Abstract
This review takes readers back to 1949, when two Australian scientists, Draper and Hodge, reported the first high-resolution electron microscopy images of striated muscle. In 1953, Jean Hanson and Hugh Huxley published phase-contrast microscopy and electron microscopy images that established the filamentous nature of the sarcomere, namely the myosin-containing thick filaments and actin-containing thin filaments. They discussed a putative third filament system, possibly a thinner actin-containing S filament, that appeared to connect one Z disc to the next. The next year, two back-to-back papers appeared in Nature, the first by Andrew Huxley and Rolf. Niedergerke, the second by Hugh Huxley with Jean Hanson. Independently, they proposed the sliding of actin filaments and myosin filaments. These two filaments quickly became firmly established in the literature and, even today, they remain the basis for the sliding filament hypothesis. The putative third filament concept was dropped, mainly through the lack of evidence but also because it was difficult to accommodate in the hypothesis where two sets of filaments maintained their lengths constant while sliding produced sarcomere shortening. The view that actin and myosin comprise more than 80% of the myofibril proteins also made it difficult to accommodate a major new protein. In the following years, using selective extraction of myosin and actin, dos Remedios (PhD thesis, University of Sydney, 1965) revealed a residual filament system in the sarcomere, and, once again, a third filament system re-entered the literature. Filaments were reported crossing the gap between the ends of thick and thin filaments in highly stretched muscle fibres. These and other early studies necessarily focussed on light and electron microscopy, and set the scene for investigations into the chemical nature and biophysical functions of the third filament system for striated muscles. Further progress had to wait for the improvement and/or development of a number of techniques. For example, in 1970, Laemmli (Nature 227:680–685, 1970) published an often cited method for improving SDS polyacrylamide gel electrophoresis. The Lowry et al. (J Biol Chem 193:265–275, 1951) protein assay method developed in 1950 was both unstable and insensitive in comparison, but we had to wait until 1976 for the development of the Bradford method (1976). Atomic force microscopy was not known before 1986, but it eventually enabled the direct measurement of single molecules of titin. This extraordinarily large (>106 Da) elastic protein became known as connectin (Maruyama in J Biochem 80:405–407, 1976) and was subsequently named titin (Wang et al. in Proc Natl Acad Sci U S A 76:3698–3702, 1979). Prior to the discovery of titin/connectin, biophysicists found it difficult to understand how a single polypeptide chain could could stretch from the Z disc to the M line, a distance of more than 1 μm. It was quite literally the ‘elephant in the room’. In this review, we follow the trail of microscopy-based reports that led to the emergence of what is now known and accepted as titin, an elastic third filamentous protein that is the focus of this Special Issue.
Keywords: Sarcomere filament structure, Connectin, Titin, Historical review, Giant proteins
1949–1953: The earliest electron microscopy of skeletal muscle
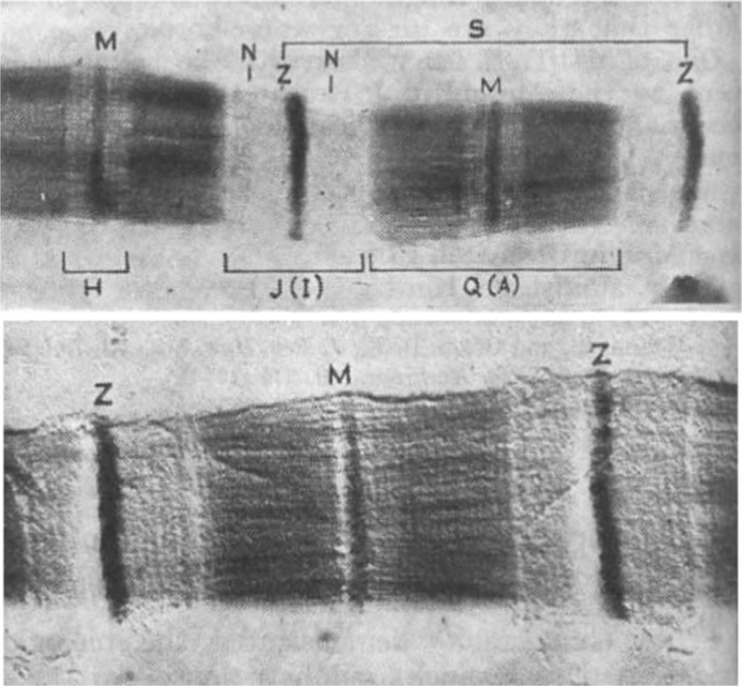
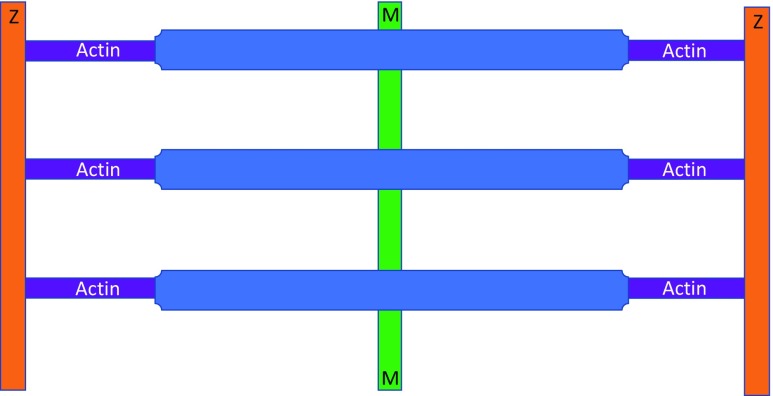
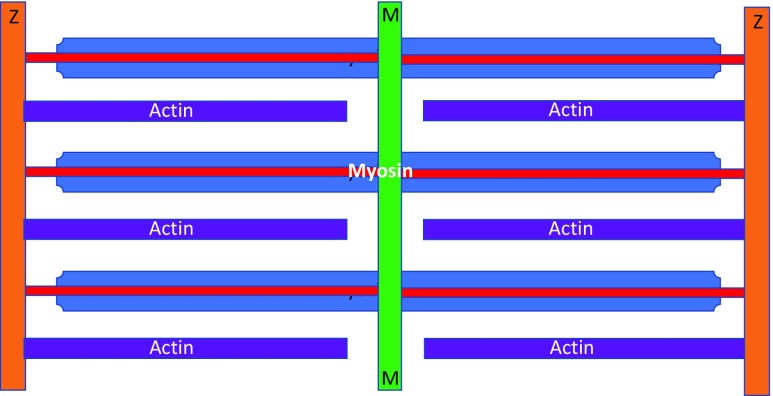
The earliest reports involving electron microscopy of myofibrils were published by Draper and Hodge (1949, 1950). Draper was a National Health and Medical Research Council Junior Research Fellow in the Physiology Department at the University of Adelaide. Hodge was at the Division of Industrial Chemistry at the Commonwealth Scientific and Industrial Research Organisation. Their stated aim was to identify metals in muscle that was ‘incinerated’ by the electron beam, but they coincidentally achieved remarkable electron microscopy images of single myofibrils by adding phosphomolybdic acid that bound to sarcomeric proteins and produced clear images of the sarcomere filaments. Figure 1 reproduces their original two figures, published in Nature. The top figure shows a single isolated, formalin-fixed, toad myofibril stained with phosphomolybdic acid. The lower figure is platinum-shadow-cast (10 Å thick) formalin-fixed toad skeletal muscle fibril at 20,000×. These images clearly show both the filamentous nature of the I and A bands, as well as distinct periodic axial repeats that were later shown to be myosin crossbridges. Figure 2 summarises their observations, suggesting that the thick filaments in the A band were continuous with the thin filaments in the I band.
Fig. 1.
Early electron microscope images of a formalin-fixed skeletal muscle myofibril published by Draper and Hodge (1950). The top figure was negatively stained with phosphomolybdic acid. The lower fibril was imaged by a platinum shadow technique
Fig. 2.
The one-filament model of sarcomere myofilament structure proposed that actomyosin filaments extend continuously between the Z disc (Draper and Hodge 1949). Z discs are orange, myosin thick filaments are blue, actin thin filaments are purple and the M line is green
Subsequently, Hodge et al. (1954) described the first transverse thin section of muscle and reported that the (130 Å diameter) thick filaments might be composed of myosin that were arranged in a hexagonal array. The lower image of Fig. 1 led them to speculate that the 400 Å repeat (Draper and Hodge 1949) may represent myosin crossbridges.
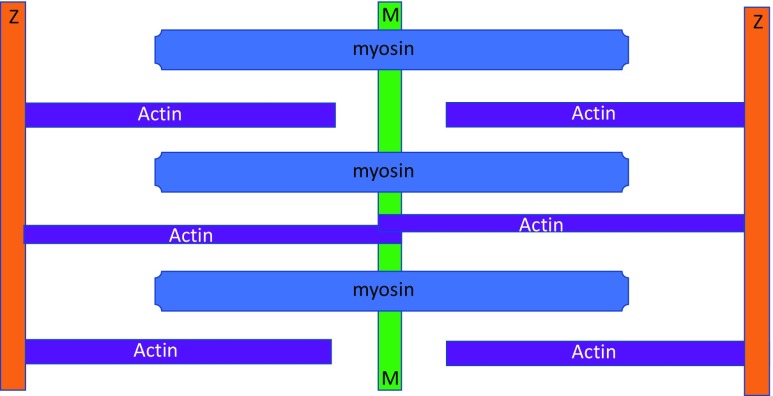
At about the same time, Hanson and Huxley (1953) published a microscopy-based paper in Nature that came very close to detecting and describing what would later become known as connectin/titin filaments (Maruyama 1976; Wang et al. 1979). Observing isolated single fibrils by phase-contrast microscopy, they showed selective extraction of an A band substance using either Guba–Straub solution (0.3 M KCl, 0.15 M phosphate, pH 6.5) or Hasselbach–Schneider solution (0.47 M KCl, 0.01 M pyrophosphate, 0.1 M phosphate, pH 6.4), both of which caused the A bands to completely dissolve. They referred to these as myosin-extracted fibrils ‘ghost’ fibrils. A similar result was seen using glycerinated muscle fibres that were fixed, embedded, sectioned and observed on microscopy, although extraction was not as complete as fibrils. They concluded that the A substance in the thick filaments was most probably myosin. The myosin-extracted sarcomeres exhibited ‘brushes’ of thin filaments bisected by the Z discs. They noted that the dissolution of the A bands did not disrupt the connections between sarcomeres in free-floating fibrils. When these ghost fibrils were mechanically disrupted, the actin thin filaments dissolved and the sarcomeres structure completely disintegrated. Importantly, these myosin-extracted ‘ghost’ fibrils were made using trypsin-treated fibrils. In 1953, they did not know that titin/connectin filaments are more sensitive to digestion than any other sarcomeric protein (Horowits and Podolsky 1987). Therefore, they concluded that there are fine filaments which cross the middle of the sarcomere that are probably composed of actin. Figure 3 summarises the conclusions of Draper and Hodge.
Fig. 3.
A diagram summarising the view of Hanson and Huxley (1953) of sarcomere filaments. Here, the sarcomere has separate thick myosin filaments and thin actin filaments, as well as a smaller number of thinner ‘S filaments’, also possibly composed of actin. These thin actin filaments cross the H band and join M line ‘microfilaments’. For an explanation of the colours, see Fig. 2
1954–1962: The sliding filament hypothesis
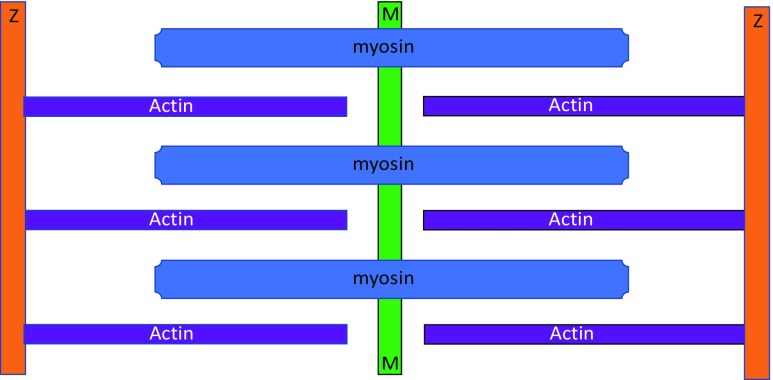
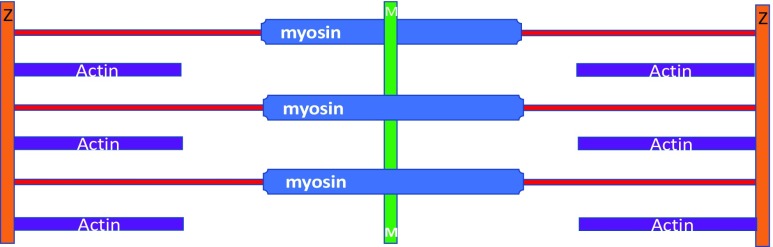
In 1954, two consecutive articles appeared in Nature (Huxley and Hanson 1954; Huxley and Niedergerke 1954) that jointly proposed the sliding filament hypothesis. These two papers provided new insights into the mechanism underlying sarcomeres in striated muscles. In essence, the Huxley and Hanson hypothesis based on electron microscopy states that the lengths of thick and thin filaments do not appreciably change during sarcomere shortening. Rather, the two sets of filaments slide relative to each other. There was no mention of S filaments (Hanson and Huxley 1953), and Hugh Huxley later formally dropped the idea (Huxley 1962). This two-filament concept is illustrated in Fig. 4 and became the basis of textbook descriptions on striated muscle structure.
Fig. 4.
The two-filament sarcomere model based on Huxley and Hanson (1954). See Fig. 2 for an explanation of the colours
1963–1967: C filaments
It was not long before evidence accumulated for filamentous connections between the ends of thick filaments and the Z discs that did not involve actin filaments. Using electron microscopy, Auber and Couteaux (1963) examined insect (Diptera) asynchronous flight muscle and reported that these muscles had clear (albeit very short) filaments that extend from the ends of the thick filaments to the Z discs. Independently, Nikoli Garamvölgyi (Garamvölgyi et al. 1964) came to the same conclusion. These C filaments had a similar diameter to actin thin filaments, and it was assumed they functioned as an elastic ‘spring’, thereby eliminating actin as a major component. C filaments were accepted by JWS Pringle (Professor of Zoology at Oxford) (Pringle 1967), but they were always seen as a special adaptation of insect flight muscle and did not apply to vertebrate striated muscles in general because skeletal muscles did not exhibit similar elastic oscillatory behaviour. Figure 5 summarises the concept of the C filaments.
Fig. 5.
The location of C filaments (red) reported for insect asynchronous flight muscle. Note the comparatively long myosin filaments in the A band which continue across the very short I bands to join the Z discs. This model assumes that the C filaments seen in the I bands are continuous with the thick filaments. For colour coding of the structures, see Fig. 2
1962–1965: Gap filaments
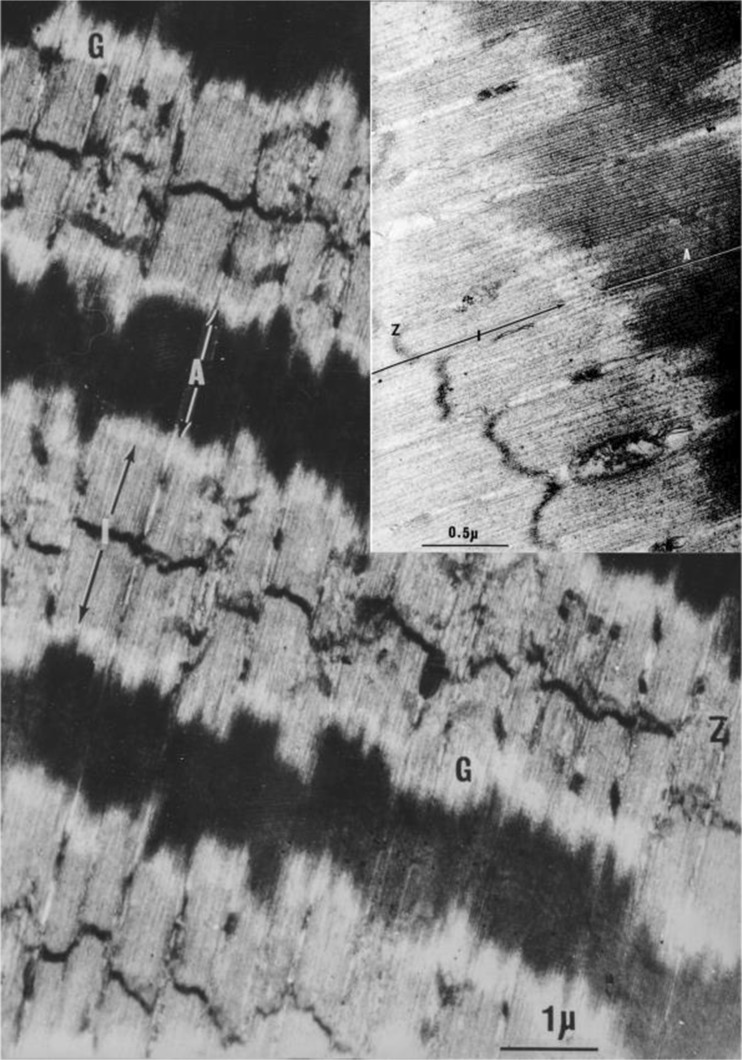
At least two groups reported ultrastructure investigations of highly stretched skeletal muscle fibres. Carlsen et al. (1965) were the first to observed muscle sarcomeres that were stretched to lengths that exceeded the sum of the thick (1.8 μm) and thin (1.0 μm) filament lengths, i.e. sarcomere lengths greater than 3.8 μm. Their principal concern was to explain how muscle fibres could produce significant contractile force at such lengths. They agreed with FS Sjöstrand (1962), the then Editor of the Journal of Ultrastructure Research, who proposed the existence of fine filaments connecting the ends of the thin filaments to the ends of the thick filaments. When we repeated their experiments (dos Remedios CG, PhD thesis, University of Sydney 1965), we also observed the presence of thin filaments leaving the ends of thick filaments and extending through to the Z discs, confirming the presence of gap filaments, which we referred to as ‘residual’ filaments because they remain even after the extraction of myosin and actin (Figure 5). The concept of gap filaments in this location was supported by AF Huxley (1980), who conceded that some kind of connection was needed in order to explain how force could be developed in the absence of an overlap of the actin and myosin filaments. Figure 6 summarises the putative connections between the ends of the thick and thin filaments that could explain the presence of fine filaments seen when sarcomeres were stretched past the point where the thick and thin filaments overlap. An electron micrograph of highly stretched rabbit psoas muscle is shown in Fig. 7, with an average sarcomere length of 4.2 μm. The insert image shows fine ‘gap’ filaments extending from the ends of the thick filaments.
Fig. 6.
The arrangement of myofilaments proposed by Carlsen et al. 1965 to explain their arrangement in highly stretched (≥4 μm) skeletal muscle sarcomeres. The red colour indicates their proposed ‘gap’ filaments. For an explanation of the colour codes, see Fig. 2)
Fig. 7.
An ultrathin section of glutaraldehyde-osmium tetroxide fixed, araldite-embedded longitudinal section of a rabbit psoas muscle stretched to a length of 4.2 μm. It was stained with uranyl acetate, followed by lead citrate. The insert figure reveals the presence of ‘gap’ filaments between the ends of the myosin thick filaments (A) in the A band and the thin filaments in the I band. Labels: “A” is the A band; “I” is the I band; “Z” is the Z disc; “G” indicates the position of fine ‘gap’ filaments that are better resolved in the insert
Selective extraction of myosin and actin from isolated myofibrils
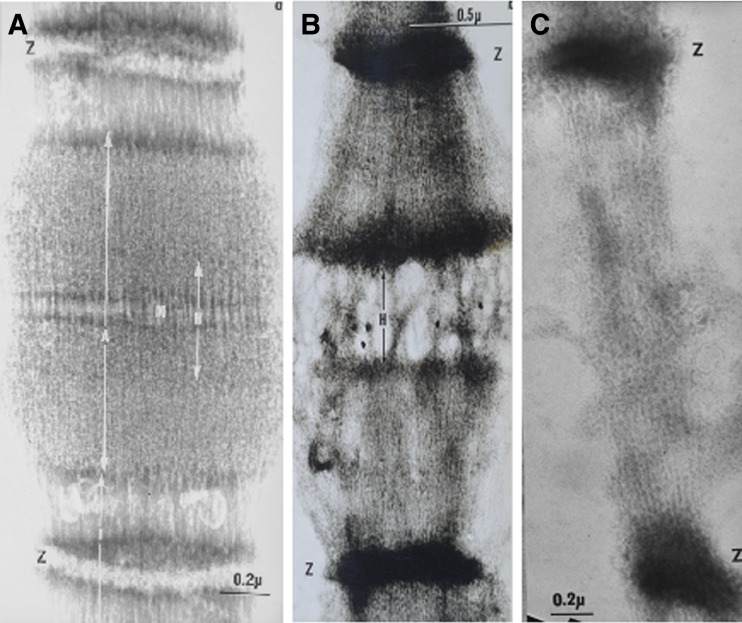
While Hanson and Huxley (1953) published very good images of isolated trypsin-treated fibrils before and after myosin extraction, they did not show images of fibrils where both myosin and actin filaments were extracted. The phase contrast images of a non-trypsin-treated, single rabbit myofibril before extraction (Fig. 8a) and after extraction with Hasselbach–Schneider solution containing 1.0 M KCl (Fig. 8b) showed Z discs and I band ‘brushes’. Both the A bands and I bands dissolved after treatment with 0.6 M KI (Fig. 8c). Note the Z discs remain in sequence but their orientation is lost when the A and I bands are removed.
Fig. 8.
Phase contrast images of a glycerinated rabbit psoas myofibril: before extraction (a), after myosin extraction with 1 M KCl (b) and after subsequent extraction with 0.6 M KI (c)
When this extraction protocol was performed on similar myofibrils on carbon-filmed electron microscope grids, the filamentous nature of the sarcomeres is illustrated in Figs. 9a, b and c, respectively. Similar images have been published (dos Remedios and Gilmour 1978).
Fig. 9.
Electron microscopy of negatively stained single myofibrils: a the filamentous nature of the A band with a clear H band showing the limits of the thick and thin filament overlap, the M band structure. Note that the ammonium-molybdate stain is excluded at the Z disc structure. In b, the myofibril was extracted with Hasselbach–Schneider solution (where the KCl concentration was increased from 0.47 M to 1.0 M). The A band has now been extracted, revealing the I band ‘brushes’ with their thin filament and positively stained Z discs. Stain is 1% uranyl acetate. In c, the fibrils were extracted with 0.6 M KI, which dissolves both myosin and actin. Filaments about the same diameter as actin thin filaments were low in number relative to actin filaments, and extend from the Z discs to the position of the original M line. There are clear thin A filaments that resisted extraction, which we named ‘residual’ filaments. Stain: 1% uranyl acetate
Selective extraction of myosin and actin from suspensions of myofilaments
In 1960, Hugh Huxley cut longitudinal ultrathin sections of rabbit psoas fibres, which also showed thick filaments tapering down to be continuous with a thin filament. Most authors have reported difficulty in completely extracting myosin and actin from glycerinated muscle fibres. Even when the conditions are optimised, extracted myofibrils may leave traces of unextracted filaments. Therefore, we homogenised glycerinated rabbit psoas muscle fibres to produce a suspension of myofilaments which might be more efficiently extracted.
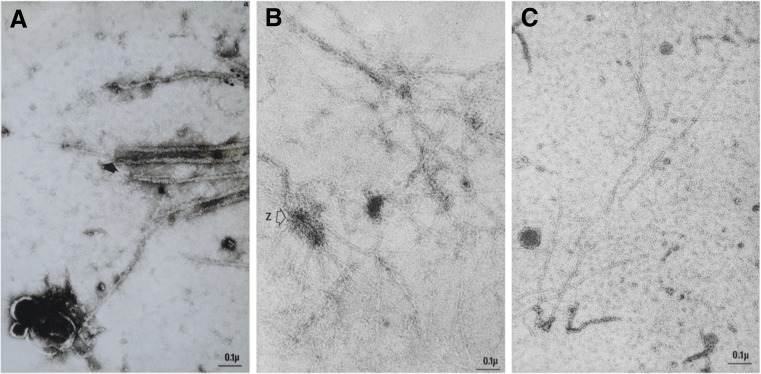
Thick and thin filaments were applied to carbon-coated electron microscope grids, extracted with several drops of augmented (1.0 M KCl) Hasselbach–Schneider solution or with 0.6 M KI, which dissolves both actin and myosin. At each stage of the extraction process, we applied a drop of 1% uranyl acetate and visualised the grids by negative staining in the electron microscope. In unextracted filament homogenates, we observed myosin thick filaments, some of which appeared to have thin filament extensions. As expected, numerous thin actin filaments were also present (Fig. 10a). When the homogenate was applied to the carbon-coated grid film, washed and then extracted with augmented (1 M KCl) Hasselbach–Schneider solution, thick filaments were completely dissolved. Only thin filaments remained, some of which appeared to be attached to the remnants of Z discs (Fig. 10b). Finally, when the homogenate was extracted with 0.6 M KI, there was a large reduction in the number of thin filaments. Those that remained were indistinguishable from the thin filaments seen in Fig. 10c. These were later formally named connectin filaments (Maruyama et al. 1976), and then, three years later, they were renamed titin filaments (Wang et al. 1979).
Fig. 10.
Electron micrographs of negatively stained myofilaments from glycerinated rabbit psoas muscle. Tissue was homogenised in the presence of 1 mM ATP using a Teflon–glass homogeniser and a drop was place on a carbon-coated grid film. a Thick and thin filaments; the arrow indicates a thick filament that appears to taper and become continuous with a thin filament. In b, the drop of suspended filaments was replaced by Hasselbach–Schneider solution containing 1.0 M KCl (5 min) and then rinsed with low salt buffer. It shows that only thin filaments were present after the myosin was extracted. The image appears to show a fragment of a Z disc with attached thin filaments. In c, myosin and actin were dissolved with 0.6 M KI for 10 min and then rinsed with low salt buffer. The number of thin filaments is substantially reduced compared to Fig. b
1960–1969: Filament counts in the A and I bands
By now, rabbit psoas muscle fibres, fibrils and filaments had become the gold standard source of muscle for most papers in this field. The numbers of thick and thin filaments in the overlap zone of both the A bands and I bands were first reported by Hugh Huxley (1960). He showed that the average ratio of thick:thin filaments in the overlap zone of the A band was 1:1.92, which is very close to the number predicted (1:2) (see Fig. 4). When Huxley counted the number of thin filaments in the I band, he reported that this number did not significantly change (1:1.88). His paper states that he used “Filament counts in neighbouring sections through the same fibril”. In other words, he used serial sections.
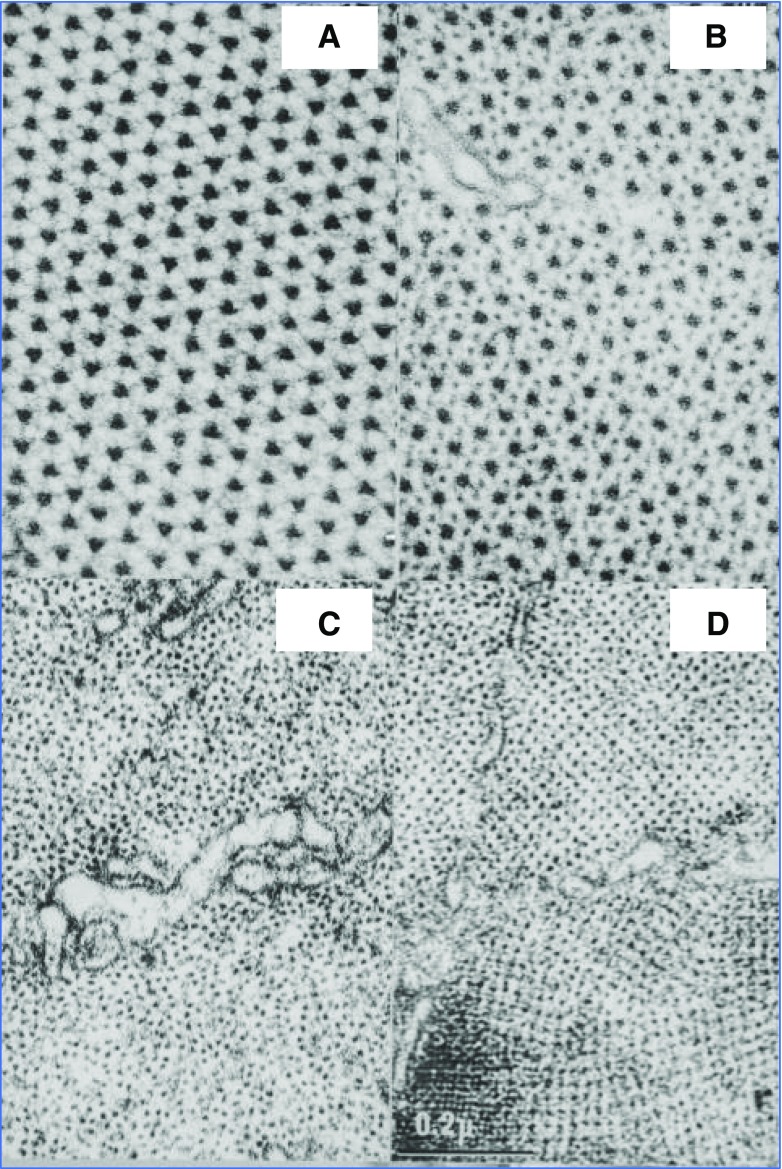
Was this the ‘death knell’ of the putative third filament? Hugh Huxley used rabbit psoas muscle that was fixed and prepared for electron microscopy in nearly the same way as the fibres shown in Fig. 11. However, there were small differences. For example, he did not use glutaraldehyde prior to osmium tetroxide fixation. He also stained his tissue blocks with phosphotungstic acid and his sections were stained with uranyl acetate. The tissue shown in Fig. 11 was fixed in glutaraldehyde (from which contaminating glutaric acid had been removed) and then post-fixed in 1% osmium tetroxide. There was no block staining with phosphotungstic acid, and sections were stained with lead citrate (Reynolds (1963), followed by 60 min. exposure to 1% uranyl acetate. In Panel A, the sarcomere is sectioned transversely through the M line; in Panel B, the section passes through the overlap region of thick and thin filaments; in Panel C, the section passes through the I band; and in Panel D, the section is cut close to the Z disc. The question of the location of a third thin filament system cannot be addressed using random sections, but it might be answered by counting serial sections through a single sarcomere.
Fig. 11.
Panel A (top left) shows a transverse section across rabbit psoas muscle fibres at the level of the M line where fine bridges can be seen connecting the thick filaments. There is no evidence for anything resembling thin filaments. In Panel B (top right), the hexagonal array of thick filaments is surrounded by a hexagonal array of thin filaments. Panel C (bottom left) shows thin filaments in the I band, where their regular hexagonal arrangement becomes more random. Sections close to the Z disc (Panel D, bottom right) were avoided because, although their tetragonal arrangement made them easier to count, as the thin filaments approached the Z discs, they appear to split into two more sub-filaments and are associated with an unknown Z disc protein
Serial transverse sections
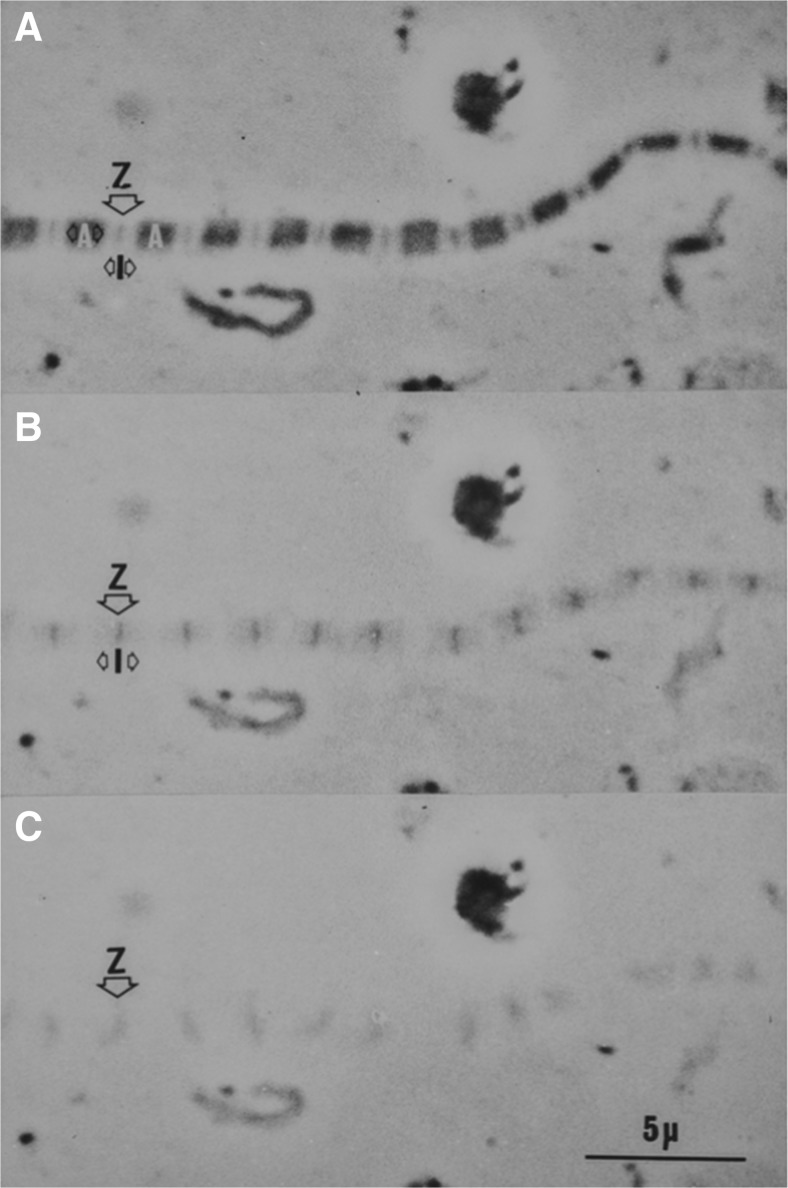
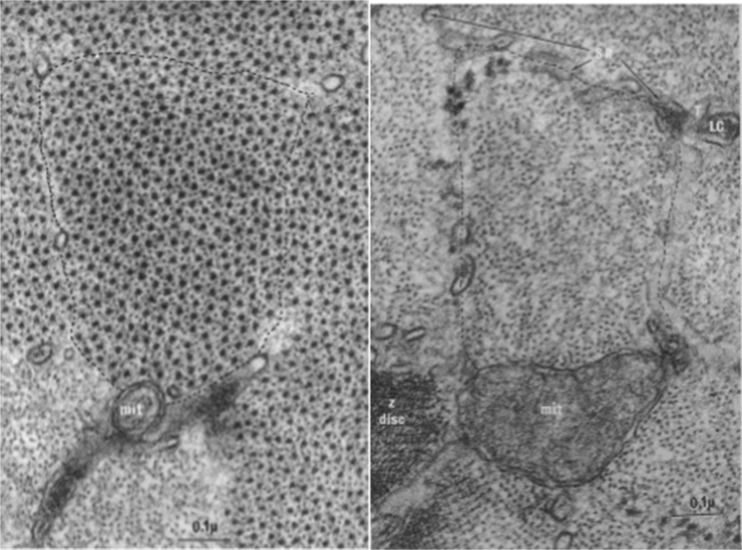
We used serial transverse sections to resolve the filament count question by examining rabbit psoas muscle prepared under the conditions described for Fig. 11. If the proposed residual filaments do indeed join the thick filaments as suggested above, they will not be seen in the A band but will be identified in the I bands. The left and right panels of Fig. 12 show sections passing through the A band and I bands, respectively, of a single sarcomere. Micrographs like these were given to an observer who had no training in biology. She was told that the images were of transverse sections through two regions of muscle where the filaments were either thick or thin. The observer was asked the decide if the filaments were either thick or thin, and then to count the numbers in the two regions.
Fig. 12.
The left panel shows a transverse section through the overlap zone of the A band. The right panel shows a serial transverse section through the same sarcomere but at the level of the I bands. In both panels, the boundaries of the fibril are indicated by the dashed line. Mitochondria (mit), lateral cisternae (lc) and sarcoplasmic reticulum (SR) assist in identifying the boundaries of the sarcomere
Thick and thin filaments were counted in 23 serial sections through sarcomeres in 14 different myofibrils. The observer confirmed Huxley’s observation that, in the overlap zone of the A band, the ratio of thick:thin filaments was 1:2.08 and that the ratio of thin filaments present in the I bands of the same sarcomere increased to 1:2.79. The 1:2.8 figure was less than the 1:3.0 ratio we predicted to be present in the I band if the third filament present in the I band joined the tip of a thick filament at the A–I junction. We examined the influence of increased print contrast and longer exposure times of the prints, but this could only account for a small fraction (2.7%) of the increase (dos Remedios 1965). We, therefore, concluded that there were significantly more thin filaments in the I band than was predicted from the two-filament model. At the time, we were unable to explain why the third filament was less than 1:3.
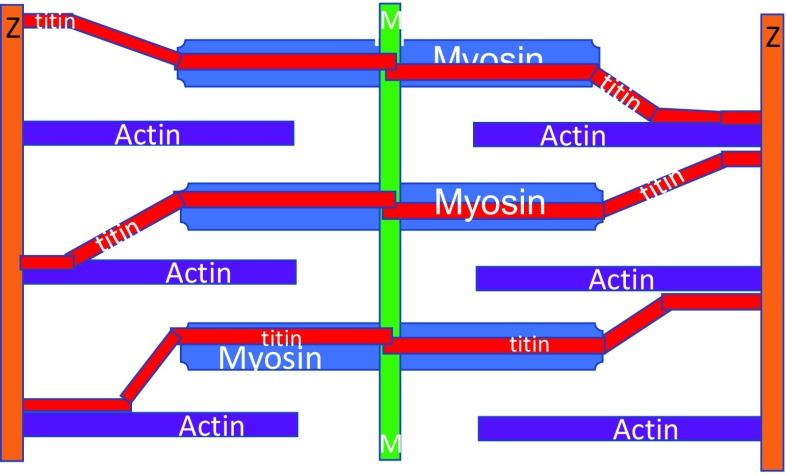
A recent review by Wolfgang Linke (2008) shows that, while titin filaments remain separated from the thin filaments along most of their length, they do bind to filamentous actin and would appear as a somewhat thicker thin filament. Perhaps this is the explanation for the slightly low 1:2.8 figure. This concept is summarised in Fig. 13.
Fig. 13.
A model of the current knowledge of the arrangement of actin (purple), myosin (blue) and titin (red) filaments in the striated muscle sarcomere. Here, titin is shown as joining the Z disc to the M line, while making variable contact with the actin filaments
Ockham’s razor
How could we explain the appearance of ‘gap’ filaments in highly stretched muscle fibres? Why did the ‘ghost’ fibrils seen under the phase-contrast microscopy not disintegrate when myosin was extracted from the thick filament? What was holding the Z discs in line as they were observed to float by the objective lens? How could we explain the electron microscope images of filaments that seemed to resist myosin and actin extraction? Why did some thick filaments appear to have thin filament extensions?
The 14th century logician William of Ockham devised the principle that entities should not be multiplied unnecessarily. We can apply this to the problem of explaining whether there are two or three sets of filaments in the striated muscle.
When we apply this principle to the data presented in this review, separate assumptions need to be explained. (1) In Fig. 7, how do we explain the so-called ‘gap’ filaments in over-stretched fibres? Answer: The titin filaments are highly elastic and can unfold to accommodate these long sarcomere lengths. (2) In Figs. 8 and 9, why do the fibrils not fall apart when actin and myosin are extracted? Answer: Because some of the proteins were denatured and remained to hold the Z discs together. (3) In the serial section filament counts, why is the ratio of thick:thin filaments neither 1:2 nor 1:3? Answer: Because when titin binds to actin thin filaments, the ratio will appear to be lower than predicted.
While separate explanations are required for each of the above observations, Ockham’s razor suggests that all of them can be explained by assuming the presence of a titin-based third filament type that is similar in diameter to the actin thin filaments.
Remaining questions
While titin, with its 38,138 amino acids approximately (∼3.8 MDa), provides the single best explanation for the protein composition of the third filament, it is conceivable that other giant proteins might lend a hand. Novex-3 (Bang et al. 2001) is a smaller isoform of titin consisting of 5604 amino acids (0.7 MDa). Its N-terminus is located in the Z disc and its C-terminus extends for some distance into the I band. Obscurin (Young et al. 2001) is another giant protein comprising 34,350 amino acids (0.7 MDa). It has been located at various sites between the Z disc and the M line. Novex-3 and obscurin are known to form a very tight complex, and, according to Bang et al. (2001), the two proteins could conceivably form an elastic Z-disc-to-I-band protein linking the Z discs and the I bands.
We know that titin mutations are the probable cause of about a quarter of cases of familiar dilated cardiomyopathy (Herman et al. 2012), but it is not known how truncation mutants cause the cardiomyopathy. Perhaps the recent measurements of single-molecule elasticity reported recently (Mártonfalvi et al. 2014) will provide new clues to this puzzle.
Acknowledgements
We are grateful to Mr. Clive Jeffery for his excellent assistance in preparing the figures.
Compliance with ethical standards
Conflicts of interest
Cris dos Remedios declares that he has no conflicts of interest. Darcy Gilmour declares that he has no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This article is part of a Special Issue on ‘Titin and its Binding Proteins in Striated Muscles’ edited by Amy Li and Cristobal G. dos Remedios.
References
- Auber J, Couteaux R. Ultrastructure de la strie Z dans des muscles de Diptères. J Microsc. 1963;2:309–324. [Google Scholar]
- Bang M-L, Centner T, Fornoff F, Geach AJ, Gotthardt M, McNabb M, Witt CC, Labeit D, Gregorio CC, Granzier H, Labeit S. The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ Res. 2001;89:1065–1072. doi: 10.1161/hh2301.100981. [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed]
- Carlsen F, Fuchs F, Knappeis GG. Contractility and ultrastructure in glycerol--extracted muscle fibers. I. The relationship of contractility to sarcomere length. J Cell Biol. 1965;27:25–34. doi: 10.1083/jcb.27.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Remedios CG (1965) Comparative studies on striated muscle. PhD thesis, University of Sydney, 300 pp
- dos Remedios CG, Gilmour D. Is there a third type of filament in striated muscles? J Biochem. 1978;84:235–238. doi: 10.1093/oxfordjournals.jbchem.a132113. [DOI] [PubMed] [Google Scholar]
- Draper MH, Hodge AJ. Sub-microscopic localization of minerals in skeletal muscle by internal ‘micro-incineration’ within the electron microscope. Nature. 1949;163:576–577. doi: 10.1038/163576a0. [DOI] [PubMed] [Google Scholar]
- Draper MH, Hodge AJ. Electron-induced microincineration with the electron microscope. I. Distribution of residual mineral content in vertebrate striated muscle. Aust J Exp Biol Med Sci. 1950;28:549–557. doi: 10.1038/icb.1950.54. [DOI] [PubMed] [Google Scholar]
- Garamvölgyi N, Kerner J, Cser-Schultz M. The cross striation of the insect flight muscle at different sarcomere lengths. Acta Physiol Acad Sci Hung. 1964;24:381–390. [PubMed] [Google Scholar]
- Hanson J, Huxley HE. Structural basis of the cross-striations in muscle. Nature. 1953;172:530–532. doi: 10.1038/172530b0. [DOI] [PubMed] [Google Scholar]
- Herman DS, Lam L, Taylor MR, Wang L, Teekakirikul P, Christodoulou D, Conner L, DePalma SR, McDonough B, Sparks E, Teodorescu DL, Cirino AL, Banner NR, Pennell DJ, Graw S, Merlo M, Di Lenarda A, Sinagra G, Bos JM, Ackerman MJ, Mitchell RN, Murry CE, Lakdawala NK, Ho CY, Barton PJ, Cook SA, Mestroni L, Seidman JG, Seidman CE. Truncations of titin causing dilated cardiomyopathy. N Engl J Med. 2012;366:619–628. doi: 10.1056/NEJMoa1110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge AJ, Huxley HE, Spiro D (1954) Electron microscope studies on ultrathin sections of muscle. J Exp Med 99(2):201–206. doi:10.1084/jem.99.2.201 [DOI] [PMC free article] [PubMed]
- Horowits R, Podolsky RJ. The positional stability of thick filaments in activated skeletal muscle depends on sarcomere length: evidence for the role of titin filaments. J Cell Biol. 1987;105:2217–2223. doi: 10.1083/jcb.105.5.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley HE. In: The cell. Brachet J, Miskey A, editors. New York: Academic Press; 1960. pp. 364–379. [Google Scholar]
- Huxley HE. In: Muscle as a tissue. Rhodahl K, Horvarth SM, editors. New York: McGraw-Hill; 1962. pp. 153–165. [Google Scholar]
- Huxley AF. Reflections on muscle. The Sherrington lectures XIV. Liverpool: Liverpool University Press; 1980. [Google Scholar]
- Huxley H, Hanson J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature. 1954;172:973–996. doi: 10.1038/173973a0. [DOI] [PubMed] [Google Scholar]
- Huxley AF, Niedergerke R. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature. 1954;172:971–973. doi: 10.1038/173971a0. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linke WA. Sense and stretchability: the role of titin and titin-associated proteins in myocardial stress-sensing and mechanical dysfunction. Cardiovasc Res. 2008;77:637–648. doi: 10.1016/j.cardiores.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mártonfalvi Z, Bianco P, Linari M, Caremani M, Nagy A, Lombardi V, Kellermayer M. Low-force transitions in single titin molecules reflect a memory of contractile history. J Cell Sci. 2014;127:858–870. doi: 10.1242/jcs.138461. [DOI] [PubMed] [Google Scholar]
- Maruyama K. Connectin, an elastic protein from myofibrils. J Biochem. 1976;80:405–407. doi: 10.1093/oxfordjournals.jbchem.a131291. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Natori R, Nonomura Y. New elastic protein from muscle. Nature. 1976;262:58–60. doi: 10.1038/262058a0. [DOI] [PubMed] [Google Scholar]
- Pringle JWS. The contractile mechanism of insect fibrillar muscle. Prog Biophys Mol Biol. 1967;17:1–12. doi: 10.1016/0079-6107(67)90003-X. [DOI] [PubMed] [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöstrand FS. The connections between A- and I-band filaments in striated frog muscle. J Ultrastruct Res. 1962;7:225–246. doi: 10.1016/S0022-5320(62)90020-5. [DOI] [PubMed] [Google Scholar]
- Wang K, McClure J, Tu AN. Titin: major myofibrillar components of striated muscle. Proc Natl Acad Sci U S A. 1979;76:3698–3702. doi: 10.1073/pnas.76.8.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P, Ehler E, Gautel M. Obscurin, a giant sarcomeric Rho guanine nucleotide exchange factor protein involved in sarcomere assembly. J Cell Biol. 2001;154:123–136. doi: 10.1083/jcb.200102110. [DOI] [PMC free article] [PubMed] [Google Scholar]