling interpersonal attitudes, regulating the synchronicity of speech during dialogue and interpersonal distance [37]. Notably, the human eye has a unique morphology: it is characterized by a depigmentated, white sclera contrasting with the dark iris [38] which might have fostered the development of the capacity to detect the gaze direction of other individuals [39]. Studies on gaze based interactions in a face-to-face set-up and in real time have been already widely used in different experimental contexts including virtual reality set-ups and live video-feeds of real interaction partners, with and without studying the underlying neural activity [35,40,41].
5. Identifying the neural mechanisms of understanding others
Cognitive processes related to communication and interaction have become a key topic in cognitive neuroscience and have defined social (cognitive) neuroscience as an autonomous scientific discipline [42,43]. One important distinction relates to two different levels of processing social information that can be either implicit or explicit [44]. So-called dual processing accounts propose that verbal descriptions of the behaviour of others use an explicit semantic code and are presented in a propositional format, whereas non-verbal behaviours do not use an explicit semantic code and are presented in a non-propositional format. It is assumed that they have different processing paths and possibly also differential neural mechanisms, although functional neuroimaging has not identified yet different subsystems for both processing formats.
Non-verbal communicative cues have a high dimensional complexity owing to the simultaneous presentation of multiple cues (e.g. smile and direct gaze) and a high processual complexity [45,46]. As a consequence, non-verbal behaviour is often produced and decoded without awareness and might hence influence impression formation intuitively, but not inferentially [47]. In other words: implicit information processing is fast and prereflexive and is employed during non-verbal behaviour. By contrast, explicit information processing is comparably slow and reflexive or inferential and is applied during information processing that is based on explicit rules, such as processing of stereotypes [48,49]. Empirical studies during the last decade focused on processes of ascribing mental states to others, ranging from ‘classical’ theory of mind studies [50] to person perception studies based on gaze behaviour [51] or gestures [52]. The creation of virtual characters that can serve as credible artificial humans provide a unique research tool to improve our knowledge about the underlying psychological processes and their neural mechanisms [4,53].
Already Fritz Heider himself dealt with the concept of animacy experience and developed displays of graphically reduced representations of moving objects [54]. Like many other animals, humans are able to detect biological motion in their environment, namely movement that is performed by biological organisms, irrespective of how this movement is presented. Phenomenally, biological motion relies on a complex perception that includes data about different aspects of the moving objects perceived. This includes, first, the physical properties of the moving object related to weight and size, second, its dependency on the physical environment such as gravity or obstacles, third, its interrelation to the social environment related to approach and avoidance and, fourth, its behavioural capacities, for instance related to the degree of efficiency during the performance of motor tasks. The variance in their movement patterns leads to the perception of a biological being and often enough even a human being that is alive and allows for meaningful inferences [55]. In this experimental context, specific movement features have already been empirically identified that contribute to the experience of animacy. A very important cue is self-propelled motion, it suggests that the initiation of movement was generated within the cognitive system in the absence of an adequate external physical cause [56]. Other indicators of animacy are related to the mutuality of the behaviour of the two interactants. These comprise, for instance, motion contingency based on both spatial and temporal synchrony between objects [57], or responsiveness to the motion of the interactant [58,59].
We developed a paradigm in which we systematically varied motion parameters of two balls in animated video sequences including self-propelled motion and different degrees of mutual responsiveness of both objects. This allowed us to parametrically vary the degree of experienced animacy [60,61]. By employing this animacy paradigm in a functional neuroimaging study with functional magnetic resonance imaging (fMRI), we were able to show that during increasing animacy experience, key regions of MENT were recruited, while decreasing experience of animacy was associated with a recruitment of MNS. At a first glance, this seems to contradict the view that MNS is engaged during motion understanding as shown in a wealth of studies [52,62,63]. However, one has to keep in mind that fMRI only provides relative signal differences so that it can by no means be excluded that MNS was also recruited during the processing of increasing animacy experience. It is highly plausible that the MNS is active during the processing of increasing animacy, too, but only to a significantly lower degree than MENT, as the experimental data suggest [60]. We argued on this basis that MNS may constitute a neural system for the ‘detection’ of socially potentially relevant actions. As a result, we showed MNS activation in the decreasing animacy condition. It can be assumed that MNS was also recruited during increasing animacy experience, but we were not able to show that because the recruitment of MENT was significantly higher and, therefore, covered MNS activity. When stimuli appeared highly animated, as in the case of movements invoking social intentions, MENT became activated, possibly responsible for the adequate interpretation or the ‘evaluation’ of relevant social cues [61].
The show case of social gaze behaviour has been studied extensively [10,33–35,38]. Early observational studies in dyadic interactions revealed subtle temporal adjustments of gaze behaviour, which despite inter-individual differences in directed gaze led to robust patterns of eye contact [32]. Emery [39] provided a taxonomy of social gaze behaviour defining mutual gaze, gaze aversion, gaze-following, joint attention and shared attention as the core processes. In a study focusing on person perception, we were able to distinguish two different subprocesses in the processing of averted and mutual gaze, namely gaze detection and gaze evaluation by the systematic variation of the experience of being gazed at by virtual characters with different durations in a study employing fMRI [51]. Gaze detection corresponding to the mere perception of being gazed at, irrespective of the duration with which a virtual character gazed at the participant, recruited fusiform and temporoparietal cortices, brain regions known to be responsible for biological motion detection. In contrast with the mere detection, the evaluative component, during which the participants had to make a sympathy rating of the virtual character at different durations of directed gaze, was accompanied by increased neural activity in the medial prefrontal cortex (mPFC) as key component of MENT. As the detection of social gaze is a necessary requirement for the successful and adequate interpretation of someone's gaze behaviour, gaze detection can be interpreted as an early stage of information processing, whereas gaze evaluation can be understood as a late stage of processing social information as shown before in a wealth of related studies focusing on the ‘meeting of minds’ [64].
In most of the studies being undertaken so far, social cognition has mostly been studied from a detached, observational perspective in tasks involving inert social stimuli (offline social cognition), which has led to a situation in which social cognition is studied without actual social interaction in isolation [65]. Recent claims emphasized that the active engagement with others in interaction (online social cognition) plays a particular role in understanding other minds [66]. Making use of the phenomenon of joint attention and by employing up-to-date eye-tracking and functional neuroimaging methods it has become possible to successfully induce the experience of a test person of being involved in an ongoing interaction with other persons based on contingent eye-movements and gaze behaviour of both persons [35,40,41,67] thereby increasing the ecological validity of social gaze behaviour in real time in contrast with purely observational paradigms [66]. Employing gaze-contingent social stimuli (similar to the virtual characters depicted in figure 1) we investigated the neural correlates of initiating and responding to joint attention in combined eye-tracking and fMRI experiments [40,41]. The experience of joint attention was associated with increased neural activity in the mPFC besides other brain regions. More specifically, in all cases, in which the test person himself was the initiator of the joint attention instantiation we also found increased neural activity in the ventral striatum as key component of the reward system [40,41]. The strength of the blood oxygen level dependent signal in this region correlated with pleasantness ratings in a post-experiment questionnaire [40] which might be interpreted as a neural correlate for the intrinsic motivation to share experiences with others [13].
6. Abduction of the functional roles of neural networks
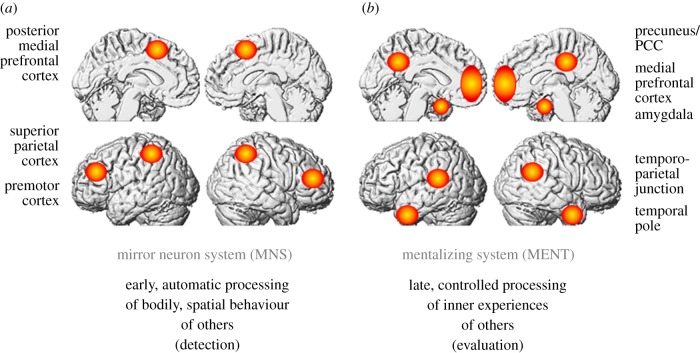
Obviously, cognitive functions are not implemented in single regions, but in neural networks, so that the strategy has to be to search for the underlying networks of activated brain regions and not only single brain areas. Social neuroscience has revealed essentially two different systems recruited during social-cognitive processes (figure 2), namely: (i) the so-called human MNS covering superior parietal and premotor regions [68], and (ii) the so-called MENT including essentially the anterior mPFC, the posterior temporal sulcus (pSTS) the temporoparietal junction (TPJ) and the temporal pole [43]. The proposal of this article for the putative functional roles of MNS and MENT is the following: as soon as the attribution of mental states to others is involved MENT is activated, whereas the MNS is recruited when a real or virtual motor component is involved, e.g. in actions, simulations or imaginations thereof [62,69,70]. An additional aspect that does not contradict the previous proposition assumes that MNS correlates with early stages of social cognition such as the detection of motor expertise and might putatively also underly the fast processing of ‘first impressions’ in social encounters that are generated on the basis of facial expressions or gestures. By contrast, MENT is assumed to be recruited during comparably ‘late’ stages of evaluation of socially relevant information [61]. The latter aspect of this hypothesis is empirically corroborated by an interesting study showing that controlled processes only affected the recruitment of MENT but not of MNS [71], suggesting that MNS is primarily responsible for automatic processing (possibly related to ‘detection’) and that MENT is recruited during controlled processes (possibly related to ‘evaluation’).
Figure 2.
Two different neural systems that appear to serve two complementary functional roles, (a) the ‘mirror neuron system’ (MNS) serves comparatively ‘early’ stages of social information processing related to spatial or bodily signals, (b) the ‘mentalizing network’ (MENT, also ‘theory of mind network’ or ‘social neural network’) is recruited during comparatively ‘late’ stages of social information processing that are related to the ‘evaluation’ of emotional and psychological states of others. (Online version in colour.)
Obviously, one cannot claim that the mPFC as one of the key regions of social cognition is specific for this cognitive domain as the mPFC is not only involved in cognitive processes associated with self-reference, interaction or communication [64,72], but is also recruited during a variety of cognitive functions including attention, multitasking, and response conflicts [73]. As individual brain regions are not specific for particular cognitive processes, we can neither ascribe a certain inner experience nor any cognitive process to a person based on the particular distribution of regions with increased neural activation. This problem has been recognized within the cognitive neuroscience community as the so-called ‘reverse inference’ [74]. The only apparent strategy is to search for something like a common ground or a common denominator which all of the cognitive functions under debate appear to share. This abductive or hypothesis-generating procedure lead Mitchell to the hypothesis that the feature of being ‘inexact, probabilistic, internally generated’ [72, p. 249] best covers at least the different socio-emotional functions of the mPFC as one key region of MENT. This can be taken to suggest that social cognition is a ‘natural kind’ and that the underlying processes can be individuated as a distinct class of cognitive processes that are related to either self-reference or interaction or communication with others [72]. This proposal shows that the identification of the functional core of a generator of ‘fuzzy’, probabilistic estimates could be a good candidate to explain the capacity of ascribing mental states to persons. Although this speculation or, at best, abductively identified hypothesis, does not formally prove anything, it is plausible as it nicely corresponds with the view of Fritz Heider presented earlier, namely the concept that the behaviour of persons as action centers is characterized by an inherent ambiguity and uncertainty [10, p. 21 and p. 77].
One of the most stimulating findings in cognitive neuroscience during the last 15 years has been the characterization of the so-called ‘default mode of the brain’ or ‘default mode network’ (DMN; [75]). The DMN has since then been studied in thousands of empirical studies. The DMN is essentially constituted by the mPFC besides the pSTS/TPJ and the posterior part of the cingulate gyrus and is systematically active during so-called resting states or baseline conditions. So-called resting state situations are characterized by the absence of an external instruction of an experimenter. During resting states participants focus on internal tasks that include the retrieval of autobiographical memory, consider future plans or take into account the inner experience of other persons, they are not otherwise engaged by any externally given tasks or instructions referring to the external world [76]. If a cognitive activity requires a higher demand (e.g. instruction by an experimenter in a formal experiment), neural activation ‘moves’, metaphorically speaking, towards the target neuronal network to be recruited, whereas at the same time medial frontal and parietal regions as part of the DMN tend to decrease their activity [75,76].
The DMN has been observed in other mammals [77] and very recently even in utero [78]. In other words, it seems to play a central role both phylogenetically and ontogenetically as a neurobiological universal: the DMN appears to be one of the most fundamental functional principles of mammalian brains. A very recent paper has corroborated the pattern of the DMN as being fundamental for the organization of the human brain showing a gradient of different cortical regions based on their connectivity characteristics [79]. This study was based on a large brain cohort of 1200 healthy adults [80]. The aim of this study was to further elucidate the relationship between the topography of the human neocortex and the cognitive capacities [79]. The empirical findings suggest a continuum between different cortical regions that range from regions responsible for primary projection cortices (sensory, motor) at the one pole to heteromodal association cortices constituting the DMN at the other pole. In other words, this study showed that the DMN is located in the brain at the largest possible distance to primary sensory and motor regions. This result demonstrates that the topographical structure is associated with cognitive capacities that range from perception and action to abstract cognitive functions that are detached from the environment [79]. This locates the DMN at the pole of the continuum of cognitive functions which is maximally unrelated either to immediate sensory input or to motor output.
Currently, we can only speculate about the function of the DMN. The group around Marc Raichle had from very early on proposed that the DMN might not only be a noisy signal, but might reflect, functionally and phenomenally speaking, a ‘continuous simulation of behaviour’ or ‘an inner rehearsal as well as an optimization of cognitive and behavioural serial programmes for the individual's future’, in short: a state of the ‘multifaceted self’ [81, p. 4263]. This speculation was recently substantiated by a study operationalizing the ‘intentional stance’ as an automatic and irresistible tendency to ascribe intentions to other persons and their behaviour. In this study, participants were asked to evaluate the appropriateness of a sentence describing the mental state of a person as opposed to a sentence describing the physical action of a person. It was shown that the DMN activity before test persons had to take the intentional stance facilitated the social task directly thereafter in the sense of a social priming procedure. The authors suggest that this is evidence for the social function of the DMN [82].
Finally, a particularly interesting aspect with respect to the difference of persons and things [10] is a substantial topographical overlap between the DMN and MENT as demonstrated in different meta-analyses [76,83]. Summarizing the evidence provided in this article, MENT is associated with social information processing referring both to oneself and to others and shows anatomical overlap with the DMN. The DMN in turn is both phylogenetically and ontogenetically one fundamental functional principle of mammalian brains. Taking these two observations together, it can be speculated that our disposition to take the intentional stance, to take the perspective of others or to mentalize is neurobiologically instantiated and implemented in a network of brain regions that can be observed as DMN. In other words, what appears as ‘state of self’ on the phenomenal level, appears as ‘default brain state’ or DMN on the neural level.
7. Psychopathology
The fundamental difference between persons and things is also reflected in the field of psychopathology as the independent scientific endeavour that aims to understand and describe the deviations and anomalies of inner experiences of persons. In his influential book ‘General Psychopathology’ Karl Jaspers distinguished two fundamentally different modes of making sense of the experience and behaviour of other persons, namely ‘understanding’ (Verstehen) and ‘explaining’ (Erklären) referring to an older terminology by Droysen & Dilthey [84, p. 301f]. Understanding in the Jaspersian sense refers to the empathic appreciation of conflicts, hopes and desires of an individual person, on the other hand, explaining relates to the attempt to take neurobiological and genetic prerequisites of mental disturbances into account and to reconstruct psychopathological conditions as consequences of impersonal natural laws. It is widely accepted that explaining is the only valid approach in natural sciences but this type of explanation is not available if we refer to the inner experience of persons, but the relation of these inner experience to their neurobiological underpinnings is unclear [84, p. 302]. This distinction lays the ground for the proposal of the ‘Psychology of Meaning’ (Verstehende Psychologie) [84, p. 301ff]. We have to understand the inner experience of persons ‘from within’ [84, p. 28]; [85]. In accordance to Heider [10], Jaspers notes that understanding is limited and that robust objective knowledge cannot be obtained [84, p. 357]. As a consequence, psychopathologists are forced to ‘interpret’ [84, p. 305].
Having this methodological restriction in mind, psychopathological conditions, generally speaking, define mental disorders as norm-deviant disturbances of subjective experiences. Norm-deviances can occur in any of the following domains: (i) changes in interactive and communicative behaviour, (ii) inadequate emotional experiences or changes in sharing emotional experiences with others, (iii) inconsistency of subjective experiences or incongruency with experiences of others leading to a loss of a sense of reality that can be shared by the majority of other persons within the same cultural background. In other words, one of the constitutive aspects of mental disorders is the fact that they are defined on the basis of norms that are generated or constituted by groups of persons, populations or social systems [86]. How we approach others—and how we are in turn perceived by them—crucially depends on the situational context which provides a set of gestures, symbols and meanings that need to be shared in the given situation in order to establish a common ground between both partners [87]. As a consequence, communication can only be studied in context, this is especially true under the conditions of psychopathological norm deviations. Psychopathological phenomena are substantially determined by cultural influences [27, p. 73 and p. 92]. The ‘cultural matrix’ is the basis for any ‘understanding of the nature of interaction between persons’ [27, p. 168].
Mentalizing in a psychopathological context is related to the experience of delusions as in psychotic disorders and deficits of communication and interaction disturbances as in autistic disorders, whereas persons suffering from delusions show an increased tendency of ascribing mental states to others that are often enough related to themselves corresponding to ‘hypermentalizing’ [88], persons with deficits in communication and interaction with others show ‘hypomentalizing’ [88]. Probably the most instructive show case in this context is the diagnostic group of persons with autism spectrum disorders (ASD), especially the group of persons with ASD without learning disabilities, referred to as high-functioning autism (HFA) or Asperger syndrome [89]. Persons with ASD suffer from ‘mindblindness’ [90], they are characterized by life-long and stable deficits in communication and social interaction, while verbal and general learning and memory abilities are in cases of HFA or Asperger syndrome independently developed and fully preserved. Individuals with ASD have difficulties in the adequate processing and integration of non-verbal communication cues into their person judgements [46,91].
This again is nicely illustrated in the domain of social gaze: persons with ASD avoid the eye region during the visual inspection of faces [92] and spend significantly less time fixating the eye region of people as compared to non-autistic controls in passive viewing studies involving social scenes [93]. Furthermore, they have difficulties with interpreting gaze as a non-verbal cue supporting the disambiguation of social scenes, thereby suggesting a more general problem in using gaze as a tool to infer the mental states of others [94]. Interestingly, autistic children appear to be able to follow someone's gaze, but tend to spend less attention to congruent objects in a gaze-following task [95]. As a consequence, they do not spontaneously attend to social information, and are thus less able to intuitively interact in social contexts [96] and to predict other persons actions in interactional contexts [97]. When confronted with non-verbal signals such as eye gaze, facial expressions or gestures, ASD individuals show atypical detection rates [98] and inadequate interpretations of such cues [99]. Generally, they seem to be less affected by them when processing a task, as compared with typically developed control persons [100], and/or they seem to use atypical strategies for social processing [101,102]. This suggests that while core processes of social gaze can be functional, they might be driven by different motives than in non-autistic individuals.
On a neural level, we could show that the processing of socially relevant information recruits significantly less MENT regions, during gaze detection and evaluation [53] and in animacy experience [102]. As a general result, these research findings show atypical processing of socially relevant information in ASD both on the behavioural and neural level. This can be interpreted as a decrease in the salience of non-verbal information for individuals with ASD compared to control persons. While the mere perception of non-verbal cues is often comparable to that of control persons ASD individuals seem to employ different strategies [103].
8. Outlook
The fundamental distinction that we make in the processing of information related to persons and related to things has been conceptualized theoretically and can be illustrated from a variety of perspectives including social psychology, social neuroscience and psychopathology, as sketched in the previous sections. A wealth of studies making use of functional neuroimaging in humans have shown plausibly that two brain networks underly the diversity of social-cognitive functions. Both systems serve different, but complementary functions so that it can be assumed that MNS and MENT both interact with each other in a dialectic manner. The claim is that MNS is related to early, automatic processing of spatial or body-related information, whereas MENT refers to late, controlled processing of information related to the inner experience of persons including oneself. In terms of their functional role MNS serves detection of potentially socially salient information, whereas MENT serves evaluation of actually socially salient information. This claim is speculative, but appears to be empirically justified [61,62,69–71].
An interesting and challenging question in this context that cannot be exhaustively answered at the moment is the following: how can the distinction between the level of processing, namely implicit versus explicit processing, be mapped onto the functional distinction of detection versus evaluation? Obviously, not all processes that are performed by MENT are controlled, and we also have to take implicit mentalizing or implicit theory of mind capacities into account [44,104]. On the other hand, we can also observe MNS activation after explicit instructions related to movement detection capacities, notably, MNS is no longer activated if the movement is unknown or hard to understand [52]. A naive hypothesis would be to assume that the two levels of processing, implicit versus explicit, and the two functions, detection and evaluation, are orthogonal to each other. In other words, it should be considered that the empirical evidence for the early, automatic mode associated with MNS and the late, controlled mode of processing associated with MENT (e.g. [61,71]) might be an oversimplification that needs to be differentiated at least with respect to the distinction between low- and high-level processing.
One of the most promising future approaches is to study communication while it is actually performed in ongoing social encounters as we suggested in the research agenda of a ‘second person neuroscience’ [66]. This approach allows us to study behavioural, cognitive and possibly neural correlates not only in a detached observers mode ‘offline’, but also ‘online’ during a truly ongoing social interaction [35,40,41]. Another methodological advancement that has already been proven to be very helpful is social interaction which builds upon the ‘plasticity’ advantage of virtual characters [4,45]. In this approach, motion is captured and rendered on a virtual character. Not only the appearance of the virtual character which can contain information on sex, identity, ethnicity or attractiveness, but also their non-verbal behaviour can be manipulated, by blending particular channels, or by modifying specific non-verbal cues. By doing this in a systematic manner, it can be determined which aspects of non-verbal behaviour are necessary and/or most efficient with regard to various social contexts. This makes it possible to systematically explore how manipulations of appearance and/or behaviour of one agent or a dyad affect the experience and the course of social interactions and possibly manipulate social encounters mediated by virtual agents or avatars—in the case of virtual characters representing human interactants [105].
As a consequent next step, the underlying neural mechanisms should also be studied in the framework of hyperscanning set-ups with the simultaneous and time-locked measurement of two persons being analysed in parallel with respect to neural and psychological synchronization measures during ongoing social interactions. Hyperscanning has been already successfully established employing fMRI [106], electro-encephalography [107], near infrared spectroscopy [108] and magnetoencephalography [109]. The study of the related phenomenon of synchronization on a neural level has already revealed a number of interesting findings. Synchronization was demonstrated more recently in simple face-to-face interactions, motor coordination and joint decision-making tasks [110–113]. However, the systematic study of experiential, behavioural and neural correlates of communication in ongoing social encounters with special consideration of psychopathological conditions has not even started. Besides the availability, conceptual questions of how best to approach and operationalize ongoing dyadic interactions empirically need to be answered first.
Approaches that would combine hyperscanning and neurofeedback could even allow to us integrate information about one's own and possibly the interaction partners brain activity: while participating in a social encounter, participants would be able not only to interact with another person, but would additionally be able to observe both their own and the interactants brain activity during the ongoing interaction in real time. This would substantially enrich simple face-to-face encounters and would allow us to perform intervention studies, for instance, non-verbal communication training in persons with ASD. Taken together, the study of communicative processes both under conditions of mental health and under psychopathological conditions is likely to foster our understanding of the underlying communicative deficits, while simultaneously providing valuable information about the neural mechanisms underlying the dynamics of social interactions.
Competing interests
I declare I have no competing interests.
Funding
No funding has been received for this article.
References
- 1.Burgoon JK. 1994. Nonverbal signals. In Handbook of Interpersonal communication (eds Knapp ML, Miller GR), pp. 229–285, 2nd edn Thousand Oaks, CA: Sage. [Google Scholar]
- 2.Argyle M. 1988. Bodily communication. Oxford, UK: Routledge. [Google Scholar]
- 3.Blakemore SJ, Frith C. 2005. The role of motor contagion in the prediction of action. Neuropsychologia 43, 260–267. ( 10.1016/j.neuropsychologia.2004.11.012) [DOI] [PubMed] [Google Scholar]
- 4.Vogeley K, Bente G. 2010. ‘Artificial humans’: psychology and neuroscience perspectives on embodiment and nonverbal communication. Neural Netw. 23, 1077–1090. ( 10.1016/j.neunet.2010.06.003) [DOI] [PubMed] [Google Scholar]
- 5.Argyle M, Salter V, Nicholson H, Wiliams M, Burgess P. 1970. The communication of inferior and superior attitudes by verbal and non-verbal signals. Br. J. Soc. Clin. Psychol. 9, 222–231. ( 10.1111/j.2044-8260.1970.tb00668.x) [DOI] [Google Scholar]
- 6.Mehrabian A, Wiener M. 1967. Decoding of inconsistent communications. J. Pers. Soc. Psychol. 6, 109–114. ( 10.1037/h0024532) [DOI] [PubMed] [Google Scholar]
- 7.Willis J, Todorov A. 2006. First impressions: making up your mind after a 100-ms exposure to a face. Psychol. Sci. 17, 592–598. ( 10.1111/j.1467-9280.2006.01750.x) [DOI] [PubMed] [Google Scholar]
- 8.Frith U, Frith CD. 2003. Development and neurophysiology of mentalizing. Phil. Trans. R. Soc. Lond. B 358, 459–473. ( 10.1098/rstb.2002.1218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Premack D, Woodruff D. 1978. Does the chimpanzee have a ‘theory of mind’? Behav. Brain Sci. 4, 515–526. ( 10.1017/S0140525X00076512) [DOI] [Google Scholar]
- 10.Heider F. 1958. The psychology of interpersonal relations. New York, NY: Jon Wiley and Sons. [Google Scholar]
- 11.Nagel T. 1986. The view from nowhere. New York, NY: Oxford University Press. [Google Scholar]
- 12.Mill JS. 1889. An examination of Sir William Hamilton's philosophy, 6th edn London, UK: Longmans. [Google Scholar]
- 13.Tomasello M, Carpenter M, Call J, Behne T, Moll H. 2005. Understanding and sharing intentions: the origins of cultural cognition. Behav. Brain Sci. 28, 675–735. ( 10.1017/S0140525X05000129) [DOI] [PubMed] [Google Scholar]
- 14.Moll H, Tomasello M. 2007. Cooperation and human cognition: the Vygotskian intelligence hypothesis. Phil. Trans. R. Soc. B 362, 639–648. ( 10.1098/rstb.2006.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrmann E, Call J, Hernàndez-Lloreda MV, Hare B, Tomasello M. 2007. Humans have evolved specialized skills of social cognition: the cultural intelligence hypothesis. Science 317, 1360–1366. ( 10.1126/science.1146282) [DOI] [PubMed] [Google Scholar]
- 16.Gallotti M, Frith CD. 2013. Social cognition in the we-mode. Trends Cogn. Sci. 17, 160–165. ( 10.1016/j.tics.2013.02.002) [DOI] [PubMed] [Google Scholar]
- 17.Tomasello M. 2008. Why we cooperate. Cambridge, MA: The MIT Press. [Google Scholar]
- 18.Vogeley K, Roepstorff A. 2009. Contextualising culture and social cognition. Trends Cogn. Sci. 13, 511–516. ( 10.1016/j.tics.2009.09.006) [DOI] [PubMed] [Google Scholar]
- 19.Sebanz N, Bekkering H, Knoblich G. 2006. Joint action: bodies and minds moving together. Trends Cogn. Sci. 10, 70–76. ( 10.1016/j.tics.2005.12.009) [DOI] [PubMed] [Google Scholar]
- 20.Shockley K, Santana M-V, Fowler CA. 2003. Mutual interpersonal postural constraints are involved in cooperative conversation. J. Exp. Psychol. Hum. Percept. Perform. 29, 326–332. ( 10.1037/0096-1523.29.2.326) [DOI] [PubMed] [Google Scholar]
- 21.Lakens D, Stel M. 2011. If they move in sync, they must feel in sync: movement synchrony leads to attributions of rapport and entitativity. Soc. Cogn. 29, 1–14. ( 10.1521/soco.2011.29.1.1) [DOI] [Google Scholar]
- 22.Lakin JL, Chartrand TL. 2003. Using nonconscious behavioral mimicry to create affiliation and rapport. Psychol. Sci. 14, 334–339. ( 10.1111/1467-9280.14481) [DOI] [PubMed] [Google Scholar]
- 23.Van Baaren RB, Maddux WW, Chartrand TL, De Bouter C, Van Knippenberg A. 2003. It takes two to mimic: behavioral consequences of self-construals. J. Pers. Soc. Psychol. 84, 1093–1102. ( 10.1037/0022-3514.84.5.1093) [DOI] [PubMed] [Google Scholar]
- 24.Markus HR, Kitayama S. 1991. Culture and the self: implications for cognition, emotion and motivation. Psychol. Rev. 98, 224–253. ( 10.1037/0033-295X.98.2.224) [DOI] [Google Scholar]
- 25.Gallagher S. 2005. How the body shapes the mind. Oxford, UK: Oxford University Press. [Google Scholar]
- 26.Searle J. 2010. Making the social world. The structure of human civilization. Oxford, UK: Oxford University Press. [Google Scholar]
- 27.Ruesch J, Bateson G. 1968(1951) Communication. The social matrix of psychiatry. New York, NY: W. W. Norton & Company Inc. [Google Scholar]
- 28.Ruesch J. 1957. Disturbed communication. The clinical assessment of normal and pathological communicative behaviour. New York, NY: Norton & Company. [Google Scholar]
- 29.Watzlawick PB, Janet H, Jackson Don D. 2011 1967 Menschliche Kommunikation - Formen, Störungen, Paradoxien (Pragmatics of human communication: a study of interactional patterns, pathologies, and paradoxes), 12th edn Bern, Switzerland: Verlag Hans Huber (W. W. Norton & Company). [Google Scholar]
- 30.Decety J, Chaminade T. 2003. When the self represents the other: a new cognitive neuroscience view on psychological identification. Consci. Cogn. 12, 577–596. ( 10.1016/S1053-8100(03)00076-X) [DOI] [PubMed] [Google Scholar]
- 31.Kelley HH. 1967. Attribution theory in social psychology. Nebr. Symp. Motiv. 15, 192–238. [Google Scholar]
- 32.Bente G, Donaghy WC, Suwelack D. 1998. Sex differences in body movement and visual attention: an integrated analysis of movement and gaze in mixed-sex dyads. J. Nonverbal Behav. 22, 31–58. ( 10.1023/A:1022900525673) [DOI] [Google Scholar]
- 33.Binetti N, Harrison C, Coutrot A, Johnston A, Mareschal I. 2016. Pupil dilation as an index of preferred mutual gaze duration. R. Soc. open sci. 3, 160086 ( 10.1098/rsos.160086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Argyle JM, Cook M. 1976. Gaze and mutual gaze. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 35.Pfeiffer UJ, Vogeley K, Schilbach L. 2013. From gaze cueing to dual eye-tracking: novel approaches to investigate the neural correlates of gaze in social interaction. Neurosci. Biobehav. Rev. 37, 2516–2528. ( 10.1016/j.neubiorev.2013.07.017) [DOI] [PubMed] [Google Scholar]
- 36.Haith MM, Bergman T, Moore MJ. 1977. Eye contact and face scanning in early infancy. Science 198, 853–855. ( 10.1126/science.918670) [DOI] [PubMed] [Google Scholar]
- 37.Argyle M, Ingham R, Alkema F, McCallin M. 1973. The different functions of gaze. Semiotica 7, 19–32. ( 10.1515/semi.1973.7.1.19) [DOI] [Google Scholar]
- 38.Kobayashi H, Kohshima S. 2001. Unique morphology of the human eye and its adaptive meaning: comparative studies on external morphology of the primate eye. J. Hum. Evol. 40, 419–435. ( 10.1006/jhev.2001.0468) [DOI] [PubMed] [Google Scholar]
- 39.Emery NJ. 2000. The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci. Biobehav. Rev. 24, 581–604. ( 10.1016/S0149-7634(00)00025-7) [DOI] [PubMed] [Google Scholar]
- 40.Schilbach L, Wilms M, Eickhoff SB, Romanzetti S, Tepest R, Bente G, Shah NJ, Fink GR, Vogeley K. 2010. Minds made for sharing: initiating joint attention recruits reward-related neurocircuitry. J. Cogn. Neurosci. 22, 2702–2715. ( 10.1162/jocn.2009.21401) [DOI] [PubMed] [Google Scholar]
- 41.Pfeiffer U, Schilbach L, Timmermans B, Kuzmanovic B, Georgescu A, Bente G, Vogeley K. 2014. Why we interact: on the functional role of the striatum in the subjective experience of social interaction. Neuroimage 101C, 124–137. ( 10.1016/j.neuroimage.2014.06.061) [DOI] [PubMed] [Google Scholar]
- 42.Cacioppo JT, Lorig TS, Nusbaum HC, Berntson GG. 2004. Social neuroscience: bridging social and biological systems. In The Sage handbook of methods in social psychology (eds Sansone C, Morf CC, Panter AT), pp. 383–404. Thousand Oaks, CA: Sage Publications. [Google Scholar]
- 43.Adolphs R. 2009. The social brain: neural basis of social knowledge. Annu. Rev. Psychol. 60, 693–716. ( 10.1146/annurev.psych.60.110707.163514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frith CD, Frith U. 2008. Implicit and explicit processes in social cognition. Neuron 60, 503–510. ( 10.1016/j.neuron.2008.10.032) [DOI] [PubMed] [Google Scholar]
- 45.Bente G, Senokozlieva M, Pennig S, Al-Issa A, Fischer O. 2008. Deciphering the secret code: a new methodology for the cross-cultural analysis of nonverbal behavior. Behav. Res. Methods 40, 269–277. ( 10.3758/BRM.40.1.269) [DOI] [PubMed] [Google Scholar]
- 46.Kuzmanovic B, Schilbach L, Lehnhardt FG, Bente G, Vogeley K. 2011. A matter of words: impact of verbal and nonverbal information on impression formation in high-functioning autism. Res. Autism Spectr. Disord. 5, 604–613. ( 10.1016/j.rasd.2010.07.005) [DOI] [Google Scholar]
- 47.Choi VS, Gray HM, Ambady N. 2005. The glimpsed world: intended communication and unintended perception. In The new unconcsious (eds Hassin RR, Uleman JS, Bargh JA), pp. 309–333. New York, NY: Oxford University Press. [Google Scholar]
- 48.Lieberman M. 2007. Social cognitive neuroscience: a review of core processes. Annu. Rev. Psychol. 58, 259–289. ( 10.1146/annurev.psych.58.110405.085654) [DOI] [PubMed] [Google Scholar]
- 49.Barsalou LW. 2008. Grounded cognition. Annu. Rev. Psychol. 59, 617–645. ( 10.1146/annurev.psych.59.103006.093639) [DOI] [PubMed] [Google Scholar]
- 50.Vogeley K, et al. 2001. Mind reading: neural mechanisms of theory of mind and self-perspective. Neuroimage 14, 170–181. ( 10.1006/nimg.2001.0789) [DOI] [PubMed] [Google Scholar]
- 51.Kuzmanovic B, Georgescu AL, Eickhoff SB, Shah NJ, Bente G, Fink GR, Vogeley K. 2009. Duration matters: dissociating neural correlates of detection and evaluation of social gaze. Neuroimage 46, 1154–1163. ( 10.1016/j.neuroimage.2009.03.037) [DOI] [PubMed] [Google Scholar]
- 52.Georgescu AL, Kuzmanovic B, Santos NS, Tepest R, Bente G, Tittgemeyer M, Vogeley K. 2014. Perceiving nonverbal behavior: neural correlates of processing movement fluency and contingency in dyadic interactions. Hum. Brain Mapp. 35, 1362–1378. ( 10.1002/hbm.22259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Georgescu AL, Kuzmanovic B, Schilbach L, Tepest R, Kulbida R, Bente G, Vogeley K. 2013. Neural correlates of ‘social gaze’ processing in high-functioning autism under systematic variation of gaze duration. Neuroimage Clin. 3, 340–351. ( 10.1016/j.nicl.2013.08.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heider F, Simmel M. 1944. An experimental study of apparent behavior. Am. J. Psychol. 57, 243–249. ( 10.2307/1416950) [DOI] [Google Scholar]
- 55.Blake R, Shiffrar M. 2007. Perception of human motion. Annu. Rev. Psychol. 58, 47–73. ( 10.1146/annurev.psych.57.102904.190152) [DOI] [PubMed] [Google Scholar]
- 56.Leslie AM. 1984. Spatiotemporal continuity and the perception of causality in infants. Perception 13, 287–305. ( 10.1068/p130287) [DOI] [PubMed] [Google Scholar]
- 57.Blakemore SJ, Boyer P, Pachot-Clouard M, Meltzoff A, Segebarth C, Decety J. 2003. The detection of contingency and animacy from simple animations in the human brain. Cereb. Cortex 13, 837–844. ( 10.1093/cercor/13.8.837) [DOI] [PubMed] [Google Scholar]
- 58.Michotte A. 1944. La perception de la causalite, 1944, Edition de l'Institut Superieur de Philosophie, Louvain and Paris, Belgium and France. [Google Scholar]
- 59.Castelli F, Happe F, Frith U, Frith C. 2000. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage 12, 314–325. ( 10.1006/nimg.2000.0612) [DOI] [PubMed] [Google Scholar]
- 60.Santos N, David N, Bente G, Vogeley K. 2008. Parametric induction of animacy experience. Conscious Cogn. 17, 425–437. ( 10.1016/j.concog.2008.03.012) [DOI] [PubMed] [Google Scholar]
- 61.Santos NS, Kuzmanovic B, David N, Rotarska-Jagiela A, Eickhoff S, Shah JN, Fink G, Bente G, Vogeley K. 2010. Animated brain: a functional neuroimaging study on the parametric induction of animacy experience. Neuroimage 53, 291–302. ( 10.1016/j.neuroimage.2010.05.080) [DOI] [PubMed] [Google Scholar]
- 62.Wheatley T, Milleville SC, Martin A. 2007. Understanding animate agents: distinct roles for the social network and mirror system. Psychol. Sci. 18, 469–474. ( 10.1111/j.1467-9280.2007.01923.x) [DOI] [PubMed] [Google Scholar]
- 63.Gobbini MI, Gentili C, Ricciardi E, Bellucci C, Salvini P, Laschi C, Guazzelli M, Pietrini P. 2011. Distinct neural systems involved in agency and animacy detection. J. Cogn. Neurosci. 23, 1911–1920. ( 10.1162/jocn.2010.21574) [DOI] [PubMed] [Google Scholar]
- 64.Amodio DM, Frith CD. 2006. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 7, 268–277. ( 10.1038/nrn1884) [DOI] [PubMed] [Google Scholar]
- 65.Becchio C, Sartori L, Castiello U. 2010. Toward you: the social side of actions. Curr. Dir. Psychol. Sci. 19, 183–188. ( 10.1177/0963721410370131) [DOI] [Google Scholar]
- 66.Schilbach L, Timmermans B, Reddy V, Costall A, Bente G, Schlicht T, Vogeley K. 2013. A second-person neuroscience in interaction. Behav. Brain Sci. 36, 341–462. ( 10.1017/S0140525X12002452) [DOI] [PubMed] [Google Scholar]
- 67.Wilms M, Schilbach L, Pfeiffer U, Bente G, Fink GR, Vogeley K. 2010. It's in your eyes. Using gaze-contingent stimuli to create truly interactive paradigms for social cognitive and affective neuroscience. Soc. Cogn. Aff. Neurosci. 5, 98–107. ( 10.1093/scan/nsq024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rizzolatti G, Craighero L. 2004. The mirror-neuron system. Annu. Rev. Neurosci. 27, 169–192. ( 10.1146/annurev.neuro.27.070203.144230) [DOI] [PubMed] [Google Scholar]
- 69.Vogeley K, May M, Ritzl A, Falkai P, Zilles K, Fink GR. 2004. Neural correlates of first-person perspective as one constituent of human self-consciousness. J. Cogn. Neurosci. 16, 817–827. ( 10.1162/089892904970799) [DOI] [PubMed] [Google Scholar]
- 70.Keysers C, Gazzola V. 2007. Integrating simulation and theory of mind: from self to social cognition. Trends Cogn. Sci. 11, 194–196. ( 10.1016/j.tics.2007.02.002) [DOI] [PubMed] [Google Scholar]
- 71.Spunt RP, Lieberman MD. 2013. The busy social brain: evidence for automaticity and control in the neural systems supporting social cognition and action understanding. Psychol. Sci. 24, 80–86. ( 10.1177/0956797612450884) [DOI] [PubMed] [Google Scholar]
- 72.Mitchell JP. 2009. Social psychology as a natural kind. Trends Cognit. Sci. 13, 246–251. ( 10.1016/j.tics.2009.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van Overwalle F. 2009. Social cognition and the brain: a meta-analysis. Hum. Brain Mapp. 30, 829–858. ( 10.1002/hbm.20547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Poldrack RA. 2006. Can cognitive processes be inferred from neuroimaging data? Trends Cogn. Sci. 10, 59–63. ( 10.1016/j.tics.2005.12.004) [DOI] [PubMed] [Google Scholar]
- 75.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. 2001. A default mode of brain function. Proc. Natl Acad. Sci. USA 98, 676–682. ( 10.1073/pnas.98.2.676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buckner RL, Andrews-Hanna JR, Schacter DL. 2008. The brain's default network: anatomy, function, and relevance to disease. Ann. NY Acad. Sci. 1124, 1–38.8 ( 10.1196/annals.1440.011) [DOI] [PubMed] [Google Scholar]
- 77.Vincent JL, et al. 2007. Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447, 83–86. ( 10.1038/nature05758) [DOI] [PubMed] [Google Scholar]
- 78.Seshamani S, Blazejewska AI, Mckown S, Caucutt J, Dighe M, Gatenby C, Studholme C. 2016. Detecting default mode networks in utero by integrated 4D fMRI reconstruction and analysis. Hum. Brain Mapp. 37, 4158–4178. ( 10.1002/hbm.23303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Margulies DS, et al. 2016. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl Acad. Sci. USA 113, 12 574–12 579. ( 10.1073/pnas.1608282113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Essen DC, Smith SM, Barch DM, Behrend TEJ, Yacoub E, Ugurbil K, WU-Minn HCP Consortium. 2013. The WU-Minn human connectome project: an overview. Neuroimage 80, 62–79. ( 10.1016/j.neuroimage.2013.05.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. 2001. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl Acad. Sci. USA 98, 4259–4264. ( 10.1073/pnas.071043098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spunt RP, Meyer ML, Lieberman MD. 2015. The default mode of human brain function primes the intentional stance. J. Cognit. Neurosci. 27, 1116–1124. ( 10.1162/jocn_a_00785) [DOI] [PubMed] [Google Scholar]
- 83.Schilbach L, Bzdok D, Timmermans B, Vogeley K, Eickhoff SB. 2012. Introspective minds: using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PLoS ONE 7, e30920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jaspers K. 1997. General psychopathology (orig. 1913). Trans. by J Hoenig, MW Hamilton, 2 vols London, UK and Baltimore MD: The John Hopkins University Press. [Google Scholar]
- 85.Vogeley K. 2013. A social cognitive perspective on ‘understanding’ and ‘explaining’. Psychopathology 46, 295–300. ( 10.1159/000351839) [DOI] [PubMed] [Google Scholar]
- 86.Vogeley K, Newen A. 2009. Consciousness of oneself and others in relation to mental disorders. In The neuropsychology of mental illness (eds Wood S, Allen N, Pantelis C), pp. 408–413. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 87.Vogeley K. In press Communication as fundamental paradigm for psychopathology. In The Oxford handbook of 4e cognition (eds Newen A, de Bruin L, Gallagher S). Oxford, UK: Oxford University Press. [Google Scholar]
- 88.Frith CD. 2004. Schizophrenia and theory of mind. Psychol. Med. 34, 385–389. ( 10.1017/S0033291703001326) [DOI] [PubMed] [Google Scholar]
- 89.Asperger H. 1944. Die ‘Autistischen Psychopathen’ im Kindesalter. Arch. Psychiatr. Nervenkrankheiten 117, 76–136. ( 10.1007/BF01837709) [DOI] [Google Scholar]
- 90.Baron-Cohen S. 1995. Mindblindness. Cambridge, MA: MIT Press. [Google Scholar]
- 91.Senju A, Southgate V, White S, Frith U. 2009. Mindblind eyes: an absence of spontaneous theory of mind in Asperger syndrome. Science 325, 883–885. ( 10.1126/science.1176170) [DOI] [PubMed] [Google Scholar]
- 92.Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. 2002. Visual scanning of faces in autism. J. Autism Dev. Disord. 32, 249–261. ( 10.1023/A:1016374617369) [DOI] [PubMed] [Google Scholar]
- 93.Klin A, Jones W, Schultz R, Volkmar F, Cohen D. 2002. Defining and quantifying the social phenotype in autism. Am. J. Psychiatry 159, 895–908. ( 10.1176/appi.ajp.159.6.895) [DOI] [PubMed] [Google Scholar]
- 94.Boraston Z, Blakemore S-J. 2007. The application of eye-tracking technology in the study of autism. J. Physiol. 581, 893–898. ( 10.1113/jphysiol.2007.133587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bedford R, Elsabbagh M, Gliga T, Pickles A, Senju A, Charman T, Johnson MH, team BASIS. 2012. Precursors to social and communication difficulties in infants at-risk for autism: gaze following and attentional engagement. J. Autism Dev. Disord. 42, 2208–2218. ( 10.1007/s10803-012-1450-y) [DOI] [PubMed] [Google Scholar]
- 96.Klin A, Jones W, Schultz R, Volkmar F. 2003. The enactive mind, or from actions to cognition: lessons from autism. Phil. Trans. R. Soc. Lond. B 358, 345–360. ( 10.1098/rstb.2002.1202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.von der Lühe T, Manera V, Barisic I, Becchio C, Vogeley K, Schilbach L. 2016. Interpersonal predictive coding, not action perception, is impaired in autism. Phil. Trans. R. Soc. B 371, 20150373 ( 10.1098/rstb.2015.0373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dratsch T, Schwartz C, Yanev K, Schilbach L, Vogeley K, Bente G. 2013. Getting a grip on social gaze: control over others’ gaze helps gaze detection in high-functioning autism. J. Autism Dev. Disord. 43, 286–300. ( 10.1007/s10803-012-1569-x) [DOI] [PubMed] [Google Scholar]
- 99.Uljarevic M, Hamilton A. 2013. Recognition of emotions in autism: a formal meta-analysis. J. Autism Dev. Disord. 43, 1517–1526. ( 10.1007/s10803-012-1695-5) [DOI] [PubMed] [Google Scholar]
- 100.Schwartz C, Bente G, Gawronski A, Schilbach L, Vogeley K. 2010. Responses to nonverbal behaviour of dynamic virtual characters in high-functioning autism. J. Autism Dev. Disord. 40, 100–111. ( 10.1007/s10803-009-0843-z) [DOI] [PubMed] [Google Scholar]
- 101.Walsh JA, Vida MD, Rutherford MD. 2014. Strategies for perceiving facial expressions in adults with autism spectrum disorder. J. Autism Dev. Disord. 44, 1018–1026. ( 10.1007/s10803-013-1953-1) [DOI] [PubMed] [Google Scholar]
- 102.Kuzmanovic B, Schilbach L, Georgescu A, Kockler H, Santos N, Shah NJ, Bente G, Fink GR, Vogeley K. 2014. Dissociating animacy processing in high-functioning autism: neural correlates of stimulus properties and subjective ratings. Soc. Neurosci. 9, 309–325. ( 10.1080/17470919.2014.886618) [DOI] [PubMed] [Google Scholar]
- 103.Georgescu AL, Kuzmanovic B, Roth D, Bente G, Vogeley K. 2014. The use of virtual characters to assess and train nonverbal communication in high-functioning autism. Front. Hum. Neurosci. 8, 807 ( 10.3389/fnhum.2014.00807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Low J, Perner J. 2012. Implicit and explicit theory of mind: state of the art. Br. J. Dev. Psychol. 30, 1–13. ( 10.1111/j.2044-835X.2011.02074.x) [DOI] [PubMed] [Google Scholar]
- 105.Roth D, Latoschik ME, Vogeley K, Bente G. 2015. Hybrid avatar-agent technology—a conceptual step towards mediated ‘social’ virtual reality and its repetitive challenges. i-com 14, 107–114. ( 10.1515/icom-2015-0030) [DOI] [Google Scholar]
- 106.Montague PR, et al. 2002. Hyperscanning: simultaneous fMRI during linked social interactions. Neuroimage 16, 1159–1164. ( 10.1006/nimg.2002.1150) [DOI] [PubMed] [Google Scholar]
- 107.Dumas G, Nadel J, Soussignan R, Martinerie J, Garnero L. 2010. Inter-brain synchronization during social interaction. PLoS ONE 5, e12166 ( 10.1371/journal.pone.0012166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cui X, Bryant DM, Reiss AL. 2012. NIRS-based hyperscanning reveals increased interpersonal coherence in superior frontal cortex during cooperation. Neuroimage 59, 2430–2437. ( 10.1016/j.neuroimage.2011.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhdanov A, et al. 2015. An internet-based real-time audiovisual link for dual MEG recordings. PLoS ONE 10, e0128485 ( 10.1371/journal.pone.0128485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hasson U, Ghazanfar AA, Galantucci B, Garrod S, Keysers C. 2012. Brain-to-brain coupling: a mechanism for creating and sharing a social world. Trends Cogn. Sci. 16, 114–121. ( 10.1016/j.tics.2011.12.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yun K. 2013. On the same wavelength: face-to-face communication increases interpersonal neural synchronization. J. Neurosci. 33, 5081–5082. ( 10.1523/JNEUROSCI.0063-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Noy L, Dekel E, Alon U. 2011. The mirror game as a paradigm for studying the dynamics of two people improvising motion together. Proc. Natl Acad. Sci. USA 108, 20 947–20 952. ( 10.1073/pnas.1108155108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nummenmaa L, Glerean E, Viinikainen M, Jääskeläinen IP, Hari R, Sams M. 2012. Emotions promote social interaction by synchronizing brain activity across individuals. Proc. Natl Acad. Sci. USA 109, 9599–9604. ( 10.1073/pnas.1206095109) [DOI] [PMC free article] [PubMed] [Google Scholar]