Abstract
Almost 40 years has passed since the discovery of giant elastic protein titin (also known as connectin) of striated and smooth muscles using gel electrophoresis. Sodium dodecyl sulfate polyacrylamide gel electrophoresis is a major technique for studying the isoform composition and content of titin. This review provides historical insights into the technical aspects of the electrophoresis methods used to identify titin and its isoforms. We particularly focus on the nuances of the technique that improve the preservation of its primary structure so that its high molecular weight isoforms can be visualized.
Keywords: Striated muscles, Titin/connectin, Titin isoforms, SDS-Page
History of the discovery and study of titin/connectin by SDS-gel electrophoresis technique
Titin was discovered in 1979 by Kuan Wang and his coauthors using gel electrophoresis. They studied chicken striated (breast), cardiac and smooth (gizzard) muscle extracts using 4% polyacrylamide gels (acrylamide/bisacrylamide ratio 50:1, Tris glycine pH 8.8; as in Etlinger et al. 1976) in which they identified another very large actin-binding protein, filamin (Wang et al. 1979). Filamin was detected in smooth muscle but not in extracts from cardiac and skeletal muscles of the chicken and rabbit in the presence of sodium dodecyl sulfate (SDS). In another polyacrylamide gel prepared as described by Etlinger et al. (1976), except that 3.2% acrylamide was used instead of 5%, three major bands were observed above the prominent myosin heavy chain. They found a closely spaced doublet and a faster moving singlet band (Wang et al. 1979). Using crosslinked myosin heavy chains (205 kDa) as standards, the authors estimated that each of these doublet bands (1 and 2) has a Mr ∼1 × 106 and that the Mr of band 3 is ∼5 × 105. Proteins 1 and 2 appeared to be immunologically identical and were named titin 1 (T1) and titin 2 (T2). The third protein was thereafter named nebulin (Wang 1982).
Independent of Kuan Wang and his coworkers, another group of investigators headed by Koscak Maruyama discovered a protein which they named connectin (Maruyama 1976; Maruyama et al. 1976). The properties of connectin as a protein candidate for the elastic filaments in sarcomeres of striated muscles of vertebrate animals were intensively explored by this group of authors in the late 1970s to early 1980s (Maruyama et al. 1977a, b, c; Matsubara and Maruyama 1977; Toyoda and Maruyama 1978). In 1981, having conducted a comparative study of electrophoretic mobility, amino acid composition, and localization in myofibrils of titin and connectin, Maruyama and coauthors showed that the major high molecular weight component of connectin was identical with that of titin (Maruyama et al. 1981). Using crosslinked myosin heavy chains as standards, and 1.8–3.0% polyacrylamide tube gels prepared according to Weber and Osborn (1969), the authors showed that the molecular weights of α-connectin (corresponding to intact molecules of titin-1) and β-connectin (corresponding to proteolytic fragments of T1 – T2) of breast muscle of the chicken were 2.8 × 106 and 2.1 × 106, respectively (Maruyama et al. 1984) (Table 1, Reference 2).
Table 1.
Types of gels for electrophoretic study of titin
| Reference | Type and size of gel, percentage of acrylamide (agarose) | Ratio of acrylamide and bis-acrylamide | Gel buffer, pH | Sample buffer, pH | Heating of samples | Tissue/muscle source | Molecular mass of titin (connectin) | Nuances |
|---|---|---|---|---|---|---|---|---|
| 1. Wang et al. 1979 | 3.2% polyacrylamide tube gel, 0.5 × 10 cm | 50/1 | 0.1 M Tris-glycine, pH 8.8, 0.4% SDS | 100 mM Tris-HCl, 10 mM EDTA, 40 mM DTT, 10% SDS, 20% glycerol, pH 8.0 | Samples were boiled for 2 min | Purified striated muscle myofibrils (chicken breast muscle, rabbit back muscle) prepared according to Etlinger et al. (1976) | Doublet bands (T1 and T2) has Mr ∼1 × 106. | This procedure was a modification of the Etlinger et al. method (1976) |
| 2. Maruyama et al. 1984 | 1.8–3.0% polyacrylamide tube gel, 0.6 × 10 cm | 37/1 | 7.8 g NaH2PO4 × H2O and 38.6 g Na2HPO4 × 7H20, pH 7.5 | 0.01 M sodium phosphate buffer, pH 7.0, 1% SDS, and 1% ß-mercaptoethanol, 8 M urea | 37 °C for 2 h | Chicken breast muscle | α-connectin – 2.8 MDa; ß-connectin – 2.1 MDa. A faint band above α-connectin is visible in gel (see fig. 2 in Maruyama et al. 1984) | Connectin was extracted from exhaustively washed myofibrils of chicken breast muscle in 0.1 M phosphate buffer, pH 6.6. |
| 3. Hu et al. 1986 | 1.8% polyacrylamide tube gel, 0.6 × 10 cm | 20/1 | 7.8 g NaH2PO4 × H2O and 38.6 g Na2HPO4 × 7H20, pH 7.5 | 10% SDS, 40 mM DTT, 10 mM EDTA, 0.1 M Tris-HCl, pH 8.0 | 2–3 min at 100 °C | Striated muscles of carp, goldfish, bull frog, newt, turtle, snake, chicken, rabbit | Decrease in mobility of α-connectin was in the following order: fish muscles > frog, newt, chicken, snake, rabbit muscles > turtle muscles. As standard, chicken breast muscle was used (MWs of α- and ß-connectins were estimated to be 2.8 and 2.1 × 106, respectively). | This procedure was a modification of the Weber–Osborn method (1969) |
| 4. Wang and Wright 1988 | 2–12% gradient polyacrylamide slab gel, 8 × 10 cm |
50/1 | 40 mM Tris-acetate, 20 mM Na-acetate, 2 mM EDTA, 0.1% SDS, pH 7.6 | 1% SDS, 5–10% sucrose, 1 mM EDTA (pH 8.0), 40 mM DTT, 10 pg/ml of pyronin Y (tracking dye), 10 mM Tris-HC1, pH 8.0 | 90 s at 95 °C | Rabbit striated muscles (longissimus dorsi, psoas, semitendinosus, soleus, diaphragm, adductor magnus, sartorius, heart) | Decrease in mobility of T1 was in the following order: heart > psoas > adductor magnus > longissimus dorsi > semitendinosus, soleus, diaphragm, sartorius. | Muscle tissues were snap-frozen in liquid nitrogen and pulverized to fine powders. Snap-frozen tissue powders were treated with SDS sample buffer preheated to 95 °C. This procedure was a modification of the Somerville and Wang method (1981) |
| 5. Fritz et al. 1989 | 10% polyacrylamide slab gel (small resolving gel, 6 × 8.2 × 0.075 cm) and 4–10–15% polyacrylamide slab gel (layer resolving gel (12 × 14 × 0.15 cm, each layer 4 cm high). | 200/1 | 0.75 M Tris, 10% glycerol, 0.1% SDS, pH 8.6, 8.8, 9.3 | 8 M urea, 2 M thiourea, 3% SDS, 75 mM DTT, 25 mM Tris-HCl, pH 6.8. | Samples were boiled for 2–8 min | Myofibrils from rabbit psoas and bovine rectus abdominis muscle | – | Myofibrils were prepared in a buffer containing 50 mM KCl, 20 mM Tris, pH 7.0, 2 mM EDTA, 4 mM MgCl2, 5 mM 2-mercaptoethanol, 0.1 mM PMSF, 1% Triton X-100. Myofibrils were stored at −20 °C in rigor buffer containing 75 mM KCl, 10 mM KH2PO4, 2 mM MgCl2, 2 mM EGTA, 50% (v/v) glycerol, pH 7.0. Migration rates of titin and nebulin in gel are especially affected by 2-mercaptoethanol inclusion in the upper buffer. |
| 6. Wang et al. 1991 | 2–12% gradient polyacrylamide slab gel, 8 × 10 cm | 50/1 | 40 mM Tris-acetate, 20 mM Na-acetate, 2 mM EDTA, 0.1% SDS, pH 7.6 | 1% SDS, 5–10% sucrose, 1 mM EDTA (pH 8), 40 mM DTT, 10 pg/ml of pyronin Y (tracking dye), 10 mM Tris-HC1, pH 8 | 90 s at 95 °C | Rabbit striated muscles (adductor magnus, psoas, longissimus dorsi, sartorius, soleus, semitendinosus, cardiac muscle) | Molecular mass of T1: AM and PS, 2.8 MDa; LD and SA, 2.88 MDa; SO and ST, 2.94 MDa. T2: 2.4 MDa. Cardiac muscle displayed the smallest titin. |
Muscle tissues were snap frozen, pulverized in liquid nitrogen, solubilized in hot SDS |
| 7. Horowits 1992 | 2–12% gradient polyacrylamide slab gel | 50/1 | 40 mM Tris-acetate, 20 mM Na-acetate, 2 mM EDTA, 0.1% SDS, pH 7.6 | 1% SDS, 10% glycerol, 1 mM EDTA (pH 8), 70 mM ß-mercaptoethanol, 0.05% bromophenol blue, 10 mM Tris-HC1, pH 8 | Samples were boiled for 1–2 min | Chemically skinned strips of rabbit psoas and soleus muscles | Soleus titin migrated in gel slightly slower than psoas titin. | Strips of rabbit psoas and soleus muscle were chemically skinned (see the article for more details). After measuring its mechanical properties and dimensions, each single fiber was dissolved in sample buffer. |
| 8. Granzier and Wang 1993 | 3.3–12% gradient polyacrylamide slab gel, 8 × 10 × 0.075 cm | 50/1 | 40 mM Tris-acetate, 20 mM Na-acetate, 2 mM EDTA, 0.1% SDS, pH 7.6 | Laemmli (1970) sample buffer (75 mM Tris-C1, 3% w/v SDS, 120 mM DTT, 55 μg/mL Pyronin Y, 15% v/v glycerol, pH 6.8) was used. Sample buffer of Fritz et al. (1989) (8 M urea, 2 M thiourea, 3% SDS, 75 mM DTT, 25 mM Tris-C1, pH 6.8) was also used. |
23–100 °C for 1–8 min. Optimal solubilization temperatures for fibers were in the range of 55–75 °C. | Skinned rabbit psoas fibers. | T1–2.8 MDa; T2–2.4 MDa; A faint band above T1 was detected. | Skinned rabbit psoas fibers were used (see the article for more details). Presoaking in a low ionic strength alkaline buffer was identified as an important step in obtaining complete solubilization of titin from purified myofibrils. |
| 9. Kawamura et al. 1994 | 2.3–4% polyacrylamide tube gel, 0.6 × 10 cm | 36.5/1 | 0.375 M Tris-HCl, 0.1% SDS, pH 8.8 | 10% (w/v) SDS, 40 mM DTT, 10 mM EDTA, 0.1 M Tris-HCl buffer, pH 8.0 | Samples were boiled for 2 min | Skeletal muscles of lamprey, electric ray, horse mackerel and chicken. | The mobility of ray α- connectin in gel was slower than that of chicken and other animals α-connectins. | SDS gel electrophoresis was carried out according to Laemmli (1970). The electrode buffer (pH 8.3) contained 0.025 M Tris, 0.192 M glycine, 0.1% SDS |
| 10. Granzier and Irving 1995 | Slab gel | – | – | 50 mM Tris-Cl, 2% SDS, 10% glycerol, 80 mM DTT, 30 μg/ml Pyronin Y, pH 6.8 | 90 s at 90–95 °C | Rat heart and rabbit skeletal muscles (semitendinosus and psoas) | 2.49 ± 0.03 MDa for rat cardiac T1 | SDS gel electrophoresis was carried out according to Granzier and Wang (1993). Muscle tissues were quick-froze in liquid nitrogen and pulverized to a fine powder. |
| 11. Tatsumi and Hattori 1995, | 2% polyacrylamide slab gel strengthened with agarose (0.5%), 8.3 × 7.2 × 0.15 cm | 20/1 (Fairbanks et al. 1971) 37.5/1 (Laemmli 1970) |
40 mM Tris-acetate, 20 mM Na-acetate, 2 mM EDTA, 0.1% SDS, pH 7.4 (Fairbanks buffer system) (1971). 0.375 M Tris-HCl, 0.1% SDS, pH 8.6 (Laemmli’s buffer system) |
1% SDS, 1% ß-mercaptoethanol, 5 mM EDTA, 5 mM Tris-HC1, pH 8.0, 10% glycerol, 30 μg/ml leupeptin | Samples were boiled for 2 min | Myofibrils of chicken breast muscle (M. pectoralis superficialis) and rabbit skeletal muscles (M. longissimus thoracis, m. soleus) | α- connectin of rabbit muscles had a lower mobility in gel than chicken muscle α- connectin. | Protein samples were frozen at −80 °C until use. Glass cell were cooled for 5 min before pouring of gel. Laemmli’s system provided higher resolution to titin and nebulin. |
| 12. Spierts et al. 1997 | 2.4–12% gradient polyacrylamide slab gel | – | – | 50 mM Tris-Cl, 2% SDS, 10% glycerol, 80 mM DTT, 30 μg/ml Pyronin Y, pH 6.8 | 90 s at 80 °C | Red and white axial muscles of carp (Cyprinus carpio L.) | Psoas and semitendinosus muscles from rabbit were used as high-molecular-mass standards, and titin of these muscles had a lower mobility than carp muscle T1. | Fiber bundles were quick frozen in liquid nitrogen and pulverized to a fine powder. |
| 13. Cazorla et al. 2000 | 2–9.5% gradient polyacrylamide slab gel | – | – | – | – | Human soleus, mouse, rat, rabbit, dog, bovine, pig, cow and human myocardium. | Two T1 bands in cardiac muscle and one T1 band in m. soleus were detected. Using the mobility of human soleus T1 (3.7 MDa) and rat cardiac titin T1 (2.97 MDa, bottom band) as standards the authors showed that molecular mass of top cardiac T1 band is ∼3.3 MDa. In addition to T1 and T2 some samples also contained a band that barely entered the gel. Western blotting with titin-specific antibodies indicated that this band is titin. The authors suggested that this is titin aggregates. |
Muscle samples were quick-frozen in liquid nitrogen, pulverized, and then rapidly solubilized. SDS gel electrophoresis was carried out according to Granzier and Irving (1995). |
| 14. Warren et al. 2003a, b | 1% agarose slab gel, 16 × 18 × 0.15 cm | – | 30% v/v glycerol, 50 mM Tris-base, 0.384 M glycine, and 0.1% w/v SDS, pH 8.5 (no pH adjustment necessary). | 8 M urea, 2 M thiourea, 3% SDS, 75 mM DTT, 0.03% bromophenol blue, and 0.05 M Tris-Cl, pH 6.8. | 100 °C for 3 min, 60 °C for 10–20 min | Rabbit tibialis anterior, semitendinosus, soleus, psoas, left ventricle, right ventricle, dog triceps, left ventricle, rat soleus. The cardiac samples contain N2BA and N2B isoforms whereas the skeletal samples contain only N2A type of titin. | Decrease in mobility of titin was in the following order: cardiac N2B > cardiac N2BA > N2A (rabbit psoas) > N2A (rabbit tibialis anterior, semitendinosus, soleus, rat soleus) > dog triceps. The rabbit psoas muscle has two differently sized N2A bands. The rabbit left and right ventricles have two N2BA bands. Two faint bands above N2B and N2BA were detected. The authors suggested that this is titin aggregates. |
Skeletal and cardiac tissue was dissected from New Zealand rabbits (2–3 kg), mongrel dogs, or Sprague Dawley rats and flash frozen in liquid nitrogen. The frozen tissue was pulverized and placed in preweighed Dounce homogenizers. The sample heating comparisons showed that the optimal temperature for solubilization of cardiac titin with minimal breakdown was 60 °C for 10 or 20 min. |
| 15. Neagoe et al. 2003 | Agarose-strengthened 2.0% polyacrylamide slab gels with a Laemmli buffer system (Tatsumi and Hattori 1995) | 37.5/1 | 0.375 M Tris-HCl, 0.1% SDS, 0.5% agarose, pH 8.6 | 1% SDS, 1% 2-mercaptoethanol, 10% glycerol, 8 μg/ml leupeptin, 6 μM bromphenol blue, 4.3 mM Tris–HCl, pH 8.8, 4.3 mM EDTA | Samples were boiled for 3 min | Frog, mouse, hamster, rat, rabbit, cat, cow and human heart, rabbit soleus (freshly excised and frozen muscle tissue). | N2B titin isoform – 3000 MDa; N2BA isoforms – 3.25-3.4 MDa. Rabbit soleus N2A-titin (3.7 MDa) and skeletal nebulin (700–800 kDa) were used as markers for molecular weight detection. |
Samples were solubilized by quickly homogenizing 30–60 mg of frozen tissue in 100 μl ice-cold relaxing buffer supplemented with 40 μg/ml leupeptin. Samples were centrifuged briefly and the pellet fraction was used for further analysis. |
| 16. Vikhlyantsev and Podlubnaya 2006 | Horizontal 1.3% polyacrylamide gel strengthened with agarose (0.5%), 8.5 × 12.5 × 0.2 cm | 37.5/1 | 0.5 M Tris-HCl, 0.1% SDS, pH 9.0 | 10 mM Tris–HCl, 1.5% SDS, 1% β -mercaptoethanol, 10% glycerol, 2.5 mM EGTA, 8 μg/ml leupeptin or E64, pH 7.0 | Fresh or frozen muscle tissues were incubated in sample buffer for 30–40 min at 20–25 °C (reference samples), after which a small amount of the protein extract was taken for subsequent heating at: 35–60 °C for 10 min, 65–75 °C for 5 min, 80–90 °C for 2–4 min, 95–100 °C for 1–4 min. | Mouse, rat, rabbit, ground squirrel and human striated muscles (freshly excised and frozen muscle tissue). | One or two titin bands (called NT) above N2A, N2BA, N2B and T2 bands were detected. Using the mobility of human and animal nebulin (770–890 kDa) and myosin heavy chain (205 kDa) as standards the authors showed that molecular weights are: 2.08–2.30 MDa (T2), 2.42–2.45 MDa (N2B), 2.56–2.8 MDa (N2A), 3.23–3.3 MDa (cardiac NT), 3.38–3.73 MDA (skeletal NT). | Fresh muscle tissues and frozen in liquid nitrogen were used. Frozen muscles were stored at −80 °C. Recommended temperature for solubilization of titin without breakdown is 35–40 °C. |
Further electrophoretic studies of titin (connectin) using different types of gels (Table 1, References 3, 4, 6, 7, 9, 10, 12) revealed differences in electrophoretic mobility of T1 (α-connectin) in cardiac and skeletal muscles of vertebrates animals (fishes, amphibians, reptiles, birds, mammals). In particular, six skeletal muscles (adductor magnus – AM, psoas – PS, longissimus dorsi – LD, sartorius – SA, soleus – SO, semitendinosus – ST) and cardiac muscle of the rabbit were analyzed on a 2–12% gradient gel (Wang et al. 1991). Plots of molecular mass versus mobility, assuming 2.8 and 2.4 MDa for T1 and T2 of the rabbit PS, respectively, yielded the following set of values for T1: AM and PS, 2.8 MDa; LD and SA, 2.88 MDa; SO and ST, 2.94 MDa (Wang et al. 1991) (Table 1, Reference 6). Cardiac muscle displayed the smallest titin. Based on data obtained the assumption on the existence of isoforms of T1 was made (Wang et al. 1991; Horowits 1992). This assumption is in agreement with the results of our experiments (Vikhlyantsev and Makarenko 2000) in which the differences in electrophoretic mobility of T1 in striated muscles of a ground squirrel were found in a 7% polyacrylamide gel, prepared according to Fritz et al. (1989). The distinguishing feature of an acceptable gel for high-molecular-mass proteins (greater than 500 kDa) is the requirement for a low concentration of bis-acrylamide (bis-acrylamide/acrylamide = 1:200) (Table 1, Reference 5).
In 1995 the complete complementary DNA sequence of human cardiac titin was determined (Labeit and Kolmerer 1995). Further studies showed that the titin gene (TTN) consists of 363 coding exons, which can be differentially spliced and theoretically could generate more than 1 million splice variants in striated and smooth muscles of mammals (Freiburg et al. 2000; Bang et al. 2001; Labeit et al. 2006; Guo et al. 2010; Gerull 2015).
Adult striated muscles express three major titin isoforms named N2A (skeletal muscles), N2B, and N2BA (cardiac muscle). Multiple splicing pathways result in titin isoform diversity (Freiburg et al. 2000). In particular, in soleus and psoas skeletal muscles, different exon-skipping pathways produce titin transcripts that code for 3.7 and 3.35 MDa isoforms, respectively (Freiburg et al. 2000). In the heart, ventricular muscle exons separated by 101 kb lead to the exclusion of 155 exons and the expression of a 2.97-MDa cardiac titin N2B isoform. The atria express exclude 90–100 exons resulting in a 3.3-MDa N2BA titin isoform (Freiburg et al. 2000).
Electrophoretic detection of titin isoforms
To confirm that muscles contain N2A, N2B and N2BA isoforms of titin, different macroporous gels have been reported by different authors (Table 1, References 13–15). These gels showed that the T1 mobility varied greatly between skeletal and cardiac muscles from different mammals (Cazorla et al. 2000; Trombitás et al. 2000; Neagoe et al. 2003; Warren et al. 2003a, b; Lahmers et al. 2004; Makarenko et al. 2004; Udaka et al. 2008; Guo et al. 2013). The major T1 bands were ascribed to the titin isoforms N2B and N2BA in cardiac muscle and the titin isoform N2A in skeletal muscles. Western blots using antibodies against the N-terminal and the C-terminal ends of titin revealed that the N2A, N2B and N2BA bands represent full-length titin molecules (T1) (Cazorla et al. 2000; Wu et al. 2000; Lahmers et al. 2004).
Using agarose-strengthened 2.0% polyacrylamide gel, isoform diversity of skeletal N2A-titin in rabbit muscles was detected (Prado et al. 2005). In particular, titin isoform analyses for 37 adult rabbit skeletal muscles showed sizes between 3.3 MDa and 3.7 MDa (Prado et al. 2005, human soleus N2A (3.7 MDa) and human cardiac N2B (3.0 MDa) and N2BA (3.3 MDa) were as markers for molecular weight detection). N2BA titin isoforms in cardiac muscle of mammals (mouse, hamster, rat, rabbit, cat, and cow) had sizes of approximately 3.25–3.4 MDa (Neagoe et al. 2003; Table 1).
With the help of the electrophoresis system using 1% vertical agarose gel (Warren et al. 2003a), at least four classes of cardiac N2BA titin isoforms were observed. In particular, two rat embryonic/neonatal forms (N2BA-N1, N2BA-N2) with apparent sizes of approximately 3710 and 3590 kDa, respectively, were found during late embryonic and immediately post-natal (Warren et al. 2004). These were gradually replaced by adult forms (N2BA-A1, N2BA-A2) with sizes of 3390 kDa and 3220 kDa, respectively (Warren et al. 2004). Similar titin isoform transformations were observed in embryonic/neonatal hearts of rat and other mammals and reported by the researchers (Lahmers et al. 2004; Opitz et al. 2004; Greaser et al. 2005; Krüger et al. 2006).
Giant titin isoforms expressed in rat striated muscles with an RBM20 autosomal dominant mutation were recently reported (Greaser et al. 2008; Li et al. 2012; Guo et al. 2012). The molecular masses of these isoforms were estimated from their electrophoretic mobility in 1% vertical agarose gel to be 3750 kDa and 3830 kDa (Li et al. 2012).
More than 10 years ago, our group headed by Zoya Podlubnaya compared the isoform composition of titin in mammalian striated muscles under conditions of hibernation, microgravity, and during the development of pathological processes. Vertical agarose-strengthened 2.1–2.3% polyacrylamide gels (8 × 10 × 0.1 cm in the Laemmli buffer system, pH 9.0) prepared according to Tatsumi and Hattori (1995; Table 1, Reference 11) were used to separate titin isoforms and their fragments. Our first experiments showed that, in addition to N2A, N2BA, N2B and T2 bands, there exist one or two more high Mr bands (named NT) (Vikhlyantsev et al. 2004a, b). Staining the gels with ethidium bromide revealed no nucleic acids in the bands, although western blots with 9D10 antibodies revealed titin bands (Vikhlyantsev et al. 2004a). Our subsequent studies showed that these titin bands appear in the electrophoregrams of striated muscles of humans (Vikhlyantsev and Podlubnaya 2008) and other mammals, including mouse, rat, rabbit (Vikhlyantsev and Podlubnaya 2006; Ulanova et al. 2015), ground squirrel (Vikhlyantsev et al. 2008,), Mongolian gerbil (Vikhlyantsev et al. 2011; Okuneva et al. 2012), and bear (Salmov et al. 2015). Titin isoform composition in striated muscles in other groups of vertebrates (amphibians and birds) revealed no NT bands (Vikhlyantsev and Podlubnaya 2012).
The content of NT titins in the muscles of small rodents was 10–20% compared to 30–40% for ground squirrel, bear and humans. Using human and animal skeletal muscle myosin heavy chain (205 kDa) and, nebulin (770–890 kDa), as well as the N2A titin isoform (∼3600 kDa and 3700 kDa) of rabbit and human soleus as standards (Wang et al. 1991; Krüger et al. 1991; Granzier and Wang 1993; Prado et al. 2005), we estimated that the NT have a Mr of ∼3.8–3.9 × 106 (Vikhlyantsev and Podlubnaya 2006). Expression of titin isoforms with these molecular weights is not excluded (Bang et al. 2001; Guo et al. 2010, 2012; Li et al. 2012), but titin aggregates in gels could not be excluded either (Granzier and Wang 1993; Cazorla et al. 2000; Warren et al. 2003a). Assuming that molecular masses of titin aggregates should considerably exceed 3800–3900 kDa, we decided to find out more about the differences in electrophoretic mobility of the observed bands.
Accordingly, we tried to increase the time of electrophoresis in the vertical agarose-strengthened 2.0–2.3% polyacrylamide gels (8 × 10 × 0.1 cm) from 2 to 4–5 h. However, this led to a strong diffusion of titin bands. Then, we tried using 1.7–1.8% instead of 2.0–2.3% polyacrylamide gels, but the attempt failed because the gels slid down between the glass plates or broke during electrophoresis. We then developed a horizontal agarose-strengthened 8.5 × 12.5 cm gel system using 1.3% polyacrylamide and 0.5% agarose (Table 1, Reference 16). The gels showed that mobility of the NT bands varied greatly between muscle sources.
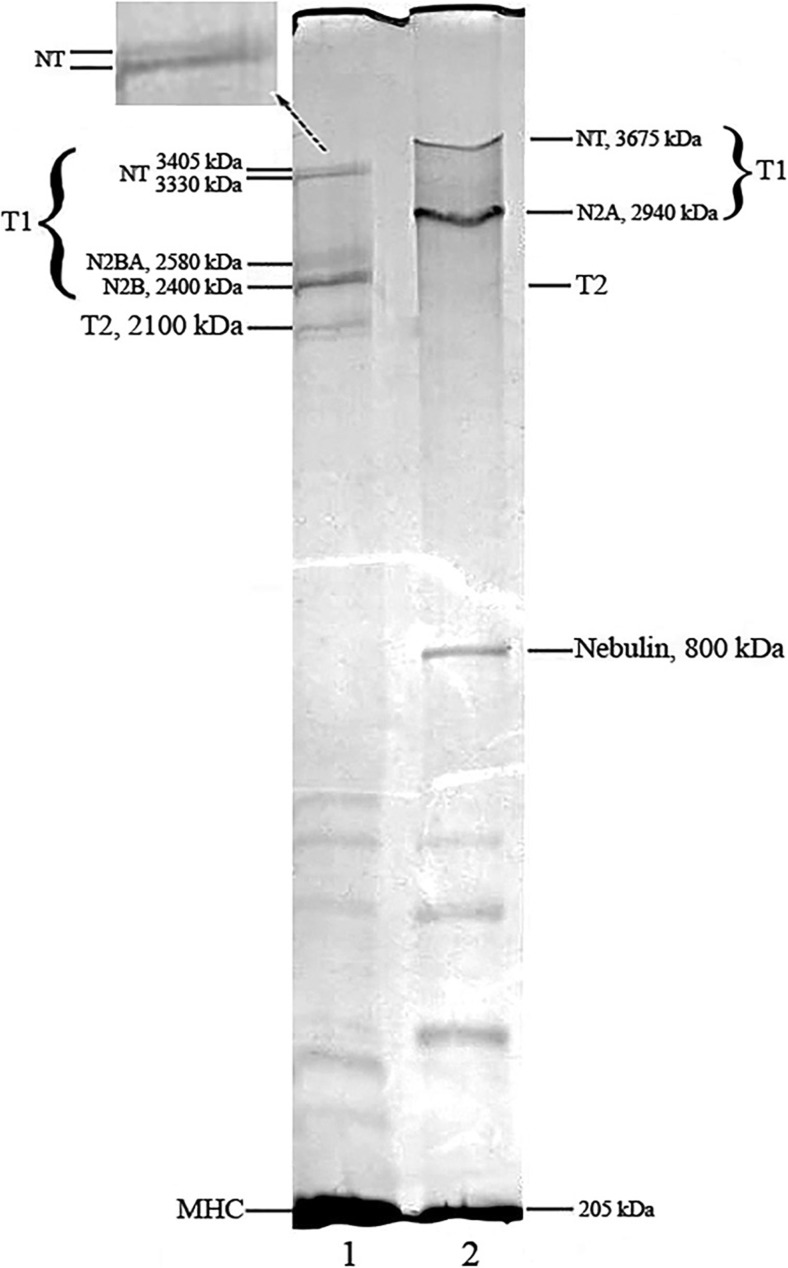
Using human and animal skeletal muscle myosin heavy chain (205 kDa) and nebulin (770–890 kDa) as standards, we estimated the molecular masses of N2A, N2BA, N2B, T2 and NT titin bands. The results obtained were impressive. The NT bands were 3230–3730 kDa, compared to the N2A, N2BA, N2B and T2 bands (Mr ∼2.1–2.8 × 106) (Table 1, Reference 16), that corresponded to a set of values for T1 (α-connectin) and T2 (β-connectin) (Maruyama et al. 1984; Wang et al. 1991; Granzier and Wang 1993). Similar data were obtained for vertical agarose-strengthened 1.9% polyacrylamide gels (14.5 × 16.0 × 0.15 cm). In Fig. 1, the gels resolved a doublet band at 3300–3400 kDa for cardiac muscle and a singlet band of 3600–3700 kDa for skeletal muscles of mammals. We therefore hypothesized that the NT bands are intact N2A, N2BA, N2B titin isoforms (Vikhlyantsev and Podlubnaya 2008).
Fig. 1.
SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) analysis of titin isoforms in striated muscles of ground squirrel (Spermophilus undulatus); a modified view from (Vikhlyantsev and Podlubnaya 2008). 1 Myocardium (left ventricle), 2 m. soleus. Protein bands are indicated: heavy chains of myosin (MHC); nebulin–cytoskeletal protein of thin filaments; proteolytic fragments of titin (T2); intact titin (T1); isoforms of T1 (N2B, N2BA, N2A, NT). Vertical agarose-strengthened 1.9% polyacrylamide gel (14.5 × 16.0 × 0.15 cm) was used to separate the titin isoforms. Enlarged fragment of the gel with NT-titin bands is in the inset. As standards for estimation of molecular weights, the MHC (205 kDa), nebulin (770–890 kDa), titin-2 (2100–2400 kDa) of rabbit and human striated muscles were used (Wang et al. 1991; Krüger et al. 1991; Granzier and Wang 1993)
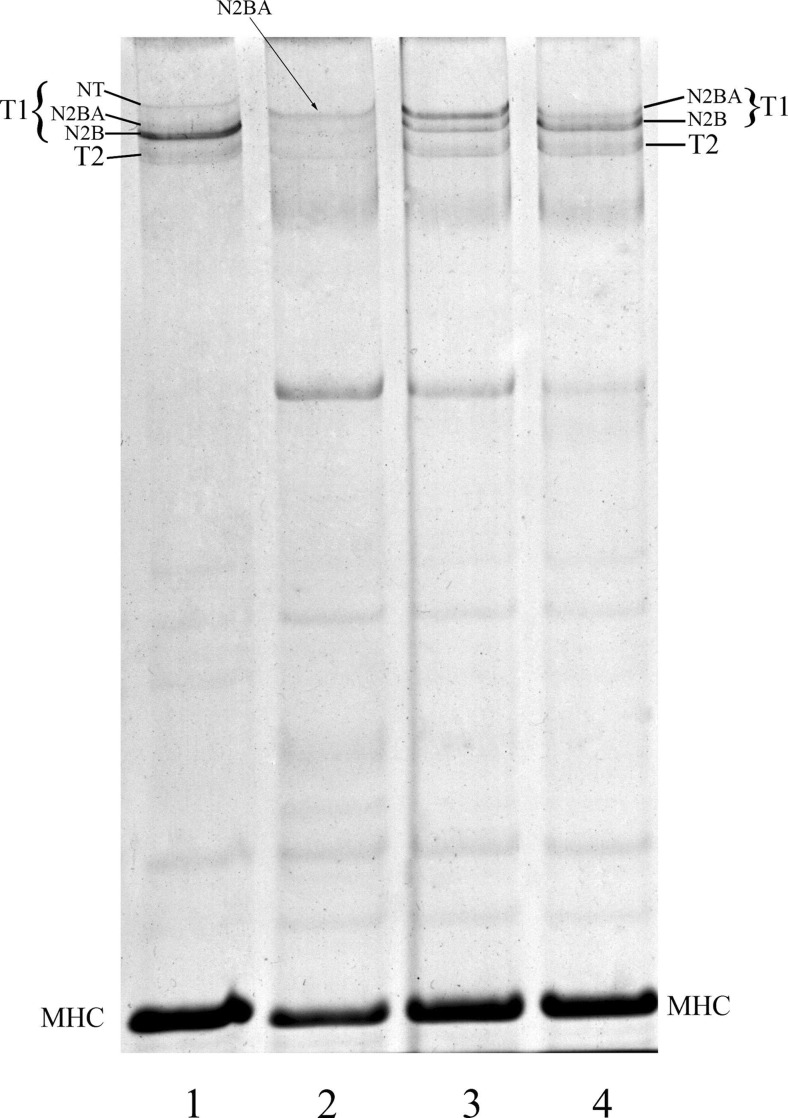
Results from western blots with Z1/Z2 antibodies against the N-terminal end and AB5 antibodies against the C-terminal end of titin revealed the NT bands were full-length titin molecules (Vikhlyantsev and Podlubnaya 2008), thereby agreeing with our hypothesis. Although this requires further research, we cannot exclude the possibility that the NT bands are the other protein immunologically identical to titin. Agarose-strengthened 2.1% polyacrylamide gels revealed no NT bands in rat heart in day 19 embryos or 2–14 days neonatal hearts (Fig. 2; unpublished data) but NT titin was detected in rats 30 days old (data not shown).
Fig. 2.
Perinatal titin isoform switching in rat heart. 1 Adult rat heart expresses N2B, N2BA and NT-titins. 2 Fetal hearts 3 day before birth (e19) expresses more long (embryonic) N2BA-isoform of titin and N2B-titin. 3, 4 Two days after birth (3) and 14 days after birth (4), N2B and N2BA-titins are expressed in rat heart. MHC Heavy chains of myosin; T1 intact titin; T2 proteolytic fragments of titin. Vertical agarose-strengthened 2.1% polyacrylamide gel (8 × 10 × 0.1 cm) was used to separate the titin isoforms
Peculiarities of sample preparation for electrophoresis study of titin
Granzier and Wang (1993) paid particular attention to sample preparation of titin. They found that titin was extraordinarily sensitive to proteolysis in situ by endogenous proteases and by proteases in buffers contaminated with bacteria and fungi. Even in SDS-solubilized myofibril samples, appreciable degradation of titin by residual protease activity can occur in a few days at room temperature (Wang 1982, 1985; Granzier and Wang 1993). To limit proteolysis, the authors suggested the inclusion of protease inhibitors in SDS samples prior to electrophoresis. Leupeptin, E-64, and a protease inhibitor cocktail (Sigma) have been used to inhibit proteolytic degradation of titin (Granzier and Irving 1995; Tatsumi and Hattori 1995; Linke et al. 1997; Neagoe et al. 2003; Warren et al. 2003a; Vikhlyantsev and Podlubnaya 2006). In order to attain better solubilization of titin, it has been proposed to use urea–thiourea SDS DTT sample buffer (Fritz et al. 1989; Warren et al. 2003a). It is conceivable that urea–thiourea may facilitate the access of SDS to titin by rapidly solubilizing other myofibrillar proteins in the sarcomere lattice.
Heat treatment of the samples is also important. SDS samples are usually prepared by heating them in boiling water for 2–5 min, a process thought to promote denaturation of proteins and facilitate disulfide reduction. Other authors have shown that boiling degrades titin (King and Kurth 1980; Granzier and Wang 1993; Mitsuhashi et al. 2002; Warren et al. 2003a). Samples heated at 100 °C had less intact titin and more breakdown products (smears migrating near the bottom of the gel) than those at 60 °C (Warren et al. 2003a). A temperature of 50–60 °C for 10–20 min has been considered optimal for the extraction and preservation of intact titin (Granzier and Wang 1993; Mitsuhashi et al. 2002; Warren et al. 2003a). We recommended heating titin in SDS at 35–40 °C for 30–40- min (Vikhlyantsev and Makarenko 2000; Vikhlyantsev and Podlubnaya 2012).
Other details of the electrophoretic study of titin
Titin has a tendency to aggregate during electrophoresis, especially in gel systems that use a stacking gel or discontinuous buffers (Granzier and Wang 1993). To prevent disulfide cross-linking, Fritz et al. (1989), as well as Greaser and Warren (2009, 2012), recommended the inclusion of β-mercaptoethanol in the top anodic buffer. Granzier and Wang (1993), using 3.3–12% linear gradient gels, noted the importance of polymerizing the gel for at least 12 h prior to sample application. Titin bands are often diffuse in non-aged gels, presumably because the low percentage acrylamide near the top took much longer to properly polymerize (Granzier and Wang 1993).
Agarose-strengthened 2% polyacrylamide gels exhibit some peculiar characteristics. To prevent polyacrylamide polymerizing before agarose is polymerized, Tatsumi and Hattori (1995) cooled the 40 °C agarose solution for 5 min in ice water. Similarly, we added the agarose solution to glass cells that were pre-cooled to 8–10 °C and left the gel for 10 min in the refrigerator at 5 °C. Then, we kept the gel for 30 min at 20 °C and then for 2–2.5 h at 27 °C.
Our recommendation is to perform electrophoresis using macroporous, agarose-strengthened polyacrylamide gels at low currents. For instance, Neagoe (of Wolfgang Linke’s group) noted that the best separation for the high molecular weight proteins was obtained by running the electrophoresis overnight at 2 mA per 8.6 × 7.7 cm gel (Neagoe 2008). Similarly, we performed the initial electrophoresis of 8.0 × 10.0 × 0.10 cm gels at 3 mA for 40 min, then increasing the current strength up to 7–8 mA. It is recommended to refresh the tank buffer once at 2.5 h to limit pH changes caused by electrolysis during electrophoresis (Granzier and Wang 1993).
Conclusion
From the above discussion, it is clear that SDS-PAGE electrophoresis of titin is a complex procedure that is certainly not routine. The combination of giant molecular mass and the susceptibility of titin to degrade during preparation compound the problem. Thus, for a successful study of titin by electrophoresis, one must follow three main rules: (1) use protease inhibitors (leupeptin, E-64, protease inhibitor cocktail); (2) do not heat SDS samples higher than 40–60 °C; and (3) judiciously select the type of the gel.
Currently, the most suitable formats for analyzing titin are: (1) vertical agarose-strengthened 2% polyacrylamide gel (Tatsumi and Hattori 1995); (2) vertical 1% agarose gel (Warren et al. 2003a); and (3) horizontal agarose-strengthened 1.3% polyacrylamide gel (Vikhlyantsev and Podlubnaya 2006).
Acknowledgements
We thank Sergey Udaltsov for helpful discussions on electrophoretic techniques. This work was financially supported by the Russian Foundation for Basic Research (project No. 14-04-00112 and 17-04-00326).
Compliance with ethical standards
Conflict of interest
Ivan M. Vikhlyantsev declares that he has no conflicts of interest. Zoya A. Podlubnaya declares that she has no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This article is part of a Special Issue on ‘Titin and its Binding Proteins in Striated Muscles’ edited by Amy Li and Cristobal G. dos Remedios.
References
- Bang ML, Centner T, Fornoff F, Geach AJ, Gotthardt M, McNabb M, Witt CC, Labeit D, Gregorio CC, Granzier H, Labeit S. The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ Res. 2001;89(11):1065–1072. doi: 10.1161/hh2301.100981. [DOI] [PubMed] [Google Scholar]
- Cazorla O, Freiburg A, Helmes M, Centner T, McNabb M, Wu Y, Trombitás K, Labeit S, Granzier H. Differential expression of cardiac titin isoforms and modulation of cellular stiffness. Circ Res. 2000;86(1):59–67. doi: 10.1161/01.res.86.1.59. [DOI] [PubMed] [Google Scholar]
- Etlinger JD, Zak R, Fischman DA. Compositional studies of myofibrils from rabbit striated muscle. J Cell Biol. 1976;68(1):123–141. doi: 10.1083/jcb.68.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G, Steck TL, Wallach DF. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Freiburg A, Trombitas K, Hell W, Cazorla O, Fougerousse F, Centner T, Kolmerer B, Witt C, Beckmann JS, Gregorio CC, Granzier H, Labeit S. Series of exon-skipping events in the elastic spring region of titin as the structural basis for myofibrillar elastic diversity. Circ Res. 2000;86(11):1114–1121. doi: 10.1161/01.res.86.11.1114. [DOI] [PubMed] [Google Scholar]
- Fritz JD, Swartz DR, Greaser ML. Factors affecting polyacrylamide gel electrophoresis and electroblotting of high-molecular-weight myofibrillar proteins. Anal Biochem. 1989;180(2):205–210. doi: 10.1016/0003-2697(89)90116-4. [DOI] [PubMed] [Google Scholar]
- Gerull B. The rapidly evolving role of titin in cardiac physiology and cardiomyopathy. Can J Cardiol. 2015;31(11):1351–1359. doi: 10.1016/j.cjca.2015.08.016. [DOI] [PubMed] [Google Scholar]
- Granzier HL, Irving TC. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J. 1995;68(3):1027–1044. doi: 10.1016/S0006-3495(95)80278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzier HL, Wang K. Gel electrophoresis of giant proteins: solubilization and silver-staining of titin and nebulin from single muscle fiber segments. Electrophoresis. 1993;14(1–2):56–64. doi: 10.1002/elps.1150140110. [DOI] [PubMed] [Google Scholar]
- Greaser ML, Warren CM. Efficient electroblotting of very large proteins using a vertical agarose electrophoresis system. Methods Mol Biol. 2009;536:221–227. doi: 10.1007/978-1-59745-542-8_24. [DOI] [PubMed] [Google Scholar]
- Greaser ML, Warren CM. Protein electrophoresis in agarose gels for separating high molecular weight proteins. Methods Mol Biol. 2012;869:111–118. doi: 10.1007/978-1-61779-821-4_10. [DOI] [PubMed] [Google Scholar]
- Greaser ML, Krzesinski PR, Warren CM, Kirkpatrick B, Campbell KS, Moss RL. Developmental changes in rat cardiac titin/connectin: transitions in normal animals and in mutants with a delayed pattern of isoform transition. J Muscle Res Cell Motil. 2005;26(6–8):325–332. doi: 10.1007/s10974-005-9039-0. [DOI] [PubMed] [Google Scholar]
- Greaser ML, Warren CM, Esbona K, Guo W, Duan Y, Parrish AM, Krzesinski PR, Norman HS, Dunning S, Fitzsimons DP, Moss RL. Mutation that dramatically alters rat titin isoform expression and cardiomyocyte passive tension. J Mol Cell Cardiol. 2008;44(6):983–991. doi: 10.1016/j.yjmcc.2008.02.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Bharmal SJ, Esbona K, Greaser ML. Titin diversity – alternative splicing gone wild. J Biomed Biotechnol. 2010;2010:753675. doi: 10.1155/2010/753675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Schafer S, Greaser ML, Radke MH, Liss M, Govindarajan T, Maatz H, Schulz H, Li S, Parrish AM, Dauksaite V, Vakeel P, Klaassen S, Gerull B, Thierfelder L, Regitz-Zagrosek V, Hacker TA, Saupe KW, Dec GW, Ellinor PT, MacRae CA, Spallek B, Fischer R, Perrot A, Özcelik C, Saar K, Hubner N, Gotthardt M. RBM20, a gene for hereditary cardiomyopathy, regulates titin splicing. Nat Med. 2012;18(5):766–773. doi: 10.1038/nm.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Pleitner JM, Saupe KW, Greaser ML. Pathophysiological defects and transcriptional profiling in the RBM20−/− rat model. PLoS ONE. 2013;8(12):e84281. doi: 10.1371/journal.pone.0084281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowits R. Passive force generation and titin isoforms in mammalian skeletal muscle. Biophys J. 1992;61(2):392–398. doi: 10.1016/S0006-3495(92)81845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu DH, Kimura S, Maruyama K. Sodium dodecyl sulfate gel electrophoresis studies of connectin-like high molecular weight proteins of various types of vertebrate and invertebrate muscles. J Biochem. 1986;99(5):1485–1492. doi: 10.1093/oxfordjournals.jbchem.a135618. [DOI] [PubMed] [Google Scholar]
- Kawamura Y, Ohtani Y, Maruyama K. Biodiversity of the localization of the epitopes to connectin antibodies in the sarcomeres of lamprey, electric ray, and horse mackerel skeletal muscles. Tissue Cell. 1994;26(5):677–685. doi: 10.1016/0040-8166(94)90052-3. [DOI] [PubMed] [Google Scholar]
- King NL, Kurth L. SDS gel electrophoresis studies of connectin. In: Parry D, Creamer LK, editors. book: Fibrous proteins: scientific, industrial and medical aspects. New York: Academic; 1980. pp. 57–66. [Google Scholar]
- Krüger M, Wright J, Wang K. Nebulin as a length regulator of thin filaments of vertebrate skeletal muscles: correlation of thin filament length, nebulin size, and epitope profile. J Cell Biol. 1991;115(1):97–107. doi: 10.1083/jcb.115.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger M, Kohl T, Linke WA. Developmental changes in passive stiffness and myofilament Ca2+ sensitivity due to titin and troponin-I isoform switching are not critically triggered by birth. Am J Physiol Heart Circ Physiol. 2006;291(2):H496–H506. doi: 10.1152/ajpheart.00114.2006. [DOI] [PubMed] [Google Scholar]
- Labeit S, Kolmerer B. Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science. 1995;270(5234):293–296. doi: 10.1126/science.270.5234.293. [DOI] [PubMed] [Google Scholar]
- Labeit S, Lahmers S, Burkart C, Fong C, McNabb M, Witt S, Witt C, Labeit D, Granzier H. Expression of distinct classes of titin isoforms in striated and smooth muscles by alternative splicing, and their conserved interaction with filamins. J Mol Biol. 2006;362(4):664–681. doi: 10.1016/j.jmb.2006.07.077. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lahmers S, Wu Y, Call DR, Labeit S, Granzier H. Developmental control of titin isoform expression and passive stiffness in fetal and neonatal myocardium. Circ Res. 2004;94(4):505–513. doi: 10.1161/01.RES.0000115522.52554.86. [DOI] [PubMed] [Google Scholar]
- Li S, Guo W, Schmitt BM, Greaser ML. Comprehensive analysis of titin protein isoform and alternative splicing in normal and mutant rats. J Cell Biochem. 2012;113(4):1265–1273. doi: 10.1002/jcb.23459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke WA, Ivemeyer M, Labeit S, Hinssen H, Rüegg JC, Gautel M. Actin-titin interaction in cardiac myofibrils: probing a physiological role. Biophys J. 1997;73(2):905–919. doi: 10.1016/S0006-3495(97)78123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarenko I, Opitz CA, Leake MC, Neagoe C, Kulke M, Gwathmey JK, del Monte F, Hajjar RJ, Linke WA. Passive stiffness changes caused by upregulation of compliant titin isoforms in human dilated cardiomyopathy hearts. Circ Res. 2004;95(7):708–716. doi: 10.1161/01.RES.0000143901.37063.2f. [DOI] [PubMed] [Google Scholar]
- Maruyama K. Connectin, an elastic protein from myofibrils. J Biochem. 1976;80(2):405–407. doi: 10.1093/oxfordjournals.jbchem.a131291. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Natori R, Nonomura Y. New elastic protein from muscle. Nature. 1976;262(5563):58–60. doi: 10.1038/262058a0. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Kimura S, Kuroda M, Handa S. Connectin, an elastic protein of muscle. Its abundance in cardiac myofibrils. J Biochem. 1977;82(2):347–350. [PubMed] [Google Scholar]
- Maruyama K, Matsubara S, Natori R, Nonomura Y, Kimura S. Connectin, an elastic protein of muscle. Characterization and function. J Biochem. 1977;82(2):317–337. [PubMed] [Google Scholar]
- Maruyama K, Murakami F, Ohashi K. Connectin, an elastic protein of muscle. Comparative Biochemistry J Biochem. 1977;82(2):339–345. [PubMed] [Google Scholar]
- Maruyama K, Kimura S, Ohashi K, Kuwano Y. Connectin, an elastic protein of muscle. Identification of “titin” with connectin. J Biochem. 1981;89(3):701–709. doi: 10.1093/oxfordjournals.jbchem.a133249. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Kimura S, Yoshidomi H, Sawada H, Kikuchi M. Molecular size and shape of beta-connectin, an elastic protein of striated muscle. J Biochem. 1984;95(5):1423–1433. doi: 10.1093/oxfordjournals.jbchem.a134750. [DOI] [PubMed] [Google Scholar]
- Matsubara S, Maruyama K. Role of connectin in the length-tension relation of skeletal and cardiac muscles. Jpn J Physiol. 1977;27(5):589–600. doi: 10.2170/jjphysiol.27.589. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi T, Kasai M, Hatae K. Detection of giant myofibrillar proteins connectin and nebulin in fish meat by electrophoresis in 3-5 gradient sodium dodecyl sulfate polyacrylamide slab gels. J Agric Food Chem. 2002;50(26):7499–7503. doi: 10.1021/jf020362l. [DOI] [PubMed] [Google Scholar]
- Neagoe C (2008) Biochemical and mechanical investigation of cardiac titin isoforms. Doctor scientiarum humanarum (Dr. sc. hum.), Ruprecht-Karls-Universität, Heidelberg
- Neagoe C, Opitz CA, Makarenko I, Linke WA. Gigantic variety: expression patterns of titin isoforms in striated muscles and consequences for myofibrillar passive stiffness. J Muscle Res Cell Motil. 2003;24(2–3):175–189. doi: 10.1023/a:1026053530766. [DOI] [PubMed] [Google Scholar]
- Okuneva AD, Vikhlyantsev IM, Shpagina MD, Rogachevskii VV, Khutzyan SS, Podlubnaya ZA, Grigoriev AI. Changes in titin and myosin heavy chain isoform composition in skeletal muscles of mongolian gerbil (Meriones unguiculatus) after 12-day spaceflight. Biophysics. 2012;57(5):581–586. [PubMed] [Google Scholar]
- Opitz CA, Leake MC, Makarenko I, Benes V, Linke WA. Developmentally regulated switching of titin size alters myofibrillar stiffness in the perinatal heart. Circ Res. 2004;94(7):967–975. doi: 10.1161/01.RES.0000124301.48193.E1. [DOI] [PubMed] [Google Scholar]
- Prado LG, Makarenko I, Andresen C, Krüger M, Opitz CA, Linke WA. Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. J Gen Physiol. 2005;126(5):461–480. doi: 10.1085/jgp.200509364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmov NN, Vikhlyantsev IM, Ulanova AD, Gritsyna YV, Bobylev AG, Saveljev AP, Makariushchenko VV, Maksudov GY, Podlubnaya ZA. Seasonal changes in isoform composition of giant proteins of thick and thin filaments and titin (connectin) phosphorylation level in striated muscles of bears (Ursidae, Mammalia) Biochemistry (Mosc) 2015;80(3):343–355. doi: 10.1134/S0006297915030098. [DOI] [PubMed] [Google Scholar]
- Somerville LL, Wang K. The ultrasensitive silver “protein” stain also detects nanograms of nucleic acids. Biochem Biophys Res Commun. 1981;102(1):53–58. doi: 10.1016/0006-291x(81)91487-x. [DOI] [PubMed] [Google Scholar]
- Spierts IL, Akster HA, Granzier HL. Expression of titin isoforms in red and white muscle fibres of carp (Cyprinus carpio L.) exposed to different sarcomere strains during swimming. J Comp Physiol B. 1997;167(8):543–551. doi: 10.1007/s003600050107. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Hattori A. Detection of giant myofibrillar proteins connectin and nebulin by electrophoresis in 2% polyacrylamide slab gels strengthened with agarose. Anal Biochem. 1995;224(1):28–31. doi: 10.1006/abio.1995.1004. [DOI] [PubMed] [Google Scholar]
- Toyoda N, Maruyama K. Fine structure of connectin nets in cardiac myofibrils. J Biochem. 1978;84(1):239–241. doi: 10.1093/oxfordjournals.jbchem.a132114. [DOI] [PubMed] [Google Scholar]
- Trombitás K, Redkar A, Centner T, Wu Y, Labeit S, Granzier H. Extensibility of isoforms of cardiac titin: variation in contour length of molecular subsegments provides a basis for cellular passive stiffness diversity. Biophys J. 2000;79(6):3226–3234. doi: 10.1016/S0006-3495(00)76555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udaka J, Ohmori S, Terui T, Ohtsuki I, Ishiwata S, Kurihara S, Fukuda N. Disuse-induced preferential loss of the giant protein titin depresses muscle performance via abnormal sarcomeric organization. J Gen Physiol. 2008;131(1):33–41. doi: 10.1085/jgp.200709888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulanova A, Gritsyna Y, Vikhlyantsev I, Salmov N, Bobylev A, Abdusalamova Z, Rogachevsky V, Shenkman B, Podlubnaya Z. Isoform composition and gene expression of thick and thin filament proteins in striated muscles of mice after 30-day space flight. Biomed Res Int. 2015;2015:104735. doi: 10.1155/2015/104735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikhlyantsev IM, Podlubnaya ZA. On the titin isoforms. Biophysics. 2006;51(5):842–848. [Google Scholar]
- Vikhlyantsev IM, Podlubnaya ZA. Composition of titin isoforms of skeletal and cardiac muscles in pathologies. Biophysics. 2008;53(6):592–597. [Google Scholar]
- Vikhlyantsev IM, Podlubnaya ZA. New titin (connectin) isoforms and their functional role in striated muscles of mammals: facts and suppositions. Biochemistry (Mosc) 2012;77(13):1515–1535. doi: 10.1134/S0006297912130093. [DOI] [PubMed] [Google Scholar]
- Vikhlyantsev IM, Makarenko IV, Khalina IN, Udaltsov SN, Malyshev SL, Podlubnaya ZA. Changes in the isoform composition of the cytoskeletal protein titin: adaptation process in hibernation. Biophysics. 2000;45(5):805–809. [PubMed] [Google Scholar]
- Vikhlyantsev IM, Podlubnaya ZA, Kozlovskaya IB. New titin isoforms in skeletal muscles of mammals. Dokl Biochem Biophys. 2004;395:111–113. doi: 10.1023/b:dobi.0000025559.14249.43. [DOI] [PubMed] [Google Scholar]
- Vikhlyantsev IM, Malyshev SL, Shenkman BS, Podlubnaya ZA. Composition of the titin family proteins in skeletal muscle of ground squirrel during hibernation and rats in simulated microgravity. Biophysics. 2004;49(6):895–900. [PubMed] [Google Scholar]
- Vikhlyantsev IM, Karaduleva EV, Podlubnaya ZA. Seasonal changes in the composition of titin isoforms in muscles of hibernating ground squirrels. Biophysics. 2008;53(6):598–603. [PubMed] [Google Scholar]
- Vikhlyantsev IM, Okuneva AD, Shpagina MD, Shumilina YV, Molochkov NV, Salmov NN, Podlubnaya ZA. Changes in isoform composition, structure, and functional properties of titin from Mongolian gerbil (Meriones unguiculatus) cardiac muscle after space flight. Biochemistry (Mosc) 2011;76(12):1312–1320. doi: 10.1134/S0006297911120042. [DOI] [PubMed] [Google Scholar]
- Wang K. Purification of titin and nebulin. Methods Enzymol. 1982;85(Pt B):264–274. doi: 10.1016/0076-6879(82)85025-8. [DOI] [PubMed] [Google Scholar]
- Wang K. Sarcomere-associated cytoskeletal lattices in striated muscle. Review and hypothesis Cell Muscle Motil. 1985;6:315–369. doi: 10.1007/978-1-4757-4723-2_10. [DOI] [PubMed] [Google Scholar]
- Wang K, Wright J. Architecture of the sarcomere matrix of skeletal muscle: immunoelectron microscopic evidence that suggests a set of parallel inextensible nebulin filaments anchored at the Z line. J Cell Biol. 1988;107(6 Pt 1):2199–2212. doi: 10.1083/jcb.107.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, McClure J, Tu A. Titin: major myofibrillar components of striated muscle. Proc Natl Acad Sci U S A. 1979;76(8):3698–3702. doi: 10.1073/pnas.76.8.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, McCarter R, Wright J, Beverly J, Ramirez-Mitchell R. Regulation of skeletal muscle stiffness and elasticity by titin isoforms: a test of the segmental extension model of resting tension. Proc Natl Acad Sci U S A. 1991;88(16):7101–7105. doi: 10.1073/pnas.88.16.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren CM, Krzesinski PR, Greaser ML. Vertical agarose gel electrophoresis and electroblotting of high-molecular-weight proteins. Electrophoresis. 2003;24(11):1695–1702. doi: 10.1002/elps.200305392. [DOI] [PubMed] [Google Scholar]
- Warren CM, Jordan MC, Roos KP, Krzesinski PR, Greaser ML. Titin isoform expression in normal and hypertensive myocardium. Cardiovasc Res. 2003;59(1):86–94. doi: 10.1016/s0008-6363(03)00328-6. [DOI] [PubMed] [Google Scholar]
- Warren CM, Krzesinski PR, Campbell KS, Moss RL, Greaser ML. Titin isoform changes in rat myocardium during development. Mech Dev. 2004;121(11):1301–1312. doi: 10.1016/j.mod.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Weber K, Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969;244(16):4406–4412. [PubMed] [Google Scholar]
- Wu Y, Cazorla O, Labeit D, Labeit S, Granzier H. Changes in titin and collagen underlie diastolic stiffness diversity of cardiac muscle. J Mol Cell Cardiol. 2000;32(12):2151–2162. doi: 10.1006/jmcc.2000.1281. [DOI] [PubMed] [Google Scholar]