Abstract
Background:
Upper airway epithelial cells produce bactericidal nitric oxide (NO) in response to both gram-positive and gram-negative bacteria. Our previous work demonstrated that T2R38, a bitter taste receptor (T2R) expressed in airway epithelium, produces NO in response to quorum-sensing molecules secreted by Pseudomonas aeruginosa. We also demonstrated that Staphylococci products elicit an NO response when using a T2R-independent pathway. When screening additional human pathogens for epithelial T2R activation, we found that the gram-positive aerobe Bacillus cereus secretes a T2R agonist that yields NO production.
Objective:
The objective of this study was to characterize the activating B. cereus product(s) and to describe the epithelial cell signaling pathway involved.
Methods:
Sinonasal air-liquid interface cultures were treated with B. cereus conditioned medium (CM), and NO production was measured by using 4-amino-5-methylamino-2′,7′-difluorofluorescein fluorescence imaging. Ciliary beat frequency (CBF) was assessed in response to B. cereus CM. Pharmacologic studies that use inhibitors of the T2R-signaling pathway were used to determine if the production of NO was mediated by a T2R. Purification studies were performed to analyze the physical properties of the activating product(s) contained in the CM.
Results:
A product(s) secreted by B. cereus induced NO production and increased CBF. The response varied markedly between individual patients and involved two important components of bitter taste signaling, phospholipase C isoform β-2 and the transient receptor potential melastatin isoform 5 ion channel.
Conclusions:
This study demonstrated that a B. cereus product(s) elicited an NO-mediated innate defense response in upper airway epithelium that seemed to be partially mediated by a T2R signaling pathway. The active product that elicited the NO response was likely a small nonpeptide compound, but further purification is required for identification. Patient variation in the NO response to B. cereus products could potentially be due to genetic differences in T2Rs.
Keywords: Chronic rhinosinusitis, innate immunity, bitter taste receptors, nitric oxide, bacteria, epithelial, signaling, genetics, sinusitis, inflammation
Bitter taste receptors (T2R) in the upper airway have emerged as novel regulators of innate immunity by detecting microbial products and mounting local responses to eliminate infection.1,2 There are ∼25 human T2Rs, and the genes that encode the receptors (TAS2R) are located on chromosomes 5, 7, and 12.3,4 One T2R, T2R38, binds quorum-sensing molecules produced by gram-negative bacteria, such as acyl-homoserine lactones (AHL) secreted by Pseudomonas aeruginosa.1,5,6 Binding of these ligands to sinonasal T2R38 results in production of nitric oxide (NO), which diffuses into the airway to kill bacteria and increase ciliary beat frequency (CBF).1,7 Genetic variation in T2R38 has been shown to contribute to individual differences in these defensive mechanisms1 and correlates with chronic rhinosinusitis (CRS) that necessitates surgical intervention,8,9 poor surgical outcomes,10 and rhinologic symptoms in patients with cystic fibrosis.11 Gram-positive bacteria secrete quorum-sensing molecules throughout their life cycles; but, unlike gram-negative bacteria, they do not use AHLs.12 Various gram-positive bacteria are implicated in CRS,13 and we previously demonstrated that gram-positive Staphylococci products elicit an NO response independent of T2Rs.14,15 However, we postulated that the host immune system could detect other gram-positive organisms via T2R signaling.
T2Rs are present outside of the oral cavity and airway, including in the gastrointestinal (GI) tract, but their specific functions are largely unknown.16,17 We believe that there is redundancy in the role of T2Rs in innate immunity in the airway and GI mucosa, and hypothesized that the products secreted by gut bacteria could trigger a T2R-mediated NO response in human sinonasal cultures. Specifically, we chose to investigate the gram-positive spore-forming bacterium Bacillus cereus, which is commonly found in the lower GI tract, with the ability to cause food poisoning in humans.18–20 An additional objective of this study was to identify and characterize the quorum-sensing molecule secreted by B. cereus that was eliciting the response if such a response was discovered. Purification of a quorum-sensing molecule that could activate a target-specific innate immune response in the upper airway could have profound therapeutic consequences for diseases such as CRS.
METHODS
Sinonasal Air-Liquid Interface Cultures
Sinonasal mucosal specimens were obtained during surgery from patients who had functional endoscopic sinus surgery at the Philadelphia Veterans Affairs Medical Center and the University of Pennsylvania. Both institutional review boards provided full study approval, and informed consent was obtained from the patients before surgery. Exclusion criteria included patients with a history of systemic diseases, such as immunodeficiencies, cystic fibrosis, granulomatosis with polyangiitis, sarcoidosis; or a history of use of antibiotics, oral corticosteroids, or biologics within 1 month of functional endoscopic sinus surgery. Air-liquid interface (ALI) cultures were prepared from the human sinonasal mucosal specimens as previously described.1,21
B. cereus Conditioned Medium
For preparation of conditioned medium (CM), B. cereus cultures were grown for 24 hours at 37°C with shaking in lysogeny broth medium. The cultures were then diluted to 0.1 optical density (log phase), grown for an additional 12 hours, and adjusted to an optical density of 1.2. The medium was centrifuged (10 minutes, 2000 × g, room temperature) and filtered by using a 0.2-μm filter. The dialyzed B. cereus CM was prepared by dialyzing for 5 hours at 4°C against a 1000-fold excess of lysogeny broth (changed once after 1.5 hours) by using a 1 kD dialysis membrane (Spectra/Por; Spectrum Medical Industries Inc., Laguna Hills, CA). The boiled B. cereus CM was prepared by heating the B. cereus CM for 1 hour at 100°C, then immediately placing it in an ice bath. The trypsin-digested B. cereus CM was prepared by incubating for 1 hour at 37°C with 250 μg/mL trypsin (Life Technologies Corp., Carlsbad, California), followed by incubation for 2 hours at 37°C with 5 mM of 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride, an irreversible serine protease inhibitor (Fisher Scientific, Waltham, MA). The proteinase K–digested B. cereus CM was prepared by incubating for 1 hour at 37°C with 200 μg/mL of proteinase K (Sigma-Aldrich, St. Louis, Missouri), followed by incubation for 2 hours at 37°C with 5 mM of 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (Fisher Scientific). All CMs were diluted in Dulbecco's phosphate-buffered saline solution to a final concentration of 25%.
Live-Cell Imaging of Reactive NO Production in Human ALI Cultures
Cellular NO production was measured by using the fluorescent reactive nitrogen species indicator 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM) (Invitrogen/Life Technologies, Inc., Carlsbad, California) and imaged as previously described 1 by using an IX-81 microscope (×10, 0.3 NA UPlanFLN objective; Olympus, Tokyo, Japan) and the 488-nm argon laser of a Fluoview FV1000 laser scanning confocal system (Olympus). DAF-FM fluorescence images were acquired every 5 seconds. Increases in DAF-FM fluorescence were expressed as arbitrary fluorescence units. The phospholipase C (PLC) β-2 inhibitor, U73122, and its inactive analog, U73343 (Cayman Chemical, Ann Arbor, Michigan) were used at 5 μM, and the transient receptor potential melastatin isoform 5 (TRPM5) inhibitor triphenylphosphine oxide (TPPO) (Sigma-Aldrich) was used at 100 μM. Fresh solutions were prepared daily in phosphate buffered saline (PBS) PBS from ×1000 dimethyl sulfoxide (DMSO) DMSO stocks. The apical side of the cultures was incubated with the inhibitors or inactive analog for 15 minutes before DAF-FM imaging. The inhibitors and inactive analog were also present in the B. cereus CM treatments.
CBF Measurement
All CBF experiments were performed on ALIs at ∼30°C by using a high-speed camera (Basler 602f, Basler, Ahrensburg, Germany; 100 frames/s) attached to an inverted Leica microscope (Leica Microsystems, Wetzlar, Germany, ×20, 0.8 NA objective) lens. CBF was measured by using the Sisson-Ammons Video Analysis system (Ammons Engineering, Clio, Michigan). Briefly, the software performs a whole-field analysis of the ciliated surface of the ALIs to provide a CBF value.1 All CBF values were then normalized to a baseline frequency of 100% with increases and decreases in CBF expressed as a percentage of the baseline frequency.
Heterologous Expression for T2R Identification
To identify the B. cereus CM responsive T2R, we used human embryonic kidney cells 293 (HEK293) (Sigma-Aldrich) cotransfected with a chimeric G protein (Gα16gustducin44) to couple specific T2R receptor activation to calcium mobilization for all 25 T2Rs separately. This expression system has previously been described.1 Increases in intracellular calcium, indicative of receptor activation, were monitored with Fluo-4AM (Invitrogen) fluorescence during stimulation by B. cereus CM, a well-established and previously described technique in our laboratory.1
Data Analysis and Statistics
Fluoview (Olympus) was used for data analysis and GraphPad Prism (GraphPad Software, Inc., La Jolla, CA) for statistical analysis with p < 0.05 considered statistically significant. One-way analysis of variance (ANOVA) with a Bonferroni posttest was used for multiple comparisons. Unpaired, two-tailed, t-tests were used for single comparisons. All data are reported as mean ± SE, unless otherwise indicated.
RESULTS
B. cereus CM Induces NO Production that Varies Among Patients
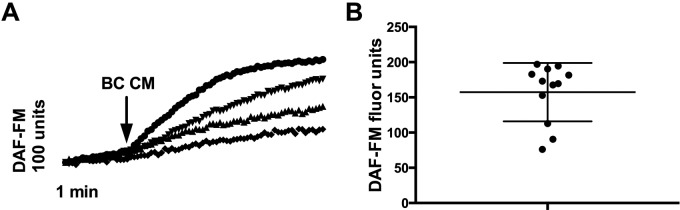
Our previous work demonstrated that NO production was induced by AHL quorum-sensing molecules secreted by P. aeruginosa and was mediated by T2R38.1 We also showed that Staphylococci products elicited a similar NO response, independent of T2Rs.14,15 We first sought to determine if B. cereus also triggered an NO response in sinonasal epithelium. By using the probe DAF-FM, which reacts with NO-derived reactive nitrogen species to form a fluorescent benzotriazole, we measured the cellular production of NO after treatment with B. cereus CM. We found that stimulating the cultures with B. cereus CM did, in fact, trigger production of NO-derived reactive nitrogen species (DAF-FM fluorescence increase) over the course of 5 minutes and that there was variability in the amount of NO produced by different patients (Fig. 1). The average DAF-FM fluorescence increases after stimulation with B. cereus CM was 157.4 ± 41.37.
Figure 1.
Bacillus cereus CM induces differential NO production in patients. (A) Representative traces of DAF-FM fluorescence in four different patients (one culture each, each symbol represents a culture from a different patient). (B) Average DAF-FM fluorescence increase over 5 minutes (n = 12 patients, one culture per patient) of cultures stimulated with B. cereus CM is 157.4 ± 41.37. The data in the graph are average ± SD. CM = conditioned medium; NO = nitric oxide; DAF-FM = 4-amino-5-methylamino-2′,7′-difluorofluorescein; SD = standard deviation.
B. cereus CM Increases CBF
In addition to being directly bactericidal, NO is known to increase CBF and thus mucociliary clearance (MCC) of respiratory bacteria.1,22–24 By using video analysis of the ciliated surface of the ALIs, we investigated the effects of B. cereus CM on CBF (Fig. 2). We found that addition of B. cereus CM to the apical surface resulted in an average percentage CBF increase of 145.7 ± 16.34% at 5 minutes compared with 102.5 ± 1.913% after PBS stimulation (p = 0.0264, two-tailed, t-test).
Figure 2.
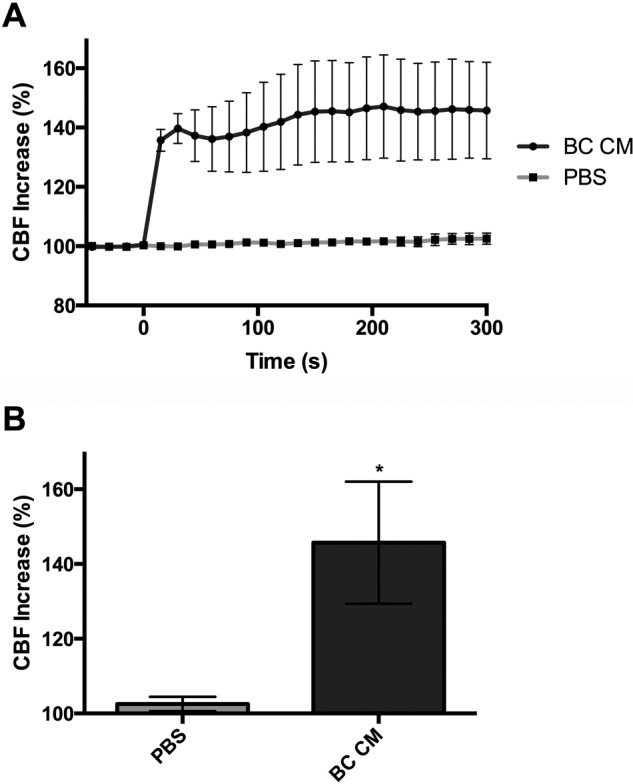
Bacillus cereus CM increases CBF. (A) Traces of CBF increase over time (n = 3–4 patients per condition) of cultures stimulated with B. cereus CM and PBS. (B) Average percentage CBF increases at 300 seconds for B. cereus CM and PBS were 145.7 ± 16.34 versus 102.5 ± 1.913 (p = 0.0264, two-tailed, t-test). The data in the graph are average ± SE; *p < 0.05. CM = conditioned medium; CBF = ciliary beat frequency; PBS = phosphate buffered saline.
B. cereus CM–induced NO Generation Potentially Involves a T2R Taste-Signaling Pathway But Is Independent of T2R38
The canonical signaling pathway for T2Rs involves G-protein coupling to activate PLCβ-2, which liberates inositol trisphosphate (IP3) and triggers downstream release of intracellular Ca+2 through the TRPM5 ion channel.1 We found that NO generation was partially blocked in the presence of TPPO, an inhibitor of the TRPM5 ion channel, and U73122, an inhibitor of PLCβ-2 (Fig. 3). The average DAF-FM fluorescence increases were 197.8 ± 17.85 with B. cereus CM alone, 93.90 ± 14.11 in the presence of TPPO (p < 0.01 by one-way ANOVA with Bonferroni posttest), 107.9 ± 18.16 in the presence of the PLCβ-2 inhibitor U73122 (p < 0.01), and 194.7 ± 20.05 in the presence of its inactive analog U73343 (not significant [n.s.]). Together, these pharmacology experiments indicated that the NO generation was at least partially dependent on a T2R signaling pathway.
Figure 3.
Bacillus cereus CM–induced NO generation is partially blocked by inhibitors of PLCβ-2 and TRPM5 dependent signaling. (A) Representative traces of DAF-FM fluorescence increases over time of cultures stimulated with B. cereus CM alone or in the presence of the PLCβ-2 inhibitor (B. cereus CM + U73122), inactive PLCβ-2 inhibitor analog (B. cereus CM + U73343), or TRPM5 blocker (B. cereus CM + TPPO). (B) Bar graphs of the average DAF-FM fluorescence (n = 5–8 cultures from five to seven patients for each condition) of cultures stimulated with B. cereus CM alone or in the presence of PLCβ-2 inhibitor (B. cereus CM + U73122), inactive PLCβ-2 inhibitor analog (B. cereus CM + U73343), or TRPM5 blocker (B. cereus CM + TPPO). After stimulation with B. cereus CM, DAF-FM fluorescence increases were 197.8 ± 17.85 (B. cereus CM) versus 194.7 ± 20.05 (B. cereus CM + U73343; n.s. by using one-way ANOVA with Bonferroni posttest) versus 107.9 ± 18.16 (B. cereus CM + U73122; p < 0.01) versus 93.90 ± 14.11 (B. cereus CM + TPPO; p < 0.01) The data in the graph are average ± SE; *p < 0.01. CM = Conditioned medium; NO = nitric oxide; PLCβ-2 = phospholipase C β-2; TRPM5 = transient receptor potential melastatin isoform 5 ion channel; DAF-FM = 4-amino-5-methylamino-2′,7′-difluorofluorescein; TPPO = triphenylphosphine oxide; n.s. = no statistical significance; ANOVA = analysis of variance.
We then investigated if the response to B. cereus CM was mediated by T2R38. There are three common polymorphisms in the TAS2R38 gene that encodes T2R38, which results in the amino acid substitutions (A49P, V262A, and I296V); these polymorphisms tend to segregate together and form two common haplotypes: AVI (“nontaster” allele) and PAV (“taster” allele). Epithelial cells from PAV/PAV genotypes exhibit markedly enhanced NO production, MCC, and bacterial killing compared with AVI/AVI or AVI/PAV cells in response to AHLs from gram-negative bacteria. However, when epithelial cells of different genotypes were exposed to B. cereus CM, there was no significant difference in levels of NO production (Fig. 4). These results indicated that the active compound(s) found in B. cereus CM increased NO production and regulated MCC in a T2R-dependent manner that did not involve T2R38.
Figure 4.
Bacillus cereus CM–induced NO generation is independent of the T2R38 signaling pathway. Average DAF-FM fluorescence (n = 3–5 patients per condition) of cultures with different T2R38 genotypes stimulated with B. cereus CM. After stimulation with B. cereus CM, DAF-FM fluorescence increases were 183.8 ± 10.71 (PAV/PAV) versus 159.5 ± 17.19 (AVI/AVI; n.s. by using one-way ANOVA with Bonferroni posttest) versus 177.6 ± 10.94 (PAV/AVI; n.s.) versus 212.4 ± 27.00 (other; n.s.). The data in the graph are average ± SE. CM = conditioned medium; NO = nitric oxide; DAF-FM = 4-amino-5-methylamino-2′,7′-difluorofluorescein; PAV = taster allele; AVI = nontaster allele; n.s. = no statistical significance; ANOVA = analysis of variance.
B. cereus CM Stimulates NO Production through a Low-Molecular-Weight, Heat, and Protease Stabile Product
We next sought to further characterize the active component(s) in the B. cereus CM that was eliciting the NO response by boiling the CM to denature any proteins, dialyzing to remove low-molecular-weight (<1 kDa) products, and treating with proteases to digest peptide bonds. B. cereus CM was not significantly affected by boiling (Fig. 5) (181.7 ± 14.85; n.s., when compared with B. cereus CM by using one-way ANOVA with Bonferroni posttest), which indicated that the active product in the B. cereus CM was heat stable and thus less likely to be a protein because proteins with a secondary or tertiary structure are often denatured by boiling. Dialyzing with a cutoff of 1 kDa reduced the CM potency nearly 20-fold (Fig. 5) (11.48 ± 10.98; p < 0.01), which demonstrated that the NO response was being activated by a low-molecular-weight product in the CM.
Figure 5.
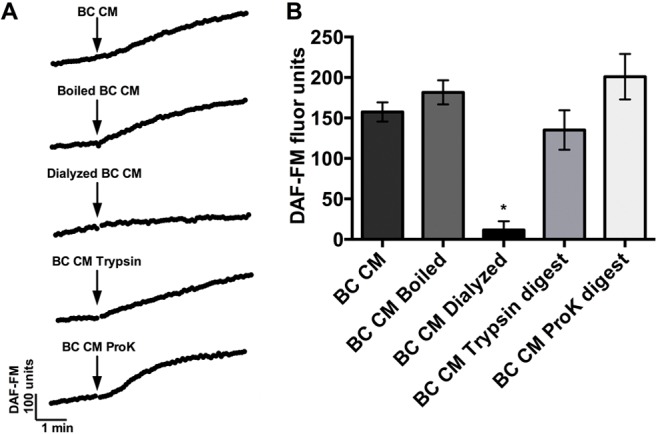
Bacillus cereus CM induces NO production through a low-molecular-weight, heat, and protease stabile product. (A) Representative traces of DAF-FM fluorescence and (B) bar graphs of average DAF-FM fluorescence (n = 3–12 cultures from 3–12 patients for each condition) for B. cereus CM, boiled B. cereus CM (100°C, 1 hour), dialyzed B. cereus CM (in LB, <1 kDa, 5 hours), trypsin digested B. cereus CM (37°C, 1 hour), and proteinase K digested B. cereus CM (37°C, 1 hour). DAF-FM fluorescence increases were 157.4 ± 11.94 (B. cereus CM) versus 181.7 ± 14.85 (B. cereus CM boiled; n.s.) versus 11.48 ± 10.98 (B. cereus CM dialyzed 1 kDa; p < 0.01) versus 135.1 ± 24.31 (B. cereus CM trypsin digest; n.s.) versus 201.0 ± 28.22 (B. cereus CM ProK digest; n.s.). The data in the graph are average ± SE. *p < 0.01, n.s. CM = Conditioned medium; NO = nitric oxide; DAF-FM = 4-amino-5-methylamino-2′,7′-difluorofluorescein; LB = lysogeny broth; n.s. = no statistical significance; ProK = proteinase K.
To determine if the small molecule was a peptide, we treated the B. cereus CM with the proteases trypsin and proteinase K, which would cleave peptides present in the CM. There was no significant difference between DAF-FM fluorescence increases for B. cereus CM with and without trypsin or proteinase K digestions (Fig. 5), which indicated that the active product was not a peptide. The average DAF-FM fluorescence increase was 135.1 ± 34.38 for B. cereus CM after trypsin digestion and 201.0 ± 28.22 after proteinase K digestion. As controls, trypsin and proteinase K alone were tested at the concentration used and were found to have no effects on NO production (not shown). By using the heterologous expression system, we attempted to identify the specific T2R being activated by the B. cereus CM. Unfortunately, the B. cereus CM caused calcium mobilization in all HEK293 cells, which made it difficult to determine if any single T2R was responsible for the T2R-dependent portion of the NO response.
DISCUSSION
The interaction between microorganisms and T2R-mediated sinonasal innate immunity plays an important role in CRS.1,8–11 In addition, T2Rs have been identified outside of the upper airway, including in the GI tract, brain, and pancreas.17,25–27 To our knowledge, this study was the first report of NO production and increased CBF in differentiated sinonasal epithelial cells in response to B. cereus, a pathogen better known for its role in the GI tract than in the respiratory tract. The pharmacologic inhibition used in this study indicated that the NO production in response to B. cereus CM may be partially mediated by a T2R. Moreover, the potential T2R that responds to the B. cereus CM was demonstrated to be separate from the previously described T2R38-mediated NO pathway triggered by gram-negative AHL quorum-sensing molecules.1
Our preceding work demonstrated that common genetic polymorphisms in the TAS2R38 gene were linked to significant differences in the ability of upper respiratory cells to produce NO and kill bacteria,1 and the nonprotective polymorphism was demonstrated to be an independent risk factor for CRS.9 As with the T2R38 response to AHLs, the response to B. cereus CM was a rapid rise in intracellular NO that lasted at least 5 minutes, with variability observed among different patients. It is entirely possible that the patient differences in NO production in response to B. cereus CM are related to genetic variability of a T2R gene and receptor activated by the product. The HEK293 heterologous expression system used in this study was ineffective in identifying any implicated T2Rs due to a global calcium response in all transfected cells; therefore, it will be necessary to repeat the experiments with the purified B. cereus CM product(s).
We demonstrated that the bacterial product that induced the response was <1 kDa in size, heat stabile, and resistant to digestion with two proteases. Together, these findings strongly indicated that the microbial product was not a peptide, the typical type of quorum-sensing molecules used by gram-positive bacteria,28 including B. cereus.29 It is possible that the product that elicited the response was not unique to B. cereus and may be shared by pathogens more commonly associated with respiratory disease. Although the specific identity of the active B. cereus CM product remains unknown, high-performance liquid chromatography (HPLC) and mass spectroscopy are feasible future options for isolation and identification.
When upper respiratory defenses fail, there is disruption of the intrinsic mucociliary transport system, stasis of sinonasal secretions, and formation of biofilms, which ultimately leads to the chronic infection and inflammation seen in intractable CRS.30–33 In the era of antibiotic resistance, treatment of CRS requires an increased understanding of the cellular responses to pathogens and effective alternative therapies. Determination of the active compound from B. cereus could lead to a novel pharmacologic treatment for CRS that could activate local NO production to kill bacteria and accelerate MCC. Moreover, compound isolation could allow for more in-depth studies of T2R genetics.
CONCLUSION
A product(s) secreted by B. cereus, a microbe typically found outside of the airway, activates an NO-mediated innate defense response in sinonasal epithelium that was potentially mediated by a T2R signaling pathway. The activating product(s) was likely a small, nonpeptide molecule, but its specific identity remained unknown. The variability in the NO response to a B. cereus product(s) could potentially be attributed to genetic differences in T2Rs.
Footnotes
Funding source: NIH Grant R01 R01DC013588
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Lee RJ, Xiong G, Kofonow JM, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest 122:4145–4159, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee RJ, Kofonow JM, Rosen PL, et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest 124:1393–1405, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chandrashekar J, Mueller KL, Hoon MA, et al. T2Rs function as bitter taste receptors. Cell 100:703–711, 2000. [DOI] [PubMed] [Google Scholar]
- 4. Matsunami H, Montmayeur JP, Buck LB. A family of candidate taste receptors in human and mouse. Nature 404:601–604, 2000. [DOI] [PubMed] [Google Scholar]
- 5. Maurer S, Wabnitz GH, Kahle NA, et al. Tasting Pseudomonas aeruginosa biofilms: Human neutrophils express the bitter receptor T2R38 as sensor for the quorum sensing molecule N-(3-oxododecanoyl)-l-homoserine lactone. Front Immunol 6:369, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gaida MM, Dapunt U, Hansch GM. Sensing developing biofilms: The bitter receptor T2R38 on myeloid cells. Pathog Dis 74: pii: ftw004, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shah AS, Ben-Shahar Y, Moninger TO, et al. Motile cilia of human airway epithelia are chemosensory. Science 325:1131–1134, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adappa ND, Howland TJ, Palmer JN, et al. Genetics of the taste receptor T2R38 correlates with chronic rhinosinusitis necessitating surgical intervention. Int Forum Allergy Rhinol 3:184–187, 2013. [DOI] [PubMed] [Google Scholar]
- 9. Adappa ND, Zhang Z, Palmer JN, et al. The bitter taste receptor T2R38 is an independent risk factor for chronic rhinosinusitis requiring sinus surgery. Int Forum Allergy Rhinol 4:3–7, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adappa ND, Farquhar D, Palmer JN, et al. TAS2R38 genotype predicts surgical outcome in nonpolypoid chronic rhinosinusitis. Int Forum Allergy Rhinol 6:25–33, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adappa ND, Workman AD, Hadjiliadis D, et al. T2R38 genotype is correlated with sinonasal quality of life in homozygous ΔF508 cystic fibrosis patients. Int Forum Allergy Rhinol 6:356–361, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ng WL, Bassler BL. Bacterial quorum-sensing network architectures. Annu Rev Genet 43:197–222, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamilos DL. Host-microbial interactions in patients with chronic rhinosinusitis. J Allergy Clin Immunol 133:640–653.e4, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carey RM, Workman AD, Chen B, et al. Staphylococcus aureus triggers nitric oxide production in human upper airway epithelium. Int Forum Allergy Rhinol 5:808–813, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carey RM, Chen B, Adappa ND, et al. Human upper airway epithelium produces nitric oxide in response to Staphylococcus epidermidis. Int Forum Allergy Rhinol 6:1238–1244, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gu F, Liu X, Liang J, et al. Bitter taste receptor mTas2r105 is expressed in small intestinal villus and crypts. Biochem Biophys Res Commun 463:934–941, 2015. [DOI] [PubMed] [Google Scholar]
- 17. Wu SV, Rozengurt N, Yang M, et al. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci U S A 99:2392–2397, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barbosa TM, Serra CR, La Ragione RM, et al. Screening for bacillus isolates in the broiler gastrointestinal tract. Appl Environ Microbiol 71:968–978, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duc le H, Hong HA, Barbosa TM, et al. Characterization of Bacillus probiotics available for human use. Appl Environ Microbiol 70:2161–2171, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stenfors Arnesen LP, Fagerlund A, Granum PE. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol Rev 32:579–606, 2008. [DOI] [PubMed] [Google Scholar]
- 21. Lai Y, Chen B, Shi J, et al. Inflammation-mediated upregulation of centrosomal protein 110, a negative modulator of ciliogenesis, in patients with chronic rhinosinusitis. J Allergy Clin Immunol 128:1207–1215.e1, 2011. [DOI] [PubMed] [Google Scholar]
- 22. Gustafsson LE, Leone AM, Persson MG, et al. Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Commun 181:852–857, 1991. [DOI] [PubMed] [Google Scholar]
- 23. Haight JS, Djupesland PG, Qjan W, et al. Does nasal nitric oxide come from the sinuses? J Otolaryngol 28:197–204, 1999. [PubMed] [Google Scholar]
- 24. Maniscalco M, Sofia M, Pelaia G. Nitric oxide in upper airways inflammatory diseases. Inflamm Res 56:58–69, 2007. [DOI] [PubMed] [Google Scholar]
- 25. Clark AA, Dotson CD, Elson AE, et al. TAS2R bitter taste receptors regulate thyroid function. FASEB J 29:164–172, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deckmann K, Filipski K, Krasteva-Christ G, et al. Bitter triggers acetylcholine release from polymodal urethral chemosensory cells and bladder reflexes. Proc Natl Acad Sci U S A 111:8287–8292, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gu F, Liu X, Liang J, et al. Bitter taste receptor mTas2r105 is expressed in small intestinal villus and crypts. Biochem Biophys Res Commun 463:934–941, 2015. [DOI] [PubMed] [Google Scholar]
- 28. Monnet V, Gardan R. Quorum-sensing regulators in gram-positive bacteria: ‘Cherchez le peptide’. Mol Microbiol 97:181–184, 2015. [DOI] [PubMed] [Google Scholar]
- 29. Grenha R, Slamti L, Nicaise M, et al. Structural basis for the activation mechanism of the PlcR virulence regulator by the quorum-sensing signal peptide PapR. Proc Natl Acad Sci U S A 110:1047–1052, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hsu J, Peters AT. Pathophysiology of chronic rhinosinusitis with nasal polyp. Am J Rhinol Allergy 25:285–290, 2011. [DOI] [PubMed] [Google Scholar]
- 31. Kern RC, Conley DB, Walsh W, et al. Perspectives on the etiology of chronic rhinosinusitis: An immune barrier hypothesis. Am J Rhinol 22:549–559, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cohen NA. Sinonasal mucociliary clearance in health and disease. Ann Otol Rhinol Laryngol Suppl 196:20–26, 2006. [DOI] [PubMed] [Google Scholar]
- 33. Gudis D, Zhao KQ, Cohen NA. Acquired cilia dysfunction in chronic rhinosinusitis. Am J Rhinol Allergy 26:1–6, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]